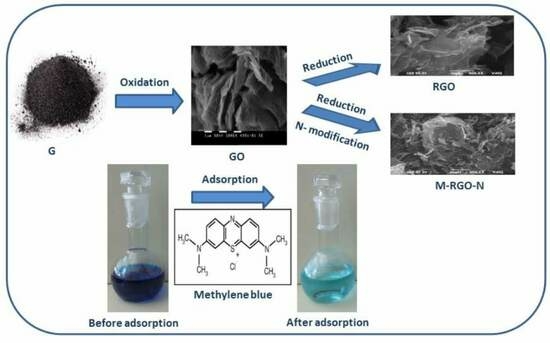
Study on the Dye Removal from Aqueous Solutions by Graphene-Based Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization
2.3. Adsorption Studies
3. Results and Discussion
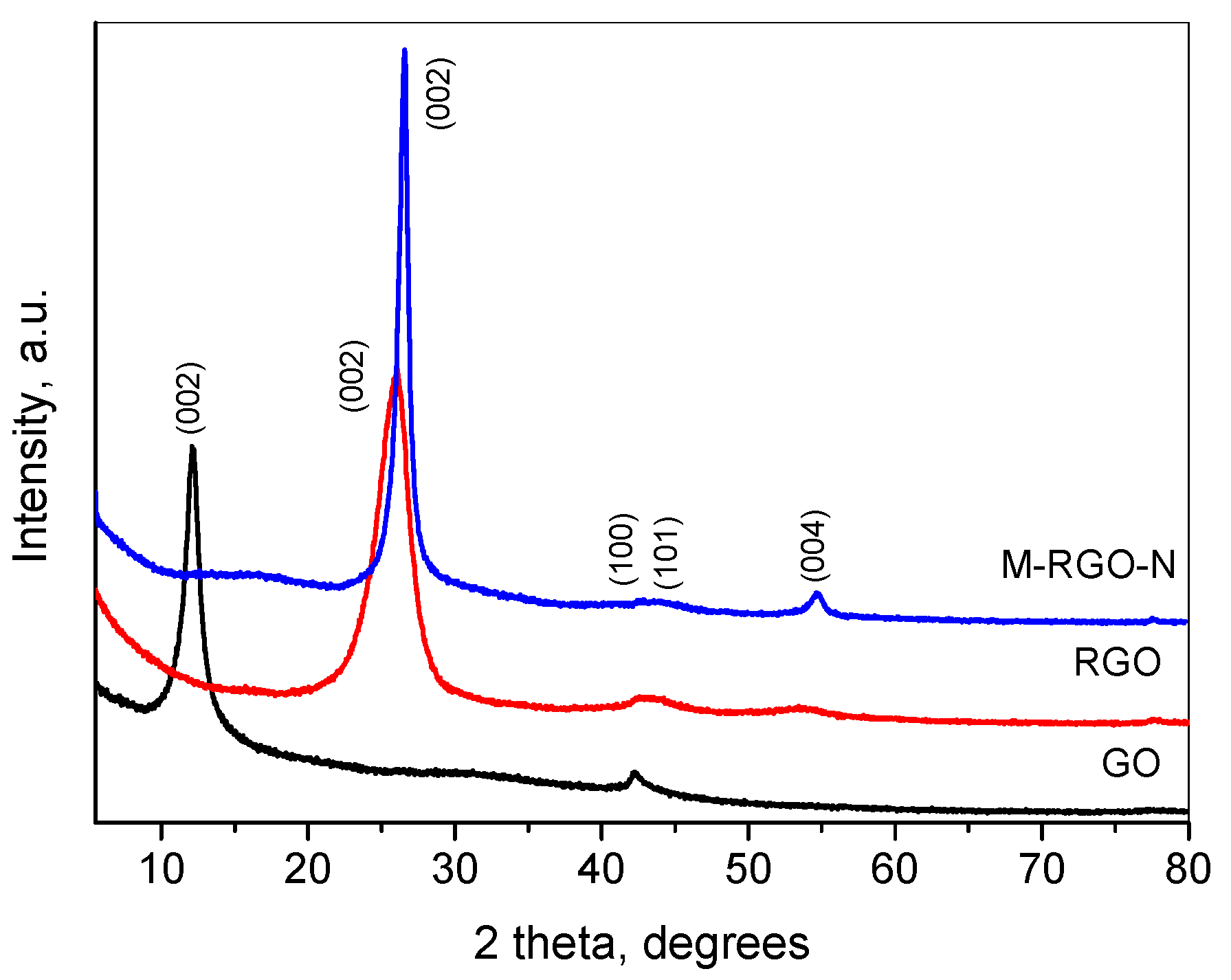
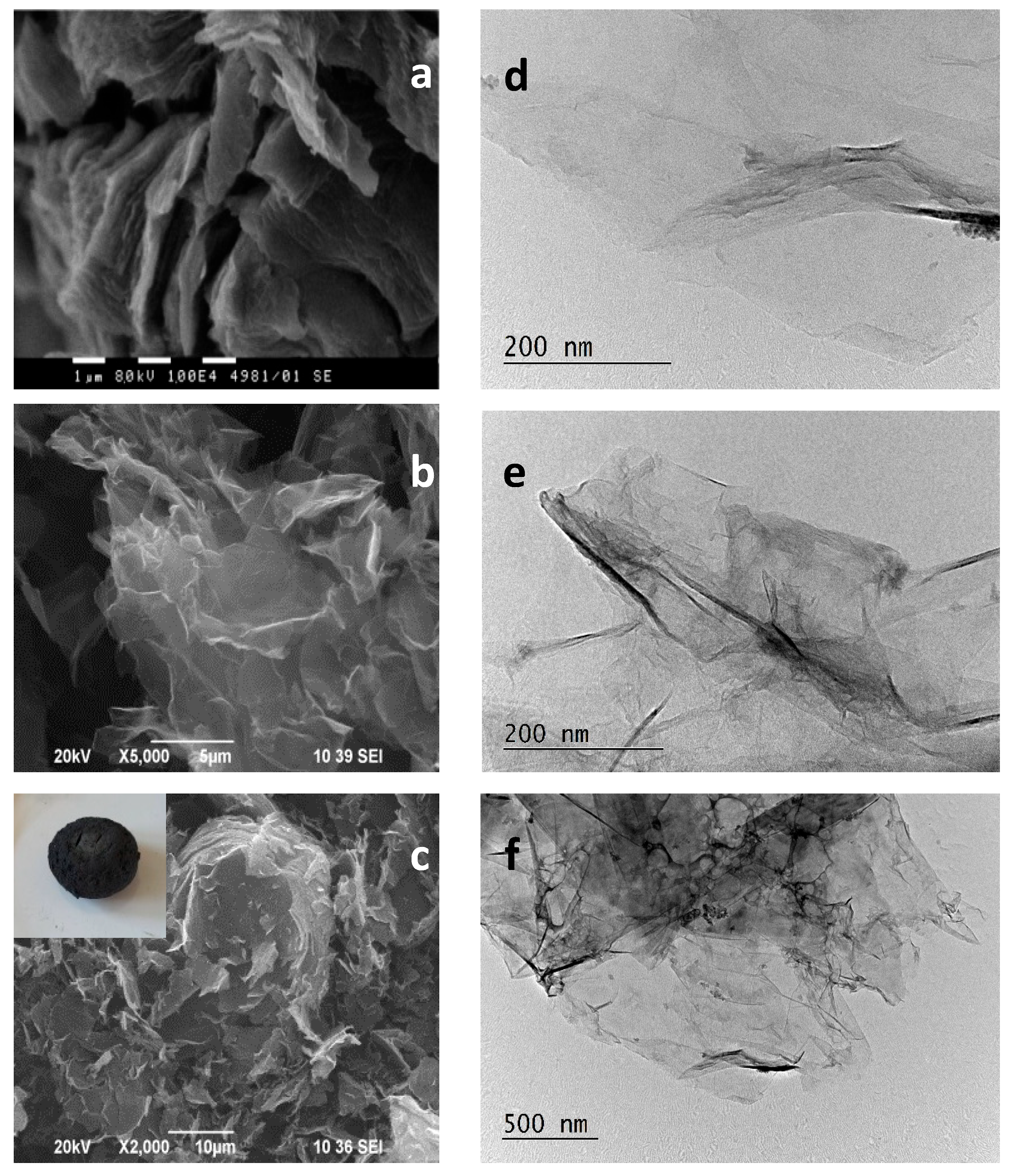
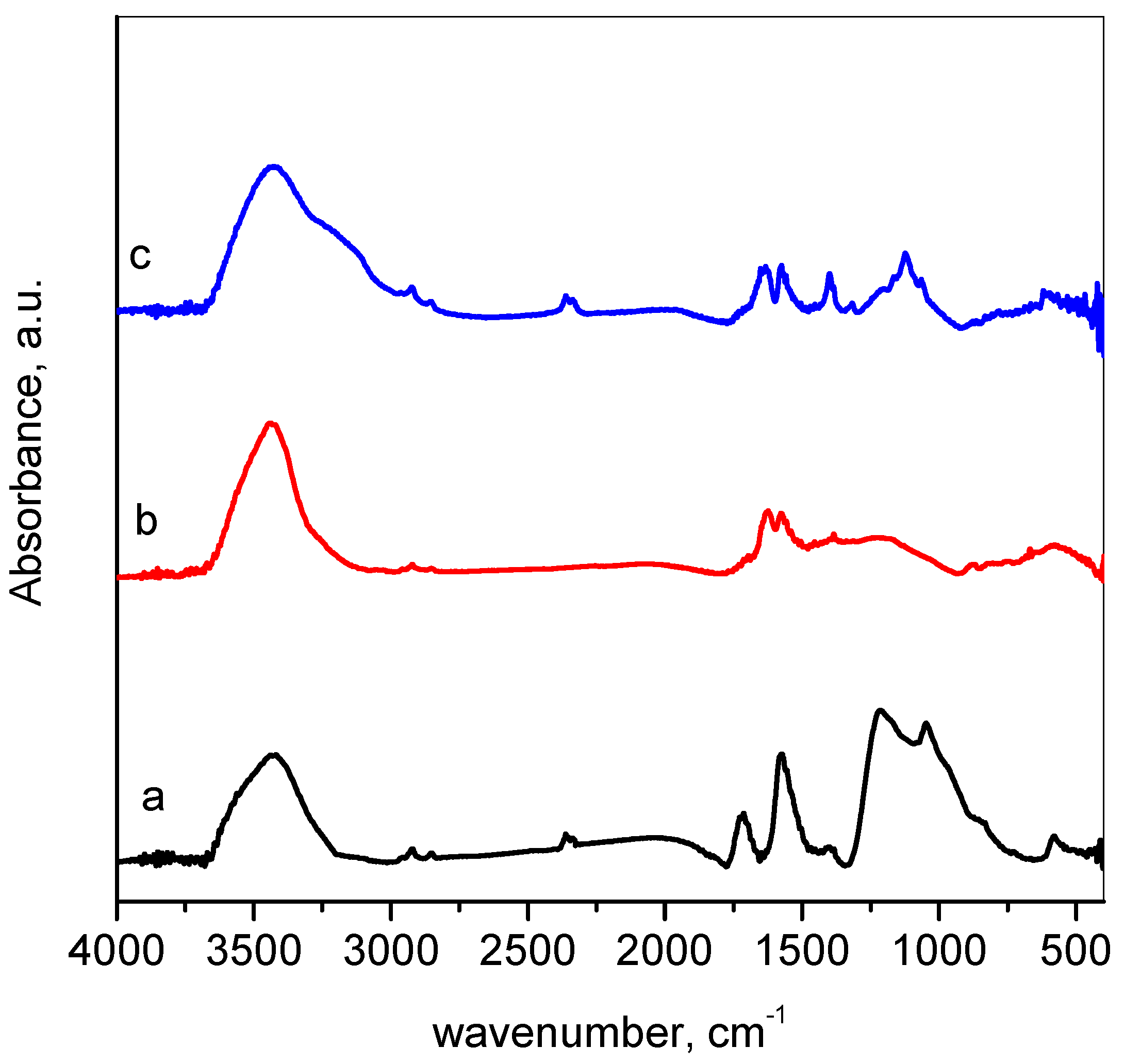
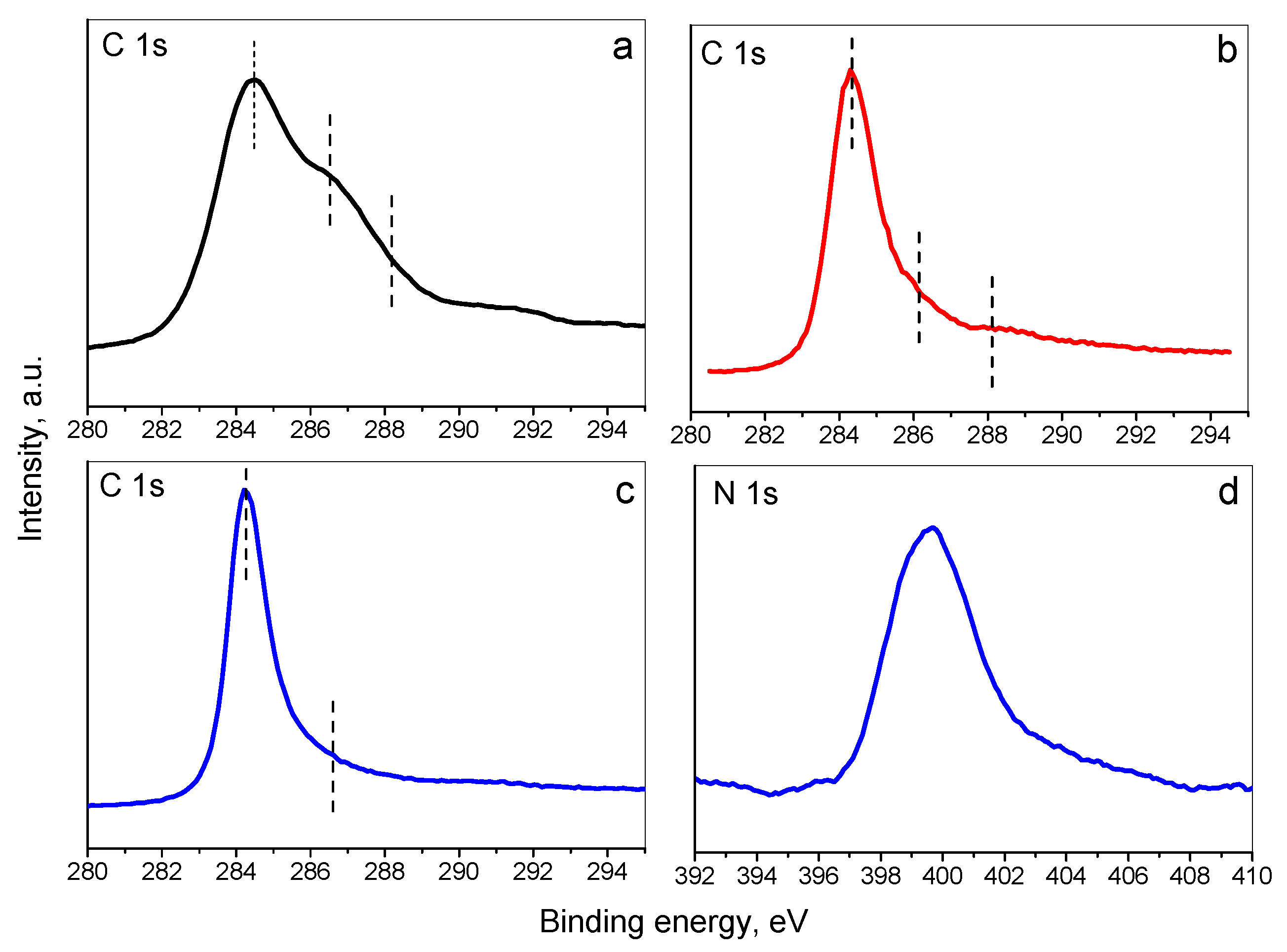
3.1. Materials Characterization
3.2. Adsorption Studies
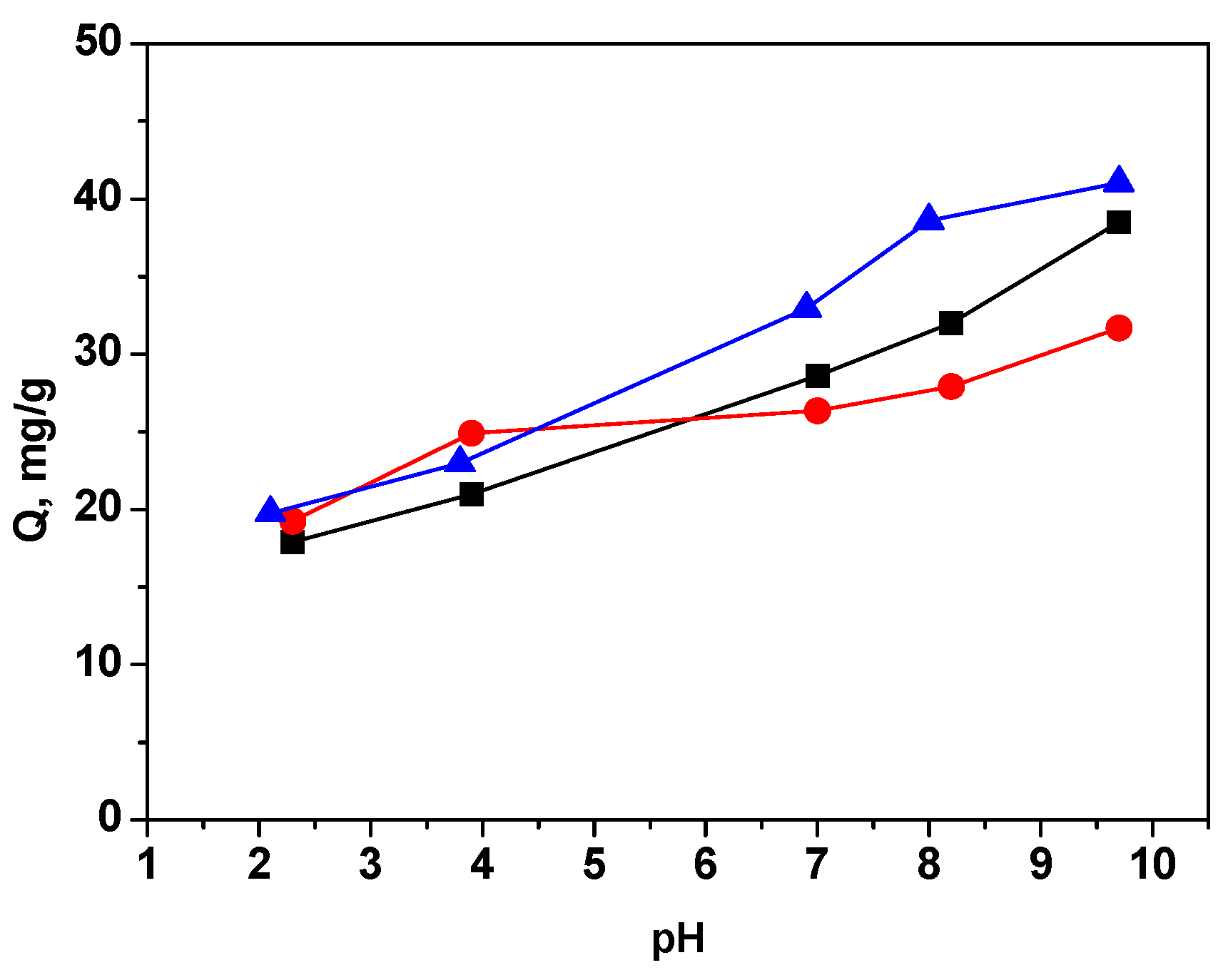
3.2.1. Effect of pH on the Adsorption Process
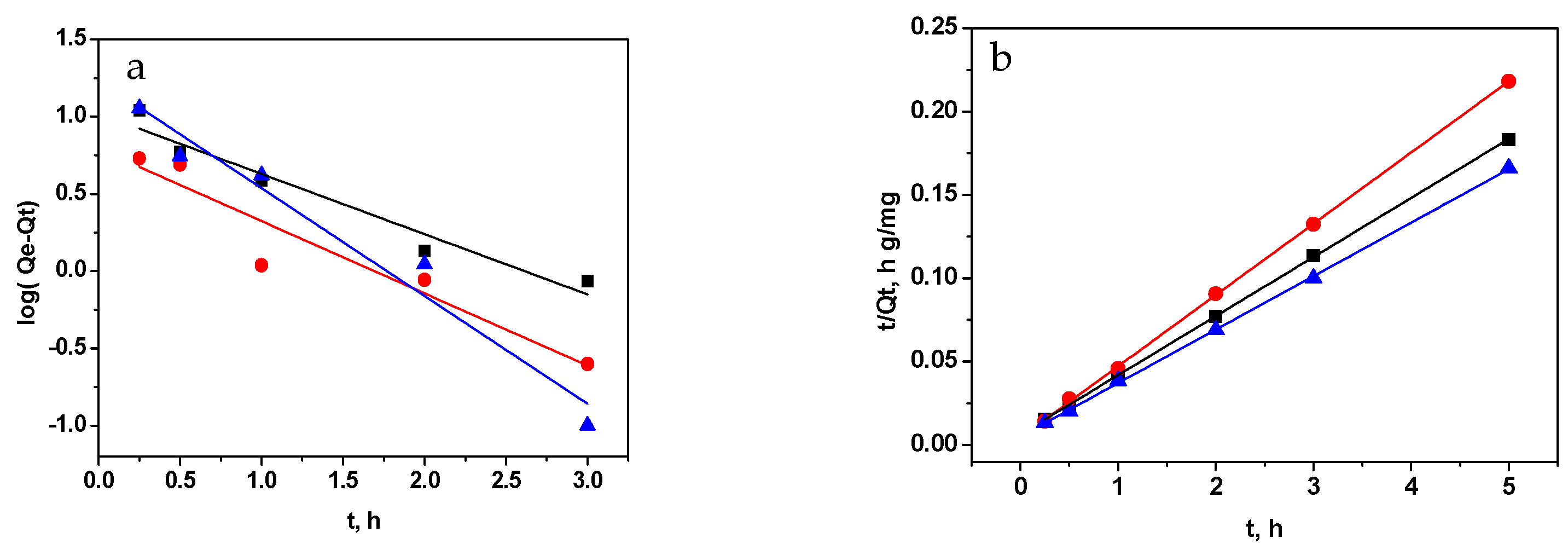
3.2.2. Effect of Contact Time and Kinetic Study
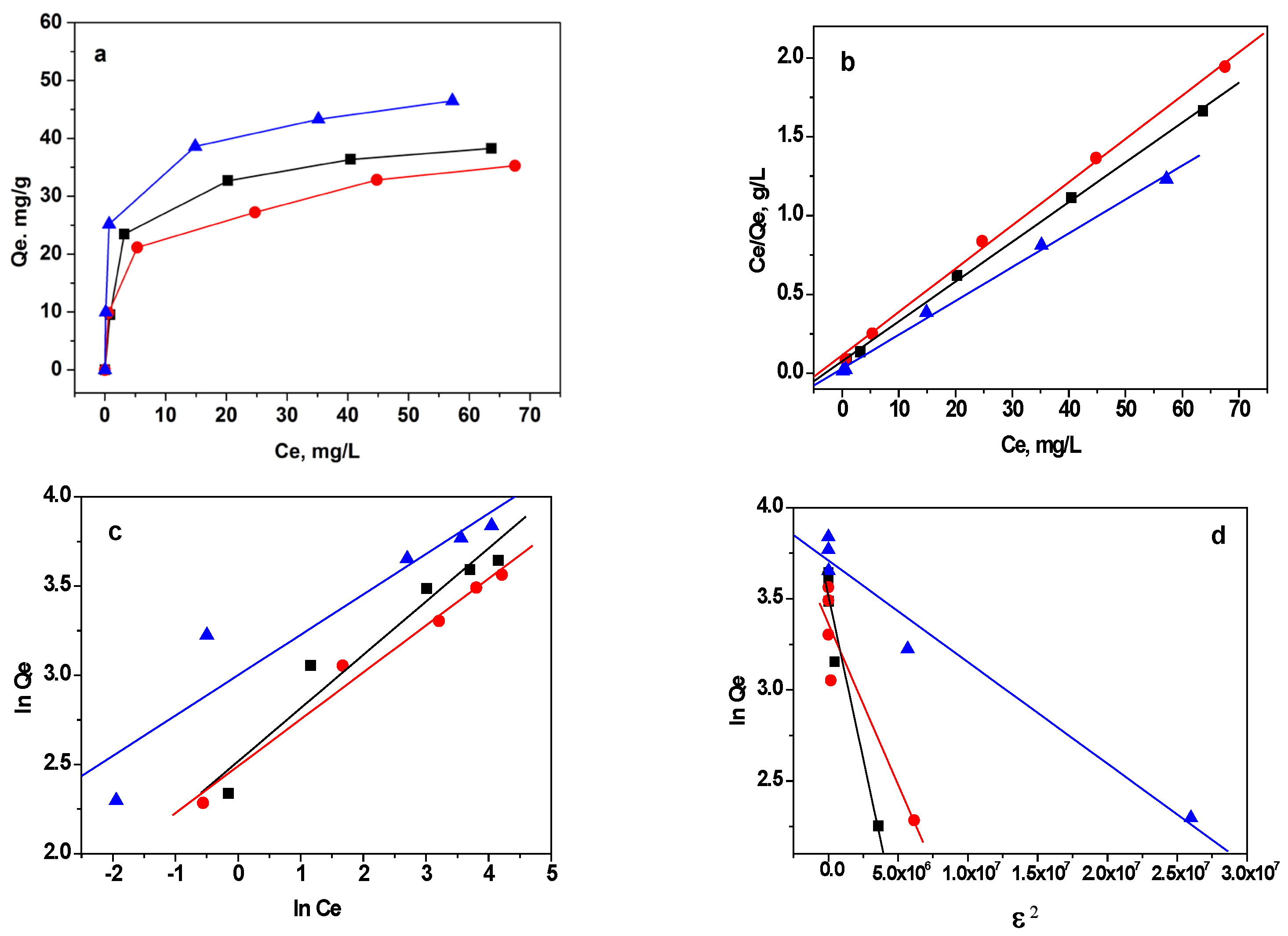
3.2.3. Adsorption Isotherms
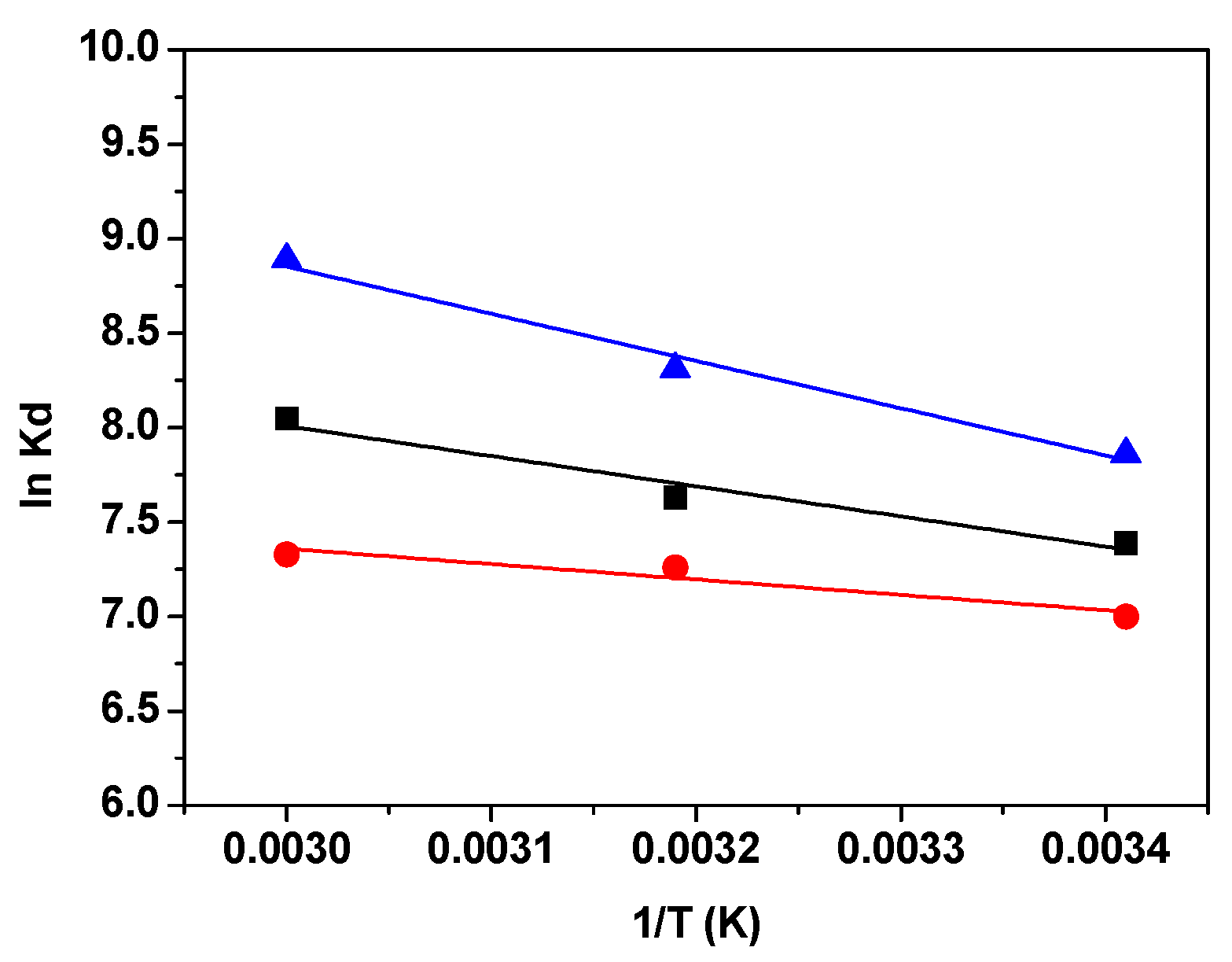
3.2.4. Thermodynamic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basha, I.K.; El-Monaem, E.M.A.; Khalifa, R.E.; Omer, A.M.; Eltawei, A.S. Sulfonated graphene oxide impregnated cellulose acetate foated beads for adsorption of methylene blue dye: Optimization using response surface methodology. Sci. Rep. 2022, 12, 9339. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Nezhad, A.A.; Alimoradi, M.; Ramezani, M. One-step Preparation of graphene oxide/polypyrrole magnetic nanocomposite and its application in the removal of methylene blue dye from aqueous solution. Mater. Res. Express 2018, 5, 025508. [Google Scholar] [CrossRef]
- Paton-Carrero, A.; Sanchez, P.; Sánchez-Silva, L.; Romero, A. Graphene-based materials behaviour for dyes adsorption. Mater. Today Commun. 2022, 30, 103033. [Google Scholar] [CrossRef]
- Shao, Y.M.; Wang, X.; Kang, Y.; Shu, Y.H.; Sun, Q.Q.; Li, L.S. Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J. Colloid Interface Sci. 2014, 429, 25–33. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Yang, Z.; Zou, J.; Tang, H. Enhanced Adsorption of Methylene Blue on Graphene Oxide by Tuning the Oxidation Degree of Graphene Oxide. J. Nanomater. Mol. Nanotechnol. 2013, S1, 003. [Google Scholar] [CrossRef]
