Hydrothermal Synthesis of Heterostructured g-C3N4/Ag–TiO2 Nanocomposites for Enhanced Photocatalytic Degradation of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of g-C3N4 Samples
2.2.2. Preparation of Ag–TiO2 Nanoparticles
2.2.3. Fabrication of g-C3N4/Ag–TiO2 Nanocomposite
2.2.4. Characterization
2.2.5. Photocatalytic Activity Testing
3. Results and Discussion
3.1. XRD Analysis
3.2. Optical Properties Analysis
3.2.1. UV/vis DRS Analysis
3.2.2. PL Analysis
3.2.3. FTIR Analysis
3.3. Morphological Analysis
3.4. Nitrogen Adsorption–Desorption Analysis
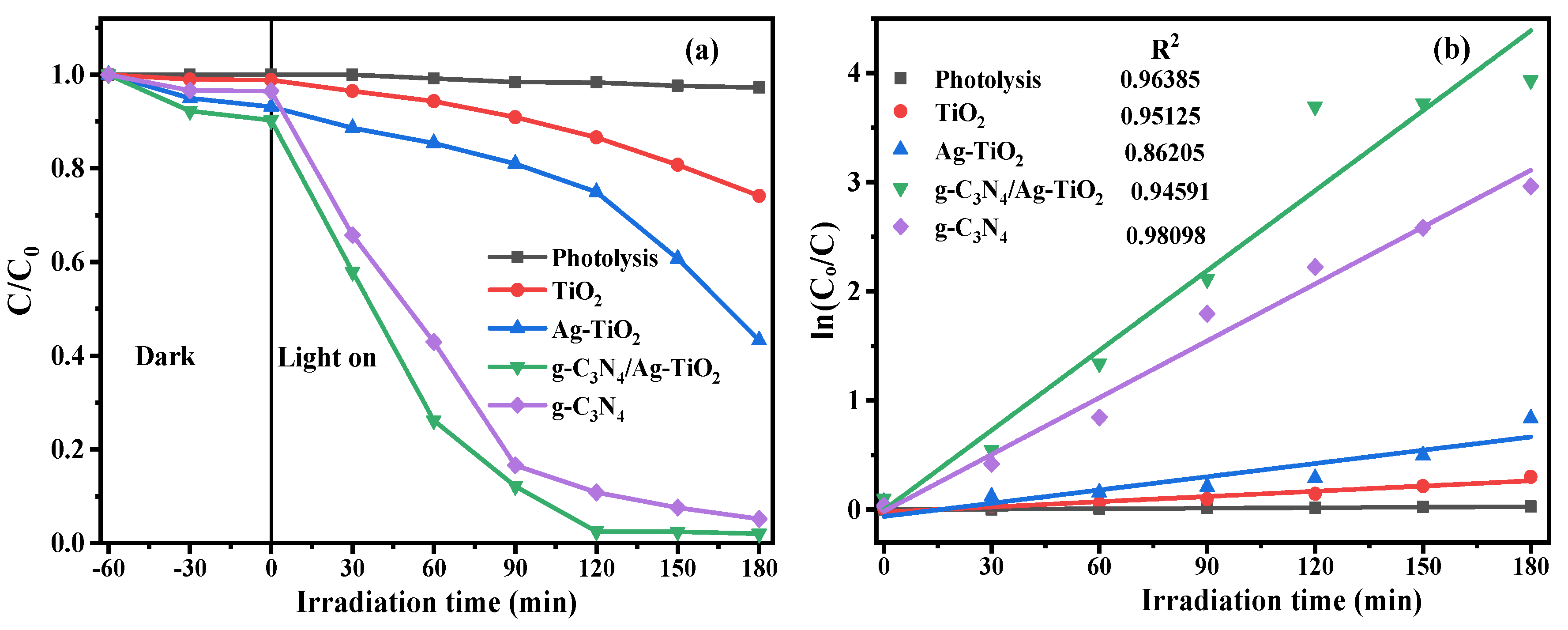
3.5. Evaluation of Photocatalytic Activity
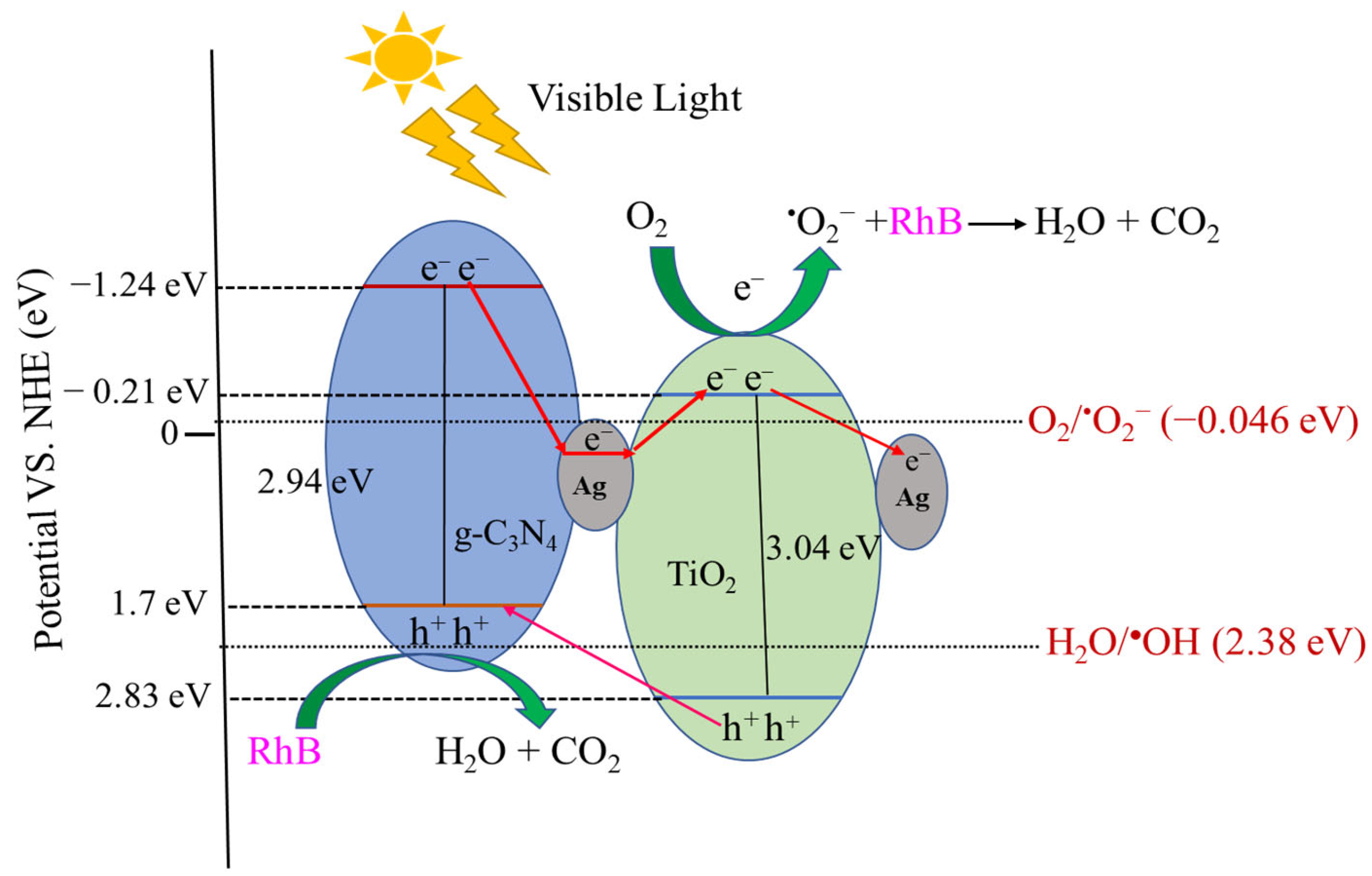
3.6. Mechanism of Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sewnet, A.; Abebe, M.; Asaithambi, P.; Alemayehu, E. Visible-Light-Driven g-C3N4/TiO2 Based Heterojunction Nanocomposites for Photocatalytic Degradation of Organic Dyes in Wastewater: A Review. Air Soil Water Res. 2022, 15. [Google Scholar] [CrossRef]
- Rafaqat, S.; Ali, N.; Torres, C.; Rittmann, B. Recent progress in treatment of dyes wastewater using microbial-electro-Fenton technology. RSC Adv. 2022, 12, 17104–17137. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M.; McKay, G. A Review of the Removal of Dyestuffs from Effluents onto Biochar. Separations 2022, 9, 139. [Google Scholar] [CrossRef]
- Cheng, N.; Tian, J.; Liu, Q.; Ge, C.; Qusti, A.H.; Asiri, A.M.; Al-youbi, A.O.; Sun, X. Au-Nanoparticle-Loaded Graphitic Carbon Nitride Nanosheets: Green Photocatalytic Synthesis and Application toward the Degradation of Organic Pollutants. ACS Appl. Mater. Interfaces 2013, 5, 4–8. [Google Scholar] [CrossRef]
- Nazir, A.; Huo, P.; Wang, H.; Weiqiang, Z.; Wan, Y. A review on plasmonic-based heterojunction photocatalysts for degradation of organic pollutants in wastewater. J. Mater. Sci. 2023, 58, 6474–6515. [Google Scholar] [CrossRef]
- Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Kalarikkal, N.; Lennartz, B. Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation. Nanomaterials 2023, 13, 762. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Yan, D.; Wu, X.; Pei, J.; Wu, C.; Wang, X.; Zhao, H. Construction of g-C3N4/TiO2/Ag composites with enhanced visible-light photocatalytic activity and antibacterial properties. Ceram. Int. 2020, 46, 696–702. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Hwang, S.H.; Jeon, S.; Hwang, B.; Jung, J.Y.; Lee, J.; Park, S.H.; Jeong, J.H. Three-dimensional plasmonic Ag/TiO2 nanocomposite architectures on flexible substrates for visible-light photocatalytic activity. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Rao, Z.; Mahmood, A.; Wang, X.; Wang, Y.; Xie, X.; Sun, J. Improved photocatalytic oxidation performance of gaseous acetaldehyde by ternary g-C3N4/Ag-TiO2 composites under visible light. J. Colloid Interface Sci. 2021, 602, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, J.; Shi, H.; Zhou, M.; Zhang, Y.; Chen, Q.; Zhang, Z. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 86, 103–110. [Google Scholar] [CrossRef]
- Abbad, S.; Guergouri, K.; Gazaout, S.; Djebabra, S.; Zertal, A.; Barille, R.; Zaabat, M. Effect of silver doping on the photocatalytic activity of TiO2 nanopowders synthesized by the sol-gel route. J. Environ. Chem. Eng. 2020, 8, 103718. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, M.; Shi, X.; Qin, X.; Chen, Q.; Jiang, L.; Jia, C. Ag–TiO2 mesocrystal-coupled g-C3N4 nanosheets with enhanced visible-light photocatalytic activity. J. Mater. Sci. 2022, 49, 101–119. [Google Scholar] [CrossRef]
- Liang, H.; Jia, Z.; Zhang, H.; Wang, X.; Wang, J. Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine B. Appl. Surf. Sci. 2017, 422, 1–10. [Google Scholar] [CrossRef]
