Abstract
Zirconium phosphate (ZrP), especially its alpha allotropic modification, appears to be a very promising sorbent material for the sorption and separation of various radionuclides due to its properties such as an extremely high ion exchange capacity and good radiation stability. Actinium-225 and its daughter nuclide 213Bi are alpha emitting radioisotopes of high interest for application in targeted alpha therapy of cancer. Thus, the main aim of this paper is to study the sorption of 225Ac on the α-ZrP surface and its kinetics, while the kinetics of the sorption is studied using natEu as a non-radioactive homologue of 225Ac. The sorption properties of α-ZrP were tested in an acidic environment (hydrochloric and nitric acid) using batch sorption experiments and characterized using equilibrium weight distribution coefficients Dw (mL/g). The modeling of the experimental data shows that the kinetics of 225Ac sorption on the surface of α-ZrP can be described using a film diffusion model (FD). The equilibrium weight distribution coefficient Dw for 225Ac in both hydrochloric and nitric acid reached the highest values in the concentration range 5.0–7.5 mM (14,303 ± 153 and 65,272 ± 612 mL/g, respectively). Considering the results obtained in radioactive static sorption experiments with 225Ac and in non-radioactive kinetic experiments with natEu, α-ZrP seems to be a very promising material for further construction of a 225Ac/213Bi generator.
1. Introduction
Zirconium phosphates have been extensively studied since the 1950s [1,2]. In recent decades, they have been reported to have many applications, including as catalysts, drug delivery agents, anticorrosive agents, flame retardants, and ion exchangers [3,4,5,6,7,8]. The extensive interest in these materials across various scientific fields can be attributed to their excellent physicochemical properties, including exceptional ion exchange capability, thermal and radiation stability, biocompatibility, numerous active sites on the surface, and easy functionalization that leads to new structures with various applications [9,10]. Although earlier studies mainly focused on the use of zirconium phosphates as catalysts, recent interest has been centered on their exceptional ion exchange properties [11,12,13].
The investigation of the properties and structure of the α-ZrP molecule was aided by the Clearfield research group, who first synthesized zirconium(IV) phosphate in its alpha allotropic modification (α-ZrP) [1,14,15,16,17,18]. The molecule is comprised of zirconium atoms that are slightly positioned above or below its midplane. Cross-linking occurs from three oxygen atoms that are attached to another zirconium atom, from each phosphate group. The interlayer distance of α-ZrP is 0.76 nm. The thickness of the α-ZrP layer is 0.66 nm, and the remaining 0.1 nm is attributed to water molecules present in the interspace between individual α-ZrP layers [15]. Covalent bonds are formed between the atoms within the individual layers, while the interaction between neighboring layers is based on van der Waals forces [17].
In the case of using of zirconium phosphates as an ion exchanger, α-ZrP is the best known and the most promising due to its high selectivity for trivalent cations [18]. Numerous studies on ion exchange separation of radioactive metals were conducted across the periodic table of elements, including from the light elements to the super-heavy elements, such as actinides [19,20,21]. The outstanding suitability of α-ZrP as an ion exchanger makes it applicable in the separation of medically significant radionuclides [13] or in the field of radioactive waste reprocessing [11]. Previous studies have explored the sorption of various metal ions using zirconium phosphate in crystalline or amorphous form, or in a composite sorbent based on a polyacrylonitrile matrix. The crystalline form of zirconium phosphate is specifically suitable for separating trivalent lanthanoids and actinoids during nuclear fuel reprocessing. The purpose of this material, especially nanocrystalline α-ZrP, was demonstrated in the separation of Eu(III)/Am(III) in nuclear fuel in the pH range of 0–3 in nitric acid. The highest separation factor of 400 was achieved at pH 1 [11]. Zirconium phosphate in its amorphous phase can also be utilized to separate different metals, particularly bivalent (Cu(II), Mn(II), Ni(II), Zn(II) and Pb(II)) and tetravalent (Th(IV)). The ability of this sorbent to separate stated metal ions was tested in various media, including 0.2 M and 0.02 M NH4NO3, HNO3, HClO4, and CH3COOH. The distribution coefficients of bivalent metal ions in the eluents depend on the pH and ionic strength. When the pH drops below the eluent’s pK value, the acid groups become mostly nonionic, leading to a decrease in the apparent capacity and distribution coefficients. The distribution coefficient shows that amorphous zirconium phosphate demonstrates higher selectivity towards Pb(II), Ni(II), and Mn(II). In 0.02 M acetic acid media, Pb(II) and Cu(II) show higher distribution coefficients than in distilled water. Except for Ni(II), Mn(II), and Th(IV), all the evaluated metal ions exhibit higher distribution coefficients in electrolyte media compared with distilled water [22]. Additionally, the amorphous form of zirconium phosphate served as a composite sorbent, based on a polyacrylonitrile matrix, to separate Co(II), Nd(III), and Dy(III). By using a single column with 1 mM nitric acid, the purity of simulated leachate increased to 87.9% for Co, 96.4% for Nd, and 40% for Dy at different stages of effluent [23].
This study aims to investigate the kinetic and equilibrium sorption of 225Ac on α-ZrP particles in the acidic environment of nitric and hydrochloric acid with future potential application in the separation of 213Bi as a medically relevant therapeutic radionuclide in targeted alpha therapy (TAT), which, together with other medical branches such as supramolecular nanochemotherapy, belongs to the modern therapeutic options for the treatment of oncological diseases [24,25]. Considering that 213Bi has been used in clinical trials, primarily on patients with leukaemia, bladder cancer, neuroendocrine tumors, melanoma, glioma, and lymphoma. Due to the very positive results of the clinical studies mentioned above, it can be assumed that this radionuclide will soon become established in normal clinical practice and the demand for its availability will increase rapidly [26,27,28,29,30,31]. The equilibrium sorption properties of α-ZrP were tested in radioactive experiments using 225Ac, while the kinetics of the sorption process on the α-ZrP particles was tested using natEu as a non-radioactive homologue of 225Ac.
