Efficient Synthesis of 2-Ethylhexanoic Acid via N-Hydroxyphthalimide Catalyzed Oxidation of 2-Ethylhexanal with Oxygen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 2-EHAL Oxidation Reaction with Oxygen or Air
2.3. Analytical Methods
3. Results
3.1. Effect of Solvent Type
3.2. Effect of the Amount of i-BuOH
3.3. Effect of the Amount of Catalyst
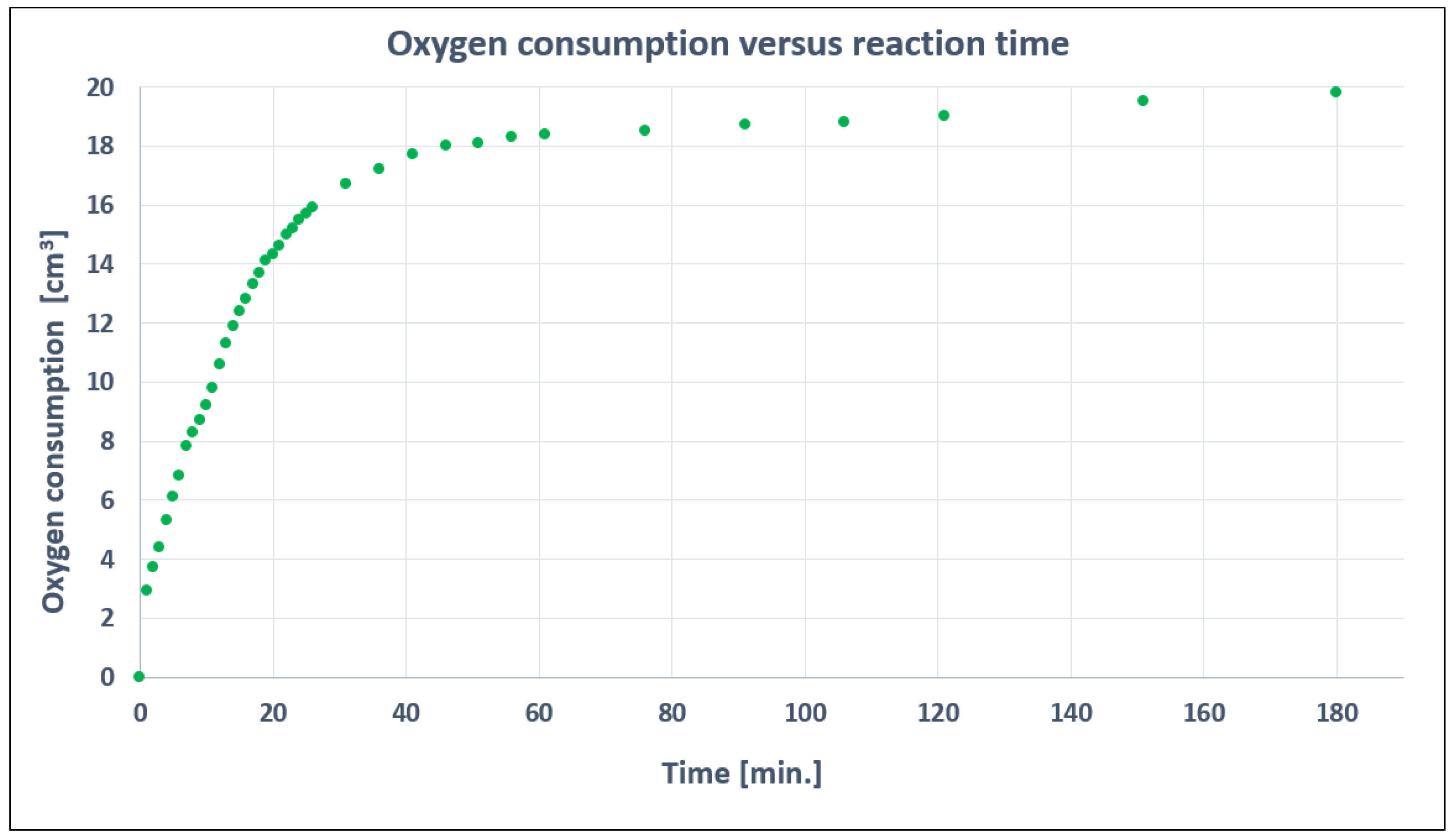
3.4. Influence of Temperature and Reaction Time
3.5. Effect of Oxidizing Agent
4. Conclusions
- (1)
- The study demonstrated the feasibility of the oxidation process of 2-EHAL to acid under mild conditions with oxygen in the presence of NHPI as a catalyst in i-BuOH as a solvent.
