Metal–Ceramic Compatibility in Dental Restorations According to the Metallic Component Manufacturing Procedure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
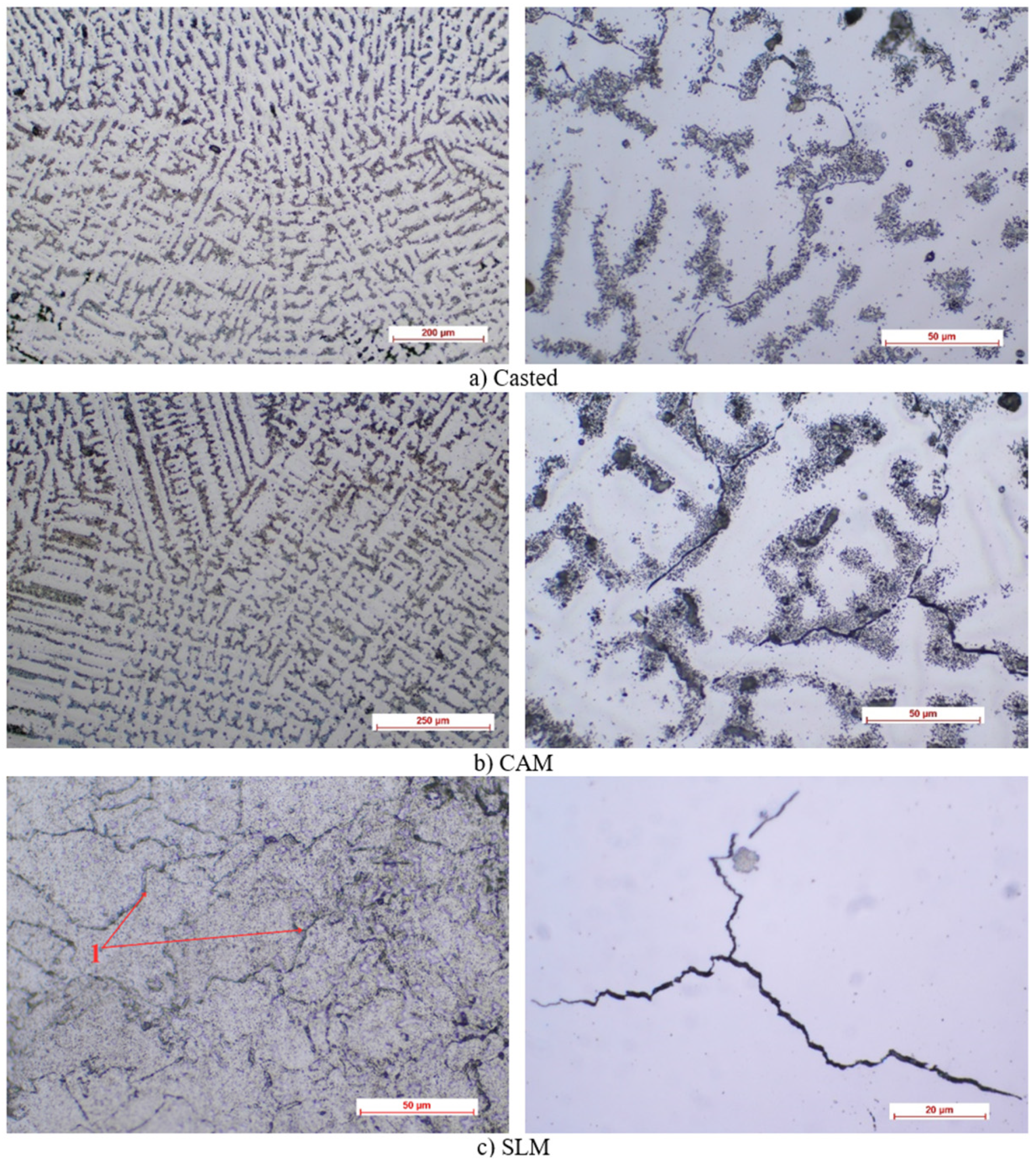
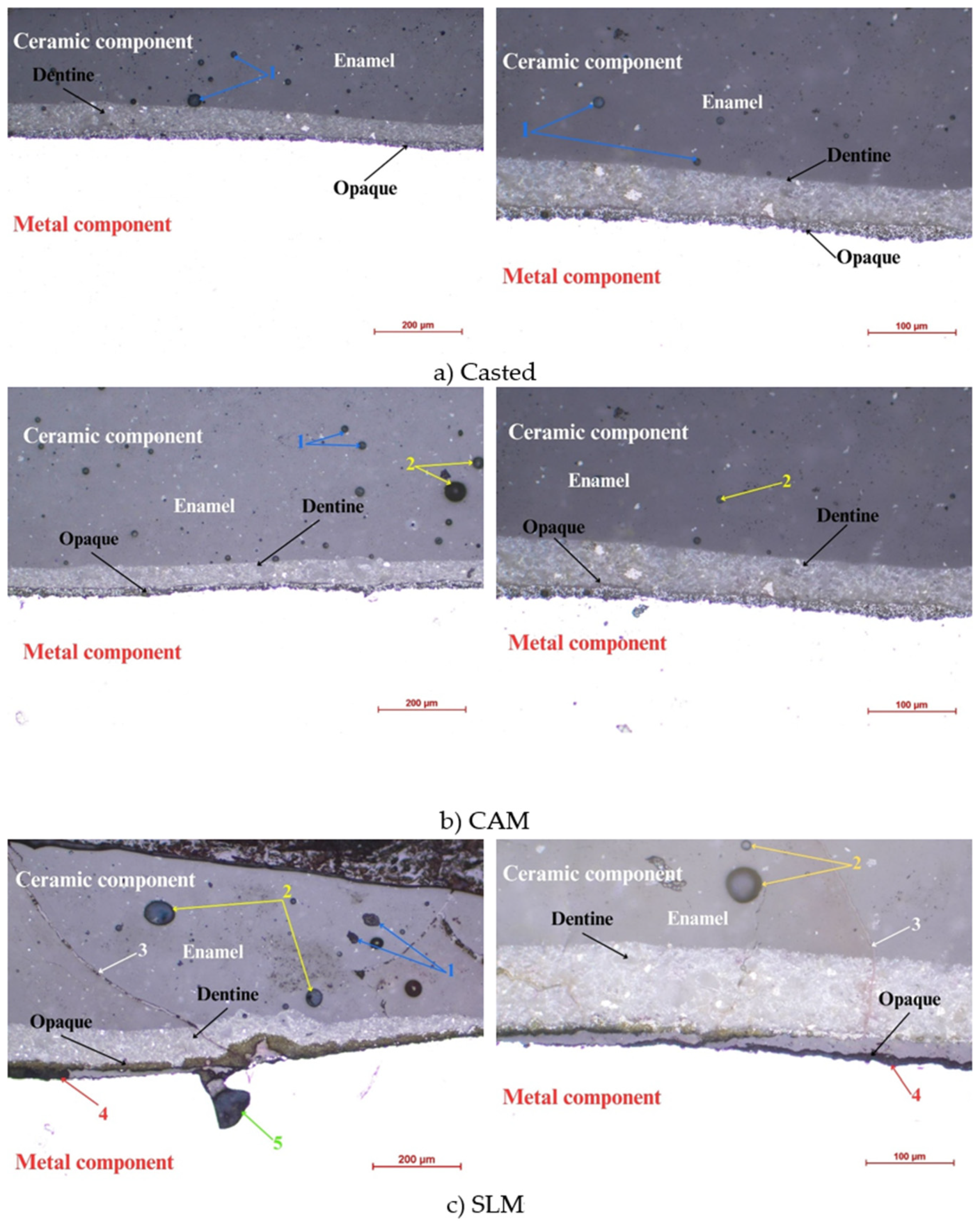
3.1. Optical Microscopy
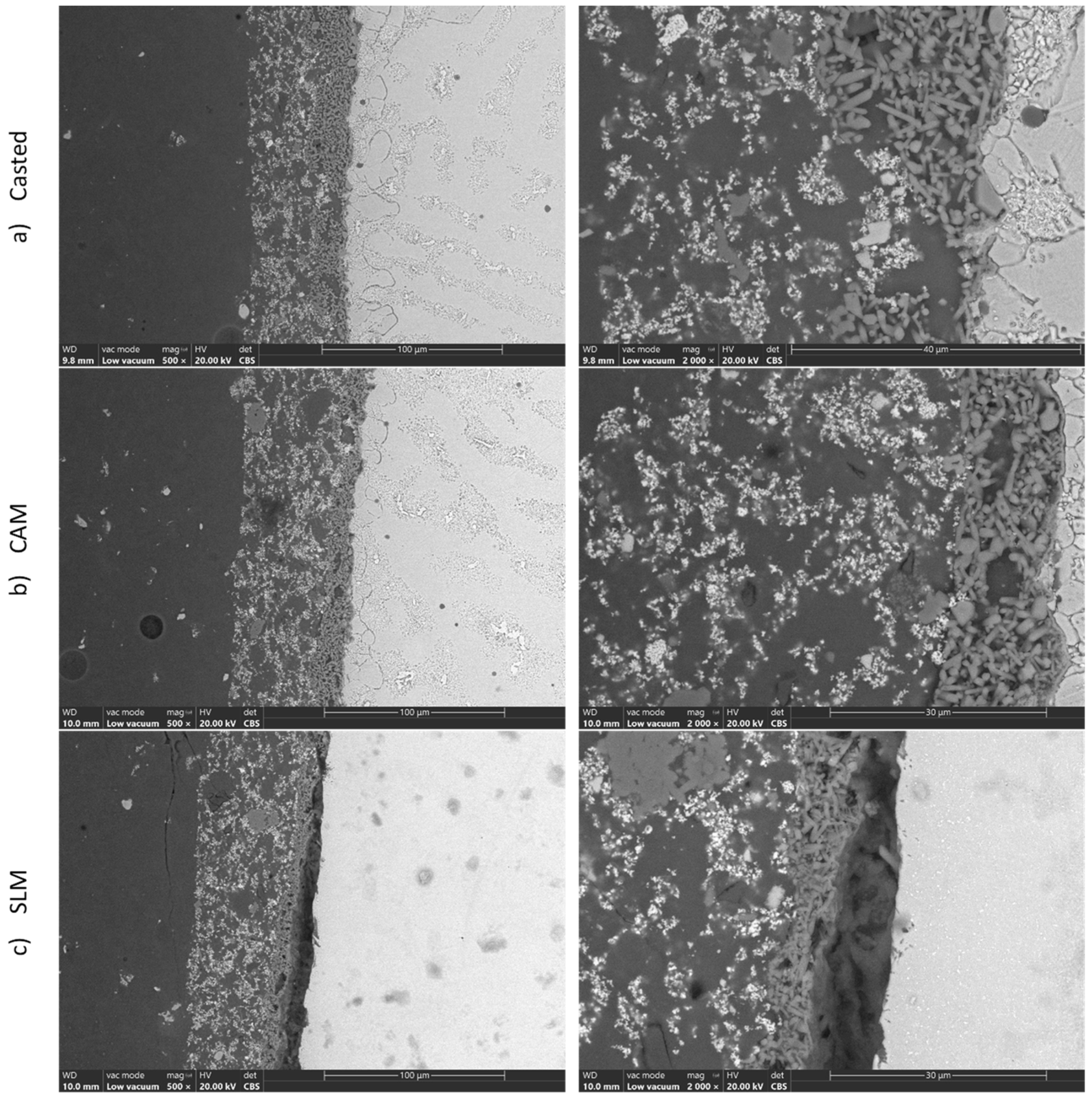
3.2. SEM/EDS
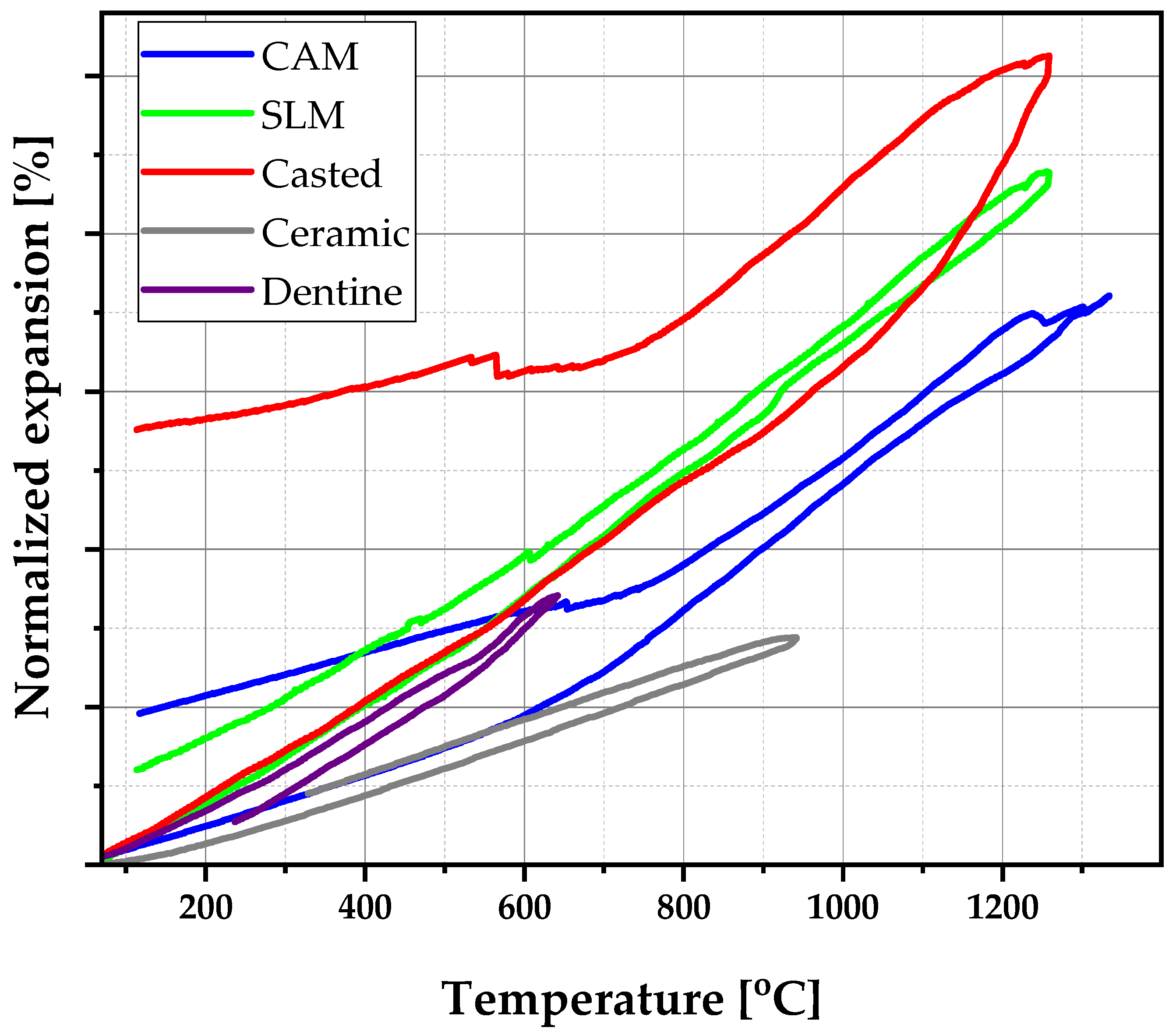
3.3. Thermal Expansion
3.4. Hardness Tests
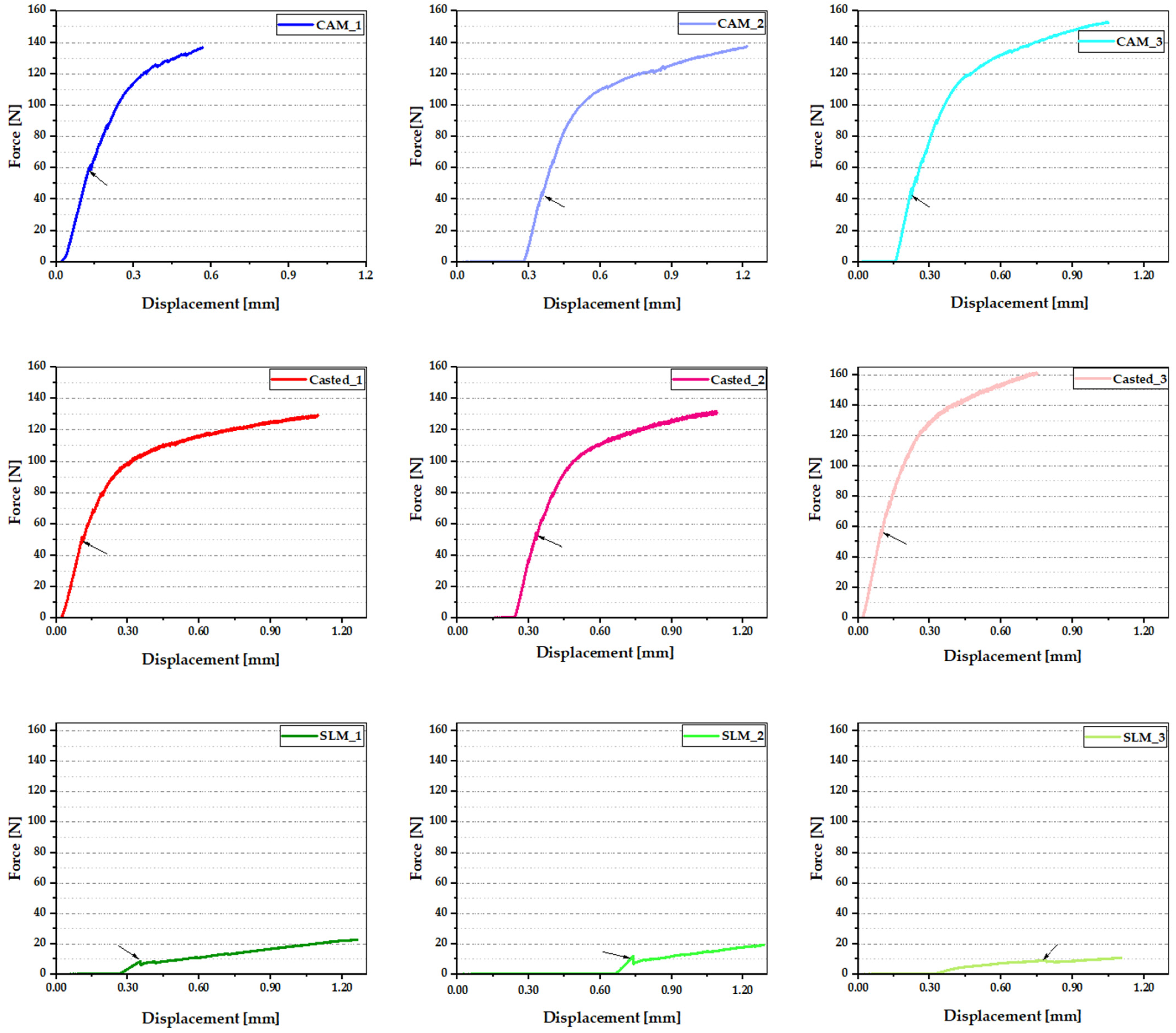
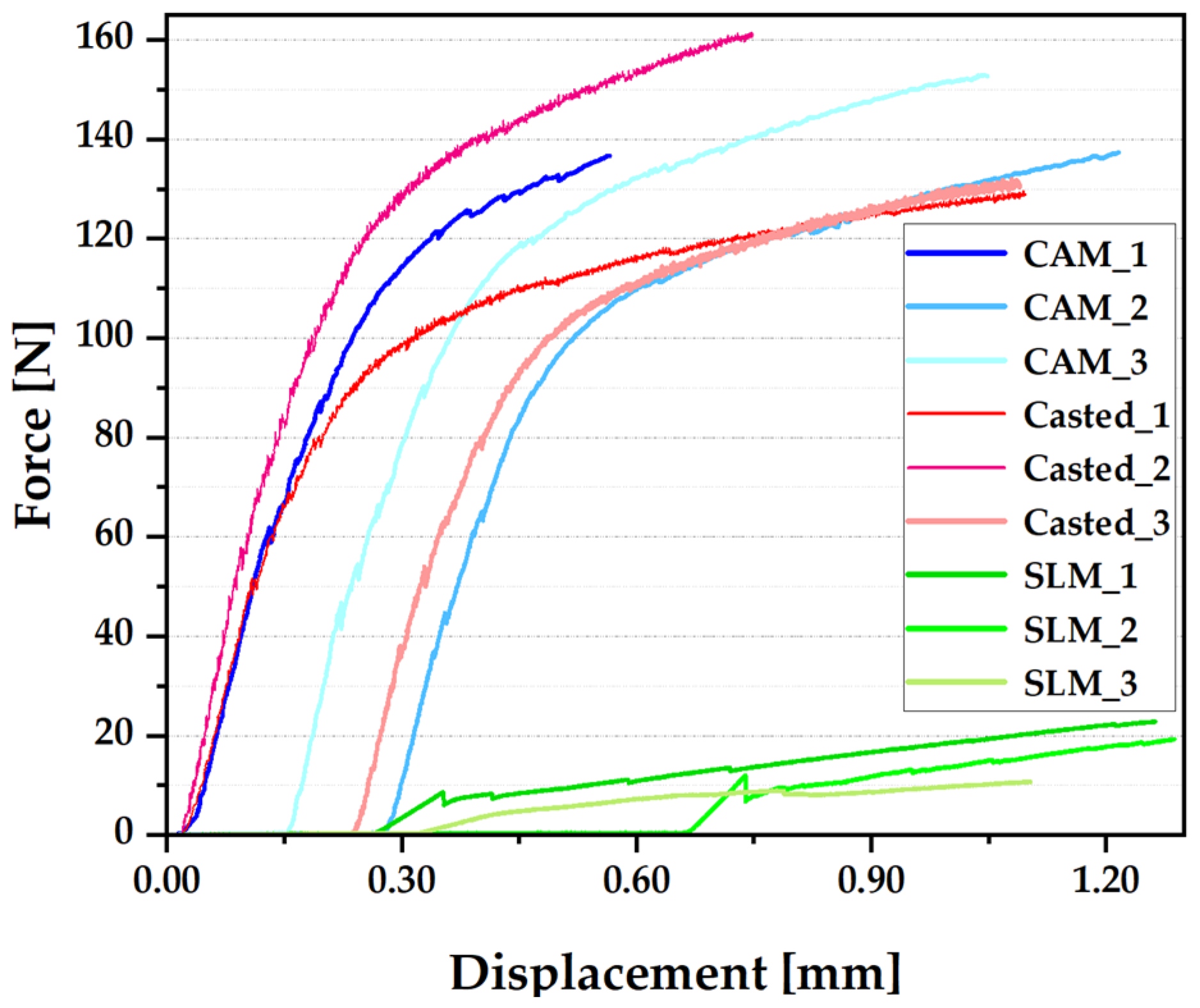
3.5. Three-Poins Bending Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zając, Z.A.; Jedynak, B. Physical and chemical properties of porcelain-fused-to-metal and porcelain-fused-to-zirconia prosthetic crowns—A literature review. Prosthodontics 2023, 73, 140–149. [Google Scholar]