- Vassileva, P.; Voykova, D.; Uzunov, I.; Uzunova, S. Methylene blue adsorption by Triticum monococcum L. husks based materials. CR Acad. Bulg. Sci. 2018, 71, 1192–1199. [Google Scholar]
- Martis, L.J.; Parushurama, N.; Sangappa, Y. Preparation, characterization, and methylene blue dye adsorption study of silk fibroin–graphene oxide nanocomposites. Environ. Sci. Adv. 2022, 1, 285–296. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Varadarajan, P.R.; Porkodi, K.; Subbhuraam, C.V. Adsorption of methylene blue onto jute fiber carbon: Kinetics and equilibrium studies. J. Colloid Interface Sci. 2005, 284, 78–82. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ethaib, S.; Zubaidi, S.L. Removal of Methylene Blue Dye from Aqueous Solution Using Kaolin. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 022030. [Google Scholar] [CrossRef]
- Mohrazi, A.; Ghasemi-Fasaei, R. Removal of methylene blue dye from aqueous solution using an efficient chitosan-pectin bio-adsorbent: Kinetics and isotherm studies. Environ. Monit. Assess. 2023, 195, 339. [Google Scholar] [CrossRef]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M.H. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef]
- Rahman, M.M.; Foisal, J.A.; Ihara, H.; Takafuji, M. Efficient removal of methylene blue dye from an aqueous solution using silica nanoparticle crosslinked acrylamide hybrid hydrogels. New J. Chem. 2021, 45, 20107–20119. [Google Scholar] [CrossRef]
- Benhachem, F.; Attar, T.; Bouabdallah, F. Kinetic study of adsorption methylene blue dye from aqueous solutions using activated carbon. Chem. Rev. Lett. 2019, 2, 33–39. [Google Scholar] [CrossRef]
- Rahman, S.; Ajmal, M.; Muhammad, S. Micron sized anionic poly (methacrylic acid) microgel particles for the adsorptive elimination of cationic water pollutants. Z. Phys. Chem. 2023, 237, 121–145. [Google Scholar] [CrossRef]