- (2)
- 2-EHA was obtained with high selectivity of 99.4% and a conversion of 59.0% (30 °C, 3 h, 0.1 MPa, 5 mol% NHPI, i-BuOH)
- (3)
- The developed method holds potential for implementation in industry due to its high selectivity, cost-effective oxidizing agent, and mild reaction conditions.
- (4)
- i-BuOH enables the dissolution of NHPI in the reaction mixture, does not undergo esterification under the reaction conditions, and facilitates heat exchange. Additionally, the use of i-BuOH as a solvent provides an opportunity for potential producers of 2-EHAL from butanal to utilize this less valuable alcohol.
- (5)
- It was observed that the use of air is feasible, however, it would require higher pressure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riemenschneider, W.; Aktiengesellschaft, H. Carboxylic acid, Aliphtalic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005; Electronic Release. [Google Scholar]
- Le Paih, J.; Frison, J.-C.; Bolm, C. Oxidation of Carbonyl Compounds. In Modern Oxidation Methods; Wiley-VCH: Weinheim, Germany, 2004; pp. 253–265. [Google Scholar]
- Yanbing, Y.; Shuai, Y.; Xin, C.; Qian, C.; Zesheng, W.; Peng, W.; Chao, L.; Ke, D.; Jiaqi, W.; Jintong, H.; et al. Nano-Gold Carbon Nitride Catalyst, Preparation Method of Catalyst, and Method for Preparing 2-Ethylhexanoic Acid from 2-Ethylhexanal. Chinese Patent CN 110773234, 14 October 2019. [Google Scholar]
- Zesheng, W.; Xin, C.; Shanjian, C.; Guangwen, H.; Chao, L.; Ke, D.; Peng, W.; Qian, C.; Yanbing, Y. Catalyst and Method for Preparinf 2-ethylhexanoic Acid by Means of Oxidizing 2-ethylhexanal and Method for Preparing Catalyst. Chinese Patent CN 109433270, 10 October 2018. [Google Scholar]
- Zesheng, W.; Shanjian, C.; Xin, C.; Guangwen, H.; Chao, L.; Ke, D.; Peng, W.; Qian, C.; Yanbing, Y. Preparation Method of Immobilized Lipase and Method for Preparing 2-ethylhexanoic Acid. Chinese Patent CN 109609488, 4 December 2018. [Google Scholar]
- Fusheng, L.; Changdong, A.; Wenguang, D. Productio Process of 2-ethylhexanoic Acid. Chinese Patent CN 1357527, 15 December 2000. [Google Scholar]
- Changdong, A.; Fusheng, L.; Qi, Y. 2-ethylhexanoic Acid Production Method. Chinese Patent CN 141040, 29 September 2001. [Google Scholar]
- Changdong, A.; Fusheng, L.; Qi, Y. 2-ethylhexanoic Acid Production Method. Chinese Patent CN 1422840, 29 November 2001. [Google Scholar]
- Liu, M.; Li, C.-J. Catalytic Fehling’s Reaction: An Efficient Aerobic Oxidation of Aldehyde Catalyzed by Copper in Water. Angew. Chem. Int. Ed. 2016, 55, 10806–10810. [Google Scholar] [CrossRef]