- Baig, N.; Barve, N.; Yeshwante, B.; Jadhav, V.; Adhapure, P.; Gorde, K. As good as new: Repair of fractured porcelain fused to metal restorations. J. Appl. Dent. Med. Sci. 2018, 4, 1. [Google Scholar]
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [PubMed]
- Volvina, M.; Sumarsongko, T.; Firman, D. The Influence of Metal Coping Design on Fracture Resistance of Porcelain-Fused-to-Metal Crown. Key Eng. Mater. 2022, 932, 147–156. [Google Scholar]
- Newaskar, P.S.; Sonkesriya, S.; Singh, R.; Palekar, U.; Bagde, H.; Dhopte, A. Evaluation and Comparison of Five-Year Survival of Tooth-Supported Porcelain Fused to Metal and All-Ceramic Multiple Unit Fixed Prostheses: A Systematic Review. Cureus 2022, 14, e30338. [Google Scholar] [PubMed]
- Revilla-León, M.; Gómez-Polo, M.; Park, S.H.; Barmak, A.B.; Özcan, M. Adhesion of veneering porcelain to cobalt-chromium dental alloys processed with casting, milling, and additive manufacturing methods: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 128, 575–588. [Google Scholar]
- Czepułkowska, W.; Wołowiec-Korecka, E.; Klimek, L. The role of mechanical, chemical and physical bonds in metal-ceramic bond strength. Arch. Mater. Sci. Eng. 2018, 92, 5–14. [Google Scholar]
- Miyazaki, T.; Hotta, Y.; Kunii, J.; Kuriyama, S.; Tamaki, Y. A review of dental CAD/CAM: Current status and future perspectives from 20 years of experience. Dent. Mater. J. 2009, 28, 44–56. [Google Scholar]
- Han, X.; Sawada, T.; Schille, C.; Schweizer, E.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Spintzyk, S. Comparative Analysis of Mechanical Properties and Metal-Ceramic Bond Strength of Co-Cr Dental Alloy Fabricated by Different Manufacturing Processes. Materials 2018, 11, 1801. [Google Scholar]
