Triboelectric Nanogenerator for Droplet Energy Harvesting Based on Hydrophobic Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of PVDF/PDMS/PTFE Hydrophobic Film
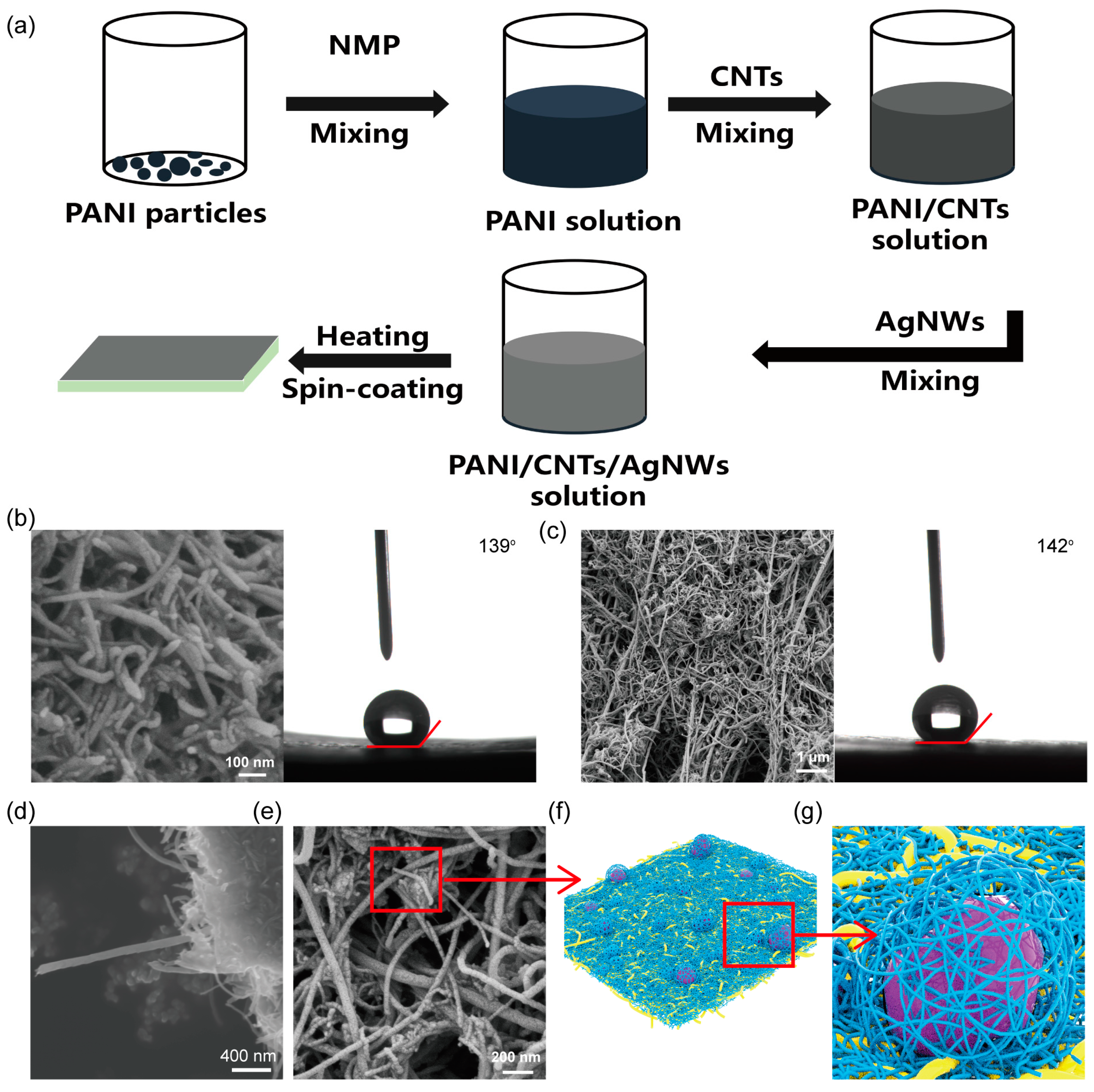
2.2. Fabrication of Hydrophobic PANI/CNTs/AgNWs Electrode
2.3. Measurements and Characterizations
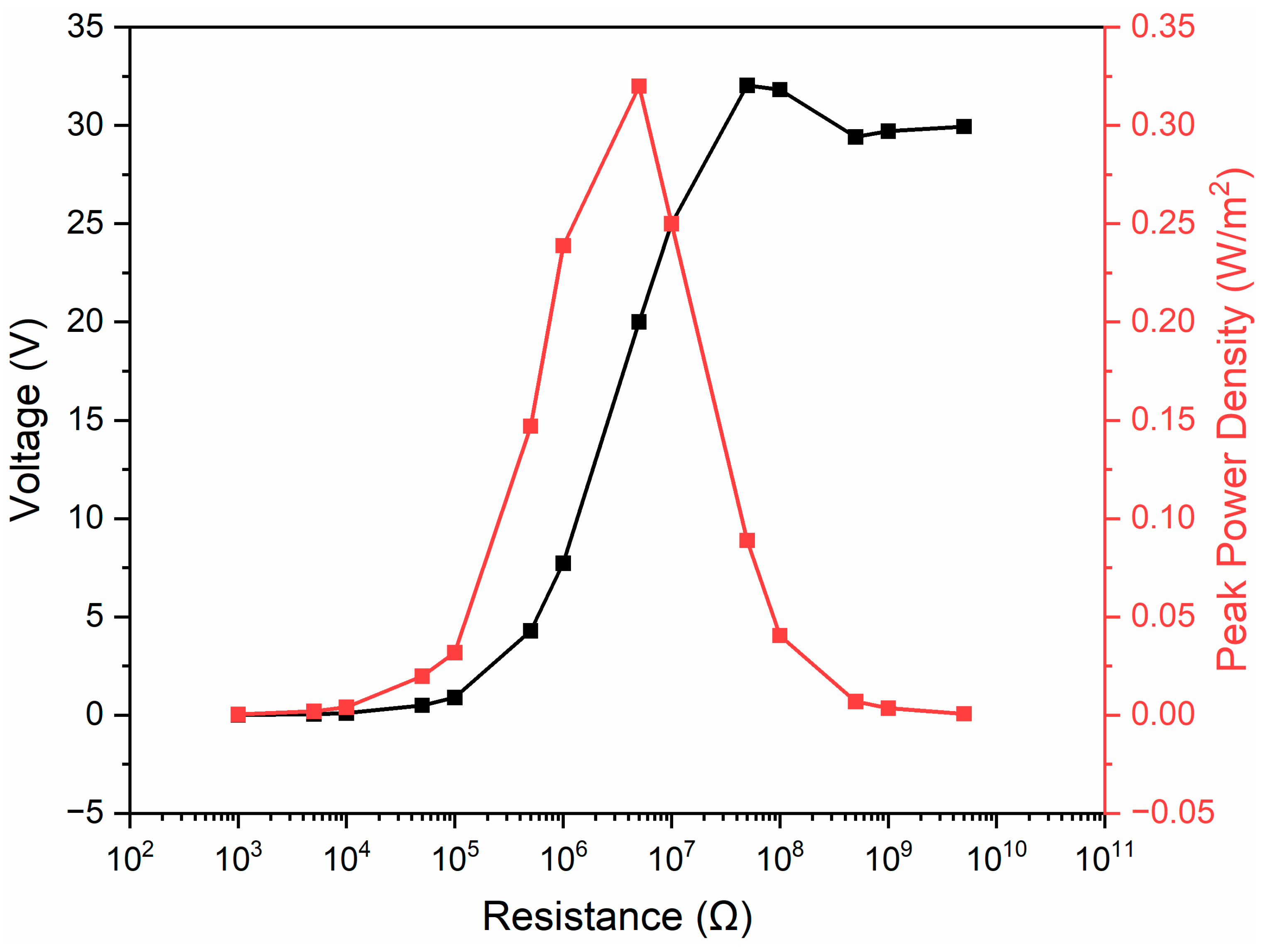
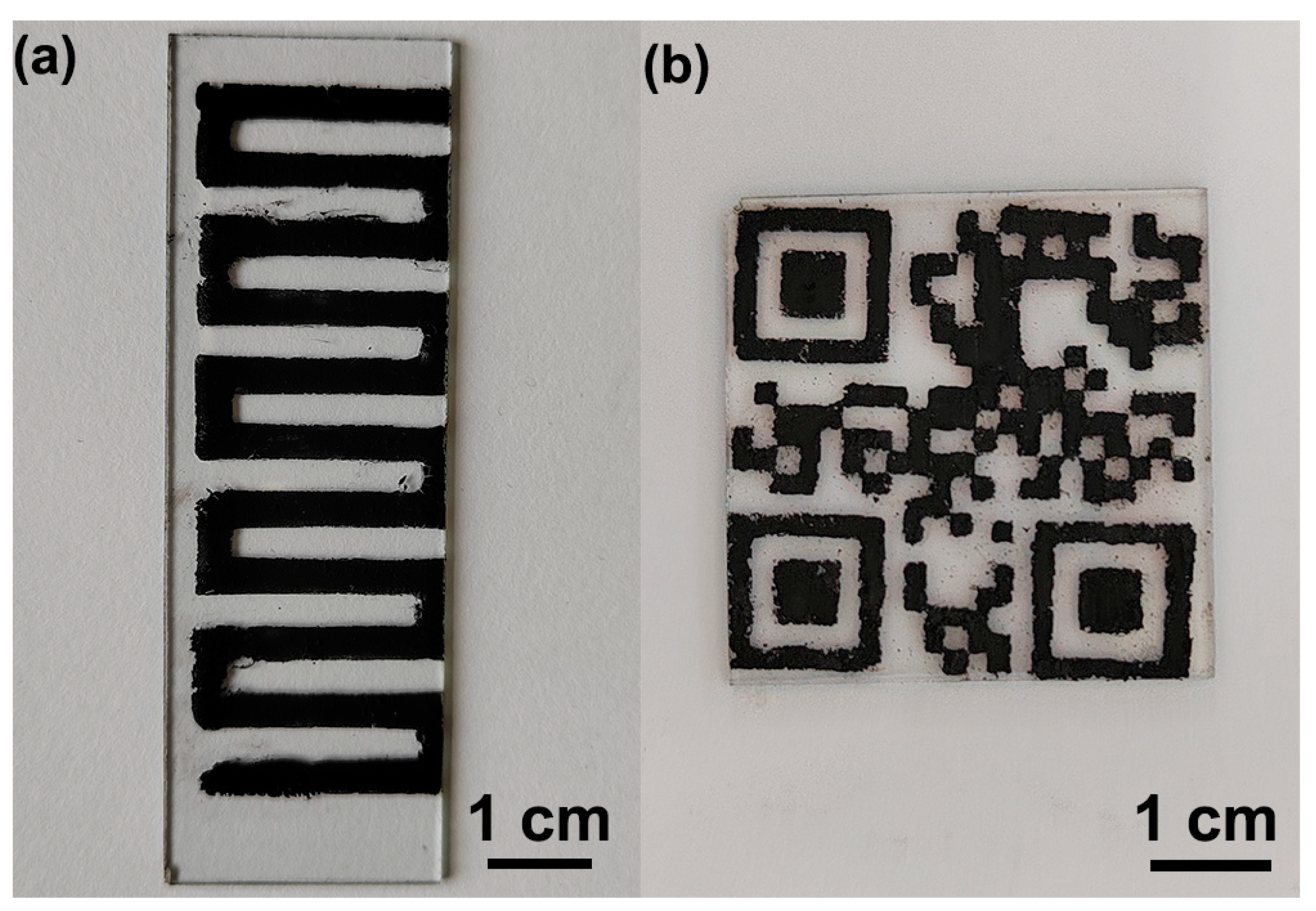
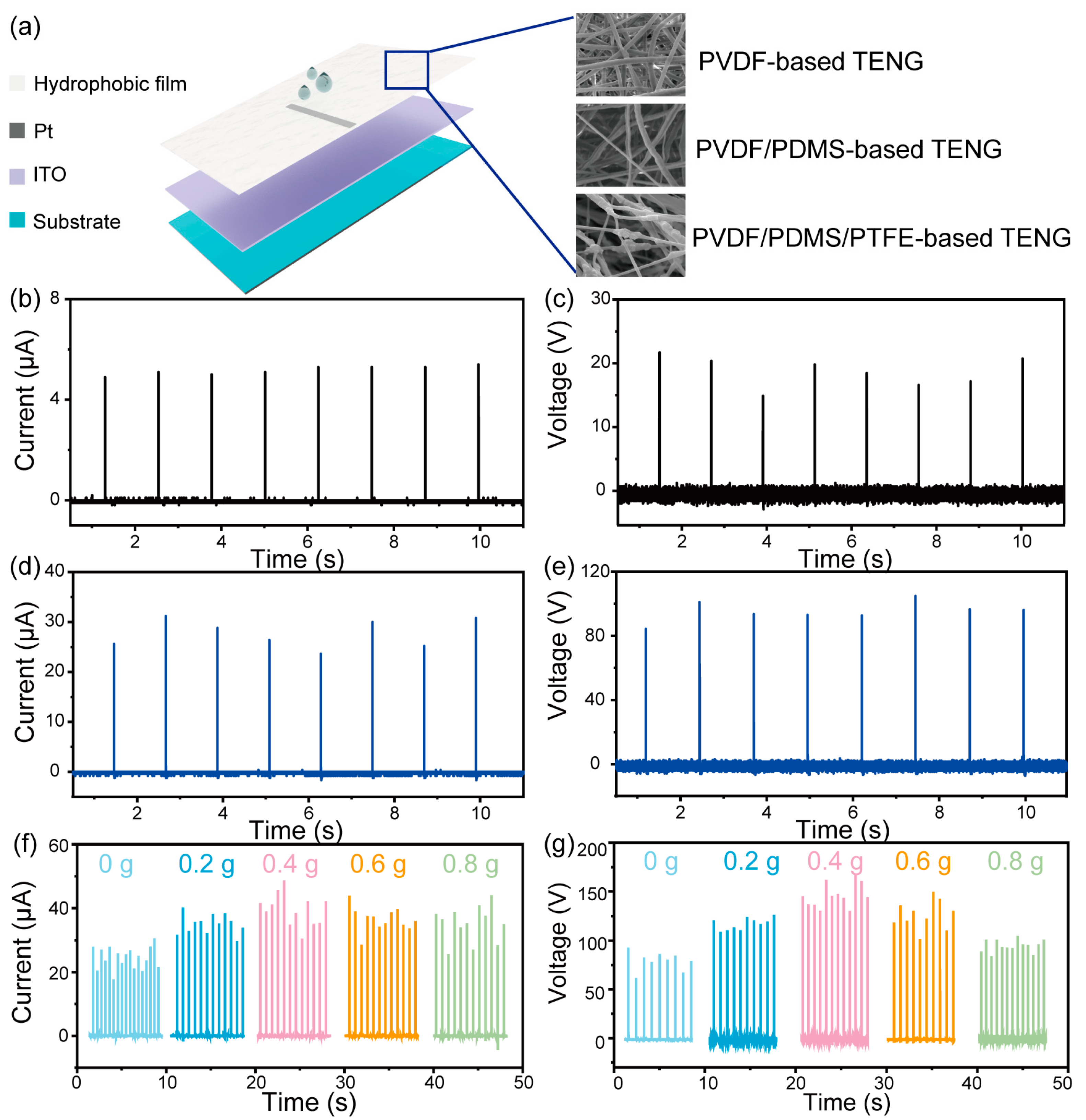
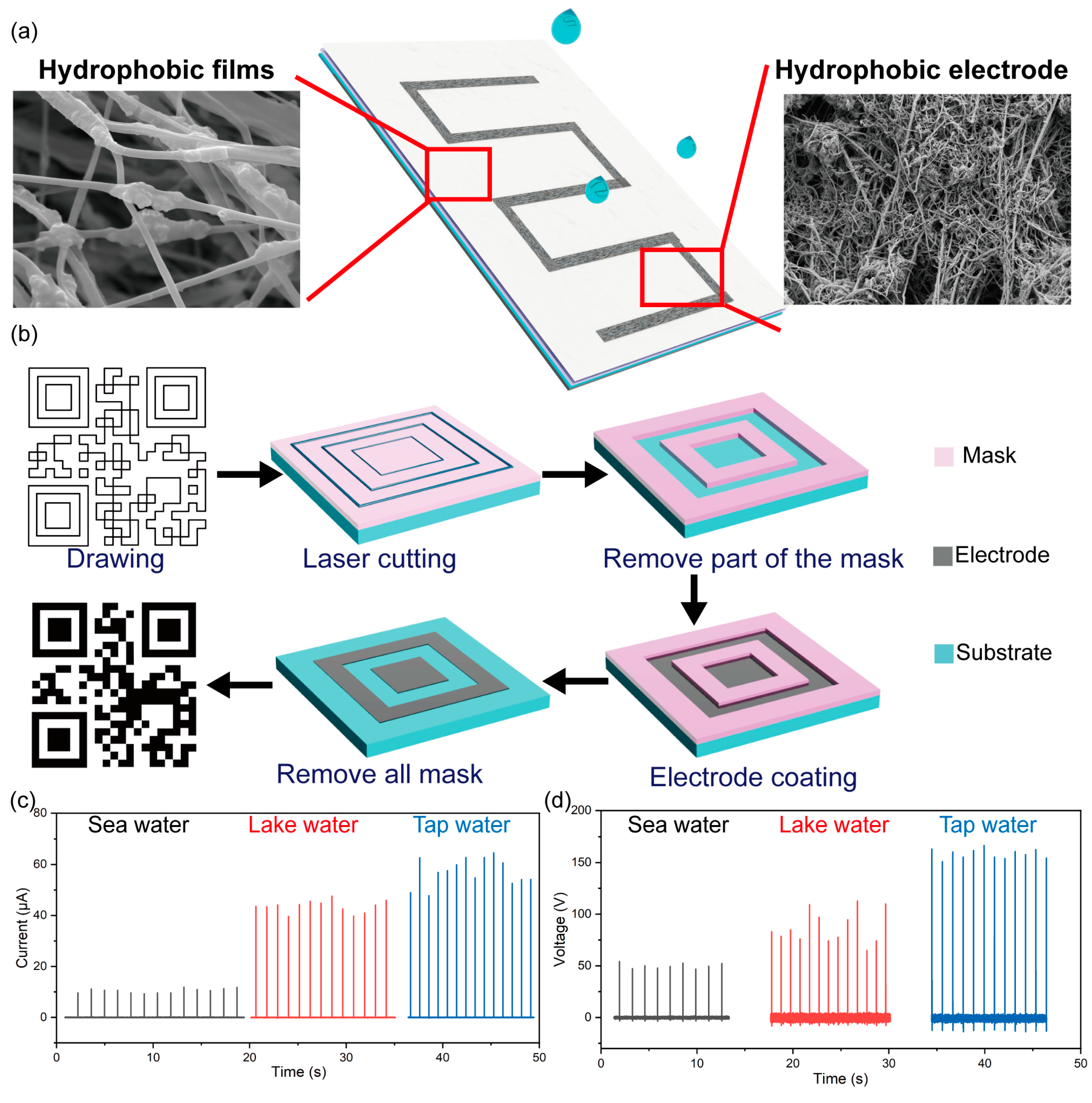
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible Triboelectric Generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Liu, R.; Wen, X.; Hou, T.-C.; Wang, Z.L. Fully Enclosed Triboelectric Nanogenerators for Applications in Water and Harsh Environments. Adv. Energy Mater. 2013, 3, 1563–1568. [Google Scholar] [CrossRef]
- Rodrigues, C.; Nunes, D.; Clemente, D.; Mathias, N.; Correia, J.M.; Rosa-Santos, P.; Taveira-Pinto, F.; Morais, T.; Pereira, A.; Ventura, J. Emerging Triboelectric Nanogenerators for Ocean Wave Energy Harvesting: State of the Art and Future Perspectives. Energy Environ. Sci. 2020, 13, 2657–2683. [Google Scholar] [CrossRef]
