Atomic Layer Deposition of Titanium Oxide-Based Films for Semiconductor Applications—Effects of Precursor and Operating Conditions
Abstract
:1. Introduction
2. Experimental Materials and Procedures
2.1. Material Preparation
2.2. ALD Deposition
2.3. Characterisation
3. Results and Discussion
3.1. TiOx Films
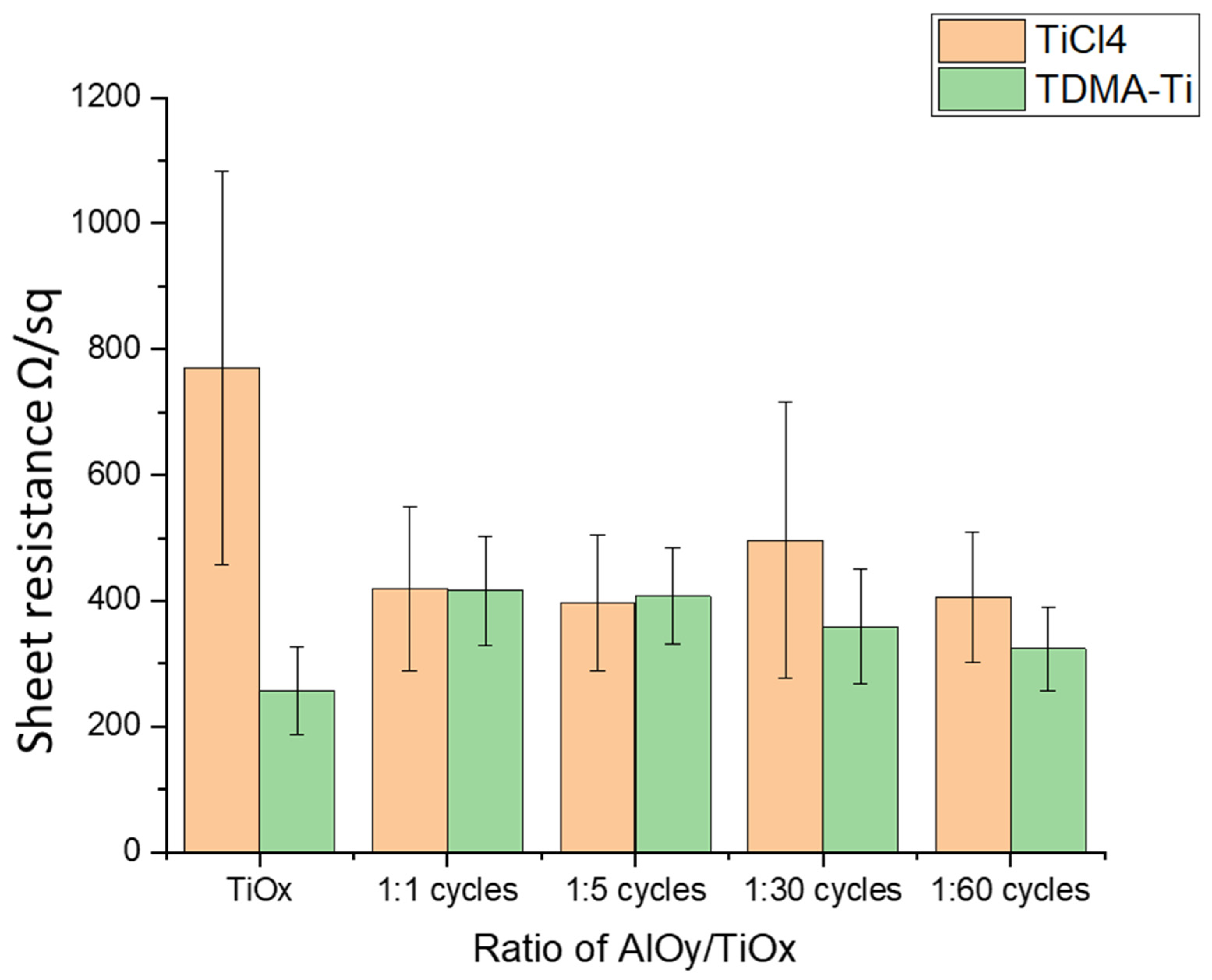
3.2. Al2O3/TiOx Stack Films
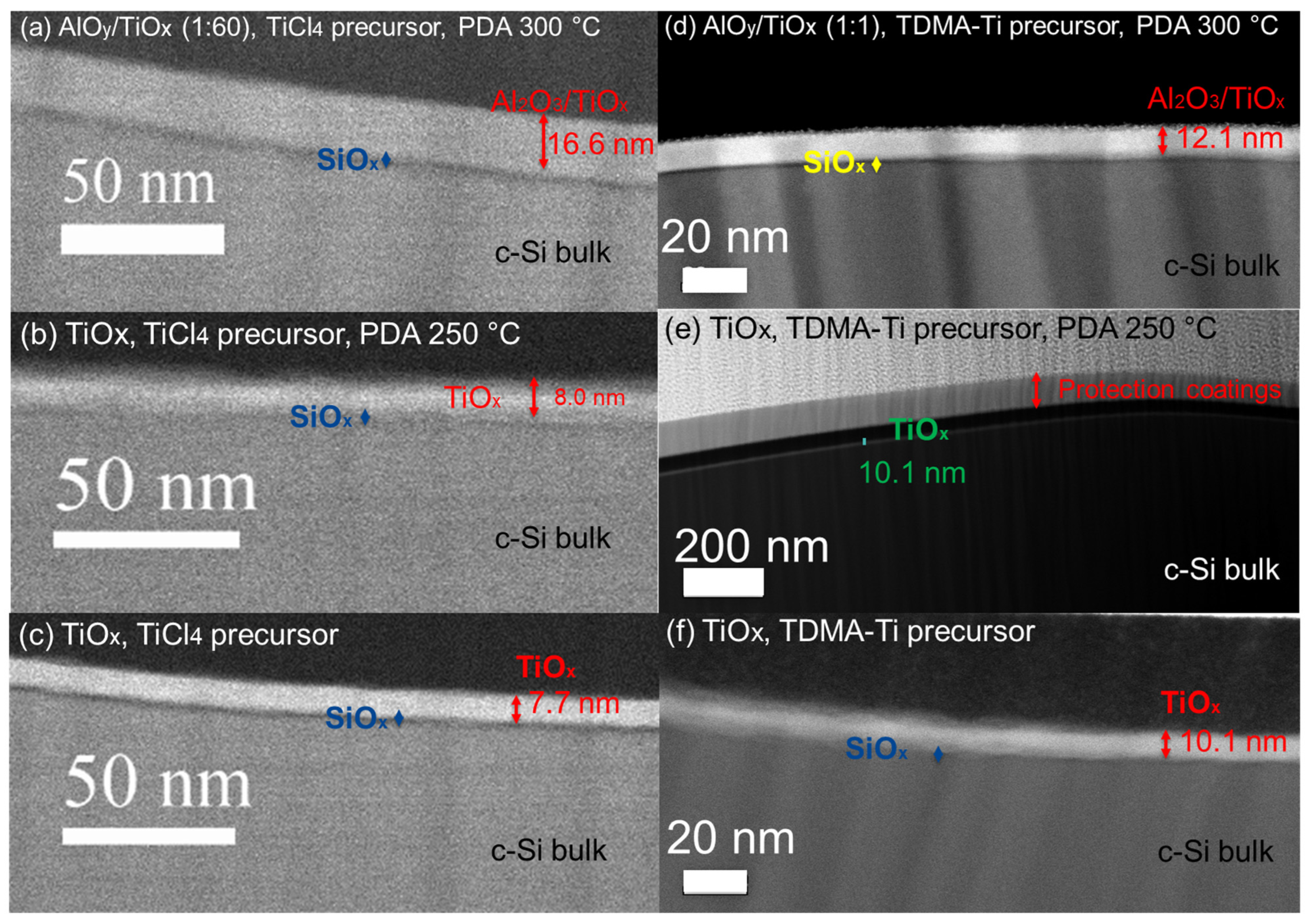
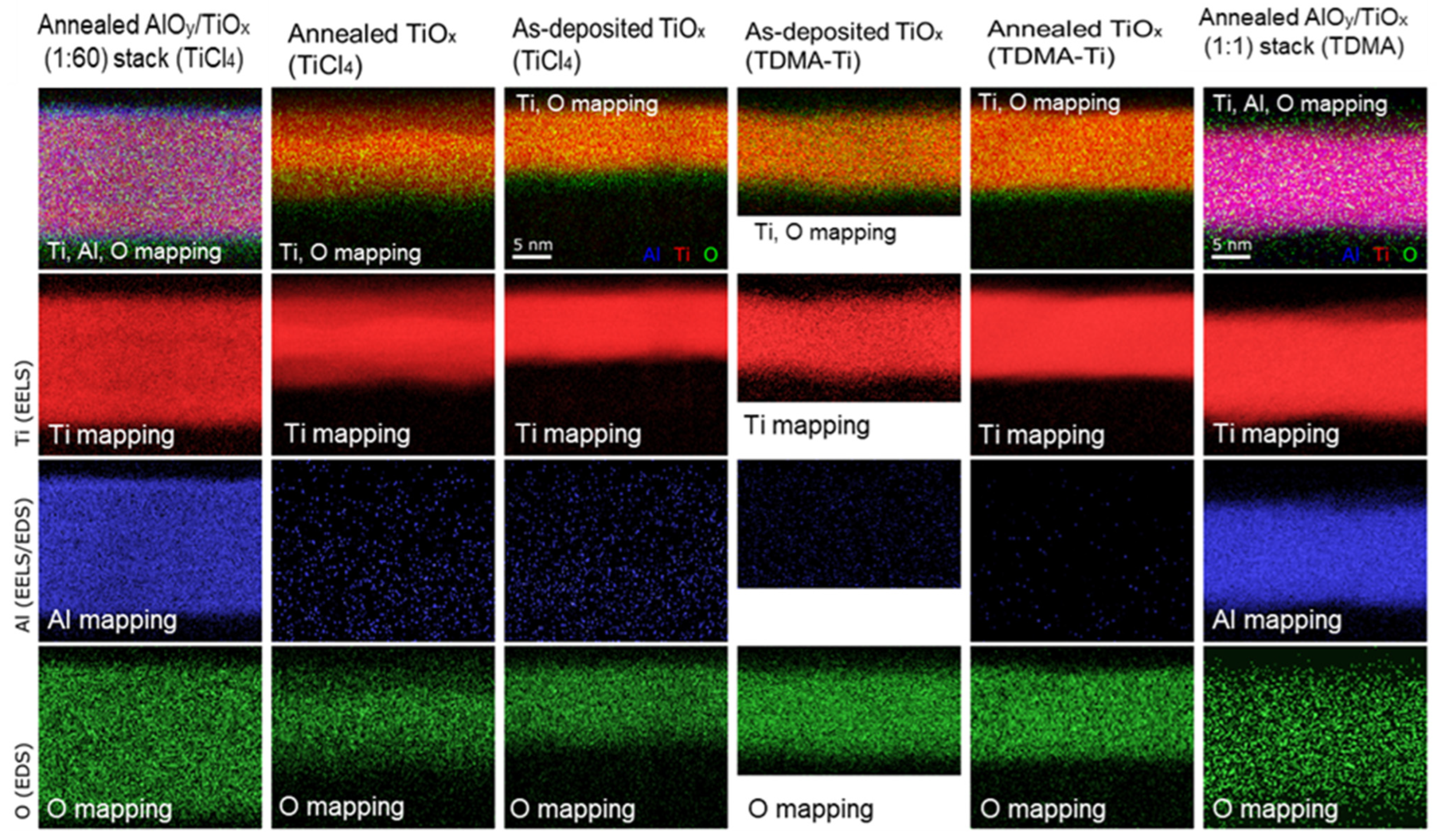
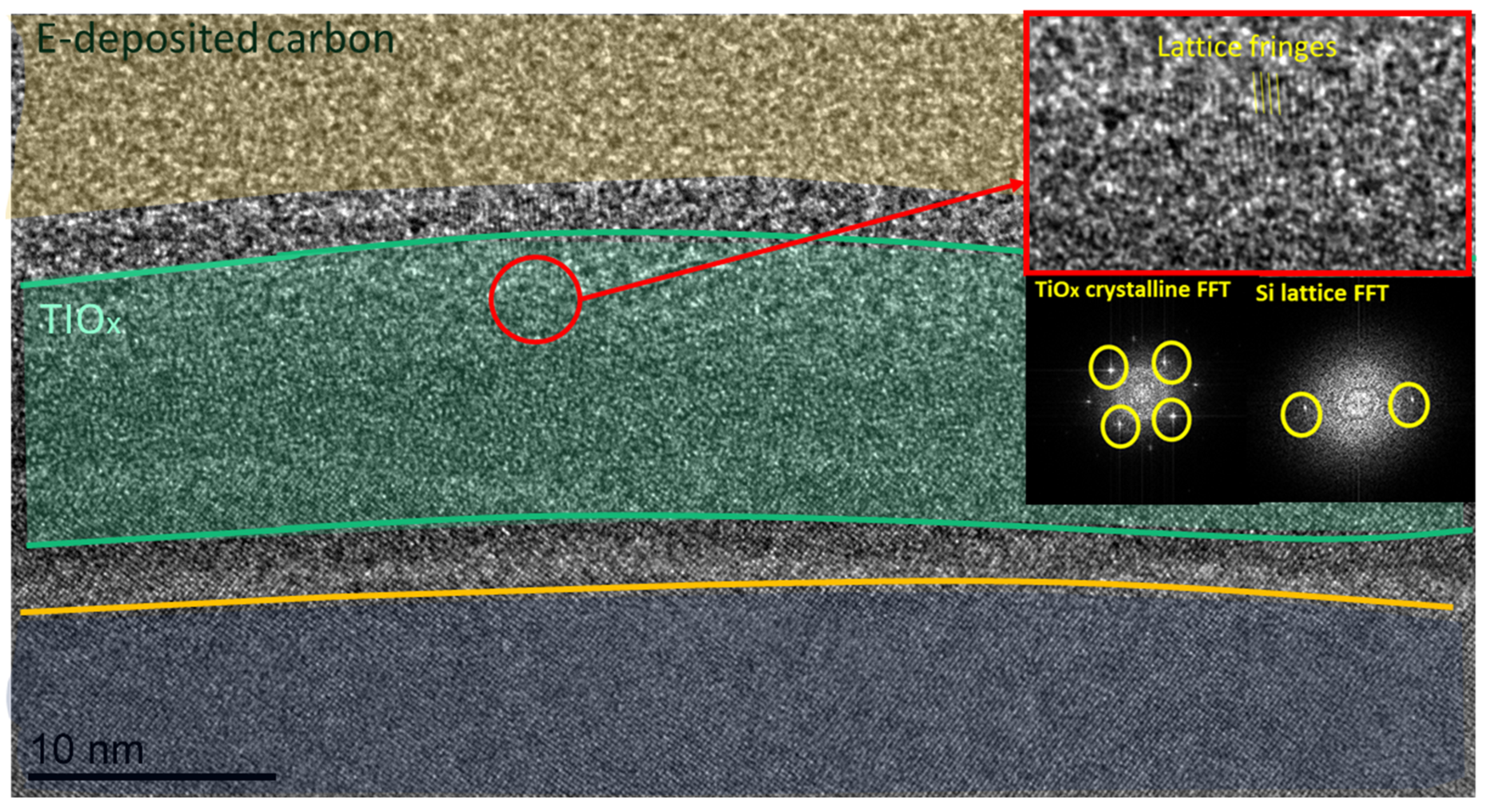
3.3. TEM Analysis of Deposited Films
4. Conclusions
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Ouyang, M.; Lu, L.; Li, J. Cycle Life of Commercial Lithium-Ion Batteries with Lithium Titanium Oxide Anodes in Electric Vehicles. Energies 2014, 7, 4895–4909. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.J.; Kopac, J.; Balazic, M.; Bombac, D.; Brojan, M.; Kosel, F. Titanium and Titanium Alloy Applications in Medicine. In Surgical Tools and Medical Devices; Ahmed, W., Jackson, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 475–517. [Google Scholar] [CrossRef]
- Li, W.; Fu, T.; Xie, F.; Yu, S.; He, S. The multi-staged formation process of titanium oxide nanotubes and its thermal stability. Mater. Lett. 2007, 61, 730–735. [Google Scholar] [CrossRef]
- Cui, J.; Allen, T.; Wan, Y.; Mckeon, J.; Samundsett, C.; Yan, D.; Zhang, X.; Cui, Y.; Chen, Y.; Verlinden, P.; et al. Titanium oxide: A re-emerging optical and passivating material for silicon solar cells. Sol. Energy Mater. Sol. Cells 2016, 158, 115–121. [Google Scholar] [CrossRef]