2. Materials and Methods
2.1. α-ZrP Preparation
α-ZrP was prepared by mixing 6.4 g of zirconium(IV) oxychloride octahydrate (Sigma-Aldrich, Darmstadt, Germany) in 20 mL of demineralized water solution and 55 g of sodium dihydrogen phosphate monohydrate (Sigma-Aldrich, Germany) in 40 mL of 3 M ultrapure hydrochloric acid solution (Sigma-Aldrich, Germany). The sodium dihydrogen phosphate monohydrate solution was added dropwise to a solution of zirconium(IV) oxychloride octahydrate at 80 °C and the mixture was stirred throughout (heating and stirring of the mixture was performed using an IKA C MAG HS 7 (IKA-Werke GmbH & Co. KG, Staufen im Breisgau, Germany)). The mixture was then refluxed at 80 °C for 30 h. Finally, the reaction mixture was left at room temperature for 2 days without stirring to promote precipitation [10]. Finally, the precipitate was filtered and washed with 200 mL of 3 M phosphoric acid (Sigma-Aldrich, Germany), and deionized water to pH 3 was reached. The precipitate was dried to a constant weight loss at 60 °C using a Binder 9630-0002 VD 56 Standard Vacuum Drying Chamber (Binder, Tuttlingen, Germany).
The prepared α-ZrP was characterized using various analytical methods such as infrared spectroscopy, X-ray powder diffraction, thermogravimetry, differential thermal analysis, and scanning and transmission electron microscopy in the work [32].
2.2. Kinetic Study of natEu Sorption on the Surface of α-ZrP
The kinetics of sorption on α-ZrP particles was studied in a non-radioactive experimental mode using natEu as a suitable and available 225Ac homologue due to the lack of a stable isotope of actinium. The most typical analog of Ac is considered to be La, although the use of Eu as a suitable analog of Ac due to its physicochemical properties corresponding to those of a typical lanthanide can be found in the literature in the context of sorption studies of actinium or actinides in general [33,34,35,36].
The kinetic study was performed with 1 g of prepared α-ZrP. The total volume of the solution of 0.1 M ultrapure hydrochloric acid (Sigma-Aldrich, Germany) with natEu (in the form of europium(III) nitrate pentahydrate (Sigma-Aldrich, Germany)) at a concentration of 10,000 ppb was 100 mL (V/m ratio 100 mL/g). The time of addition of the sorbent to the Eu solution was marked as time t = 0. The sorbent suspension was continuously stirred throughout the experiment using an IKA NANOSTAR 7.5 digital (IKA-Werke GmbH & Co. KG, Germany). To determine the instantaneous residual concentration of Eu in the suspension, 0.2 mL aliquots of the stirred suspension were taken at times 0, 0.5, 1, 5, 10, 15, 20, 25, 30, 45, 60, 90, and 120 min. All aliquots taken were filtered through a microfiber glass filter and then diluted 30× with 5% solution of ultrapure nitric acid (Sigma-Aldrich, Germany) for ICP-MS analysis of Eu content.
ICP-MS analysis of the prepared samples was performed on an Agilent 7500 Series ICP-MS (Agilent Technologies Inc., Santa Clara, CA, USA) with a radiofrequency source power of 1550 W, a sampling rate of 0.1 rps, a carrier gas flow rate of 1.0 L/min, and a sampling cone distance of 7 mm in collisionless gas mode. The data were processed using the MassHunter program (version B.01.01).
Finally, the experimental data were evaluated using several models for two-phase systems. The kinetic models used are summarized in Table 1 [37]. The models reflect the following different rate-controlling processes: mass transfer (DM), film diffusion (FD), diffusion in inert layer (ID), diffusion in reacted layer (RLD), chemical reaction (CR), and gel diffusion (GD).

Table 1.
Kinetic models of sorption/extraction taking place in two-phase systems [37].
The following balance and equilibrium equations apply:
and
where c0 is the starting concentration of the component in the aqueous phase. And finally:
and
where Kd is the distribution coefficient.
The procedure of experimental data evaluation by means of the above-mentioned models has been described and demonstrated in detail by Suchánková et al. [38]. For this evaluation, in the course of which the values of the total mass transfer coefficients, e.g., KFD, are sought, the Newton–Raphson multidimensional non-linear regression method, combined with the solution of the differential equation under given boundary conditions (by means of the Runge–Kutha method), was used. The code P60.fm (in the software product FAMULUS (version 3.5), code package STAMB 2016) was used. Of course, the quantities Kd (i.e., distribution coefficient), the mean radius of solid phase particle, R, and the initial concentrations, c0 and q0, must be known. The input data for the calculations of the kinetic model parameters and the modeling of the 225Ac sorption kinetics in the case of this paper were as follows: KD = 2.51 × 103 L/kg; C0 = 6.80 × 10−5 M; q0 = 0; Cexp = f(t); r = V/m = 100 mL/g.
The quantity WSOS/DF (weighted sum of squares of differences divided by number of degrees of freedom) is used as the criterion of goodness-of-fit (for this particular criterion, the fit is considered acceptable if 0.1 < WSOS/DF < 20). Its calculation is based on the χ2 test calculated according to Equation (17):
where (SSx)i is the i-th square of the deviation of i-th experimental value from the corresponding calculated one, and (sq)i is the estimate of standard deviation (uncertainty) of the i-th experimental point.
Then the WSOS/DF is obtained by means of Equation (18):
where nd is defined by means of Equation (19):
where nd is the number of degrees of freedom, np is the number of experimental points, and n is the number of model parameters sought during the regression procedure.