- Guo, X.; He, J.; Zheng, Y.; Wu, J.; Pan, C.; Zi, Y.; Cui, H.; Li, X. High-Performance Triboelectric Nanogenerator Based on Theoretical Analysis and Ferroelectric Nanocomposites and Its High-Voltage Applications. Nano Res. Energy 2023, 2, e9120074. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Zeng, Q.; Zhang, Y.; Guo, H.; Zhang, X.; He, W.; Luo, Y.; Wang, X.; Wang, Z.L. A Mutual Boosting Self-Excitation Hybrid Cell for Harvesting High Entropy Energy at 32% Efficiency. Small 2022, 18, 2205704. [Google Scholar] [CrossRef]
- Li, X.; Tao, J.; Wang, X.; Zhu, J.; Pan, C.; Wang, Z.L. Networks of High Performance Triboelectric Nanogenerators Based on Liquid–Solid Interface Contact Electrification for Harvesting Low-Frequency Blue Energy. Adv. Energy Mater. 2018, 8, 1800705. [Google Scholar] [CrossRef]
- Zhai, N.; Wen, Z.; Chen, X.; Wei, A.; Sha, M.; Fu, J.; Liu, Y.; Zhong, J.; Sun, X. Blue Energy Collection toward All-Hours Self-Powered Chemical Energy Conversion. Adv. Energy Mater. 2020, 10, 2001041. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, L.; Yu, Y.; Wang, D.; Sun, W.; Liu, Y.; Yu, J.; Zhang, J.; Wang, K.; Hu, H.; et al. Liquid-Liquid Triboelectric Nanogenerator Based on the Immiscible Interface of an Aqueous Two-Phase System. Nat. Commun. 2022, 13, 5316. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, A.; Zhang, X.; Luo, Y.; Tan, L.; Wang, X. A Dual-Functional Triboelectric Nanogenerator Based on the Comprehensive Integration and Synergetic Utilization of Triboelectrification, Electrostatic Induction, and Electrostatic Discharge to Achieve Alternating Current/Direct Current Convertible Outputs. Adv. Mater. 2023, 35, 2208139. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Zhou, G.; Qin, F.; Jing, M.; Li, L.; Song, W.; Sun, Z. A High-Efficiency Bioinspired Photoelectric-Electromechanical Integrated Nanogenerator. Nat. Commun. 2020, 11, 6158. [Google Scholar] [CrossRef]
- Li, C.; Guo, H.; Wu, Z.; Wang, P.; Zhang, D.; Sun, Y. Self-Healable Triboelectric Nanogenerators: Marriage between Self-Healing Polymer Chemistry and Triboelectric Devices. Adv. Funct. Mater. 2023, 33, 2208372. [Google Scholar] [CrossRef]
- Lei, D.; Wu, J.; Zi, Y.; Pan, C.; Cui, H.; Li, X. Self-Powered Sterilization System for Wearable Devices Based on Biocompatible Materials and Triboelectric Nanogenerator. ACS Appl. Electron. Mater. 2023, 5, 2819–2828. [Google Scholar] [CrossRef]