- Amjad, S.; Shaukat, S.; Rahman, H.M.A.; Usman, M.; Farooqi, Z.H.; Nazar, M.F. Application of anionic-nonionic mixed micellar system for solubilization of methylene blue dye. J. Mol. Liq. 2023, 369, 120958. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Ghufran, M.; Muhammad, Z.R.; Najeeb, J.; Irfan, A.; Al-Sehemi, A.G. Poly(N-isopropylmethacrylamide-acrylic acid) microgels as adsorbent for removal of toxic dyes from aqueous medium. J. Mol. Liq. 2018, 268, 229–238. [Google Scholar] [CrossRef]
- Sindona, A.; Pisarra, M.; Gomez, C.V.; Riccardi, P.; Falcone, G.; Bellucci, S. Calibration of the fine-structure constant of graphene by time-dependent density-functional theory. Phys. Rev. B 2017, 96, 201408. [Google Scholar] [CrossRef]
- Coello-Fiallos, D.; Tene, T.; Guayllas, J.L.; Haro, D.; Haro, A.; Gomez, C.V. DFT comparison of structural and electronic properties of graphene and germanene: Monolayer and bilayer systems. Mater. Today Proc. 2017, 4, 6835–6841. [Google Scholar] [CrossRef]
- Abdelhalim, A.O.E.; Semenov, K.N.; Nerukh, D.A.; Murin, I.V.; Maistrenko, D.N.; Molchanov, O.E.; Sharoyko, V.V. Functionalisation of graphene as a tool for developing nanomaterials. J. Mol. Liq. 2022, 348, 118368. [Google Scholar] [CrossRef]
- Ghulam, A.N.; dos Santos, O.A.L.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.T.; Feng, S.; Ma, Q.; Peng, X.; Wu, D. Preparation and application of carboxylated graphene oxide sponge in dye removal. Int. J. Environ. Res. Public Health 2017, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Rahman, D.A.; Helmy, Q.; Syafila, M.; Gumilar, A. Adsorption of Dyes Using Graphene Oxide-Based Nano-Adsorbent: A Review. Jurnal Presipitasi Media Komunikasi Pengembangan Teknik Lingkungan 2022, 19, 384–397. [Google Scholar] [CrossRef]