- Sui, G.; Li, J.; Du, L.; Zhuang, Y.; Zhang, Y.; Zou, Y.; Li, B. Preparation and characterization of g-C3N4/Ag–TiO2 ternary hollowsphere nanoheterojunction catalyst with high visible light photocatalytic performance. J. Alloys Compd. 2020, 823, 153851. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Demirci, S.; Dikici, T.; Yurddaskal, M.; Gultekin, S.; Toparli, M.; Celik, E. Synthesis and characterization of Ag doped TiO2 heterojunction films and their photocatalytic performances. Appl. Surf. Sci. 2016, 390, 591–601. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Zeng, J.; Guo, J.; Wang, H. Enhancing visible-light photocatalytic activity of Ag-TiO2 nanowire composites by one-step hydrothermal process. Mater. Lett. 2020, 279, 128506. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K. TiO2-modified g-C3N4 nanocomposite for photocatalytic degradation of organic dyes in aqueous solution. Heliyon 2022, 8, e10683. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, X.; Li, Q.; Yang, J. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction. Appl. Catal. B Environ. 2017, 212, 106–114. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Zeng, Y.; Liu, F.; Yuan, J.; Li, X.; Yu, Y.; Zhu, X.; Xiong, Z.; Yu, H.; et al. TiO2/Graphitic Carbon Nitride Nanosheets for the Photocatalytic Degradation of Rhodamine B under Simulated Sunlight. ACS Appl. Nano Mater. 2019, 2, 7255–7265. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, B.; Chen, Q.; Shi, H.; Zhang, Y.; Li, D.; Yang, X.; Zhao, W.; Liu, C.; Wei, G.; et al. Improved visible light photocatalytic activity on Z-scheme g-C3N4 decorated TiO2 nanotube arrays by a simple impregnation method. Mater. Res. Bull. 2019, 124, 110757. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Shen, C.; Tang, G.; Zhao, S.; Zhang, J.; Chen, D.; Wei, Q.; Dong, X. Facile Construction of g-C3N4 Nanosheets/TiO2 Nanotube Arrays as Z-Scheme Photocatalyst with Enhanced Visible-Light Performance. ChemCatChem 2016, 8, 3064–3073. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Dong, X.; Zhang, J.; Qin, L. In-situ construction of all-solid-state Z-scheme g-C3N4/TiO2 nanotube arrays photocatalyst with enhanced visible-light-induced properties. Sol. Energy Mater. Sol. Cells 2016, 157, 399–405. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.; Ren, J.; Li, X.; Ma, Y.; Huang, W.; Zhao, X. Enhanced photocatalytic hydrogen evolution over TiO2/g-C3N4 2D heterojunction coupled with plasmon Ag nanoparticles. Ceram. Int. 2020, 46, 5725–5732. [Google Scholar] [CrossRef]
- Qin, H.; Lv, W.; Bai, J.; Zhou, Y.; Wen, Y.; He, Q.; Tang, J.; Wang, L.; Zhou, Q. Sulfur-doped porous graphitic carbon nitride heterojunction hybrids for enhanced photocatalytic H2 evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Sutar, R.S.; Barkul, R.P.; Delekar, S.D.; Patil, M.K. Sunlight assisted photocatalytic degradation of organic pollutants using g-C3N4-TiO2 nanocomposites. Arab. J. Chem. 2020, 13, 4966–4977. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Hu, X.; Hu, J.; Wang, Z.; Yin, Y.; Sun, C.; Zhang, X. Two-dimensional g-C3N4/TiO2 nanocomposites as vertical Z-scheme heterojunction for improved photocatalytic water disinfection. Catal. Today 2018, 335, 243–251. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Raja, V.; Jaffar Ali, B.M. Synergy of photon up-conversion and Z-scheme mechanism in graphitic carbon nitride nanoparticles decorated g-C3N4-TiO2. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125862. [Google Scholar] [CrossRef]
- Bi, X.; Yu, S.; Liu, E.; Liu, L.; Zhang, K.; Zang, J.; Zhao, Y. Construction of g-C3N4/TiO2 nanotube arrays Z-scheme heterojunction to improve visible light catalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125193. [Google Scholar] [CrossRef]