- Afzal, A.; Habib, A.; Ulhasan, I.; Shahid, M.; Rehman, A. Antireflective Self-Cleaning TiO2 Coatings for Solar Energy Harvesting Applications. Front. Mater. 2021, 8, 687059. [Google Scholar] [CrossRef]
- Melskens, J.; van de Loo, B.W.H.; Macco, B.; Black, L.E.; Smit, S.; Kessels, W.M.M. Passivating Contacts for Crystalline Silicon Solar Cells: From Concepts and Materials to Prospects. IEEE J. Photovoltaics 2018, 8, 373–388. [Google Scholar] [CrossRef] [Green Version]
- Richards, B.S. Novel Uses of Titanium Dioxide for Silicon Solar Cells; UNSW Sydney: Kensington, Australia, 2002. [Google Scholar]
- Horprathum, M.; Chindaudom, P.; Limsuwan, P. A Spectroscopic Ellipsometry Study of TiO2 Thin Films Prepared by dc Reactive Magnetron Sputtering: Annealing Temperature Effect. Chin. Phys. Lett. 2007, 24, 1505–1508. [Google Scholar] [CrossRef]
- Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. A spectroscopic ellipsometry study of TiO2 thin films prepared by ion-assisted electron-beam evaporation. Curr. Appl. Phys. 2009, 9, 707–712. [Google Scholar] [CrossRef]
- Hitosugi, T.; Yamada, N.; Nakao, S.; Hirose, Y.; Hasegawa, T. Properties of TiO2-based transparent conducting oxides. Phys. Status Solidi (a) 2010, 207, 1529–1537. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Sharma, R.; Biris, A.S.; Zhang, J.; Calle, C.; Zahn, M. Self-Cleaning Transparent Dust Shields for Protecting Solar Panels and Other Devices. Part. Sci. Technol. 2007, 25, 5–20. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, J. Oxide Semiconductors for Solar Energy Conversion: Titanium Dioxide; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Zeng, Y.; Tong, H.; Quan, C.; Cai, L.; Yang, Z.; Chen, K.; Yuan, Z.; Wu, C.-H.; Yan, B.; Gao, P.; et al. Theoretical exploration towards high-efficiency tunnel oxide passivated carrier-selective contacts (TOPCon) solar cells. Sol. Energy 2017, 155, 654–660. [Google Scholar] [CrossRef]