- Ederer, J.; Ecorchard, P.; Slušná, M.; Tolasz, J.; Smržová, D.; Lupínková, S.; Janoš, P. A Study of Methylene Blue Dye Interaction and Adsorption by Monolayer Graphene Oxide. Adsorpt. Sci. Technol. 2022, 2022, 7385541. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Elabiad, M.A.; Mohamed, A.A. Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100719. [Google Scholar] [CrossRef]
- Khurana, I.; Bharti, A.S.; Khurana, J.M.; Rai, P.K. Removal of Dyes Using Graphene-Based Composites: A Review. Water Air Soil Pollut. 2017, 228, 180. [Google Scholar] [CrossRef]
- Verma, S.; Kim, K.-H. Graphene-based materials for the adsorptive removal of uranium in aqueous solutions. Environ. Int. 2022, 158, 106944. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.H.; Razali, N.F.; Sajab, M.S.; Zakaria, S.; Huang, N.M.; Lim, H.N. Methylene blue adsorption on graphene oxide. Sains Malays. 2013, 42, 819–826. [Google Scholar]
- Chen, L.; Yang, J.; Zeng, X.; Zhang, L.; Yuan, W. Adsorption of methylene blue in water by reduced graphene oxide: Effect of functional groups. Mater. Express 2013, 3, 281–290. [Google Scholar] [CrossRef]
- Chilakapati, R.B.; Kumar, S.H.; Satyanarayana, S.V.; Behara, D.K. Adsorptive removal of methylene blue (MB) and malachite green (MG) dyes from aqueous solutions using graphene oxide (GO). Z. Phys. Chem. 2021, 235, 1645–1660. [Google Scholar] [CrossRef]
- Vinh, L.T.; Khiem, T.N.; Chinh, H.P.; Tuan, P.V.; Tan, V.T. Adsorption capacities of reduced graphene oxide: Effect of reductants. Mater. Res. Express 2019, 6, 075615. [Google Scholar] [CrossRef]
- Soudagar, S.; Akash, S.; Venkat, M.S.; Poiba, V.R.; Vangalapati, M. Adsorption of methylene blue dye on nano graphene oxide-thermodynamics and kinetic studies. Mater. Today Proc. 2022, 59, 667–672. [Google Scholar] [CrossRef]