- Wang, W.; Sun, W.; Du, Y.; Zhao, W.; Liu, L.; Sun, Y.; Kong, D.; Xiang, H.; Wang, X.; Li, Z.; et al. Triboelectric Nanogenerators-Based Therapeutic Electrical Stimulation on Skin: From Fundamentals to Advanced Applications. ACS Nano 2023, 17, 9793–9825. [Google Scholar] [CrossRef]
- Pang, Y.; Fang, Y.; Su, J.; Wang, H.; Tan, Y.; Cao, C. Soft Ball-Based Triboelectric–Electromagnetic Hybrid Nanogenerators for Wave Energy Harvesting. Adv. Mater. Technol. 2023, 8, 2201246. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Yan, H.; Jiang, H.; Luo, J.; Zhang, C.; Pang, Y.; Tan, Y. Biodegradable, Transparent, and Antibacterial Alginate-Based Triboelectric Nanogenerator for Energy Harvesting and Tactile Sensing. Chem. Eng. J. 2023, 468, 143572. [Google Scholar] [CrossRef]
- Pang, Y.; Huang, Z.; Fang, Y.; Xu, X.; Cao, C. Toward Self-Powered Integrated Smart Packaging System—Desiccant-Based Triboelectric Nanogenerators. Nano Energy 2023, 114, 108659. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.-X.; Xie, G.; Ma, J.; Lv, T.; Du, K.; Hu, H.; Zhang, J.; Li, Y.; Long, Y.-Z.; et al. Preparation and Piezoelectric Catalytic Performance of Flexible Inorganic Ba1−xCaxTiO3 via Electrospinning. J. Mater. Chem. A 2021, 9, 24695–24703. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, C. Quantifying Electron-Transfer in Liquid-Solid Contact Electrification. Sci. Bull. 2020, 65, 868–869. [Google Scholar] [CrossRef]