- Kamisaka, H.; Adachi, T.; Yamashita, K. Theoretical study of the structure and optical properties of carbon-doped rutile and anatase titanium oxides. J. Chem. Phys. 2005, 123, 084704. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Kamai, T.-A.; Tsubota, T.; Ohno, T. Novel hydrothermal preparation of pure brookite-type titanium(IV) oxide nanocrystal under strong acidic conditions. Catal. Commun. 2009, 10, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.A.; Said, N.D.M.; Abdullah, S.A.; Rahim, M.S. Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. AIP Conf. Proc. 2017, 1788, 030030. [Google Scholar] [CrossRef] [Green Version]
- Kukli, K.; Ritala, M.; Schuisky, M.; Leskelä, M.; Sajavaara, T.; Keinonen, J.; Uustare, T.; Hårsta, A. Atomic layer deposition of titanium oxide from TiI4 and H2O2. Chem. Vap. Depos. 2000, 6, 303–310. [Google Scholar] [CrossRef]
- Jang, H.K.; Whangbo, S.W.; Choi, Y.K.; Chung, Y.D.; Jeong, K.; Whang, C.N.; Lee, Y.S.; Lee, H.-S.; Choi, J.Y.; Kim, G.H.; et al. Titanium oxide films on Si(100) deposited by e-beam evaporation. J. Vac. Sci. Technol. A 2000, 18, 2932–2936. [Google Scholar] [CrossRef]
- César, R.; Barros, A.; Doi, I.; Diniz, J.; Swart, J. Thin titanium oxide films obtained by RTP and by sputtering. In Proceedings of the 2014 29th Symposium on Microelectronics Technology and Devices (SBMicro), Aracaju, Brazil, 1–5 September 2014; pp. 1–4. [Google Scholar]