- Gliński, M.; Kijeński, J. Oxidation of 2-ethylhexanal in liquid phase: II Catalytic pathway. React. Kinet. Catal. Lett. 1995, 55, 319–323. [Google Scholar] [CrossRef]
- Leshina, T.V.; Zakharov Nartsissov, O.I.; Beer, A.A.; Kalitaeva, I.A. Method of Preparing 2-ethylhexanoic Acid. Chinese Patent SU 557566, 22 September 1975. [Google Scholar]
- Ing, K.J.; Ing, M.V. Process for Preparing Industrial 2-Ethylhexanoic Acid. Chinese Patent CZ 278403, 30 October 1991. [Google Scholar]
- Gliński, M.; Kijeński, J. The vapor phase oxidation of 2-ethylhexanal over oxidecapacists. React. Kinet. Catalan. Lett. 1995, 55, 305–309. [Google Scholar] [CrossRef]
- Gliński, M.; Kijeński, J. Oxidation of 2-ethylhexanal in liquid phase: I Non-catalytic pathway. React. Kinet. Catal. Lett. 1995, 55, 311–318. [Google Scholar] [CrossRef]
- Lehtinen, C.; Brunov, G. Factors affecting the selectivity of air oxidation of 2-ethyhexanal, an α-Branched Aliphatic Aldehyde. Org. Process Res. Dev. 2000, 4, 544–549. [Google Scholar] [CrossRef]
- Giannandrea, R.; Mastrorilli, P.; Nobile, C.F.; Suranna, G.P. Aerobic oxidation of aldehydes, ketones, sulfides, alcohols and alkanes catalysed by polymerizable pketoesterate complexes of iron(III), nickel(II) and cobalt(II). J. Mol. Cat. 1994, 94, 27–36. [Google Scholar] [CrossRef]
- Ko, D.; Lee, S.; Know, O.; Moon, J.; Kim, D.; Choi, J.; Hong, B.; Eom, S. Preparation or Organic Acids from Aldehyde Compounds by Means of Liquid Phase Oxidation Reaction. US Patent US 0010688, 7 September 2006. [Google Scholar]
- Vanoye, L.; Pablos, M.; Smith, N.; Bellefon, C.; Ravre-Reguillon, A. Aerobics oxidation of aldehydes: Selectivity improvement uising sequential pulse experimentation in continuous flow microreactor. RSC Adv. 2014, 4, 57159–57163. [Google Scholar] [CrossRef]
- Vanoye, L.; Wang, J.; Pablos, M.; Philippe, R.; Bellefon, C.; Favre-Reguillon, A. Continuous, Fast and Safe Aerobics Oxidation of 2-Ethylhexanal: Pushing The Limits of The Simple Tube Reactor for a Gas/Liquid Reaction. Org. Process Res. Dev. 2016, 20, 90–94. [Google Scholar] [CrossRef]
- Sato, K.; Hyodo, M.; Takagi, J.; Aoki, M.; Noyori, R. Hydrogen peroxide oxidation of aldehydes this carboxylic acids: An organic solvent-, halide- and metal-free procedure. Tetrahedron Lett. 2000, 41, 1439–1442. [Google Scholar] [CrossRef]
- Gliński, M.; Kijeński, J. Method of Manufacturing 2-ethylhexane Acid. Chinese Patent PL 177034, 28 October 1994. [Google Scholar]