- Zhu, G.; Su, Y.; Bai, P.; Chen, J.; Jing, Q.; Yang, W.; Wang, Z.L. Harvesting Water Wave Energy by Asymmetric Screening of Electrostatic Charges on a Nanostructured Hydrophobic Thin-Film Surface. ACS Nano 2014, 8, 6031–6037. [Google Scholar] [CrossRef]
- Li, X.; Tao, J.; Guo, W.; Zhang, X.; Luo, J.; Chen, M.; Zhu, J.; Pan, C. A Self-Powered System Based on Triboelectric Nanogenerators and Supercapacitors for Metal Corrosion Prevention. J. Mater. Chem. A 2015, 3, 22663–22668. [Google Scholar] [CrossRef]
- Yang, G.; Wu, H.; Li, Y.; Wang, D.; Song, Y.; Zhou, Y.; Hao, J.; Zi, Y.; Wang, Z.; Zhou, G. Direct Ink Writing of Fluoropolymer/CNT-Based Superhydrophobic and Corrosion-Resistant Electrodes for Droplet Energy Harvesters and Self-Powered Electronic Skins. Nano Energy 2021, 86, 106095. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Jia, T.; Wu, Q.; Dong, Y.; Wang, D. Triboelectric Nanogenerator with a Seesaw Structure for Harvesting Ocean Energy. Nano Energy 2022, 102, 107622. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, N.; Liu, J.; Wen, Z.; Sun, X.; Lee, S.