- Mathur, S.; Kuhn, P. CVD of titanium oxide coatings: Comparative evaluation of thermal and plasma assisted processes. Surf. Coatings Technol. 2006, 201, 807–814. [Google Scholar] [CrossRef]
- Meng, L.-J.; dos Santos, M. Investigations of titanium oxide films deposited by d.c. reactive magnetron sputtering in different sputtering pressures. Thin Solid Films 1993, 226, 22–29. [Google Scholar] [CrossRef]
- Liao, B.; Hoex, B.; Aberle, A.G.; Chi, D.; Bhatia, C.S. Excellent c-Si surface passivation by low-temperature atomic layer deposited titanium oxide. Appl. Phys. Lett. 2014, 104, 253903. [Google Scholar] [CrossRef]
- Liu, Y.; Sang, B.; Hossain, A.; Gao, K.; Cheng, H.; Song, X.; Zhong, S.; Shi, L.; Shen, W.; Hoex, B.; et al. A novel passivating electron contact for high-performance silicon solar cells by ALD Al-doped TiO2. Sol. Energy 2021, 228, 531–539. [Google Scholar] [CrossRef]
- Yu, I.-S.; Chang, I.-H.; Cheng, H.-E.; Lin, Y.-S. Surface passivation of c-Si by atomic layer deposition TiO2 thin films deposited at low temperature. In Proceedings of the 2014 IEEE 40th Photovoltaic Specialist Conference (PVSC), Denver, CO, USA, 8–13 June 2014; pp. 1271–1274. [Google Scholar] [CrossRef]
- Yu, I.-S.; Wang, Y.-W.; Cheng, H.-E.; Yang, Z.-P.; Lin, C.-T. Surface Passivation and Antireflection Behavior of ALD on n-Type Silicon for Solar Cells. Int. J. Photoenergy 2013, 2013, 431614. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-H.; Chen, K.-T.; Huang, P.-H.; Wu, W.-Y.; Zhang, X.-Y.; Wang, C.; Liang, L.-S.; Gao, P.; Qiu, Y.; Lien, S.-Y.; et al. Effect of Annealing Temperature on Spatial Atomic Layer Deposited Titanium Oxide and Its Application in Perovskite Solar Cells. Nanomaterials 2020, 10, 1322. [Google Scholar] [CrossRef]