The experimental kinetic dependence was fitted step by step by all six models (shown in Table 1), resulting in the values of the goodness-of-fit criterion, WSOS/DF, and of the values of the overall kinetic coefficients.
2.3. Batch Experiments of 225Ac Sorption on the Surface of α-ZrP
The samples were mixed using an orbital shaker, KS250 basic (IKA-Werke GmbH & Co. KG, Germany), during the experiment investigating the sorption of 225Ac and 213Bi under static conditions in an acidic environment.
A phase separation of α-ZrP in nitric acid suspension to prepare a sample for gamma counting was performed using a coil of a Whatman GF/C microfiber glass filter (GE HealthCare, Chicago, IL, USA) in a 5 mL polypropylene tip. The activity of the samples was measured using an Ortec HPGe–Dspec Junior 2.0 (Ortec, Oak Ridge, TN, USA) gamma spectrometer. The measured spectra were evaluated using MAESTRO Multichannel Analyzer Emulation Software (version 7.01) (Ortec, United States). The evaluation of the measured gamma spectra was performed using a line of 213Bi with an energy 440 keV. Gamma spectrometry of all experimentally obtained samples was performed after establishing a permanent radioactive equilibrium between 225Ac and 213Bi.
Actinium-225 was purchased dry from the Joint Research Center, European Commission in Karlsruhe, Germany. Before use, 225Ac was dissolved in 0.1 M hydrochloric acid.
Batch experiments were performed by mixing a defined amount of prepared α-ZrP as an ion exchanger (100 mg) with a defined amount of nitric acid solution in the concentration range of 0.0001 to 1 M (10 mL). A defined amount of 225Ac (10 μL of stock solution in 0.1 M hydrochloric acid, corresponding to approximately 10 kBq of 225Ac, a molar concentration of Ac3+ of approximately 2 pmol/L) was added to the prepared α-ZrP in nitric or hydrochloric acid suspensions. The prepared suspensions were shaken for 2 h and then filtered through a glass fiber filter. For each sample, a corresponding standard solution was prepared (the only difference