Effect of Ball Milling Time on the Microstructure and Properties of High-Silicon–Aluminum Composite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
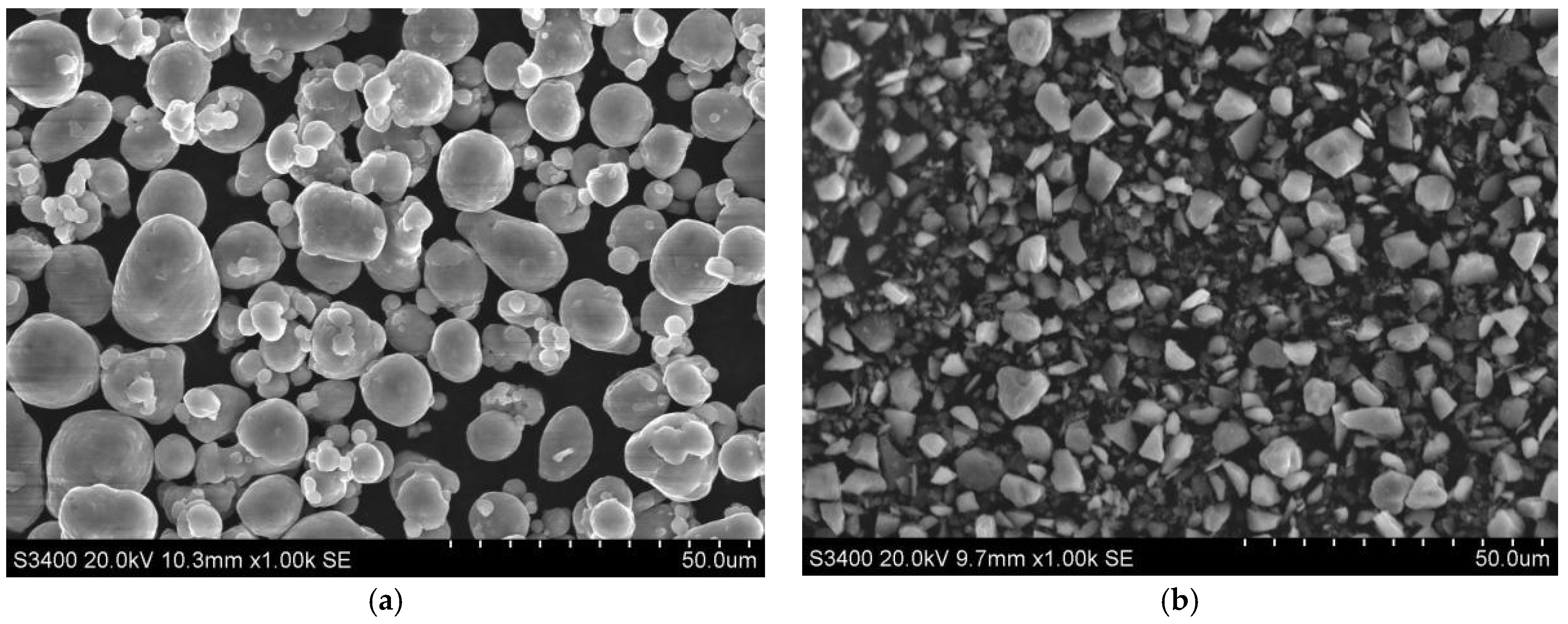
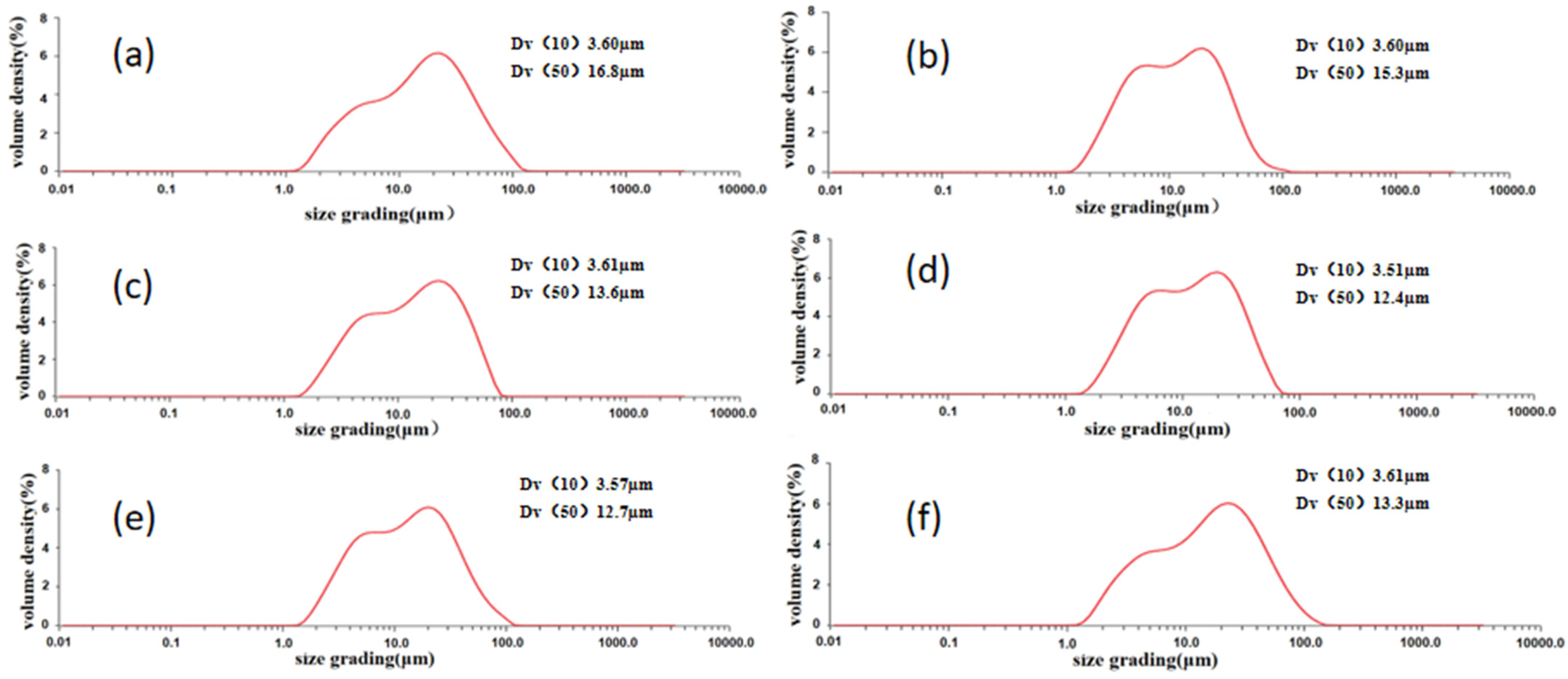
3.1. Effect of Milling Time on Particle Size, Morphology, and Oxygen Content of the Powder
3.2. Effect of Milling Time on Microstructure and Properties of Materials
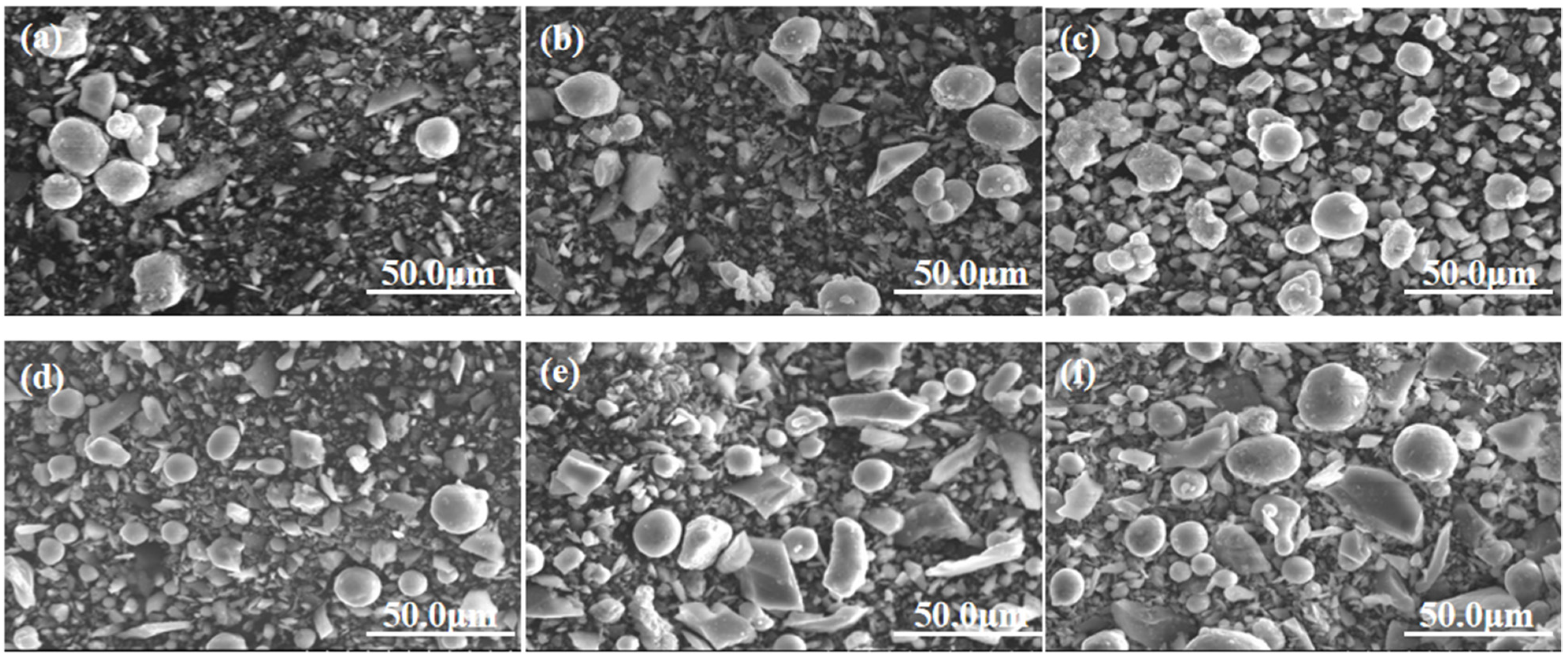
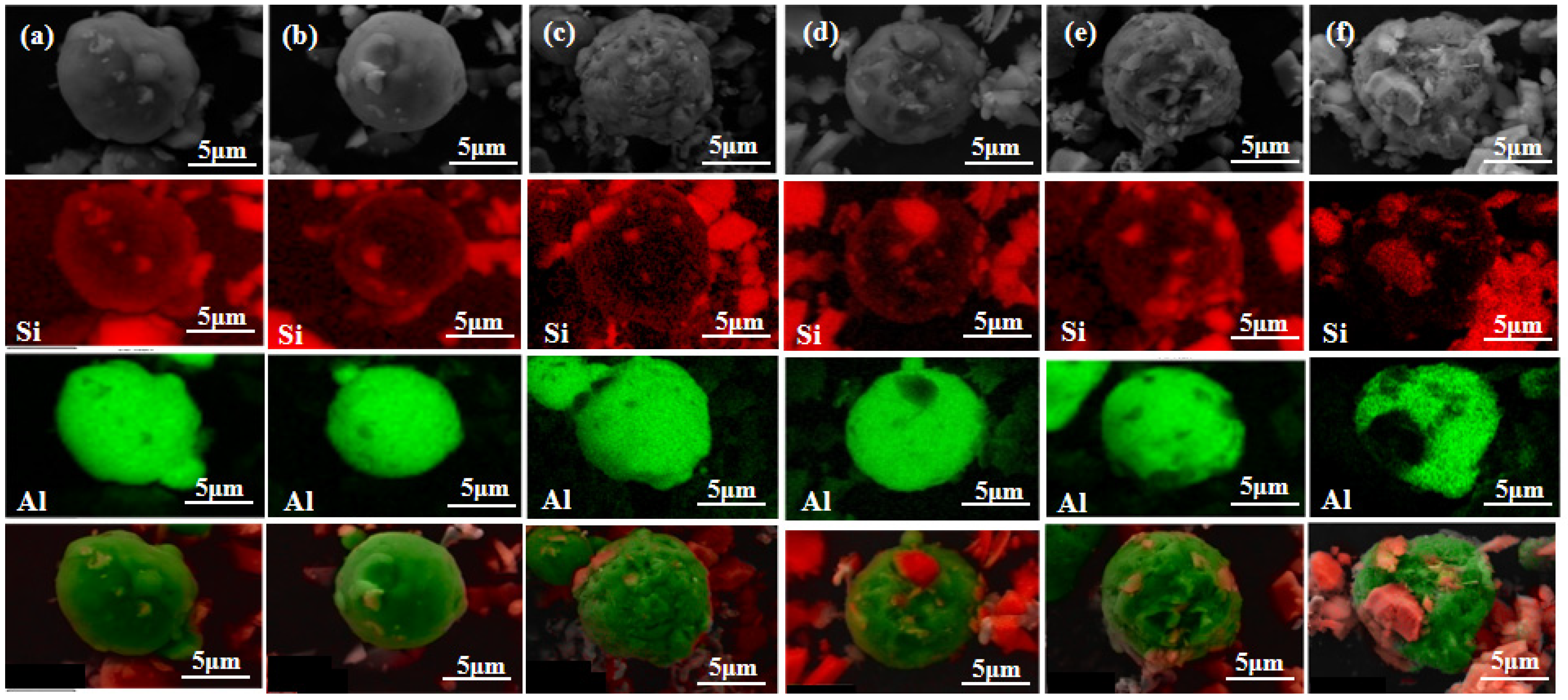
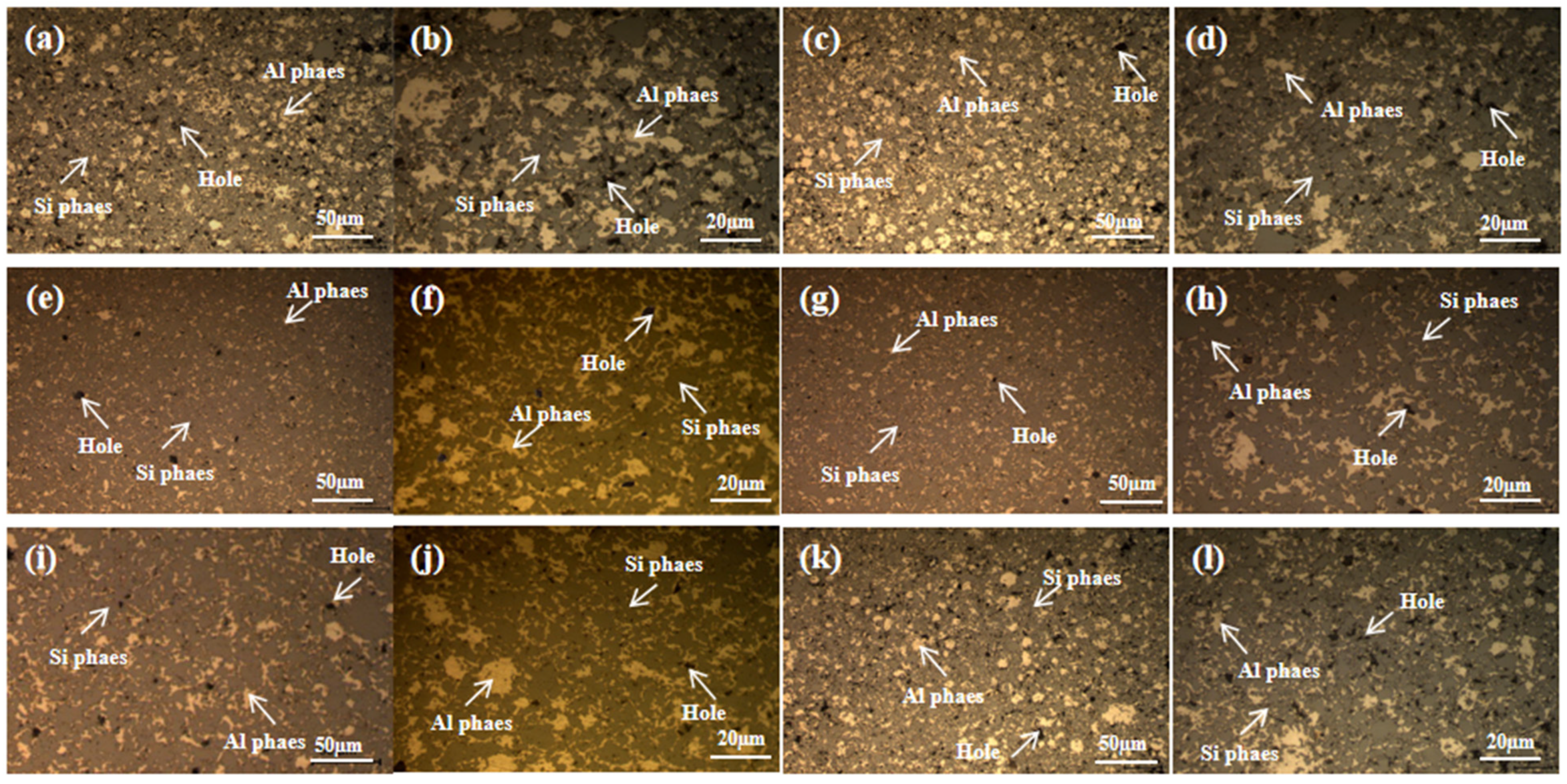
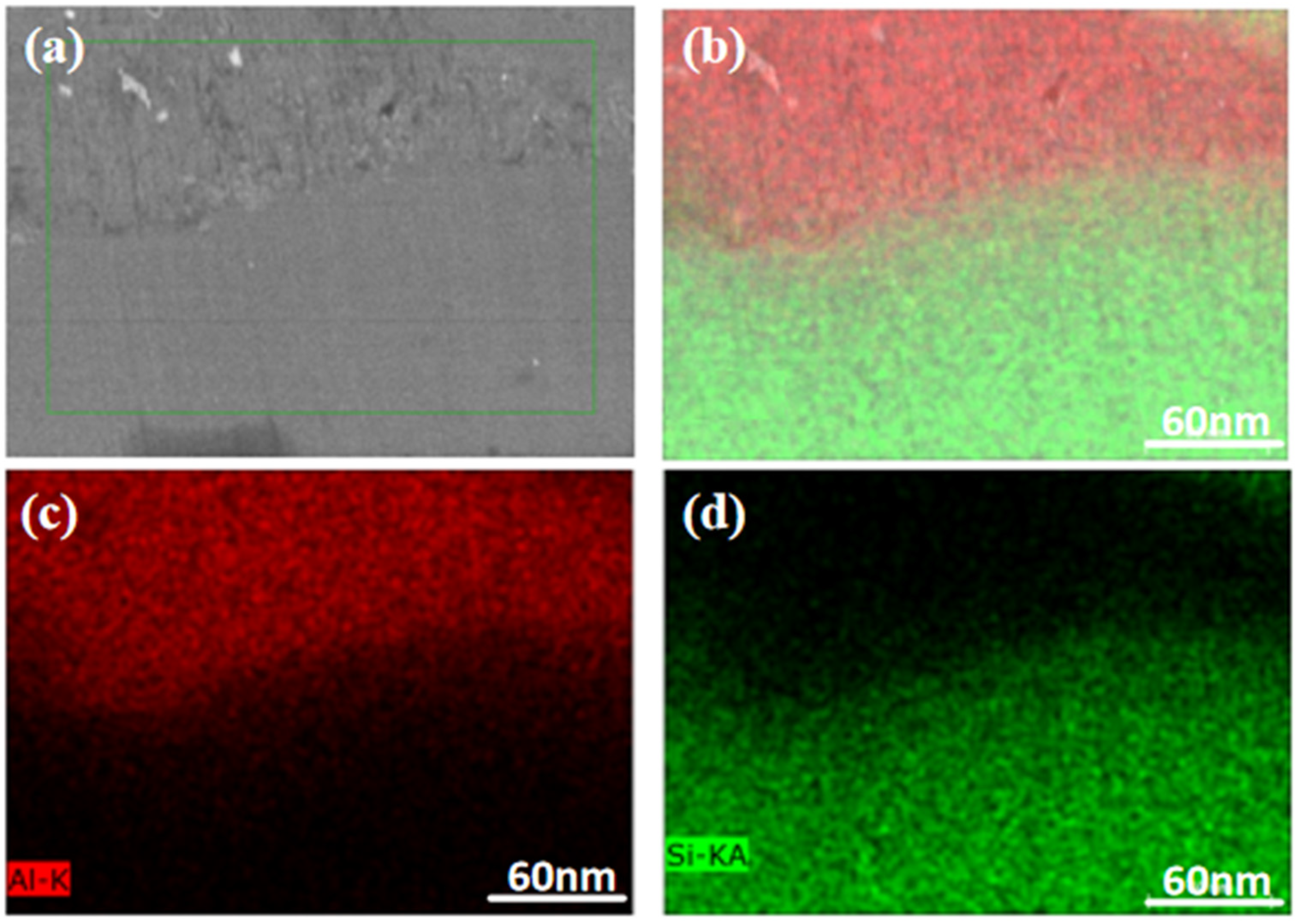
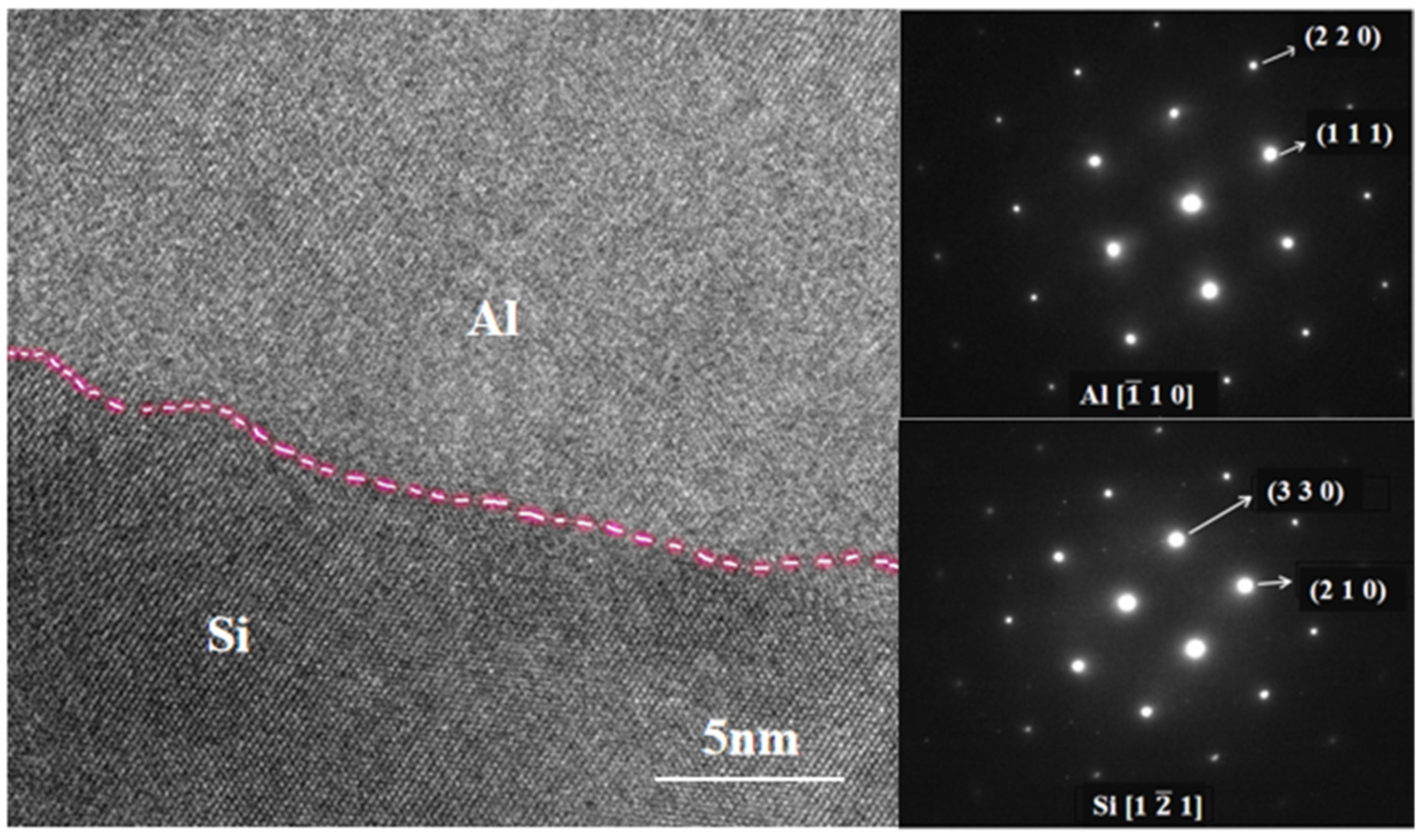
3.2.1. Effect of Milling Time on Microstructure
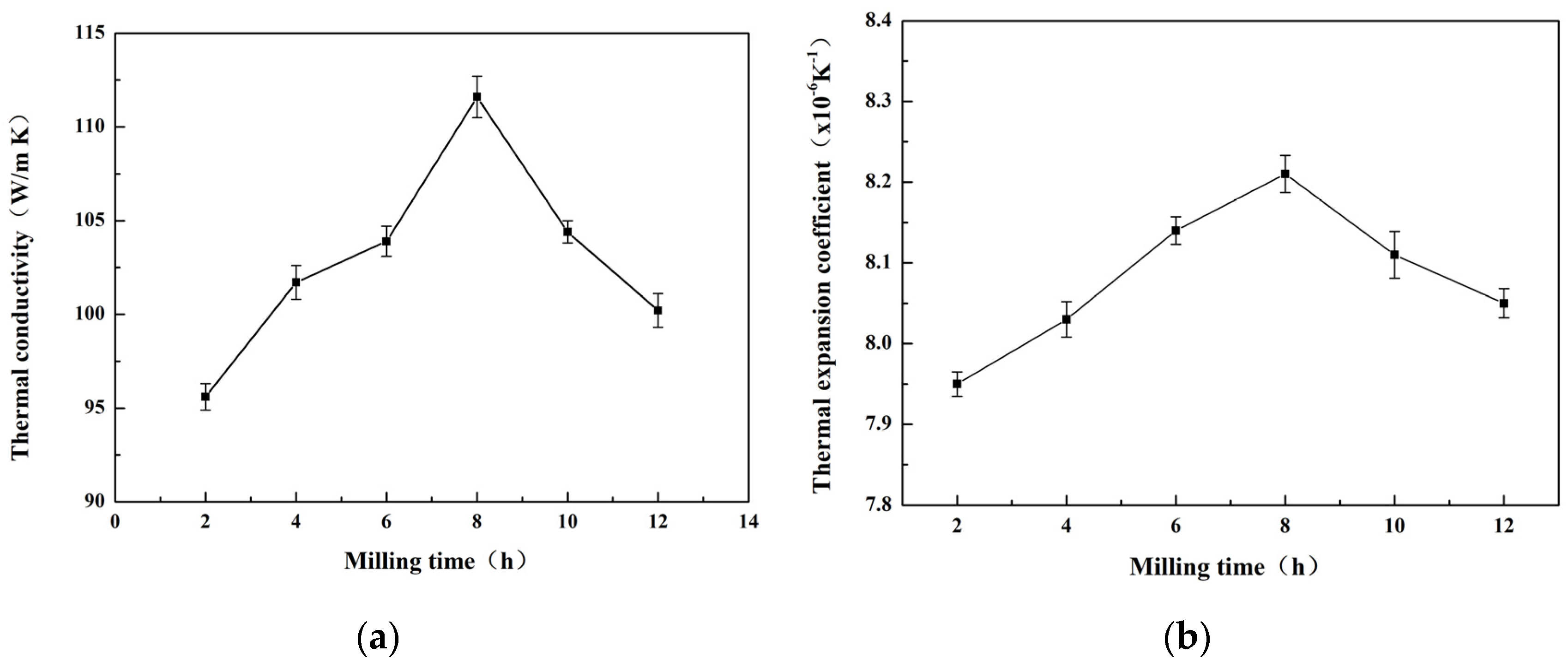
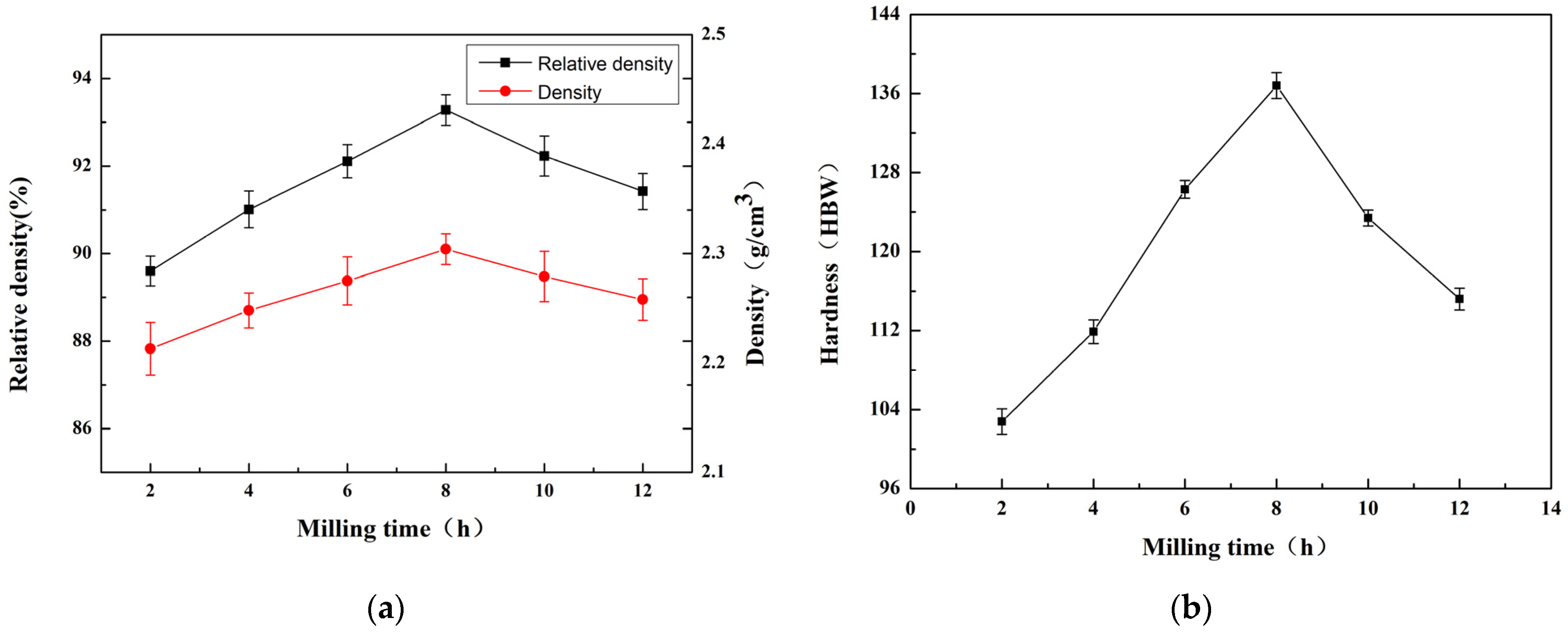
3.2.2. Effect of the Milling Time on Properties of Materials
4. Conclusions
- (1)
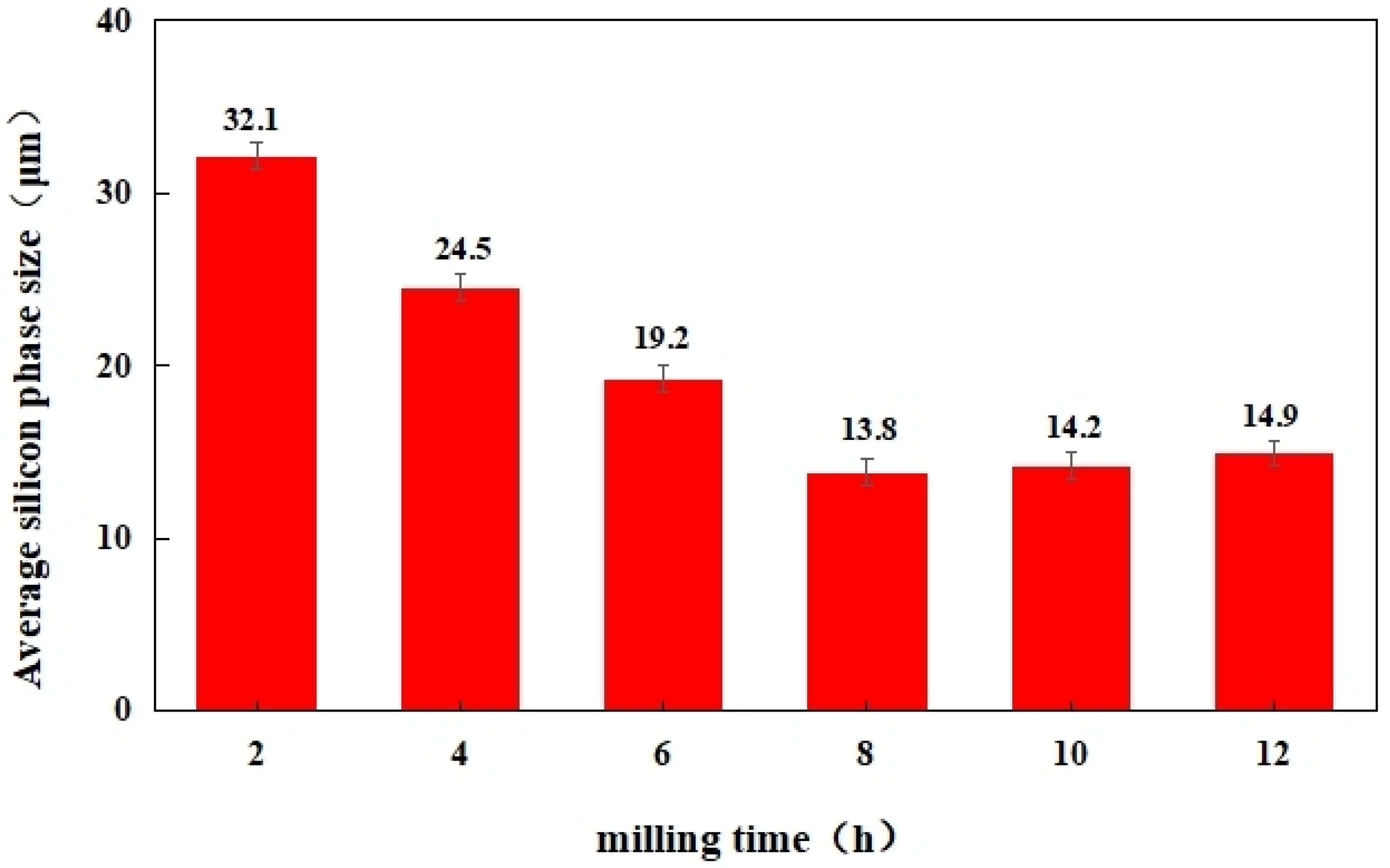
- The oxygen content in the powder increased with both the increase in pellet ratio and the extension of milling time. The observed rise in oxygen content was considerably greater than that seen in the powder exposed to air oxidation at elevated temperatures over an equivalent period. The milling procedure resulted in a marked decrease