-T.; Sun, B. Integrating a Silicon Solar Cell with a Triboelectric Nanogenerator via a Mutual Electrode for Harvesting Energy from Sunlight and Raindrops. ACS Nano 2018, 12, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Cheng, G.; Lee, S.; Pradel, K.C.; Wang, Z.L. Harvesting Water Drop Energy by a Sequential Contact-Electrification and Electrostatic-Induction Process. Adv. Mater. 2014, 26, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, S.; Zhang, L.; Wang, L.; Xue, H.; Wang, Z.L. Non-Contact and Liquid–Liquid Interfacing Triboelectric Nanogenerator for Self-Powered Water/Liquid Level Sensing. Nano Energy 2020, 72, 104703. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A Droplet-Based Electricity Generator with High Instantaneous Power Density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Xie, L.; Yin, L.; Liu, Y.; Liu, H.; Lu, B.; Zhao, C.; Khattab, T.A.; Wen, Z.; Sun, X. Interface Engineering for Efficient Raindrop Solar Cell. ACS Nano 2022, 16, 5292–5302. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, S.; Wang, Z.L. Quantifying Contact-Electrification Induced Charge Transfer on a Liquid Droplet after Contacting with a Liquid or Solid. Adv. Mater. 2021, 33, 2102886. [Google Scholar] [CrossRef]