- Arias, F.A.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Gomez, C.V.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Haşimoğlu, A.; Özdemir, O.K. Synthesis, characterization and an application of graphene oxide nanopowder: Methylene blue adsorption and comparison between experimental data and literature data. J. Dispers. Sci. Technol. 2021, 42, 771–783. [Google Scholar] [CrossRef]
- Khiam, G.K.; Karri, R.R.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Rahman, M.E. Modelling and optimization for methylene blue adsorption using graphene oxide/chitosan composites via artificial neural network particle swarm optimization. Mater. Today Chem. 2022, 24, 100946. [Google Scholar] [CrossRef]
- Sharma, P.; Hussain, N.; Borah, D.J.; Das, M.R. Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: A comparative study. J. Chem. Eng. Data 2013, 58, 3477–3488. [Google Scholar] [CrossRef]
- Yun, S.; Lee, H.; Lee, W.-E.; Park, H.S. Multiscale textured, ultralight graphene monoliths for enhanced CO2 and SO2 adsorption capacity. Fuel 2016, 174, 36–42. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rao, G.-S.; Nabipour, H.; Zhang, P.; Wang, X.; Xing, W.; Song, L.; Hu, Y. Lightweight, hydrophobic and recyclable carbon foam derived from lignin–resorcinol–glyoxal resin for oil and solvent spill capture. J. Mater. Technol. 2020, 9, 4655–4664. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Van Khai, T.; Na, H.G.; Kwak, D.S.; Kwon, Y.J.; Ham, H.; Shim, K.B.; Kim, H.W. Comparison study of structural and optical properties of boron-doped and undoped graphene oxide films. Chem. Eng. J. 2012, 211–212, 369–377. [Google Scholar] [CrossRef]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Preparation of Nitrogen-Doped Graphene Sheets by a Combined Chemical and Hydrothermal Reduction of Graphene Oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Arafa, W.A.A.; Ahmed, I.M.; El-Shanshory, A.A.; Abu-Saied, M.A.; Soliman, H.M.A.; Abdelgawad, M.A.; Ali, H.M.; Bräse, S. Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater. Polymers 2022, 14, 4236. [Google Scholar] [CrossRef]