between the sample and the standard solution was the presence/absence of the α-ZrP in the vessel). Thanks to this experimental design, the sorption of 225Ac on the vessel walls had no influence on the values of the experimentally obtained weight distribution coefficients Dw. Aliquots of 1 mL volume were prepared from all samples and standards for gamma counting with the above-mentioned gamma spectrometer.
The weight distribution coefficients for 225Ac in each used concentration of nitric or hydrochloric acid were calculated by Equation (20):
where Ast is activity of standard [imp/s], A is activity of filtrate [imp/s], V is volume of aqueous phase [ml] and m is weight of α-ZrP [g].
3. Results and Discussion
3.1. Kinetic Study of natEu Sorption on the Surface of α-ZrP
Experimental data of natEu kinetic sorption on the α-ZrP surface are given in Table 2 as a fraction F of Eu cations remaining in the solution in time.

Table 2.
Values of fraction F of Eu cations remaining in the solution in time.
The fraction F was defined as:
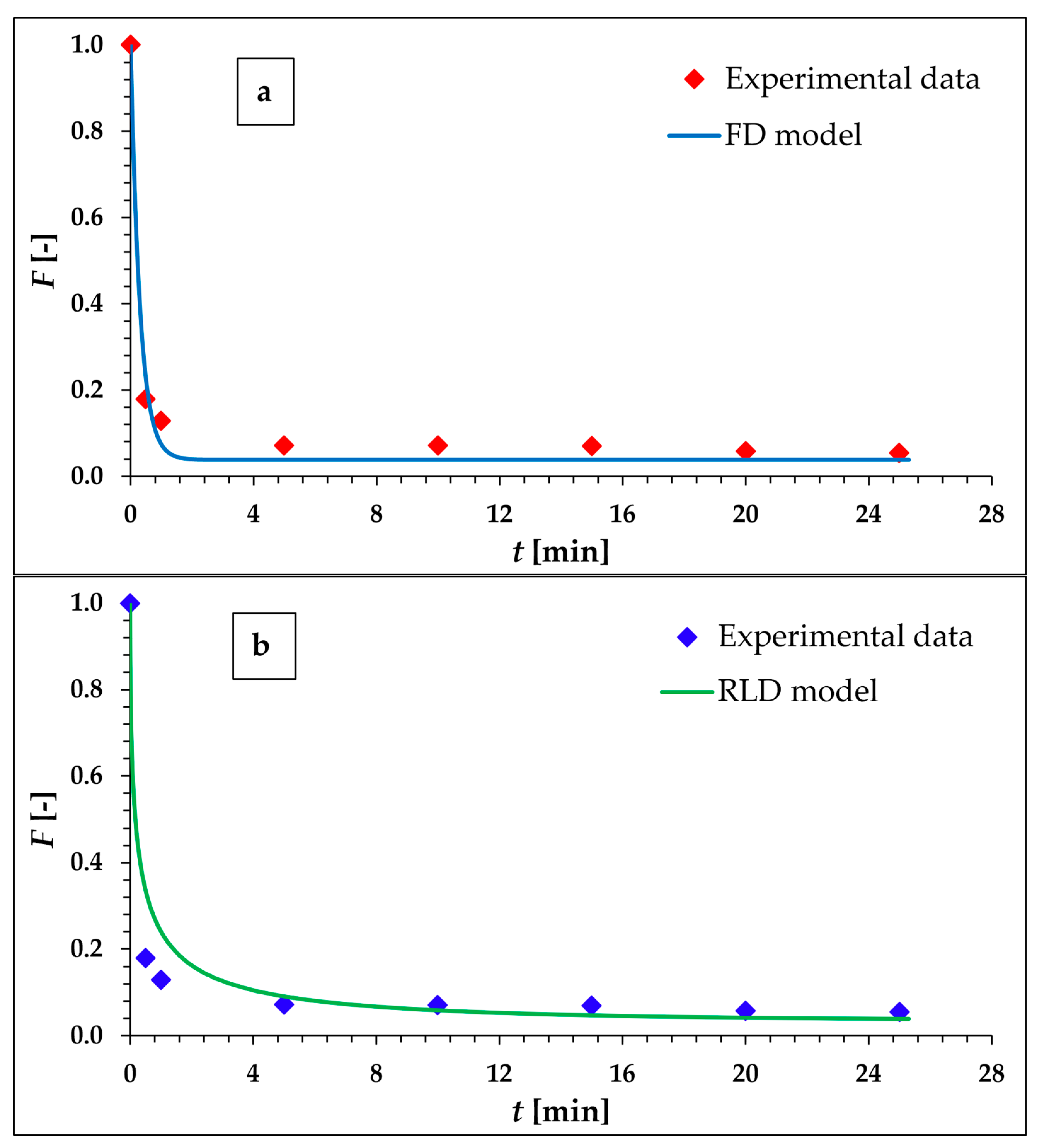
As can be seen from the kinetics data presented in Table 2, the kinetics of Eu sorption on the surface of α-ZrP is quite fast and in 5 min, 93% of the Eu cations are sorbed by α-ZrP. Based on the assumption that Eu is homologous to Ac, this makes α-ZrP a promising material in the case of 225Ac sorption, and modeling of the kinetic process will follow, with the aim of finding the control step of the sorption process. The results of the values of the goodness-of-fit criterion, WSOS/DF, and of the values of the overall kinetic mass transfer coefficients Kmodel resulting from the stepwise fitting of the experimental kinetic data using all six models mentioned and defined above, are presented in Table 3. The two most promising models (FD and RLD) are shown graphically in Figure 1a,b.

Table 3.
Values of goodness-of-fit criterion, WSOS/DF, and overall kinetic mass transfer coefficients, Kmodel.
Figure 1.
Evaluation of kinetic experimental data by means of FD (a) and RLD (b) kinetic models.