- Liu, R.; Bie, Y.; Qiao, Y.; Liu, T.; Song, Y. Design of g-C3N4/TiO2 nanotubes heterojunction for enhanced organic pollutants degradation in waste water. Mater. Lett. 2019, 251, 126–130. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Chaveanghong, S.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Photocatalytic Activity of TiO2/g-C3N4 Nanocomposites for Removal of Monochlorophenols from Water. Nanomaterials 2022, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.L.; Reis-machado, A.S.; Rodrigues, J.; Deuermeier, J.; Pimentel, A.; Fortunato, E.; Martins, R.; Nunes, D. Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts. Nanomaterials 2023, 13, 1090. [Google Scholar] [CrossRef]
- Zhou, B.; Hong, H.; Zhang, H.; Yu, S.; Tian, H. Heterostructured Ag/g-C3N4/TiO2 with enhanced visible light photocatalytic performances. J. Chem. Technol. Biotechnol. 2019, 94, 3806–3814. [Google Scholar] [CrossRef]
- Hoa, D.T.N.; Tu, N.T.T.; Thinh, H.Q.A.; Van Thanh Son, L.; Son, L.V.T.; Quyen, N.D.V.; Son, L.L.; Tuyen, T.N.; Thong, P.L.M.; Diem, L.H.; et al. TiO2/g-C3N4 Visible-Light-Driven Photocatalyst for Methylene Blue Decomposition. J. Nanomater. 2023, 2023, 9967890. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Wu, X.; Lee, P.H.; Shih, K. Fabrication of Heterostructured g-C3N4/Ag-TiO2 Hybrid Photocatalyst with Enhanced Performance in Photocatalytic Conversion of CO2 under Simulated Sunlight Irradiation. Appl. Surf. Sci. 2017, 402, 198–207. [Google Scholar] [CrossRef]
- Zang, M.; Shi, L.; Liang, L.; Li, D.; Sun, J. Heterostructured g-C3N4/Ag-TiO2 composites with efficient photocatalytic performance under visible-light irradiation. RSC Adv. 2015, 5, 56136–56144. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sattayaporn, S.; Saito, N.; Sujaridworakun, P. Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV–Visible light irradiation. Appl. Surf. Sci. 2022, 573, 151617. [Google Scholar] [CrossRef]
- Tangwongputti, C.; Reubroycharoen, P.; Sujaridworakun, P. Facile synthesis of heterostructured g-C3N4/Ag-TiO2 photocatalysts with enhanced visible-light photocatalytic performance. J. Met. Mater. Miner. 2022, 32, 48–54. [Google Scholar] [CrossRef]