- Viderščak, D.; Schauperl, Z.; Šolić, S.; Ćatić, A.; Godec, M.; Kocijan, A.; Paulin, I.; Donik, Č. Additively Manufactured Commercial Co-Cr Dental Alloys: Comparison of Microstructure and Mechanical Properties. Materials 2021, 14, 7350. [Google Scholar]
- Mocanu, A.C.; Miculescu, F.; Stan, G.E.; Tite, T.; Miculescu, M.; Țierean, M.H.; Pascu, A.; Ciocoiu, R.T.; Butte, T.M.; Ciocan, L.T. Development of ceramic coatings on titanium alloy substrate by laser cladding with pre-placed natural derived-slurry: Influence of hydroxyapatite ratio and beam power. Ceram. Int. 2023, 49, 10445–10454. [Google Scholar]
- Ciocan, L.T.; Miculescu, F.; Miculescu, M.; Patrascu, I. Biological reactions to dental implants. In Implant Dentistry—A Rapidly Evolving Practice; Turkyilmaz, I., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Miculescu, F.; Ciocan, L.T.; Meghea, D.; Miculescu, M. Morphologic characterization of ceramic-ceramic dental systems failure. Key Eng. Mater. 2014, 614, 140–143. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. (Eds.) Craig’s Restorative Dental Materials-E-Book, 13th ed.; Elsevier Mosby: Maryland Heights, MO, USA, 2012. [Google Scholar]
- Lohbauer, U. Dental glass ionomer cements as permanent filling materials?—Properties, limitations, future trends. Materials 2009, 3, 76–96. [Google Scholar] [CrossRef] [Green Version]
- Koutsoukis, T.; Zinelis, S.; Eliades, G.; Al-Wazzan, K.; Rifaiy, M.A.; Al Jabbari, Y.S. Selective Laser Melting Technique of