- Shapiro, N.; Vigalok, A. Highly Efficient Organic Reactions “on Water”, “in Water”, and Both, Angew. Chem. Int. Ed. 2008, 47, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Marteau, C.; Ruyffelaere, F. Oxidative degradation of fragrant aldehydes. Autoxidation by molecular oxygen. Tetrahedron 2013, 69, 2268–2275. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M.D.R.S. Organocatalysis Meets Hydrocarbon Oxyfunctionalization: The Role of N-Hydroxyimides. Eur. J. Org. Chem. 2021, 2021, 4715–4727. [Google Scholar] [CrossRef]
- Dai, P.F.; Qu, J.P.; Kang, Y.B. Organocatalyzed Aerobic Oxidation of Aldehydes to Acids. Org. Lett. 2019, 21, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Moiseevich, K.S.; Sergeevich, K.N.A.; Samojlovich, M. A Method of Producing 2-ethylhexanoic Acid. Chinese Patent RU 2426721, 29 April 2010. [Google Scholar]
- Yida, Y. Pharmaceutical Intermediate 2-ethylhezanoivhexanoic Acis Synthesis Method. Chinese Patent CN 108238892, 4 May 2017. [Google Scholar]
- Vanoye, L.; Favre-Reguillon, A.; Aloui, A.; Philippe, R.; Bellephone, C. Insights in the aerobic oxidation of aldehydes. RSC Adv. 2013, 3, 18931–18937. [Google Scholar] [CrossRef]
- Lehtinen, C.; Nevalainen, V.; Brunow, G. Experimental and Computational Studies on Substituent Effects In reactions of Peracid–Aldehyde Adducts. Tetrahedron 2000, 56, 9375–9382. [Google Scholar] [CrossRef]
- Lehtinen, C.; Nevalainen, V.; Brunow, G. Experimental and computational studies on solvent effects in reactions of peracid-aldehyde adducts. Tetrahedron 2001, 57, 4741–4751. [Google Scholar] [CrossRef]
- Vanoye, L.; Abdelaal, M.; Grundhauser, K.; Guicheret, B.; Fongarland, P.; De Bellefon, C.; Favre-Réguillon, A. Reinvestigation of the Organocatalyzed Aerobic Oxidation of Aldehydes to Acids. Org. Lett. 2019, 21, 10134–10138. [Google Scholar] [CrossRef] [PubMed]



| Entry | Solvent | Conv. 2-EHAL [%] | Sel. 2-EHA [%] | Sel. Heptane [%] | Sel. 3H=O [%] | Sel. 3HFE [%] | Sel. 3H-OL [%] |
|---|---|---|---|---|---|---|---|
| 1 | AcOH | 99.9 | 61.9 | 1.6 | nd | 8.5 | 6.1 |
| 2 | MeCN | 99.8 | 47.1 | nd | 1.5 | 1.9 | 8.5 |
| 3 | PhCH3 | 99.5 | 60.7 | 0.7 | 0.5 | 21.7 | 0.5 |
| 4 | Heptane | 98.9 | 68.7 | ** | 0.4 | 14.4 | 2.2 |
| 5 | Decane | 98.8 | 71.5 | 0.8 | 0.2 | 16.0 | 1.0 |
| 6 | 2-ethylhexanol | 55.2 | 93.0 | 1.6 | 2.1 | 0.8 | 6.3 |
| 7 | i-BuOH | 47.8 | 92.6 | nd | 0.9 | 0.8 | 1.0 |
| 8 | n-butanol | 42.7 | 97.0 | nd | * | 0.7 | 4.1 |
| 9 | MeOH | 0.0 | nd | nd | nd | nd | nd |
| Entry | Volume of Solvent (cm3) | Conc. of 2-EHAL (mol/dm3) | Conv. 2-EHAL (%) | Sel. 2-EHA (%) | Sel. Heptane (%) | Sel. 3H=O (%) | Sel. 3HFE (%) | Sel. 3H-OL (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 4 | 47.8 | 92.6 | nd | 0.9 | 0.8 | 1.0 |
| 2 | 6 | 3 | 59.1 | 95.5 | nd | 1.0 | 1.0 | 0.7 |
| 3 | 4 | 2 | 61.3 | 94.9 | nd | 0.7 | 1.1 | 0.1 |