- Ajmal, M.; Demirci, S.; Siddiq, M.; Aktas, N.; Sahiner, N. Amidoximated poly (acrylonitrile) particles for environmental applications: Removal of heavy metal ions, dyes, and herbicides from water with different sources. J. Appl. Polym. Sci. 2016, 133, 7. [Google Scholar] [CrossRef]
- Vassileva, P.; Uzunov, I.; Voykova, D. Kinetics, equilibrium and thermodynamics of Congo red removal by cationized cellulose obtained from cereals by-product. Cellul. Chem. Technol. 2022, 56, 1117–1128. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Juang, R.-S. Efficient removal of methylene blue dye by a hybrid adsorption–photocatalysis process using reduced graphene oxide/titanate nanotube composites for water reuse. J. Ind. Eng. Chem. 2019, 76, 296–309. [Google Scholar] [CrossRef]
- El-Sharkaway, E.A.; Kamel, R.M.; El-Sherbiny, I.M.; Gharib, S.S. Removal of methylene blue from aqueous solutions using polyaniline/graphene oxide or polyaniline/reduced graphene oxide composites. Environ. Technol. 2020, 41, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.; Cheng, R. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, G.K.; Kumara, A.V.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Zanella, R.; Kowenje, C.O.; Donato, F.; Bandeira, N.M.G.; Prestes, O.D. Single and binary adsorption of sulfonamide antibiotics onto iron-modified clay: Linear and nonlinear isotherms, kinetics, thermodynamics, and mechanistic studies. Appl. Water Sci. 2018, 8, 175. [Google Scholar] [CrossRef]
- Sahmoune, M.N. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ. Chem. Lett. 2019, 17, 697–704. [Google Scholar] [CrossRef]