in particle size. After milling for 8 h, the powder exhibited the most uniform distribution, with an average particle size of 12.4 µm. Moreover, the prepared materials featured the smallest average size of the silicon phase in their microstructure.
- (2)
- At a milling time of 8 h, the Al matrix demonstrated a continuous network distribution, while the silicon phase displayed a skeleton structure with minimal internal voids. The material exhibited a density of 2.304 g/cm3, a maximum thermal conductivity of 111.6 W/m·K, an average thermal expansion coefficient of 8.21 × 10−6 K−1 from room temperature to 100 °C, and a hardness of 136.8 HBW.
- (3)
- The pretreatment of powder using ball milling effectively enhanced the quality of the powder. When utilizing a ball milling duration of 8 h, a ball-to-material ratio of 10:1, and a milling speed of 200 r/min, the most favorable outcomes were obtained. Under these parameters, the material demonstrated superior performance, aligning with the standards for electronic packaging materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, W.K.; Yılmaz, F.; Kim, H.S.; Koo, J.M.; Hong, S.J. Fabrication of Al–20 wt%Si powder using scrap Si by ultra high-energy milling process. J. Alloys Compd. 2012, 536, S45–S49. [Google Scholar] [CrossRef]
- Wang, K.; Wei, M.; Zhang, L.; Du, Y. Morphologies of Primary Silicon in Hypereutectic Al-Si composites: Phase-Field Simulation Supported by Key Experiments. Metall. Mater. Trans. A 2016, 47, 1510–1516. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.P. Microstructure Evolution of a Fine Grain Al-50wt%Si composite Fabricated by High Energy Milling. Key Eng. Mater. 2011, 479, 54–61. [Google Scholar] [CrossRef]
- Hong, S.J.; Suryanarayana, C. Mechanical properties and fracture behavior of an ultrafine-grained Al-20 wt pct Si alloy. Exp. Metall. Mater. Trans. A 2005, 36, 715–723. [Google Scholar] [CrossRef]
- Lv, G.; Bao, Y.; Zhang, Y.; He, Y.; Ma, W.; Lei, Y. Effects of electromagnetic directional solidification conditions on the separation of primary silicon from Al-Si composite with high Si content. Mat. Sci. Semicon. Proc. 2018, 81, 139–148. [Google Scholar] [CrossRef]
- Liou, T.H. Kinetics study of thermal decomposition of electronic packaging material. Chem. Eng. J. 2004, 98, 39–51. [Google Scholar]