According to the WSOS/DF values, it is obvious that there is only one model suitable for modeling and describing the experimental data, namely the FD model. It is the model derived under the assumption that diffusion in the liquid film covering the surface of the solid sorbent particle is the rate-controlling process. The low value of the standard uncertainty (about 0.05%) of the KFD quantity also confirms the suitability of the FD model. Considering the relationships for the FD model in Table 1, it is clear that the rate of kinetics is inversely proportional to the surface film thickness and the particle size of the sorbent. It is directly proportional to the value of the partition coefficient Kd, which determines the value of the parameter c*, i.e., the magnitude of the driving force of the transport process.
The obtained result for the FD model agrees well with the sorption kinetics on classical ion exchangers, especially on cation exchangers, under conditions characterized by sorption from solutions with a very low concentration of the monitored component [37]. Finally, the studied sorbent is generally considered to be a cation exchanger.
3.2. Batch Experiment of 225Ac Sorption on the Surface of α-ZrP
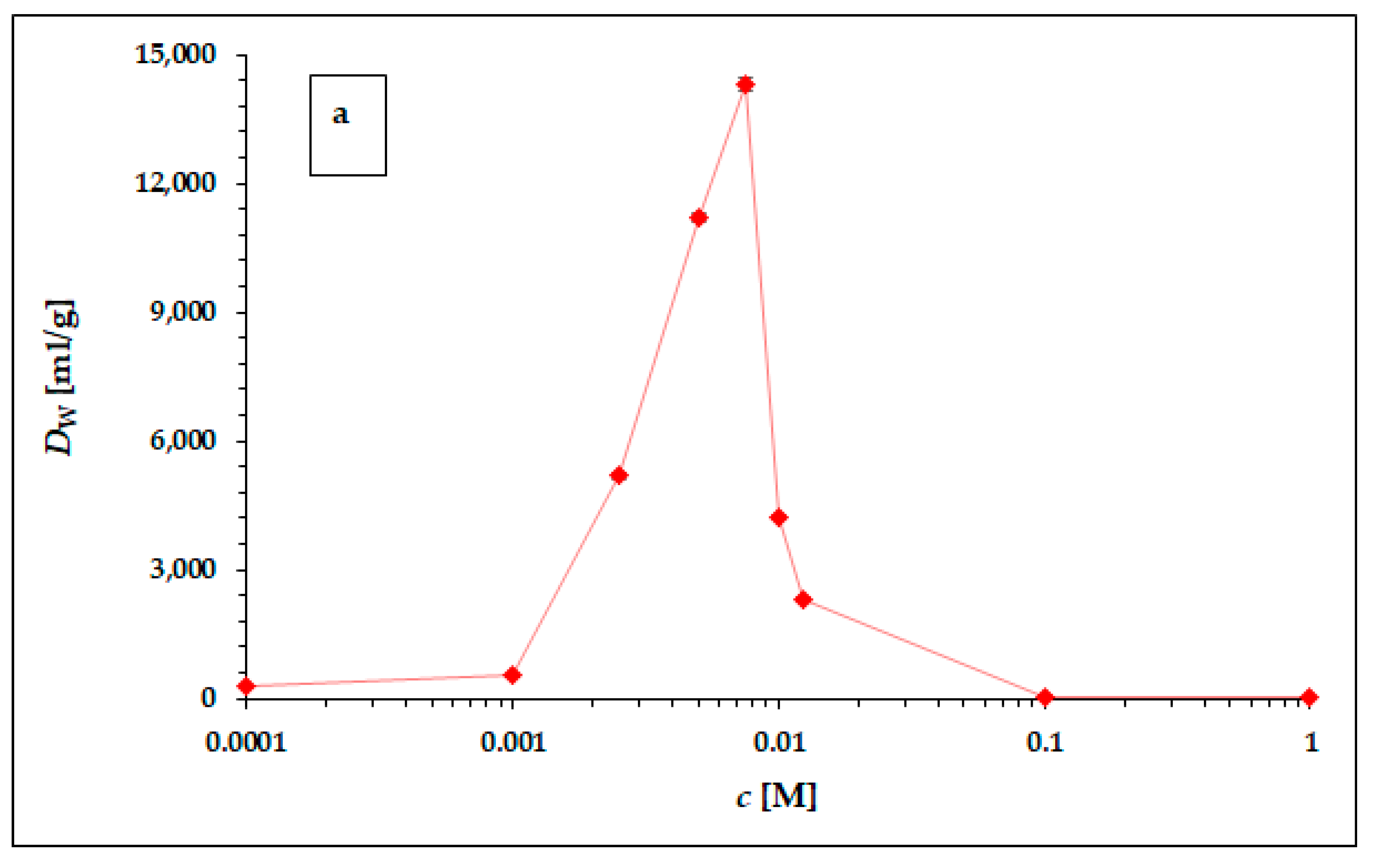
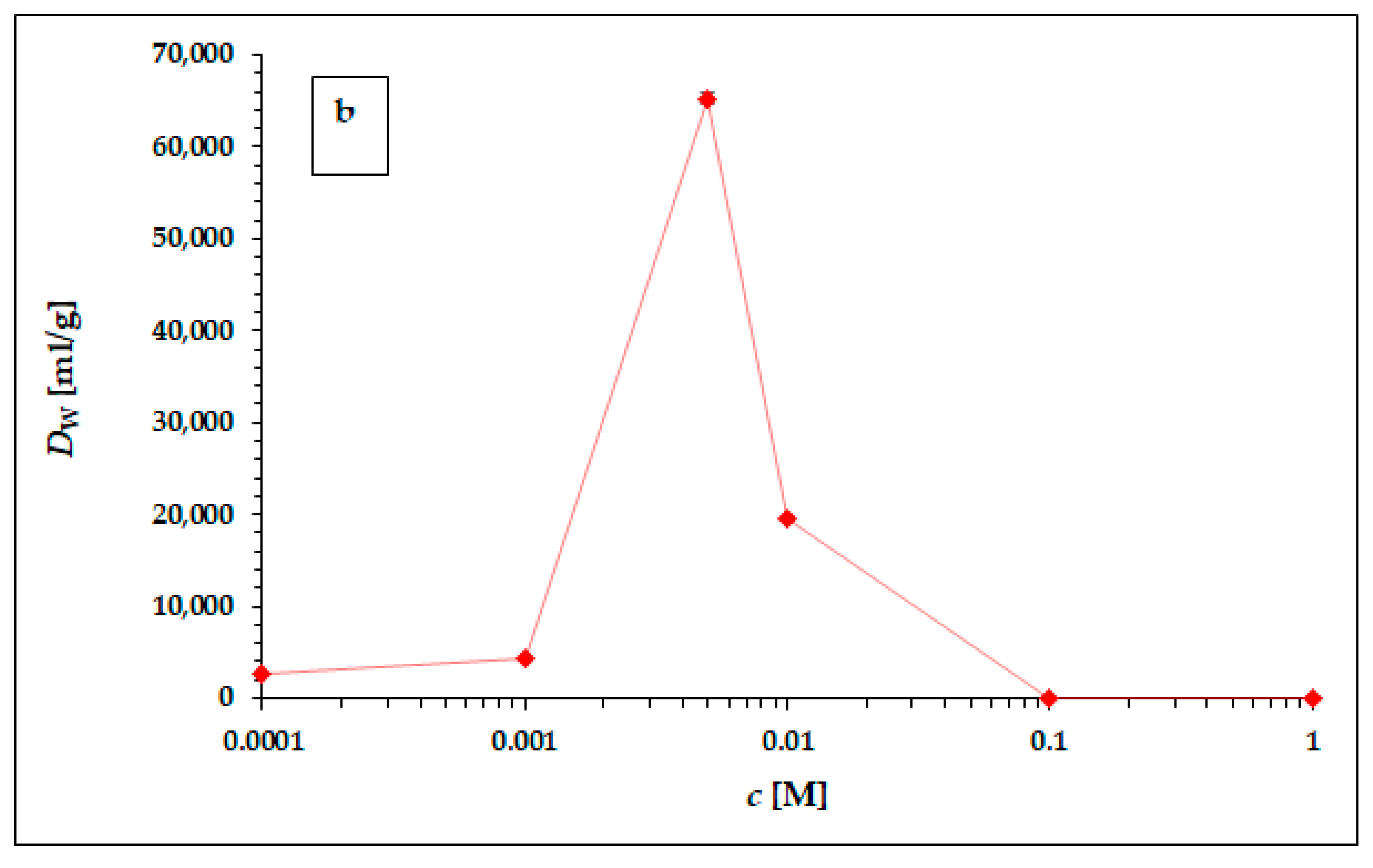
In Table 4 (results obtained in hydrochloric acid) and Table 5 (results obtained in nitric acid), the experimentally obtained results of 225Ac sorption on the particles of the α-ZrP static study, carried out according to the experimental setup described above, are presented as coefficients Dw. The graphical representation of the results obtained is shown in Figure 2a,b.

Table 4.
Values of experimentally obtained 225Ac weight distribution coefficients, Dw, in hydrochloric acid and its deviation, σDw, calculated based on error propagation law.

Table 5.
Values of experimentally obtained 225Ac weight distribution coefficients, Dw, in nitric acid and its deviation, σDw, calculated based on error propagation law.