- Lang, J.; Takahashi, K.; Kubo, M. Preparation of TiO2-CNT-Ag Ternary Composite Film with Enhanced Photocatalytic Activity via Plasma-Enhanced Chemical Vapor Deposition. Catalysts 2022, 12, 508. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. [Google Scholar] [CrossRef]
- Sabir, A.; Sherazi, T.A.; Xu, Q. Porous polymer supported Ag-TiO2 as green photocatalyst for degradation of methyl orange. Surf. Interfaces 2021, 26, 101318. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Li, Z.; Wu, X.; Hu, M.; Yan, X.; Zhu, Q. Mesoporous black TiO2-x/Ag nanospheres coupled with g-C3N4 nanosheets as 3D/2D ternary heterojunctions visible light photocatalysts. J. Hazard. Mater. 2018, 343, 181–190. [Google Scholar] [CrossRef]
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Ni, S.; Liu, J.; Xie, S.; Liu, Y. Construction of g-C3N4/Ag/TiO2 Z-scheme photocatalyst and Its improved photocatalytic U(VI) reduction application in water. Water Sci. Technol. 2022, 85, 2639–2651. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Ma, X.; Chen, Q.; Liu, G.; Zhou, Y.; Ma, D.; Cui, C.; Ma, J.; Xin, Y. In situ synthesis of ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst with enhanced photocatalytic activity. Sep. Purif. Technol. 2020, 247, 116932. [Google Scholar] [CrossRef]
- Hu, F.; Sun, S.; Xu, H.; Li, M.; Hao, X.; Shao, G.; Wang, H.; Chen, D.; Lu, H.; Zhang, R. Investigation on g-C3N4/rGO/TiO2 nanocomposite with enhanced photocatalytic degradation performance. J. Phys. Chem. Solids 2021, 156, 110181. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of Tri-S-Triazine Based g-C3N4 Photocatalyst for Cationic Rhodamine B Degradation under Visible Light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible-light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Ren, Y.; Xing, S.; Wang, J.; Liang, Y.; Zhao, D.; Wang, H.; Wang, N.; Jiang, W.; Wu, S.; Liu, S.; et al. Weak-light-driven Ag–TiO2 photocatalyst and bactericide prepared by coprecipitation with effective Ag doping and deposition. Opt. Mater. 2022, 124, 111993. [Google Scholar] [CrossRef]