- Peng, T.; Xiao, X.; Ren, F.; Xu, J.; Zhou, X.; Mei, F.; Jiang, C. Influence of annealing temperature on the properties of TiO2 films annealed by ex situ and in situ TEM. J. Wuhan Univ. Technol. Sci. Ed. 2012, 27, 1014–1019. [Google Scholar] [CrossRef]
- Parsons, G.N.; George, S.M.; Knez, M. Progress and future directions for atomic layer deposition and ALD-based chemistry. MRS Bull. 2011, 36, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Jiang, Y.-L.; Detavernier, C.; Deduytsche, D.; Van Meirhaeghe, R.L.; Ru, G.-P.; Li, B.-Z.; Qu, X.-P. Atomic layer deposition of TiO2 from tetrakis-dimethyl-amido titanium or Ti isopropoxide precursors and H2O. J. Appl. Phys. 2007, 102, 083521. [Google Scholar] [CrossRef]
- Park, H.K.; Yang, B.S.; Park, S.; Kim, M.S.; Shin, J.C.; Heo, J. Purge-time-dependent growth of ZnO thin films by atomic layer deposition. J. Alloys Compd. 2014, 605, 124–130. [Google Scholar] [CrossRef]
- Saarenpää, H.; Niemi, T.; Tukiainen, A.; Lemmetyinen, H.; Tkachenko, N. Aluminum doped zinc oxide films grown by atomic layer deposition for organic photovoltaic devices. Sol. Energy Mater. Sol. Cells 2010, 94, 1379–1383. [Google Scholar] [CrossRef]
- Merisalu, J.; Arroval, T.; Kasikov, A.; Kozlova, J.; Rähn, M.; Ritslaid, P.; Aarik, J.; Tamm, A.; Kukli, K. Engineering of atomic layer deposition process for titanium-aluminum-oxide based resistively switching medium. Mater. Sci. Eng. B 2022, 282. [Google Scholar] [CrossRef]