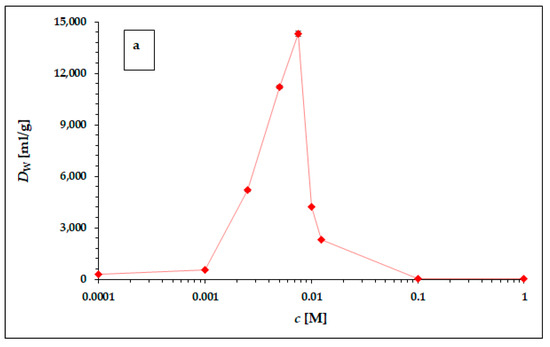
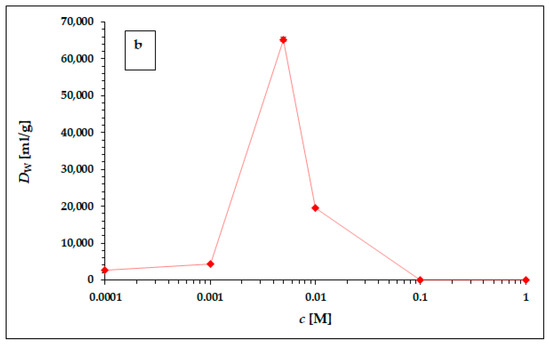
Figure 2.
The dependence of 225Ac weight distribution coefficient Dw in hydrochloric (a) and nitric (b) acid. The error bars corresponding to the deviations σDw presented in Table 4 and Table 5 are not visible due to the scale of the y-axis. The line chart was used for better orientation and following the dependence.
The dependence of the weight distribution coefficient Dw on the molarity c of the acid used, shown in Figure 2a,b, shows a sharp maximum in the concentration range around 0.005 M for both hydrochloric acid and nitric acid. As can be seen in Figure 2a,b, in the range of lower concentrations (from 0.0001 to 0.001 M) and higher concentrations (from 0.1 M to 1 M) for both acids, there is no visible or very little sorption of 225Ac. The values of the equilibrium weight distribution coefficient Dw for 225Ac in both hydrochloric and nitric acid reached values 14,303 ± 153 mL/g and 65,272 ± 612 mL/g respectively, in the concentration range of 5–7.5 mM. Therefore, the concentration range around 0.005 mM seems to be appropriate for the next applications (e.g., 225Ac/213Bi radionuclide generator construction). However, a comparison of the results obtained with the two acids used clearly shows that nitric acid is a more suitable medium for the sorption of 225Ac on the surface of α-ZrP.
The following possibilities are offered to explain this phenomenon:
- (a)
- In the range of hydrochloric and nitric acid concentrations higher than 0.01 M, i.e., at pH < 2, the cationic sorption capacity of α-ZrP decreases significantly, as shown not only by our results from the evaluation of the corresponding titration curve [32], but also by data in the literature devoted to the properties of α-ZrP, e.g., [39].
- (b)
- Increasing the acid concentration increases the ionic strength, which generally leads to a decrease in the equilibrium constants, eventually also of Dw.
- (c)
- The difference between the hydrochloric and nitric acid environments is probably due to the generally higher complexation efficiency of the chloride ligand compared with the nitrate ligand, resulting in a reduction of the cationic form in solution and a decrease of its sorption capacity. Unfortunately, the values of the stability constants for Ac3+-Cl− and Ac3+-NO3− complexation could not be found in the available literature. However, there is a significant difference in the values of the dissociation constants of these acids; in the case of hydrochloric acid, the pKa has value −6, and in the case of nitric acid, the pKa has value −1.32. Thus, the concentration of the ligand in the dissociated state is an order of magnitude higher in the case of hydrochloric acid than in the case of nitric acid, which may contribute to a higher complexation efficiency of the cationic form of 225Ac3+ and consequently to a lower sorption capacity of α-ZrP in the hydrochloric acid environment compared with the corresponding values in the nitric acid environment [40].
- (d)
- In the range of concentrations below 0.001 M, i.e., at pH > 3 and especially at pH > 4, a gradual hydrolysis of the cationic forms and an increase in the concentration of hydroxo-complexes in solution and a decrease in the sorption of the cationic forms can be expected.
4. Conclusions
The modeling of the experimental data shows that the kinetics of the model Eu sorption on the particles of α-ZrP can be described in the most satisfactory way using a model of film diffusion (FD). Taking into account the kinetic equations for FD, it is clear that the rate of kinetics is inversely proportional to the thickness of the surface film and the particle size of the sorbent (the particle size of the used particles was determined to be 150 nm in the previous work [32]). The results of the static sorption experiments show that the equilibrium weight distribution coefficients Dw for 225Ac in both hydrochloric and nitric acid reached the highest values of 14,303 ± 153 mL/g and 65,272 ± 612 mL/g, respectively, in the concentration range from 5.0 to 7.5 mM. From the comparison of these two results obtained for the equilibrium weight distribution coefficients Dw of 225Ac in both used acids, it can be concluded that nitric acid is a more promising environment for 225Ac sorption on the α-ZrP particles, and thus the use of nitric acid and α-ZrP can be considered as a very interesting combination for further research of 225Ac/213Bi generator construction. The developed material seems to be very promising even in comparison with other separation systems often described as generator systems in the literature. Compared to the extraction agents used, their use for the purpose of separating 213Bi from the parent 225Ac is described by some authors in the literature as a two-step process, where in the second step of the separation, the eluent is concentrated or the extractant molecule is removed and 213Bi is converted into a salt form [41,42]. Generator separation systems for the separation of 213Bi often appear in the literature, but they work as reverse systems, i.e., 213Bi is filtered on the columns while the parent 225Ac flows through the column without retention to the column material and the 213Bi is subsequently washed out with another eluent [42,43]. Even in comparison to these systems, the developed α-ZrP should be more advantageous, since it allows a classical separation in a direct arrangement. Compared with the above-mentioned separation systems, our developed material shows parameters and properties based on which it can be promised that the future generator system based on developed material combined with 5 mM nitric acid as eluent will give better results in terms of 213Bi acquisition rate, ease of carrying, and, of course, price.