- Bahadur, J.; Agrawal, S.; Parveen, A.; Jawad, A.; Ashraf, S.S.Z.; Ghalib, R.M. Micro-Structural, Optical and Dielectric Properties of Ag Doped TiO2 Synthesized by Sol–Gel Method. Mater. Focus 2015, 4, 134–141. [Google Scholar] [CrossRef]
- Abdulkadhim, W.K. Synthesis titanium dioxide nanoparticles doped with silver and Novel antibacterial activity. J. Phys. Conf. Ser. 2021, 1999, 012033. [Google Scholar] [CrossRef]
- Santos, L.M.; Machado, W.A.; França, M.D.; Borges, K.A.; Paniago, R.M.; Patrocinio, A.O.T.; Machado, A.E.H. Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity. RSC Adv. 2015, 5, 103752–103759. [Google Scholar] [CrossRef]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of Heterostructured g-C3N4/Ag/TiO2 Microspheres with Enhanced Photocatalysis Performance under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Alothman, A.A.; Ayub, A.; Hachim, S.K.; Mohammed, B.M.; Hussain, F.; Altaf, M.; Kadhim, Z.J.; Lafta, H.A.; Alnassar, Y.S.; Shams, M.A.; et al. Facile synthesis and comparative study of the enhanced photocatalytic degradation of two selected dyes by TiO2-g-C3N4 composite. Environ. Sci. Pollut. Res. 2022, 30, 37332–37343. [Google Scholar] [CrossRef] [PubMed]
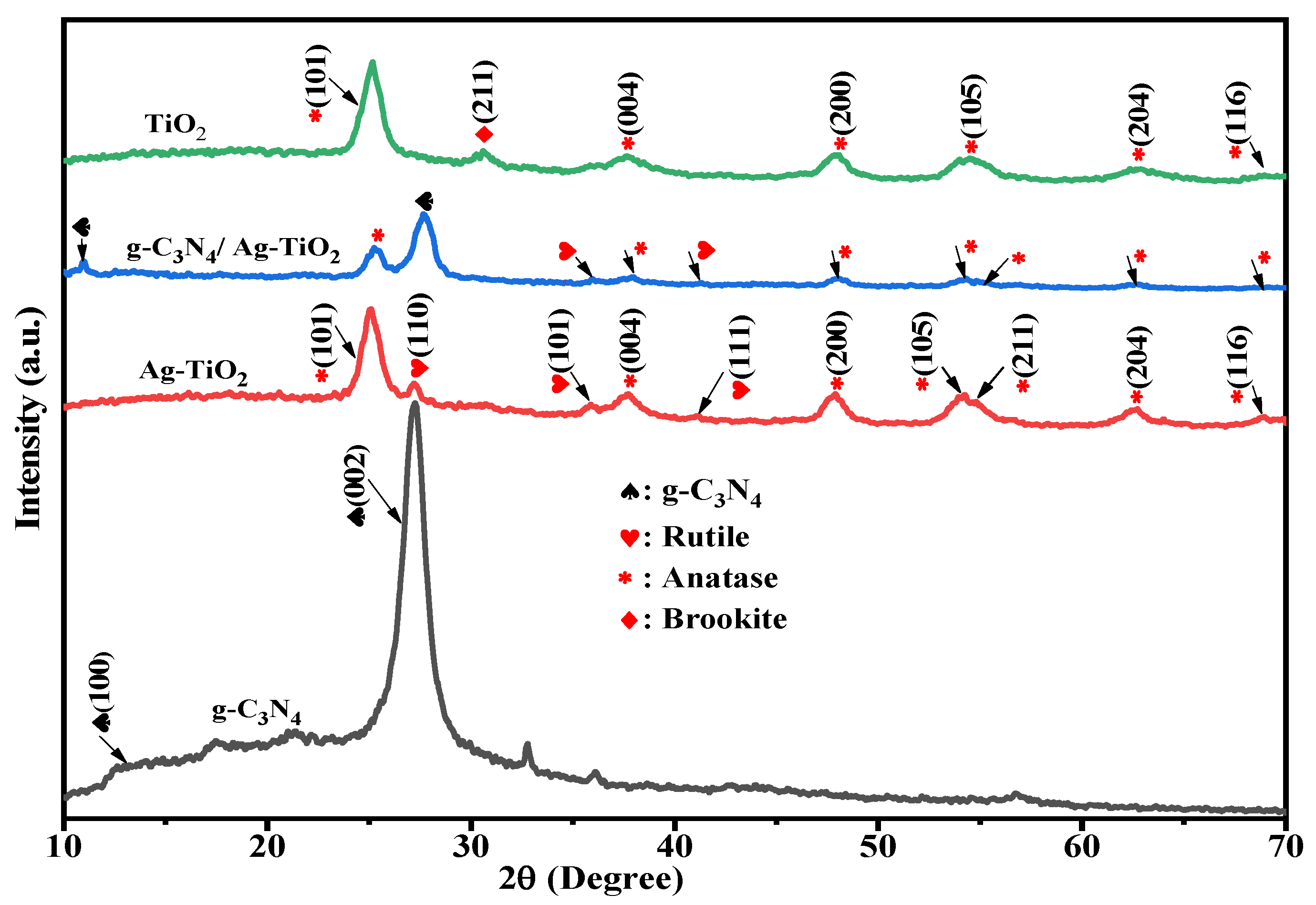
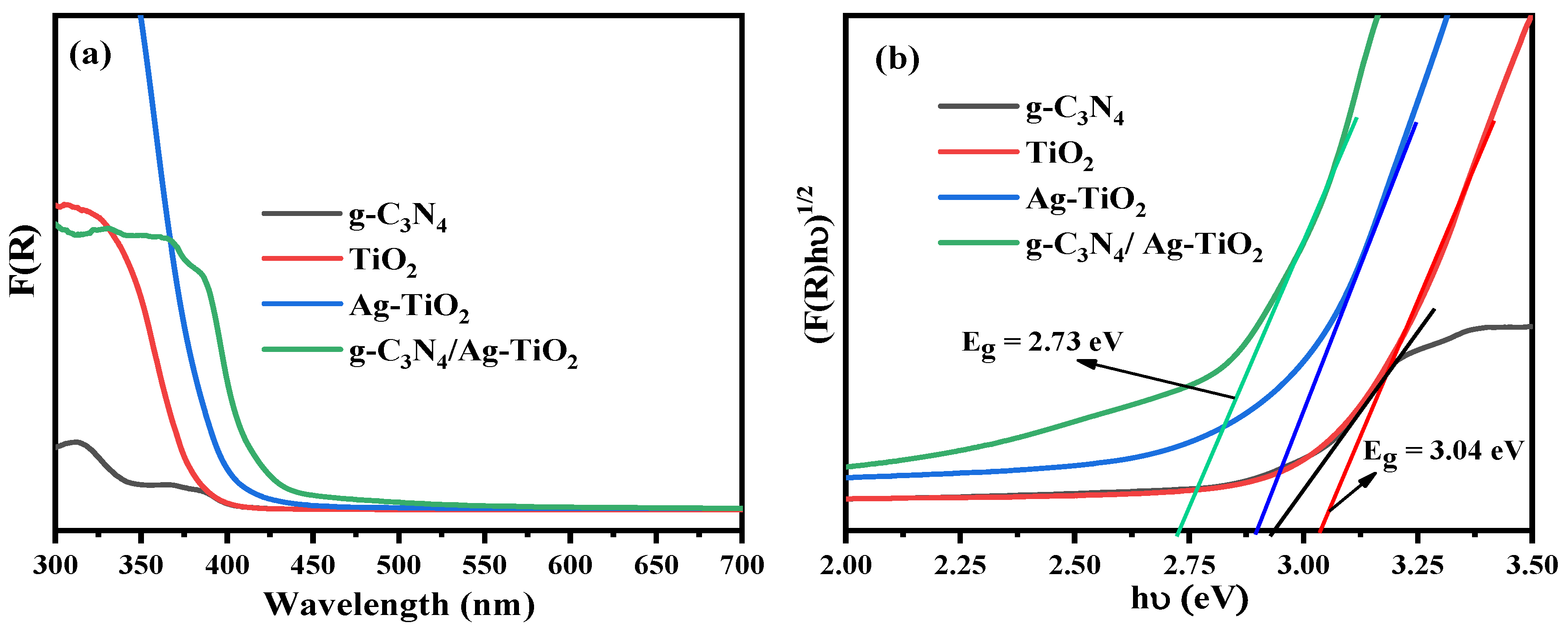
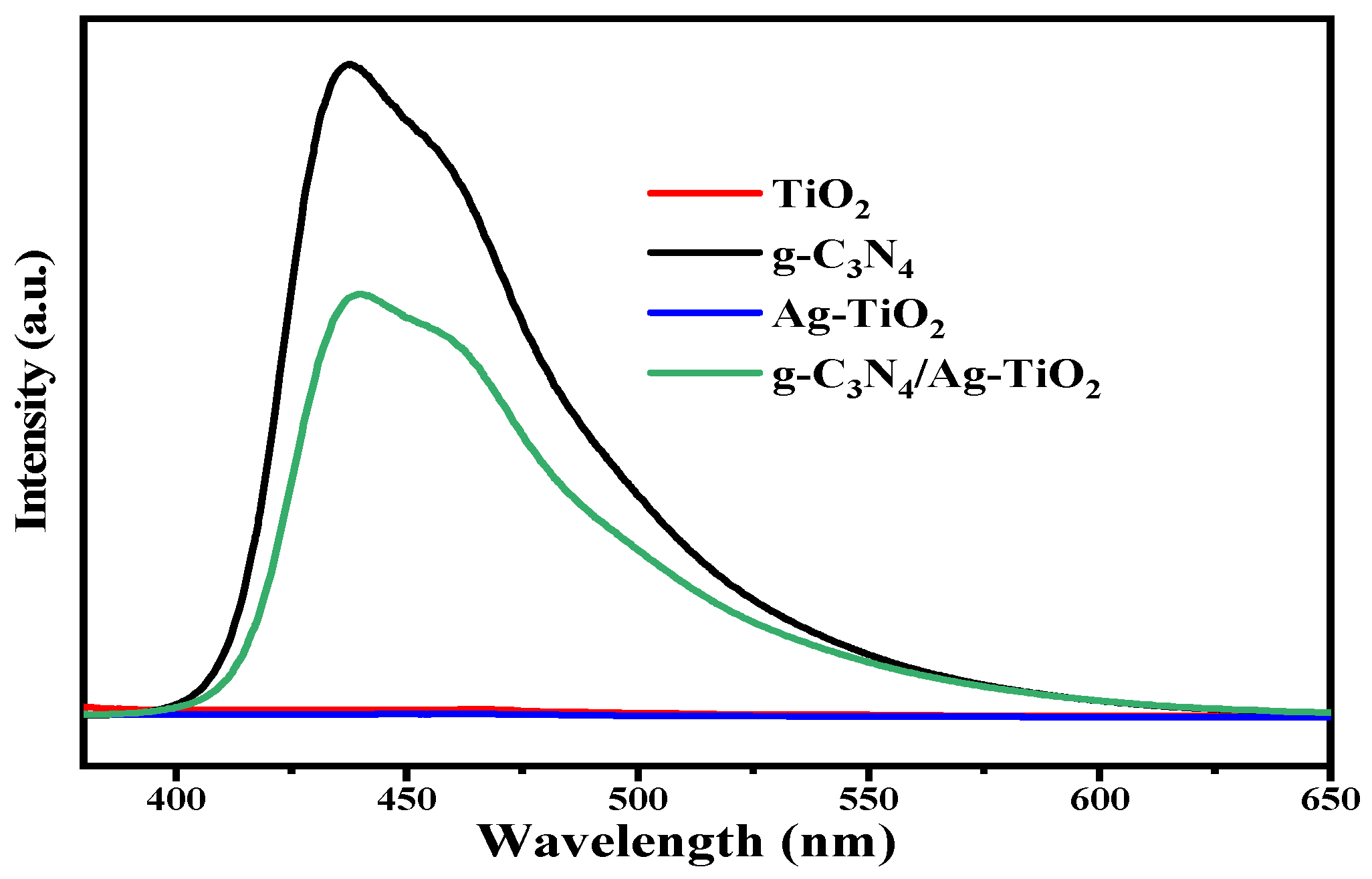
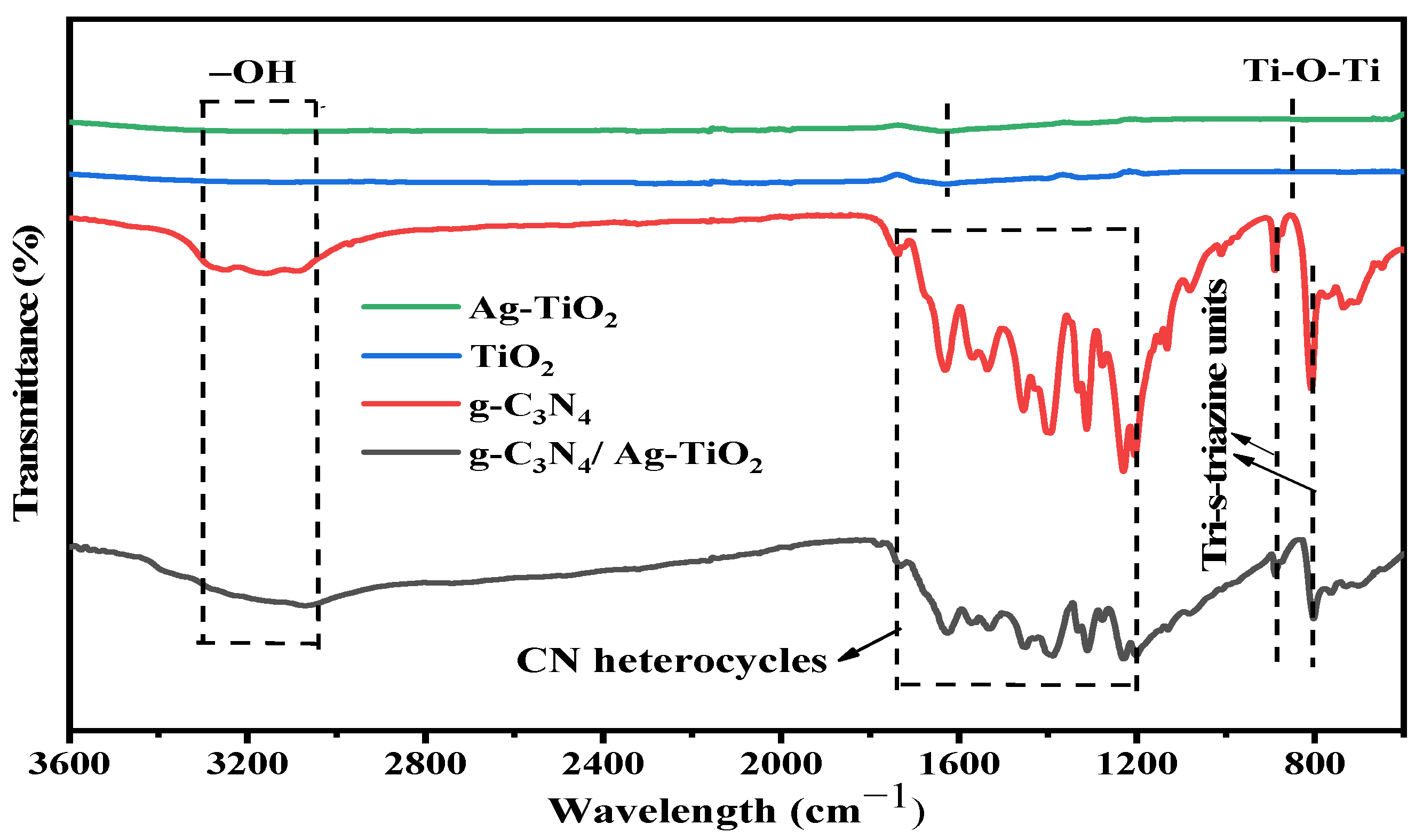

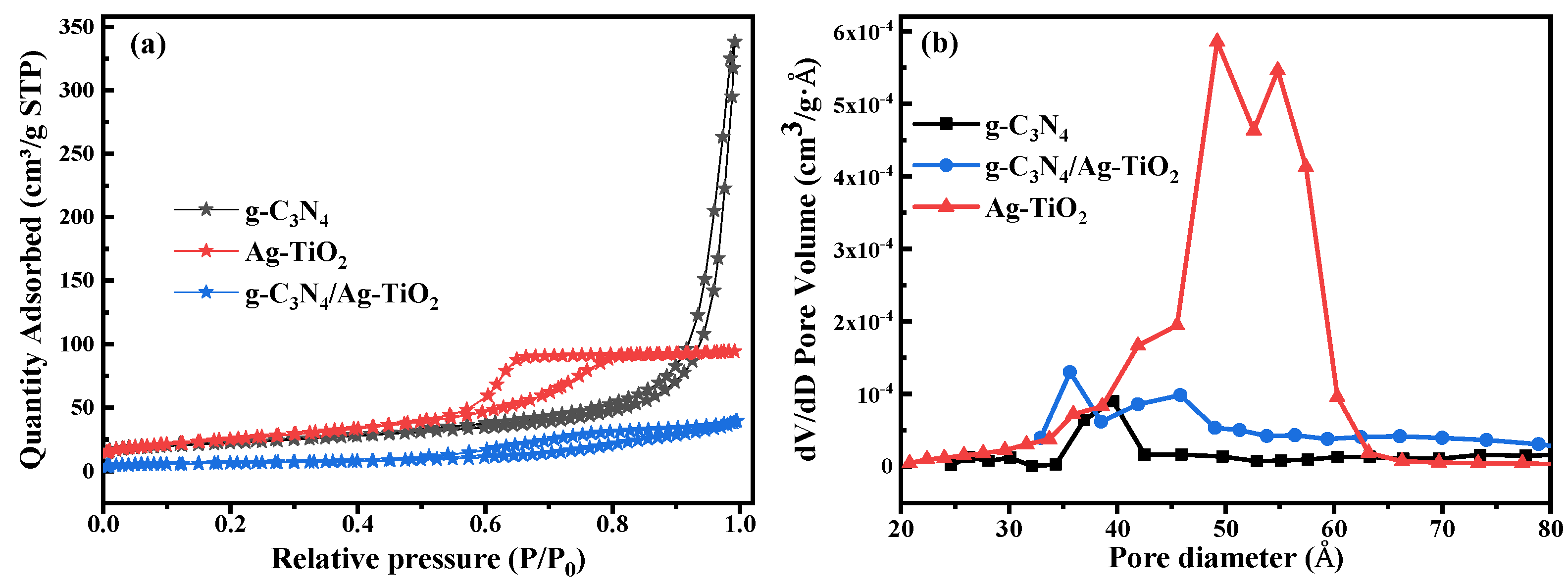
| Photocatalysts | Crystallite Size (nm) | Eg (eV) | SBET (m2·g−1) | Pore Size (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|---|---|
| Ag–TiO2 | 9.1 | 2.89 | 90.8 | 6.5 | 0.15 |
| g-C3N4 | 4.3 | 2.94 | 79.5 | 25.3 | 0.52 |