- Sun, Y.; Zhao, D.; Song, J.; Wang, C.; Zhang, Z.; Huang, L.; Liu, J.; Liu, Z. Rapid Fabrication of Superhydrophobic High-Silicon Aluminum composite Surfaces with Corrosion Resistance. Results Phys. 2018, 12, 1082–1088. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Fan, J.Z.; Hao, X.X.; Wei, S.H.; Nie, J.H.; Ma, Z.L.; Liu, M.K.; Wang, Y.B. Advanced hermetic electronic packaging based on lightweight silicon/aluminum composite produced by powder metallurgy technique. Rare Met. 2020, 39, 1307–1313. [Google Scholar] [CrossRef]
- Wang, X.F.; Wu, G.H.; Wang, R.C.; Xiu, Z.Y.; Yu, K. Fabrication properties of Si/Al interpenetrating phase composites for electronic packaging. Trans. Nonferrous Met. Soc. China 2007, 17, 1039–1042. [Google Scholar]
- Yang, F.L.; Gan, W.P.; Chen, Z.K. A few preparation methods and their delication mechanism for high-silicon aluminum composite. Mat. Res. 2004, 18, 79–82. [Google Scholar]
- Li, C.J.; Zhang, J.D.; Zhu, X.K.; Shi, B.C.; Tang, H.L.; Li, J. Preparation of pure Aluminum nanocrystalline materials by High energy ball milling. Power Met. 2006, 24, 457–459. [Google Scholar]
- Hua, Y.; Zhang, L.; Cheng, L.; Wang, J. Silicon carbide whisker reinforced silicon carbide composites by chemical vapor infiltration. Mat. Sci. Eng. A 2006, 428, 346–350. [Google Scholar] [CrossRef]