- Sun, M.; Lu, Q.; Wang, Z.L.; Huang, B. Understanding Contact Electrification at Liquid–Solid Interfaces from Surface Electronic Structure. Nat. Commun. 2021, 12, 1752. [Google Scholar] [CrossRef]
- Nie, J.; Ren, Z.; Xu, L.; Lin, S.; Zhan, F.; Chen, X.; Wang, Z.L. Probing Contact-Electrification-Induced Electron and Ion Transfers at a Liquid–Solid Interface. Adv. Mater. 2020, 32, 1905696. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Xu, W.; Li, W.; Jin, Y.; Gao, S.; Yang, S.; Wu, C.; Wang, S.; Wang, Z. Harvesting Energy from High-Frequency Impinging Water Droplets by a Droplet-Based Electricity Generator. EcoMat 2021, 3, e12116. [Google Scholar] [CrossRef]
- Sun, X.; Feng, Y.; Wang, B.; Liu, Y.; Wu, Z.; Yang, D.; Zheng, Y.; Peng, J.; Feng, M.; Wang, D. A New Method for the Electrostatic Manipulation of Droplet Movement by Triboelectric Nanogenerator. Nano Energy 2021, 86, 106115. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, T.; Wu, J.; Xu, T.; Wang, X.; Han, X.; Cui, H.; Xu, X.; Pan, C.; Li, X. Energy Conversion Analysis of Multilayered Triboelectric Nanogenerators for Synergistic Rain and Solar Energy Harvesting. Adv. Mater. 