Author Contributions
Conceptualization, L.O., K.O.F. and M.V.; methodology, L.O., K.O.F. and M.V.; software, K.Š.; investigation, L.O. and K.O.F.; resources, L.O. and K.O.F.; data curation, L.O.; writing—original draft preparation, L.O.; writing—review and editing, K.O.F., M.V., F.B., A.M., K.Š. and J.K.; supervision, M.V. and J.K.; project administration, L.O. and K.O.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Technology Agency of the Czech Republic, grant number TJ04000129, and the Czech Technical University in Prague, grant number SGS22/188/OHK4/3T/14. We are also grateful to Watrex Praha, s.r.o. for their material support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
There are no further data provided.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CR | chemical reaction in reaction zone |
| DM | mass transfer |
| FD | film diffusion |
| GD | gel diffusion |
| ID | diffusion in inert layer |
| ICP-MS | inductively coupled plasma mass spectrometry |
| RLD | diffusion in reacted layer |
| TAT | targeted alpha therapy |
| ZrP | zirconium(IV) phosphate |
References
- Clearfield, A.; Stynes, J.A. The preparation of crystalline zirconium phosphate and some observations on its ion exchange behavior. J. Inorg. Nucl. Chem. 1964, 26, 117–129. [Google Scholar] [CrossRef]
- Amphlett, C.B.; McDonald, L.A.; Redman, M.J. Synthetic inorganic ion-exchange materials—I zirconium phosphate. J. Inorg. Nucl. Chem. 1958, 6, 220–235. [Google Scholar] [CrossRef]
- Alberti, G.; Constantino, U. Recent progress in the field of synthetic inorganic exchangers having a layered or fibrous structure. J. Chromatogr. A 1974, 102, 5–29. [Google Scholar] [CrossRef]
- Clearfield, A.; Thakur, D.S. Zirconium and titanium phosphates as catalysts: A review. Appl. Catal. 1984, 26, 1–26. [Google Scholar] [CrossRef]
- Pica, M. Zirconium Phosphate Catalysts in the XXI Century: State of the Art from 2010 to Date. Catalysts 2017, 7, 190. [Google Scholar] [CrossRef]
- Colón, J.L.; Casañas, B. Drug carriers based on zirconium phosphate nanoparticles. In Tailored Organic-Inorganic Materials; Wiley: Hoboken, NJ, USA, 2015; pp. 395–437. [Google Scholar]
- Alongi, J.; Frache, A. Flame retardancy properties of α-zirconium phosphate based composites. Polym. Degrad. Stab. 2010, 95, 1928–1933. [Google Scholar] [CrossRef]
- Huang, T.C.; Lai, G.H.; Li, C.E.; Tsai, M.H.; Wan, P.Y.; Chung, Y.H.; Lin, M.H. Advanced anti-corrosion coatings prepared from α-zirconium phosphate/polyurethane nanocomposites. RSC Adv. 2017, 7, 9908–9913. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. Zirconium phosphate (ZrP)-based functional materials: Synthesis, properties and applications. Mater. Des. 2018, 155, 19–35. [Google Scholar] [CrossRef]
- Wiikinkoski, E.W.; Harjula, R.O.; Lehto, J.K.; Kemell, M.L.; Koivula, R.T. Effects of synthesis conditions on ion exchange properties of α-zirconium phosphate for Eu and Am. Radiochim. Acta 2017, 105, 1033–1042. [Google Scholar] [CrossRef][Green Version]
- Wiikinkoski, E.W.; Rautsola, I.; Xu, J.; Koivula, R. Column Separation of Am (III) and Eu (III) by α-Zirconium Phosphate Ion Exchanger in Nitric Acid. Chem. Eng. 2020, 4, 14. [Google Scholar] [CrossRef]
- Wiikinkoski, E.W.; Xu, J.; Zhang, W.; Hietala, S.; Koivula, R.T. Modification of α-Zirconium Phosphate Synthesis–Effects of Crystallinity and Acidity on Eu (III) and Am (III) Ion Exchange. ChemistrySelect 2018, 3, 9583–9588. [Google Scholar] [CrossRef]
- Lee, J.Y.; Vyas, C.K.; Kim, B.R.; Kim, H.J.; Hur, M.G.; Yang, S.D.; Park, J.H.; Kim, S.W. Acid resistant zirconium phosphate for the long term application of 68Ge/68Ga generator system. Appl. Radiat. Isot. 2016, 118, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. Group IV phosphates as catalysts and catalyst supports. J. Mol. Catal. 1984, 27, 251–262. [Google Scholar] [CrossRef]
- Troup, J.; Clearfield, A. Mechanism of ion exchange in zirconium phosphates. 20. Refinement of the crystal structure of α-zirconium phosphate. Inorg. Chem. 1977, 16, 3311–3314. [Google Scholar] [CrossRef]
- Clearfield, A. Inorganic ion exchangers with layered structures. Annu. Rev. Mater. Sci. 1984, 14, 205–229. [Google Scholar] [CrossRef]
- Albertsson, J.; Oskarsson, A.; Tellgren, R.; Thomas, J. Inorganic ion exchangers. 10. A neutron powder diffraction study of the hydrogen bond geometry in α-zirconium bis(monohydrogen orthophosphate) monohydrate. A model for the ion exchange. J. Phys. Chem. 1977, 81, 1574–1578. [Google Scholar] [CrossRef]
- Clearfield, A.; Smith, G.D. The crystallography and structure of α-zirconium bis(monohydrogenorthophosphate) monohydrate. Inorg. Chem. 1969, 8, 431. [Google Scholar] [CrossRef]