- Steedman, G.; Bishop, D.P.; Caley, W.F.; Kipouros, G.J. Surface porosity investigation of aluminum–silicon PM alloys. Powder Technol. 2012, 226, 225–230. [Google Scholar] [CrossRef]
- Yoshizawa, Y.I.; Toriyama, M.; Kanzaki, S.; Saito, F. Strength of Sintered Alumina at Low Temperature Due to Ball Mill Abrasion Powder Seeding. J. Ceram. Soc. Jpn. 2010, 106, 444–446. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Hu, Z.; Xu, Y.; Wang, J.; Schützendübe, P.; Huang, Y.; Liu, Y.; Wang, Z. Microstructure evolution interface structure of Al-40 wt% Si composites produced by high-energy ball milling. J. Mater. Sci. Technol. 2019, 35, 512–519. [Google Scholar] [CrossRef]
- Choi, Y.; Kataoka, Y.; Zu, Z.; Matsugi, K.; Sasaki, G. Manufacturing Process of Carbon-Alumina Short Fiber Hybrid Reinforced Aluminum Matrix Composites by Low Pressure Casting. Mater. Trans. 2017, 58, 1097–1099. [Google Scholar]
- Carreño-Gallardo, C.; Estrada-Guel, I.; López-Meléndez, C.; Martínez-Sánchez, R. Dispersion of silicon carbide nanoparticles in a AA2024 aluminum alloy by a high-energy ball mill. J. Alloys Compd. 2013, 586, S68–S72. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Jung, H.S.; Kim, J.B. Effect of ball size and powder loading on the milling efficiency of a laboratory-scale wet ball mill. Ceram. Int. 2013, 39, 8963–8968. [Google Scholar] [CrossRef]
- Cambronero, L.E.G.; Sánchez, E.; Ruiz-Roman, J.M.; Ruiz-Prieto, J.M. Mechanical characterisation of AA7015 aluminium alloy reinforced with ceramics. J. Mater. Process. Technol. 2003, 143, 378–383. [Google Scholar] [CrossRef]
- Son, H.T.; Kim, T.S.; Suryanarayana, C.; Chun, B.S. Homogeneous dispersion of graphite in a 6061 aluminum alloy by ball milling. Mat. Sci. Eng. A 2003, 348, 163–169. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, B.K. Effects of lifter bars on the ball motion and aluminum foil milling in tumbler ball mill. Mater. Lett. 2002, 57, 275–279. [Google Scholar] [CrossRef]
- Fang, R.R.; He, Y.Z.; Zhang, K.; Li, H. Melting Behavior of Aluminum Nanowires in Carbon Nanotube. J. Phys. Chem. C 2014, 118, 7622–7629. [Google Scholar] [CrossRef]
- Jargalsaikhan, B.; Bor, A.; Lee, J.; Choi, H. Al/CNT nanocomposite fabrication on different property of raw material using a planetary ball mill. Adv. Powder Technol. 2020, 31, 1957–1962. [Google Scholar] [CrossRef]