- Momina; Rafatullah, M.; Ismail, S.; Ahmad, A. Optimization study for the desorption of methylene blue dye from clay based adsorbent coating. Water 2019, 11, 1304. [Google Scholar] [CrossRef]
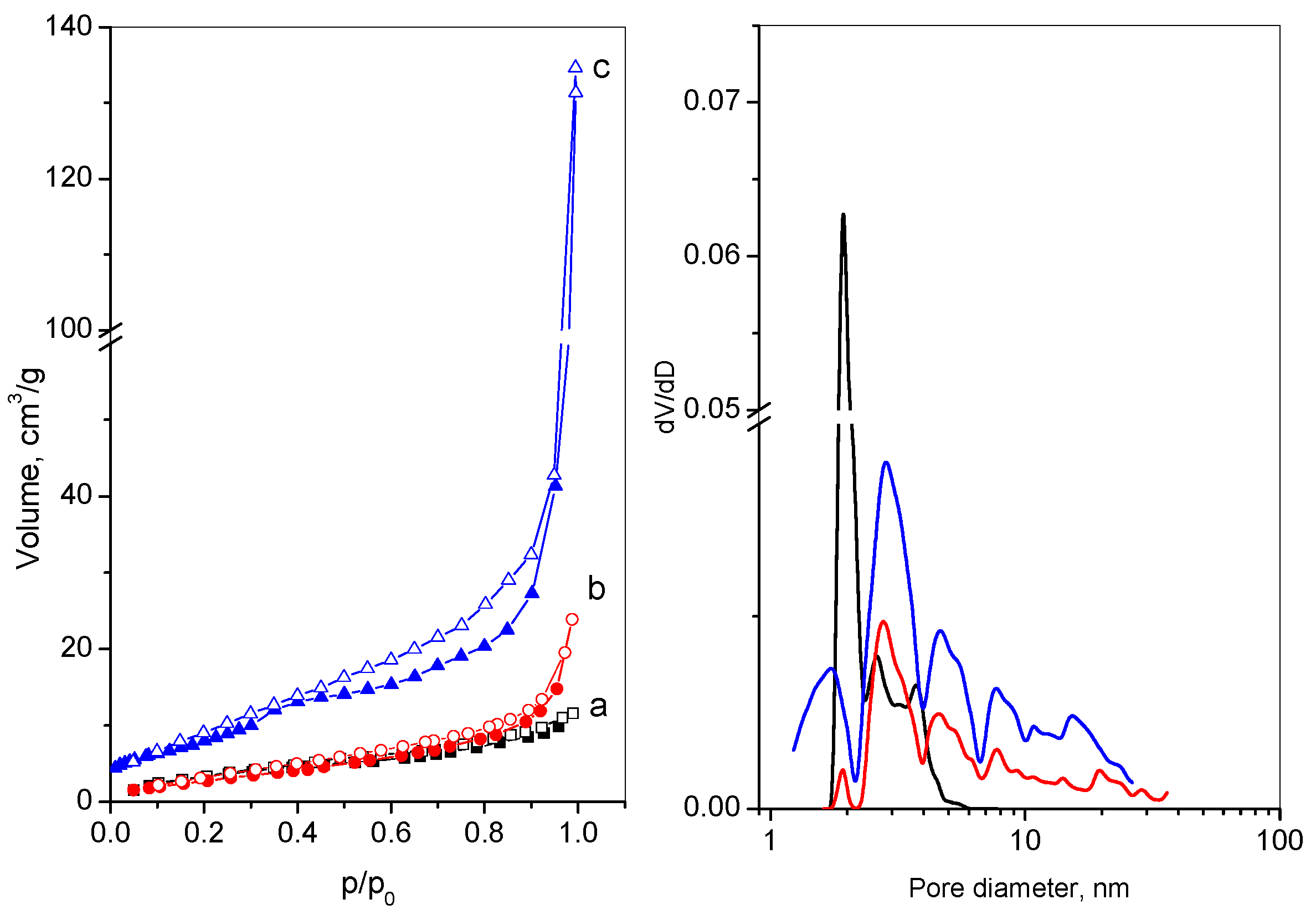


| Sample | S 1 m2/g | V 2 cm3/g | Dav 3 nm | V 4mi cm3/g |
|---|---|---|---|---|
| GO | 14 | 0.02 | 5 | - |
| RGO | 18 | 0.05 | 12 | - |
| M-RGO-N | 40 | 0.18 | 18 | 0.02 |
| Sample | Pseudo-First-Order Constants | Pseudo-Second-Order Constants | Intraparticle Diffusion Constants | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | k1 (h−1) | r2 | Qe (mg g−1) | k2 (g mg−1h−1) | r2 | kid (mg g−1 h−1/2) | C (mg g−1) | r2 | |
| GO | 10.46 | 0.169 | 0.9399 | 28.24 | 5.531 | 0.9996 | 5.650 | 16.33 | 0.8695 |
| RGO | 6.19 | 0.203 | 0.8820 | 23.41 | 8.173 | 0.9997 | 3.499 | 16.02 | 0.8728 |
| M-RGO-N | 17.18 | 0.303 | 0.9545 | 31.15 | 6.275 | 0.9996 | 5.912 | 11.12 | 0.8808 |
| Sample | Langmuir Parameters 1 | Freundlich Parameters 2 | D-R Parameters 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q0 (mg g−1) | K1 (L mg−1) | r2 | kF (mg1−nLn g−1) | n (L mg−1) | r2 | Qm (mg g−1) | E (kJ mol−1) | r2 | |
| GO | 39.81 | 0.335 | 0.9984 | 12.39 | 3.344 | 0.9546 | 32.46 | 1.20 | 0.9352 |
| RGO | 36.63 | 0.241 | 0.9977 | 12.07 | 3.802 | 0.9512 | 27.99 | 1.74 | 0.8227 |
| M-RGO-N | 49.38 | 0.711 | 0.9958 | 20.09 | 4.415 | 0.9508 | 33.32 | 3.78 | 0.9567 |
| Adsorbent | Qmax (mg/g) | Conditions | Reference/ Year |
|---|---|---|---|
| Monolayer GO | 440.5−803.7 | pH = 3, 7, 11; 2.35 mg GO; MB range: 0.05–0.475 g/L; 298 K | [28] (2022) |
| rGO | 81 | 283 K, 10–50 mg/L, 0.01 g adsorbent | [34] (2013) |
| GO | 119.0 | 293 K, 180 min, pH = 4, 600 mgL−1 | [35] (2021) |
| rGO | 64.8 and 80.1 | MB 20−50 mgL−1 dosage of 50 mgL−1; pH = 4 | [36] (2019) |
| GO | 429.5 | 5–25 ppm MB, 10 mg adsorbent, 313 K, 15 min, pH 8 | [37] (2019) |