- Peron, M.; Cogo, S.; Bjelland, M.; Bin Afif, A.; Dadlani, A.; Greggio, E.; Berto, F.; Torgersen, J. On the evaluation of ALD TiO2, ZrO2 and HfO2 coatings on corrosion and cytotoxicity performances. J. Magnes. Alloy. 2021, 9, 1806–1819. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Macco, B.; Deligiannis, D.; Smit, S.; van Swaaij, R.A.C.M.M.; Zeman, M.; Kessels, W.M.M. Influence of transparent conductive oxides on passivation of a-Si:H/c-Si heterojunctions as studied by atomic layer deposited Al-doped ZnO. Semicond. Sci. Technol. 2014, 29, 122001. [Google Scholar] [CrossRef]
- Musschoot, J.; Xie, Q.; Deduytsche, D.; Berghe, S.V.D.; Van Meirhaeghe, R.; Detavernier, C. Atomic layer deposition of titanium nitride from TDMAT precursor. Microelectron. Eng. 2009, 86, 72–77. [Google Scholar] [CrossRef]
- Fox, G.R.; Potrepka, D.M.; Polcawich, R.G. Dependence of {111}-textured Pt electrode properties on TiO2 seed layers formed by thermal oxidation. J. Mater.Sci. Mater. Electron. 2018, 29, 412–426. [Google Scholar] [CrossRef]
- Temperton, R.H.; Gibson, A.; O’Shea, J.N. In situ XPS analysis of the atomic layer deposition of aluminium oxide on titanium dioxide. Phys. Chem. Chem. Phys. 2018, 21, 1393–1398. [Google Scholar] [CrossRef]
- Cheng, X.; Marstein, E.S.; Haug, H.; Di Sabatino, M. Double Layers of Ultrathin a-Si:H and SiNx for Surface Passivation of n-type Crystalline Si Wafers. Energy Procedia 2016, 92, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Weckman, T.; Laasonen, K. First principles study of the atomic layer deposition of alumina by TMA–H 2 O-process. Phys. Chem. Chem. Phys. 2015, 17, 17322–17334. [Google Scholar] [CrossRef] [Green Version]
- Matkivskyi, V.; Lee, Y.; Seo, H.S.; Lee, D.-K.; Park, J.-K.; Kim, I. Electronic-beam evaporation processed titanium oxide as an electron selective contact for silicon solar cells. Curr. Appl. Phys. 2021, 32, 98–105. [Google Scholar] [CrossRef]