- Kullberg, L.; Clearfield, A. Mechanism of ion exchange in zirconium phosphates. 32. Thermodynamics of alkali metal ion exchange on crystalline α-ZrP. J. Phys. Chem. 1981, 85, 1585–1589. [Google Scholar] [CrossRef]
- Möller, T.; Bestaoui, N.; Wierzbicki, M.; Adams, T.; Clearfield, A. Separation of lanthanum, hafnium, barium and radiotracers yttrium-88 and barium-133 using crystalline zirconium phosphate and phosphonate compounds as prospective materials for a Ra-223 radioisotope generator. Appl. Radiat. Isot. 2011, 69, 947–954. [Google Scholar] [CrossRef]
- Mimura, H.; Akiba, K. Adsorption properties of europium on granulated α-zirconium phosphate. J. Nucl. Sci. Technol. 1996, 33, 592–596. [Google Scholar] [CrossRef]
- Jayswal, A.; Chudasama, U. Synthesis and Characterization of a New Phase of Zirconium Phosphate for the Separation of Metal Ions. J. Iran. Chem. Soc. 2007, 4, 510–515. [Google Scholar] [CrossRef]
- Xu, J.; Virolainen, S.; Zhang, W.; Kuva, J.; Sainio, T.; Koivula, R. Polyacrylonitrile-encapsulated amorphous zirconium phosphate composite adsorbent for Co, Nd and Dy separations. J. Chem. Eng. 2018, 351, 832–840. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host–Guest Interactions. Adv. Mater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted α particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Allen, B.J.; Raja, C.; Rizvi, S.; Li, Y.; Tsui, W.; Graham, P.; Thompson, J.; Reisfeld, R.; Kearsley, J.; Morgenstern, A.; et al. Intralesional targeted alpha therapy for metastatic melanoma. Cancer Biol. Ther. 2005, 4, 1318–1324. [Google Scholar] [CrossRef]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local targeting of malignant gliomas by the diffusible peptidic vector 1, 4, 7, 10-tetraazacyclododecane-1-glutaric acid-4, 7, 10-triacetic acid-substance p. Clin. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef]
- Kellerbauer, A.; Bruchertseifer, F.; Malmbeck, R.; Morgenstern, A. Targeted α therapy with 213Bi and 225Ac. J. Phys. Conf. Ser. 2020, 1643, 012205. [Google Scholar] [CrossRef]
- Ondrák, L.; Ondrák-Fialová, K.; Sakmár, M.; Vlk, M.; Štamberg, K.; Drtinová, B.; Šlouf, M.; Bruchertseifer, F.; Morgenstern, A.; Kozempel, J. Preparation and characterization of α-zirconium phosphate as a perspective material for separation of 225Ac and 213Bi. J. Radioanal. Nucl. Chem. 2023, 332, 1527–1532. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Zhang, D.; Wang, S.; Qiu, M.; Song, G.; Fu, D.; Hu, B.; Wang, X. MXenes as emerging nanomaterials in water purification and environmental remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef] [PubMed]
- Missana, T.; Alonso, U.; García-Gutiérrez, M. Evaluation of component additive modeling approach for europium adsorption on 2:1 clays: Experimental, thermodynamic databases, and models. Chemosphere 2021, 272, 129877. [Google Scholar] [CrossRef] [PubMed]
- Rabung, T.; Pierret, M.C.; Bauer, A.; Geckeis, H.; Bradbury, M.H.; Baeyens, B. Sorption of Eu (III)/Cm (III) on Ca-montmorillonite and Na-illite. Part 1: Batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim. Cosmochim. 2005, 69, 5393–5402. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B.; Geckeis, H.; Rabung, T. Sorption of Eu (III)/Cm (III) on Ca-montmorillonite and Na-illite. Part 2: Surface complexation modeling. Geochim. Cosmochim. 2005, 69, 5403–5412. [Google Scholar] [CrossRef]
- Beneš, P.; Stamberg, K.; Štegman, R. Study of the Kinetics of Interaction of Cs-137 and Sr-85 with Soils using a Batch Method: Methodological Problems. Radiochim. Acta 1994, 66, 315–321. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Determination, modeling and evaluation of kinetics of 223Ra sorption on hydroxyapatite and titanium dioxide nanoparticle. Materials 2020, 13, 1915. [Google Scholar] [CrossRef]
- Manecke, G. Ionenaustauscher, Band I: Grundlagen. Struktur—Herstellung—Theorie, von F. Helfferich. Verlag Chemie GmbH, Weinheim/Bergstraße 1959. 1. Aufl., VIII, 520 S., 153 Abb., 14 Tab., geb. DM 48.–. Angew. Chem. 1962, 74, 596. [Google Scholar] [CrossRef]
- Starý, J.; Kyrš, M.; Marhol, M. Separační metody v radiochemii (Separation methods in radiochemistry); Academia: Praha, Czech Republic, 1975; 253p. [Google Scholar]
- Wu, C.; Brechbiel, M.W.; Gansow, O.A. An improved generator for the production of 213Bi from 225Ac. Radiochim. Acta 1997, 79, 141–144. [Google Scholar] [CrossRef]
- McAlister, D.R.; Horwitz, E.P. Automated two column generator systems for medical radionuclides. Appl. Radiat. Isot. 2009, 67, 1985–1991. [Google Scholar] [CrossRef]
- Bray, L.A.; Tingey, J.M.; DesChane, J.R.; Egorov, O.B.; Tenforde, T.S.; Wilbur, D.S.; Hamlin, D.K.; Pathare, P.M. Development of a unique bismuth (Bi-213) automated generator for use in cancer therapy. Ind. Eng. Chem. Res. 2000, 39, 3189–3194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).