| 4 | 2 | 1 | 59.0 | 99.4 | nd | 0.6 | 1.4 | 0.2 |
| Entry | NHPI (mol%) | Conv. 2-EHAL (%) | Sel. 2-EHA (%) | Sel. Heptane (%) | Sel. 3H=O (%) | Sel. 3HFE (%) | Sel. 3H-OL (%) |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 61.2 | 96.5 | nd | 0.2 | 1.5 | nd |
| 2 | 6 | 62.1 | 95.2 | nd | 0.3 | 1.5 | nd |
| 3 | 5 | 59.0 | 99.4 | nd | 0.6 | 1.4 | 0.2 |
| 4 | 4 | 55.8 | 94.4 | nd | 0.2 | 1.4 | nd |
| 5 | 2 | 47.7 | 96.8 | nd | 0.3 | 1.5 | nd |
| 6 | - | 22.9 | 23.4 | nd | nd | 0.2 | nd |
| Entry | Temp. (°C) | Time (h) | Conv. 2-EHAL (%) | Sel. 2-EHA (%) | Sel. Heptane (%) | Sel. 3H=O (%) | Sel. 3HFE (%) | Sel. 3H-OL (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 3 | 59.0 | 99.4 | nd | 0.6 | 1.4 | 0.2 |
| 2 | 35 | 3 | 70.3 | 75.0 | nd | 0.1 | 1.2 | 0.0 |
| 3 | 40 | 3 | 73.9 | 85.2 | nd | 0.4 | 1.6 | 0.1 |
| 4 | 50 | 3 | 76.0 | 71.4 | nd | 0.6 | 1.9 | 0.4 |
| 5 | 60 | 3 | 99.9 | 59.3 | 0.1 | 0.3 | 1.8 | 0.8 |
| 6 | 35 | 0.5 | 59.7 | 76.2 | nd | 0.2 | 1.1 | 0.2 |
| 7 | 35 | 1 | 62.8 | 63.0 | nd | 0.1 | 0.6 | 0.0 |
| 8 | 35 | 2 | 69.2 | 71.0 | nd | 0.1 | 1.1 | 0.0 |
| 9 | 35 | 3 | 70.3 | 75.0 | nd | 0.1 | 1.2 | 0.0 |
| Entry | Temp. (°C) | Oxidizing Agent | Conv. 2-EHAL (%) | Sel. 2-EHA (%) | Sel. Heptane (%) | Sel. 3H=O (%) | Sel. 3HFE (%) | Sel. 3H-OL (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 30 | oxygen | 59.0 | 99.4 | nd | 0.6 | 1.4 | 0.2 |
| 2 | 30 | air | 48.0 | 86.6 | nd | 1.1 | 1.3 | 0.5 |
| 3 | 40 | oxygen | 73.9 | 85.2 | nd | 0.4 | 1.6 | 0.1 |
| 4 | 40 | air | 58.1 | 63.4 | 0.1 | 0.4 | 1.0 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czieszowic, Ł.; Orlińska, B.; Lisicki, D.; Pankalla, E. Efficient Synthesis of 2-Ethylhexanoic Acid via N-Hydroxyphthalimide Catalyzed Oxidation of 2-Ethylhexanal with Oxygen. Materials 2023, 16, 5778. https://doi.org/10.3390/ma16175778
Czieszowic Ł, Orlińska B, Lisicki D, Pankalla E. Efficient Synthesis of 2-Ethylhexanoic Acid via N-Hydroxyphthalimide Catalyzed Oxidation of 2-Ethylhexanal with Oxygen. Materials. 2023; 16(17):5778. https://doi.org/10.3390/ma16175778
Chicago/Turabian StyleCzieszowic, Łukasz, Beata Orlińska, Dawid Lisicki, and Ewa Pankalla. 2023. "Efficient Synthesis of 2-Ethylhexanoic Acid via N-Hydroxyphthalimide Catalyzed Oxidation of 2-Ethylhexanal with Oxygen" Materials 16, no. 17: 5778. https://doi.org/10.3390/ma16175778
APA StyleCzieszowic, Ł., Orlińska, B., Lisicki, D., & Pankalla, E. (2023). Efficient Synthesis of 2-Ethylhexanoic Acid via N-Hydroxyphthalimide Catalyzed Oxidation of 2-Ethylhexanal with Oxygen. Materials, 16(17), 5778. https://doi.org/10.3390/ma16175778