Co-Cr Dental Alloys: A Review of Structure and Properties and Comparative Analysis with Other Available Techniques. J. Prosthodont. 2015, 24, 303–312. [Google Scholar]
- Miculescu, M.; Bane, M.; Miculescu, F.; Ciocan, L.T.; Preda, O.; Antoniac, V.I. A study on metal-ceramic interface on metal base dental alloys. Key Eng. Mater. 2015, 638, 14–19. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, S.H.; Kim, Y.K.; Son, J.S.; Min, B.K.; Kim, K.H.; Kwon, T.Y. Microstructures and mechanical properties of Co-Cr dental alloys fabricated by three CAD/CAM based processing techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; Yanan, Z.; Xin, D.; Ning, L.; Jiazhen, Y.; Haojiang, S.; Sheng, X.; Biao, Z. Metal-ceramic interface characteristics of Co-Cr alloy fabricated by selective laser melting. Chin. J. Tissue Eng. Res. 2021, 25, 521–525. [Google Scholar]
- Fairhurst, C.W.; Anusavice, K.J.; Hashinger, D.T.; Ringle, R.D.; Twiggs, S.W. Thermal expansion of dental alloys and porcelains. J. Biomed. Mat. Res. 1980, 14, 435–446. [Google Scholar] [CrossRef]
- Hammad, I.A.; Talic, Y.F. Designs of bond strength tests for metal-ceramic complexes: Review of the literature. J. Prosthet. Dent. 1996, 75, 602–608. [Google Scholar] [PubMed]
- Oilo, G.; Johansson, B.; Syverud, M. Bond strength of porcelain to dental alloys—An evaluation of two test methods. Scand. J. Dent. Res. 1981, 89, 289–296. [Google Scholar]
- ISO 9693:2019; Dentistry—Compatibility Testing for Metal-Ceramic and Ceramic-Ceramic Systems. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/75855.html (accessed on 10 June 2023).