- Rafique, R.; Tonny, K.N.; Sharmin, A.; Mahmood, Z.H. Study on the Effect of Varying Film Thickness on the Transparent Conductive Nature of Aluminum Doped Zinc Oxide Deposited by Dip Coating. Mater. Focus 2018, 7, 707–713. [Google Scholar] [CrossRef]
- Kale, A.S.; Nemeth, W.; Harvey, S.P.; Page, M.; Young, D.L.; Agarwal, S.; Stradins, P. Effect of silicon oxide thickness on polysilicon based passivated contacts for high-efficiency crystalline silicon solar cells. Sol. Energy Mater. Sol. Cells 2018, 185, 270–276. [Google Scholar] [CrossRef]
- Hussin, R.; Choy, K.L.; Hou, X.H. Growth of TiO2 Thin Films by Atomic Layer Deposition (ALD). Adv. Mater. Res. 2016, 1133, 352–356. [Google Scholar] [CrossRef]
- Chen, X.Y.; Lu, Y.F.; Tang, L.J.; Wu, Y.H.; Cho, B.J.; Xu, X.J.; Dong, J.R.; Song, W.D. Annealing and oxidation of silicon oxide films prepared by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2005, 97, 014913. [Google Scholar] [CrossRef]
- Dingemans, G.; Kessels, W. Status and prospects of Al2O3-based surface passivation schemes for silicon solar cells. J. Vac. Sci. Technol. A Vac.Surf. Film. 2012, 30, 040802. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.; Zhou, Y.; Postill, A. A novel technique for the deposition of aluminium-doped zinc oxide films. Thin Solid Films 2003, 426, 111–116. [Google Scholar] [CrossRef]
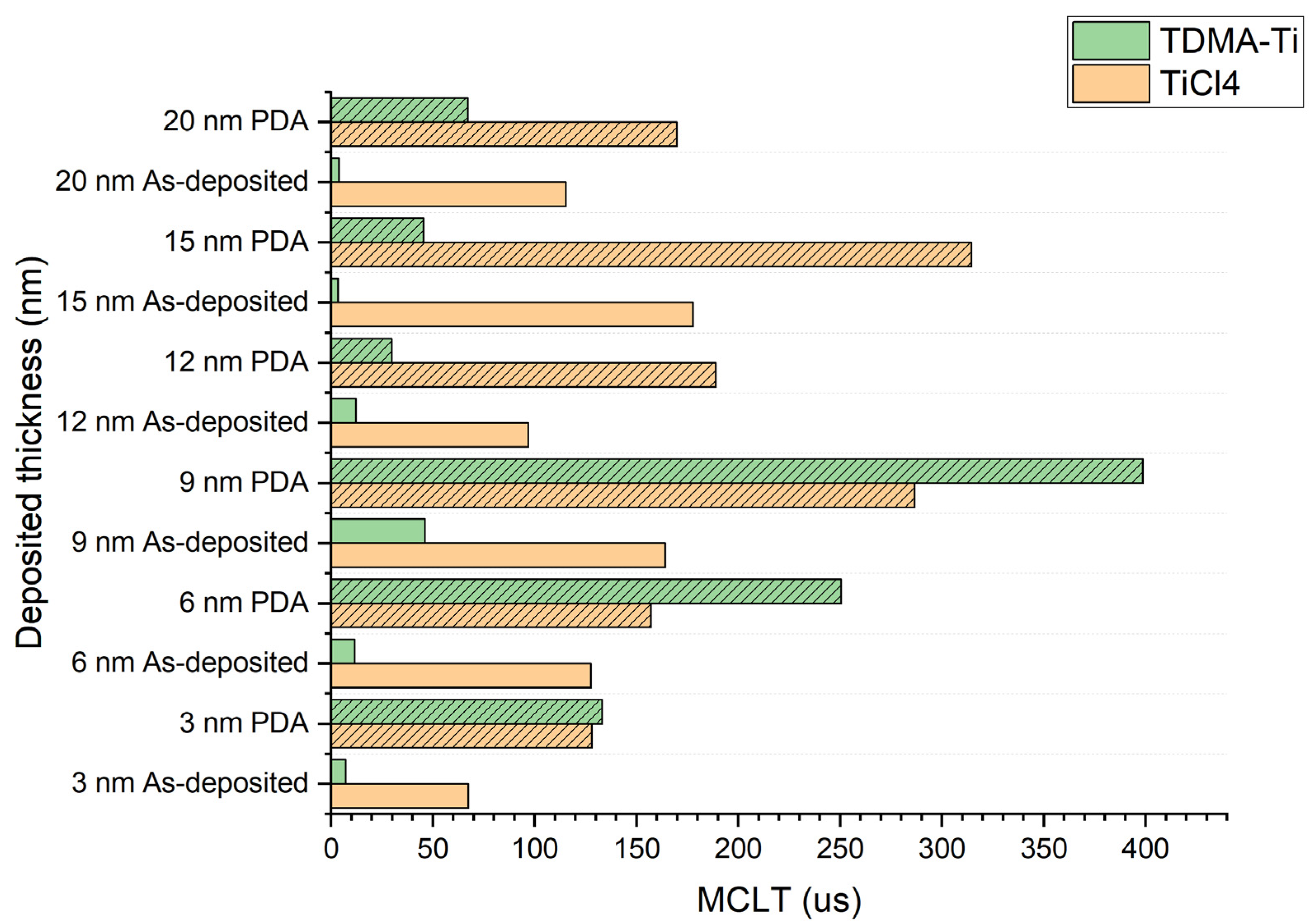


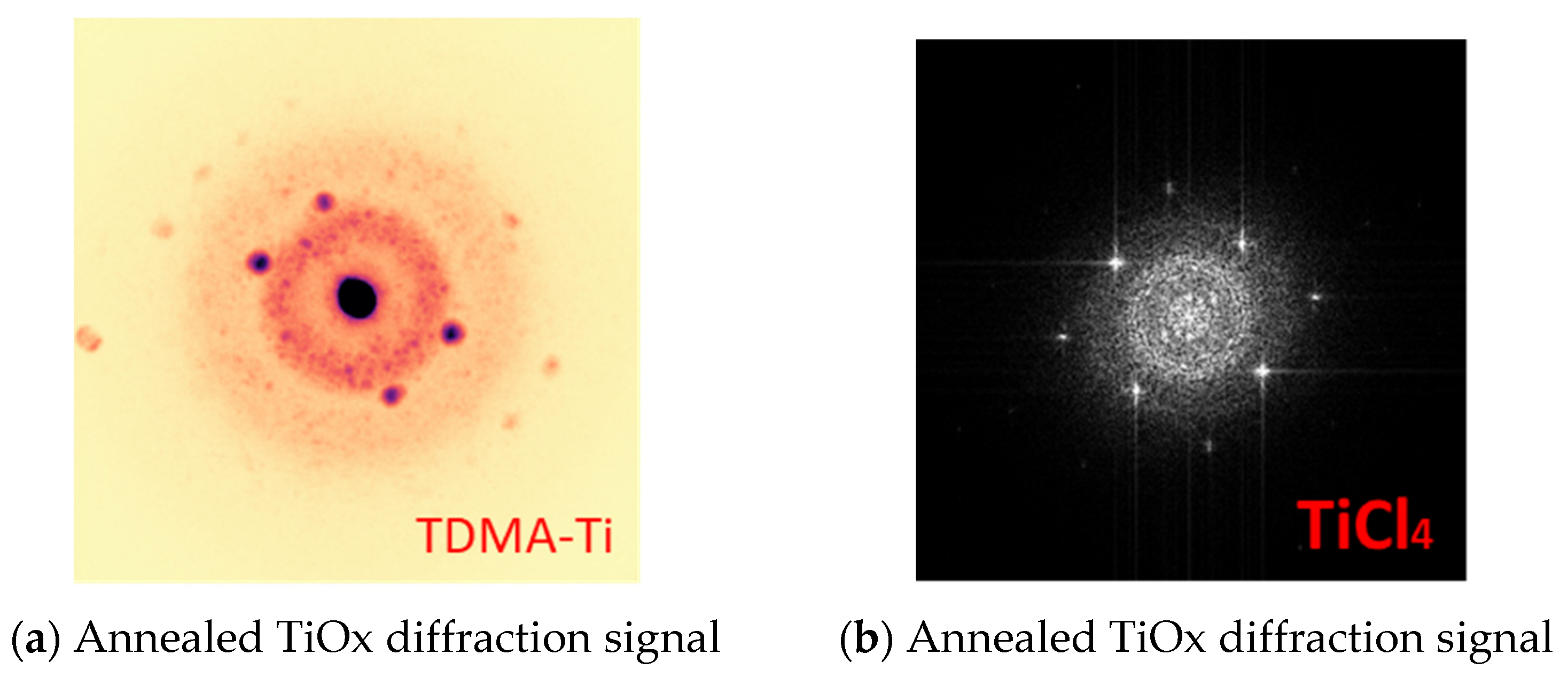
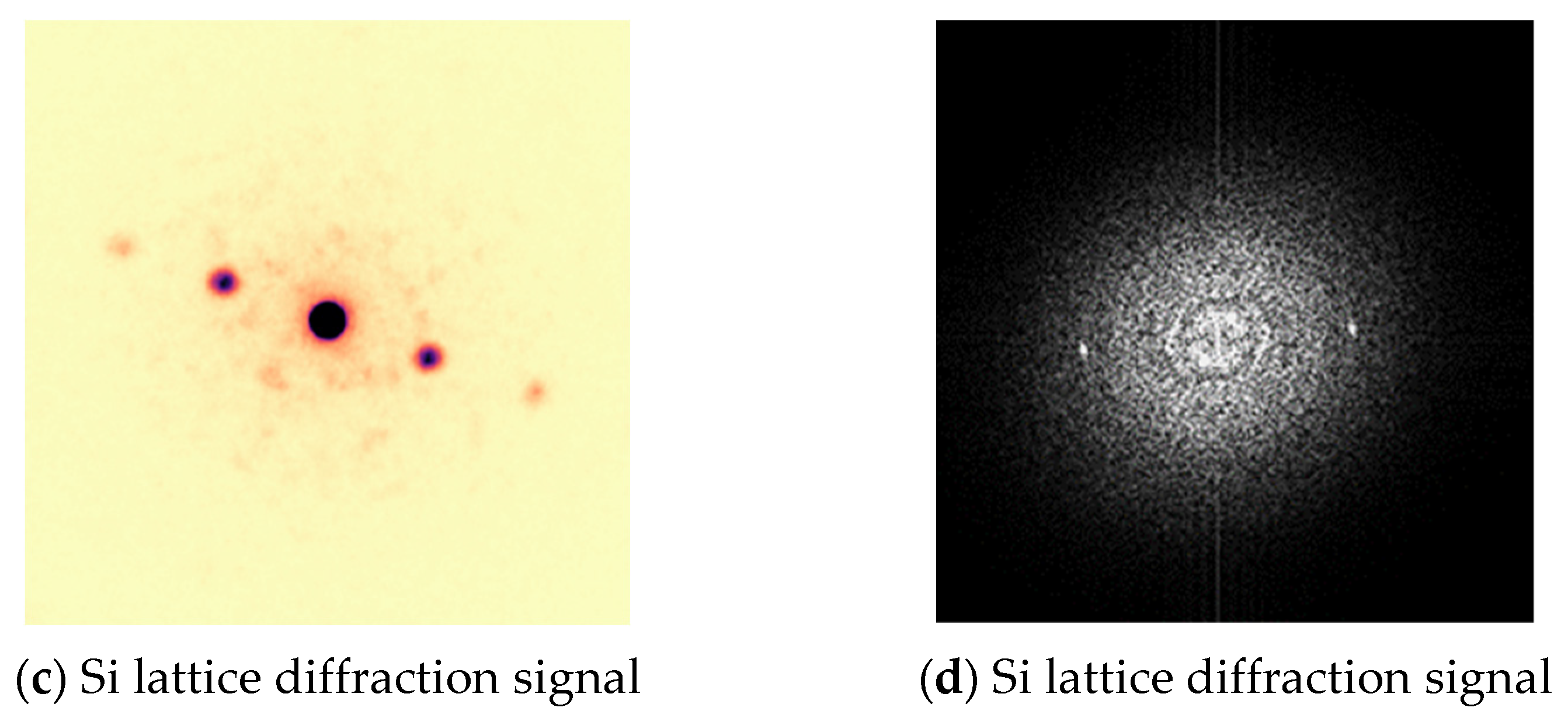
| Deposited Thickness of TiOx (nm) | 3, 6, 9,12, 15, 20 |
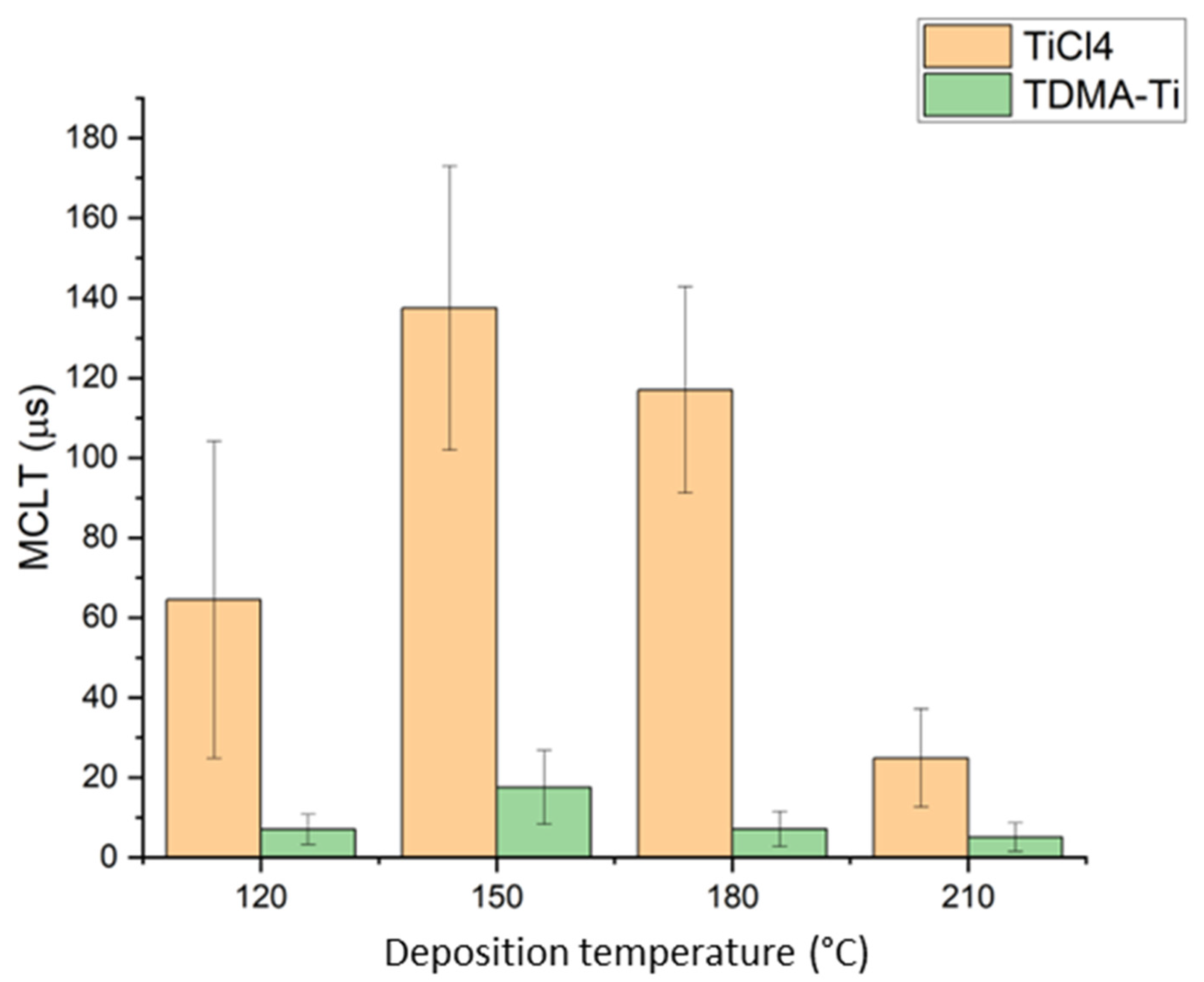