- Teng, F.; Yu, K.; Luo, J.; Fang, H.J.; Shi, C.L.; Dai, Y.L.; Xiong, H.Q. Microstructures and properties of Al-50%SiC composites for electronic packaging applications. Trans. Nonferrous Met. Soc. China 2016, 26, 2647–2652. [Google Scholar] [CrossRef]
- Mujahid, M.; Friska, I. Influence of Mechanical Milling on the SiC Particulate Size in an Al-SiC Composite. J. Mater. Eng. Perform. 2005, 14, 69–74. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.; Ahn, B. Microstructure and properties of in-situ Al–Si/Al2O3composites prepared by displacement reaction. Powder Metall. 2021, 64, 192–197. [Google Scholar] [CrossRef]
- Lee, H.; Sharma, A.; Ahn, B. Exploring strengthening mechanism of FeCoNiAl high-entropy alloy by non-metallic silicon addition produced via powder metallurgy. J. Alloys Compd. 2023, 947, 169545. [Google Scholar] [CrossRef]
- Salur, E.; Acarer, M.; Savkliyildiz, I. Improving mechanical properties of nano-sized TiC particle reinforced AA7075 Al alloy composites produced by ball milling and hot pressing. Mater. Today Commun. 2021, 27, 102202. [Google Scholar] [CrossRef]