| rGO | 121.9 | pH = 6, 298 K, 10–100 mg/L MB, 200 mg adsorbent−1 | [38] (2020) |
| GO | 311.7 | 6–100 ppm MB, 0.5 gL−1 adsorbent, 293 K, pH = 10 | [39] (2021) |
| GO-CS composites | 7.5 | MB 10 mg/L, pH = 5, adsorbent 0.143 g/L, 125 min | [40](2022) |
| Chitosan-Functionalized GO | 23.3 | MB 10−50 mg/L, 35 min, GO@CS 50 mg/10 mL, pH = 7, 25 °C | [49] (2022) |
| rGO/TNT | 26.3 | MB 1−60 mg/L, 25 °C, 0.1 g/10 mL adsorbent, pH 6.8 | [52] (2019) |
| GO/polyaniline | 14.2 | MB 10−80 mg/L, 270 min, 0.3–0.4 g adsorbent, pH = 6.5 | [53] (2019) |
| rGO/polyaniline | 19.2 | ||
| GO | 48.8–598.8 | 298 K, pH = 7, 0.015 g GO, MB 5–600 mg dm−3 | [54] (2014) |
| GO | 17.3 | MB 10−50 mg/L, 25 °C, 0.22 mg/mL adsorbent | [55] (2011) |
| GO | 39.8 | MB 10−100 mg/L, 293 K, pH = 8, 0, 10 mg adsorbent | Present study |
| RGO | 36.6 | ||
| M-RGO-N | 49.4 |
| Adsorbent | ΔH0, kJ mol−1 | ΔS0, J mol−1 K−1 | ΔG0, kJ mol−1 | ||
|---|---|---|---|---|---|
| 293 K | 313 K | 333 K | |||
| GO | 13.72 | 107.82 | −17.87 | −18.95 | −22.18 |
| RGO | 7.28 | 83.14 | −17.05 | −17.91 | −20.36 |
| M-RGO-N | 21.41 | 138.01 | −19.03 | −20.41 | −24.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassileva, P.; Tumbalev, V.; Kichukova, D.; Voykova, D.; Kovacheva, D.; Spassova, I. Study on the Dye Removal from Aqueous Solutions by Graphene-Based Adsorbents. Materials 2023, 16, 5754. https://doi.org/10.3390/ma16175754
Vassileva P, Tumbalev V, Kichukova D, Voykova D, Kovacheva D, Spassova I. Study on the Dye Removal from Aqueous Solutions by Graphene-Based Adsorbents. Materials. 2023; 16(17):5754. https://doi.org/10.3390/ma16175754
Chicago/Turabian StyleVassileva, Paunka, Vencislav Tumbalev, Diana Kichukova, Dimitrinka Voykova, Daniela Kovacheva, and Ivanka Spassova. 2023. "Study on the Dye Removal from Aqueous Solutions by Graphene-Based Adsorbents" Materials 16, no. 17: 5754. https://doi.org/10.3390/ma16175754