- Hong, J.K.; Kim, S.K.; Heo, S.J.; Koak, J.Y. Mechanical Properties and Metal-Ceramic Bond Strength of Co-Cr Alloy Manufactured by Selective Laser Melting. Materials 2020, 13, 5745. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, M.; Vlăsceanu, R.; Baciu, F.; Miculescu, F. Study on the Metal-Ceramic Bonding Strength Improvement by Unconventional Methods. U.P.B. Sci. Bull. Ser. B 2016, 78, 193–200. [Google Scholar]
- ISO 6507-1:2018; Metallic Materials—Vickers Hardness Test—Part 1: Test Method. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6507:-1:ed-4:v1:en (accessed on 10 June 2023).
- Miculescu, M.; Ion, O.A. Regulation and Certification of (Bio)Medical Engineers: A Case Study on Romania. Int. J. Environ. Res. Public Health 2022, 19, 9004. [Google Scholar] [CrossRef]
- Bezzon, O.L.; de Mattos, M.G.; Ribeiro, R.F.; Rollo, J.M. Effect of beryllium on the castability and resistance of metal-ceramic bonds în nickel chromium alloys. J. Prosthet. Dent. 1998, 80, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Mackert, J.R., Jr.; Ringle, R.D.; Fairhurst, C.W. High-temperature behavior of a Pd-Ag alloy for porcelain. J. Dent. Res. 1983, 62, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiang, N.; Wei, B. A comparison of corrosion resistance of cobalt-chromium-molybdenum metal ceramic alloy fabricated with selective laser melting and traditional processing. J. Prosthet. Dent. 2014, 112, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; van Noort, R.; Stokes, C.W. Surface analysis of porcelain fused to metal systems. Dent. Mater. 2006, 22, 330–337. [Google Scholar] [CrossRef] [PubMed]


| Alloy | Element | ||||||
|---|---|---|---|---|---|---|---|
| Co | Cr | Mo | W | Mn | Si | N | |
| Herenium | 59.0 | 25.0 | 4.0 | 10.0 | 0.8 | 1.0 | 0.2 |
| Temperature Range [°C] | Coefficient of Thermal Expansion (×10−6/°C) | ||
|---|---|---|---|
| CAM | SLM | CASTING | |
| 20–300 | 13.31 | 12.79 | 14.02 |
| 300–600 | 18.23 | 13.93 | 12.25 |
| 600–900 | 19.41 | 18.91 | 15.75 |
| 900–1200 | 23.38 | 20.39 | 17.15 |
| Temperature Range [°C] | Coefficient of Thermal Expansion (×10−6/°C) | |
| Enamel | Dentine | |
| 20–300 | 15.1 | 13.5 |
| 300–600 | 17.4 | 14.8 |
| 600–900 | 22.1 | 20.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawod, N.; Miculescu, M.; Antoniac, I.V.; Miculescu, F.; Agop-Forna, D. Metal–Ceramic Compatibility in Dental Restorations According to the Metallic Component Manufacturing Procedure. Materials 2023, 16, 5556. https://doi.org/10.3390/ma16165556
Dawod N, Miculescu M, Antoniac IV, Miculescu F, Agop-Forna D. Metal–Ceramic Compatibility in Dental Restorations According to the Metallic Component Manufacturing Procedure. Materials. 2023; 16(16):5556. https://doi.org/10.3390/ma16165556
Chicago/Turabian StyleDawod, Nazem, Marian Miculescu, Iulian Vasile Antoniac, Florin Miculescu, and Doriana Agop-Forna. 2023. "Metal–Ceramic Compatibility in Dental Restorations According to the Metallic Component Manufacturing Procedure" Materials 16, no. 16: 5556. https://doi.org/10.3390/ma16165556