- Sübütay, H.; Savklıyıldız, I. The relationship between structural evolution and high energy ball milling duration in tin reinforced Mg alloys. Mater. Today Commun. 2023, 32, 105868. [Google Scholar] [CrossRef]





| Element | Purity (wt.%) | Median Particle Size (µm) |
|---|---|---|
| Al | ≥99.5 | 25 |
| Si | ≥99.5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Z.; Wang, Z.; Chen, B.; Li, Y.; Li, R. Effect of Ball Milling Time on the Microstructure and Properties of High-Silicon–Aluminum Composite. Materials 2023, 16, 5763. https://doi.org/10.3390/ma16175763
Kong Z, Wang Z, Chen B, Li Y, Li R. Effect of Ball Milling Time on the Microstructure and Properties of High-Silicon–Aluminum Composite. Materials. 2023; 16(17):5763. https://doi.org/10.3390/ma16175763
Chicago/Turabian StyleKong, Zhaoyang, Zhipeng Wang, Bin Chen, Yingmin Li, and Runxia Li. 2023. "Effect of Ball Milling Time on the Microstructure and Properties of High-Silicon–Aluminum Composite" Materials 16, no. 17: 5763. https://doi.org/10.3390/ma16175763
APA StyleKong, Z., Wang, Z., Chen, B., Li, Y., & Li, R. (2023). Effect of Ball Milling Time on the Microstructure and Properties of High-Silicon–Aluminum Composite. Materials, 16(17), 5763. https://doi.org/10.3390/ma16175763