| g-C3N4/Ag–TiO2 | 7.6 | 2.73 | 22.5 | 10.5 | 0.06 |
| Photocatalyst | Catalyst Dosage | Pollutant Concentration | Light Source (λ ≥ 420 nm) | Irradiation Time | Degradation Efficiency | Refs. |
|---|---|---|---|---|---|---|
| g-C3N4/Ag–TiO2 | 50 mg | RhB, 10 mg/L | 300 W Xe lamp | 30 min | 98.13% | [42] |
| g-C3N4/Ag–TMCs | 20 mg | RhB, 20 mg/L | 300 W Xe lamp | 15 min | 100% | [15] |
| g-C3N4/Ag–TiO2 | 50 mg | RhB, 5 mg/L | 500 W Xe lamp | 105 min | 92.7% | [17] |
| g-C3N4/Ag–TiO2 | 50 mg | RhB,10 mg/L | 300 W Xe lamp | 60 min | 100% | [43] |
| g-C3N4/Ag–TiO2 | 50 mg | RhB, 5 mg/L | 300 W Xe lamp | 30 min | 96% | [41] |
| Ag/g-C3N4/TiO2 | 20 mg | RhB, 10 mg/L | 300 W Xe lamp | 120 min | 100% | [38] |
| g-C3N4/Ag–TiO2 | 50 mg | RhB, 10 mg/L | 50 W LED lamp | 180 min | 98.04% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Lennartz, B. Hydrothermal Synthesis of Heterostructured g-C3N4/Ag–TiO2 Nanocomposites for Enhanced Photocatalytic Degradation of Organic Pollutants. Materials 2023, 16, 5497. https://doi.org/10.3390/ma16155497
Sewnet A, Alemayehu E, Abebe M, Mani D, Thomas S, Lennartz B. Hydrothermal Synthesis of Heterostructured g-C3N4/Ag–TiO2 Nanocomposites for Enhanced Photocatalytic Degradation of Organic Pollutants. Materials. 2023; 16(15):5497. https://doi.org/10.3390/ma16155497
Chicago/Turabian StyleSewnet, Agidew, Esayas Alemayehu, Mulualem Abebe, Dhakshnamoorthy Mani, Sabu Thomas, and Bernd Lennartz. 2023. "Hydrothermal Synthesis of Heterostructured g-C3N4/Ag–TiO2 Nanocomposites for Enhanced Photocatalytic Degradation of Organic Pollutants" Materials 16, no. 15: 5497. https://doi.org/10.3390/ma16155497
APA StyleSewnet, A., Alemayehu, E., Abebe, M., Mani, D., Thomas, S., & Lennartz, B. (2023). Hydrothermal Synthesis of Heterostructured g-C3N4/Ag–TiO2 Nanocomposites for Enhanced Photocatalytic Degradation of Organic Pollutants. Materials, 16(15), 5497. https://doi.org/10.3390/ma16155497


_Low.png)