2022, 34, 2202238. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, K.; Cui, P.; Qi, H.; Qin, H.; Gu, G.; Shang, W.; Wang, S.; Cheng, G.; Du, Z. Hybrid Energy Harvester with Bi-Functional Nano-Wrinkled Anti-Reflective PDMS Film for Enhancing Energies Conversion from Sunlight and Raindrops. Nano Energy 2019, 66, 104188. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Li, J.; Xu, T.; Cui, H.; Li, X. Triboelectric Nanogenerator for Droplet Energy Harvesting Based on Hydrophobic Composites. Materials 2023, 16, 5439. https://doi.org/10.3390/ma16155439
Zheng Y, Li J, Xu T, Cui H, Li X. Triboelectric Nanogenerator for Droplet Energy Harvesting Based on Hydrophobic Composites. Materials. 2023; 16(15):5439. https://doi.org/10.3390/ma16155439
Chicago/Turabian StyleZheng, Yang, Jingjing Li, Tiantian Xu, Hongzhi Cui, and Xiaoyi Li. 2023. "Triboelectric Nanogenerator for Droplet Energy Harvesting Based on Hydrophobic Composites" Materials 16, no. 15: 5439. https://doi.org/10.3390/ma16155439
APA StyleZheng, Y., Li, J., Xu, T., Cui, H., & Li, X. (2023). Triboelectric Nanogenerator for Droplet Energy Harvesting Based on Hydrophobic Composites. Materials, 16(15), 5439. https://doi.org/10.3390/ma16155439