| Deposition temperature of TiOx from TDMA-Ti (°C) | 120, 140, 150, 180, 200, 210 |
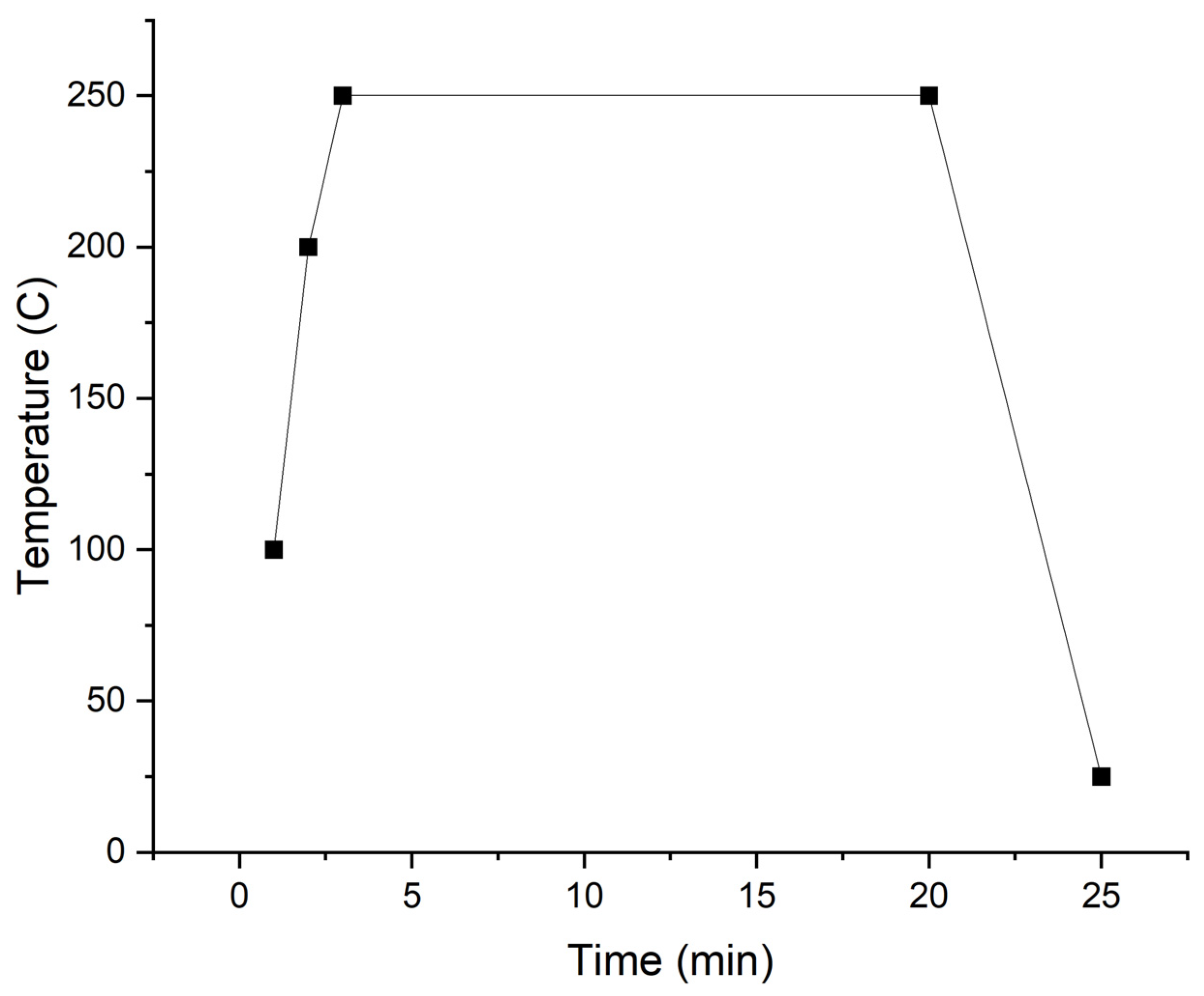
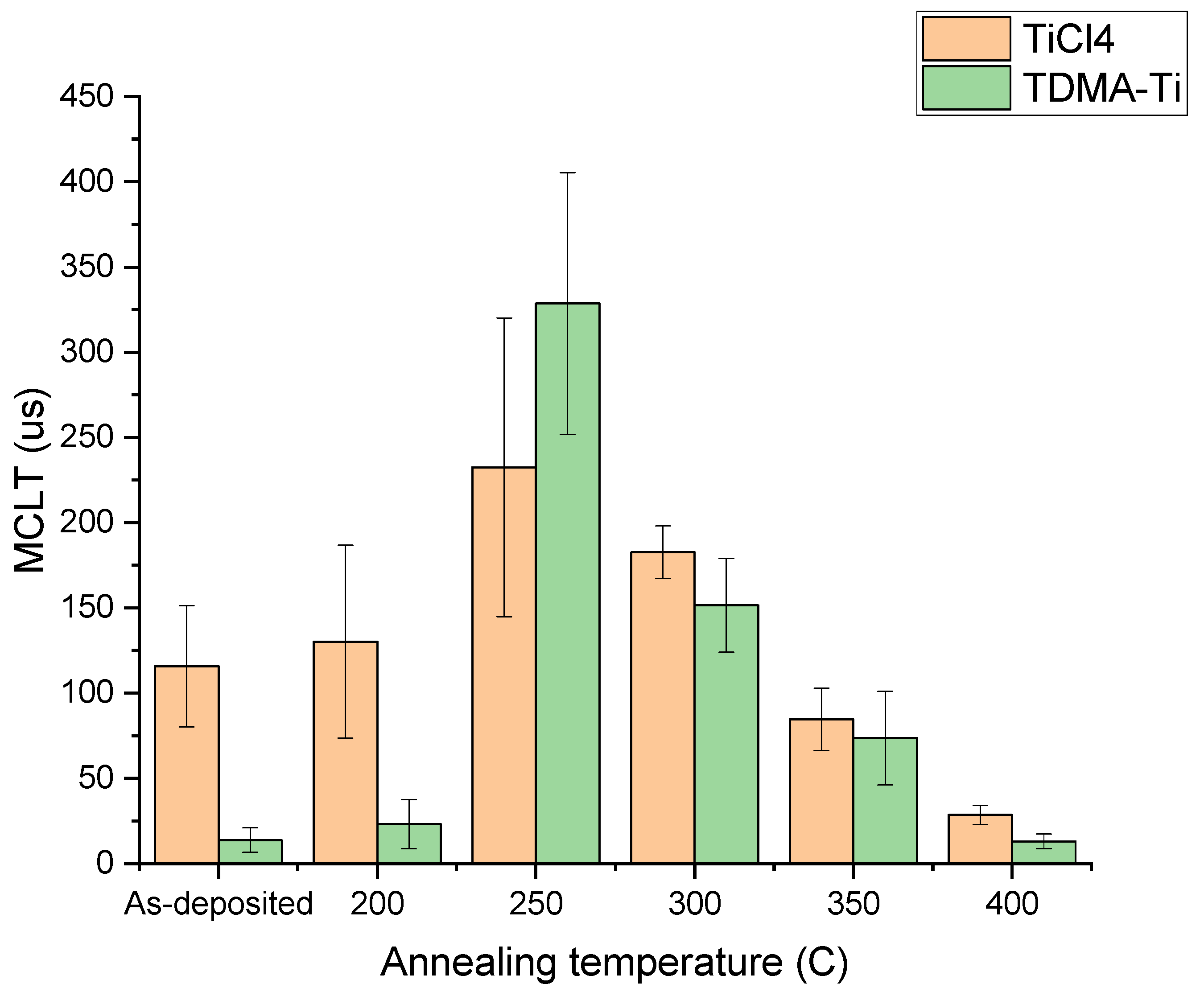
| Post deposition annealing temperature (°C) | 200, 250, 300, 350, 400 |
| Deposition temperature of TiOx from TiCl4 precursor (°C) | 120, 150, 180, 210 |
| Deposition layer stack ration AlOy:TIOx | 1:1, 1:5, 1:30, 1:60 |
| Ti, Atomic % | O, Atomic % | |
| As-deposited TiOx (TiCl4) | 22.83 ± 0.25 | 77.17 ± 0.78 |
| As-deposited TiOx (TDMA-Ti) | 29.53 ± 0.21 | 70.47 ± 0.47 |
| Annealed TiOx (TiCl4) | 20.76 ± 0.31 | 79.24 ± 0.99 |
| Annealed TiOx (TDMA-Ti) | 26.09 ± 0.20 | 73.80 ± 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matkivskyi, V.; Leiviskä, O.; Wenner, S.; Liu, H.; Vähänissi, V.; Savin, H.; Di Sabatino, M.; Tranell, G. Atomic Layer Deposition of Titanium Oxide-Based Films for Semiconductor Applications—Effects of Precursor and Operating Conditions. Materials 2023, 16, 5522. https://doi.org/10.3390/ma16165522
Matkivskyi V, Leiviskä O, Wenner S, Liu H, Vähänissi V, Savin H, Di Sabatino M, Tranell G. Atomic Layer Deposition of Titanium Oxide-Based Films for Semiconductor Applications—Effects of Precursor and Operating Conditions. Materials. 2023; 16(16):5522. https://doi.org/10.3390/ma16165522
Chicago/Turabian StyleMatkivskyi, Vladyslav, Oskari Leiviskä, Sigurd Wenner, Hanchen Liu, Ville Vähänissi, Hele Savin, Marisa Di Sabatino, and Gabriella Tranell. 2023. "Atomic Layer Deposition of Titanium Oxide-Based Films for Semiconductor Applications—Effects of Precursor and Operating Conditions" Materials 16, no. 16: 5522. https://doi.org/10.3390/ma16165522







