Human Bone-Marrow-Derived Stem-Cell-Seeded 3D Chitosan–Gelatin–Genipin Scaffolds Show Enhanced Extracellular Matrix Mineralization When Cultured under a Perfusion Flow in Osteogenic Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Three-Dimensional Chitosan–Gelatin–Genipin Scaffolds
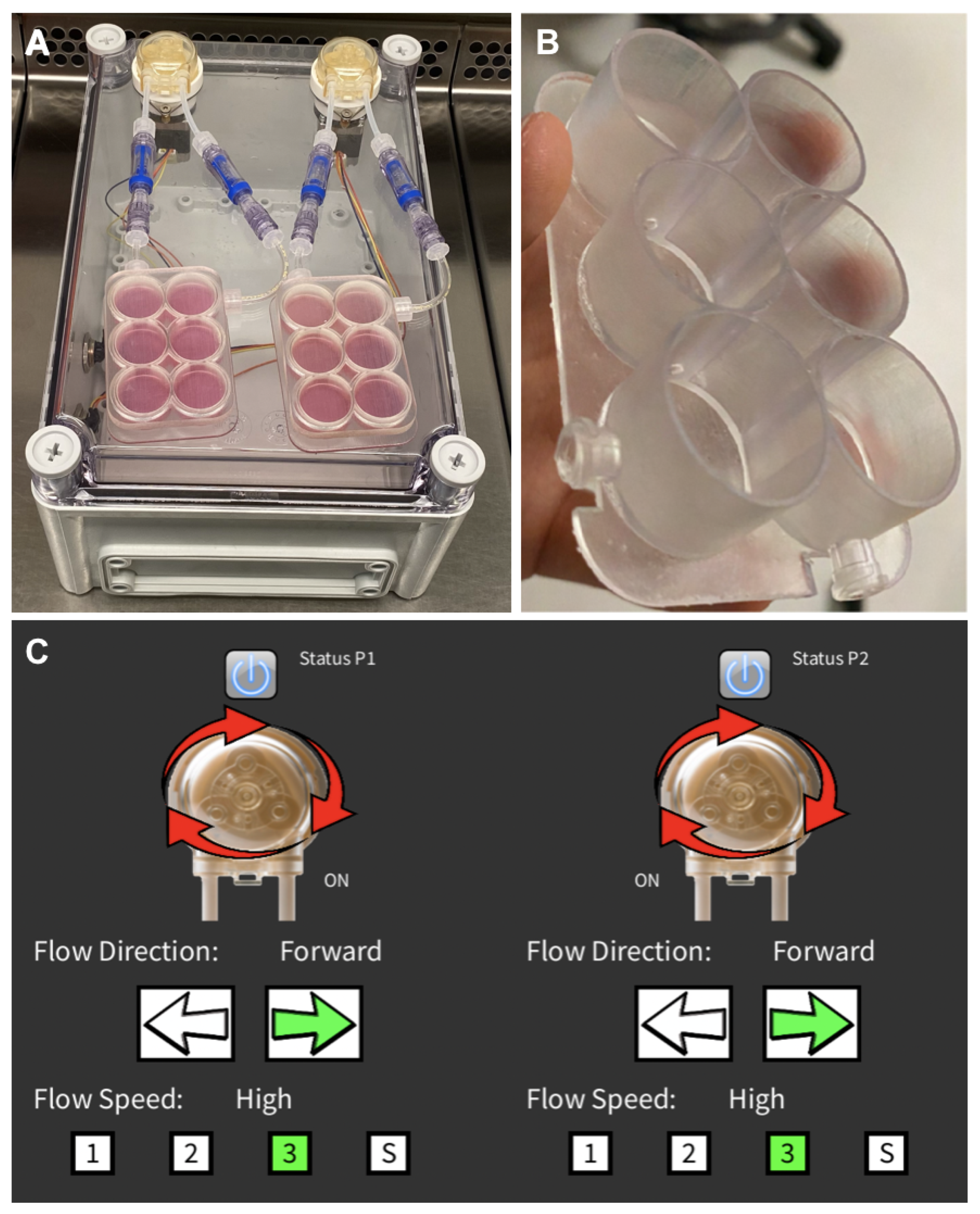
2.2. Perfusion Bioreactor
2.2.1. Three-Dimensional-Printed Culture Plates
2.2.2. Graphical User Interface
2.3. Human Mesenchymal Stem Cell Culture
2.4. Cell Culture
Osteogenic Differentiation
2.5. Micro-Computer Tomography (μCT)
2.6. Histochemical Assay and Image Analisys
3. Results and Discussion
3.1. Characterization of CGG Scaffolds
3.2. Matrix Mineralization upon Dynamic Culture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone Tissue Engineering Graft Substitutes: What Is the Future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. S1), 36–42. [Google Scholar] [CrossRef]
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O.C. Biofabrication of Bone Tissue: Approaches, Challenges and Translation for Bone Regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Novel bone-mimetic nanohydroxyapatite/collagen porous scaffolds biomimetically mineralized from surface silanized mesoporous nanobioglass/collagen hybrid scaffold: Physicochemical, mechanical and in vivo evaluations. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110660. [Google Scholar] [CrossRef]
- Silva, J.C.; Carvalho, M.S.; Udangawa, R.N.; Moura, C.S.; Cabral, J.M.S.; Lda Silva, C.; Ferreira, F.C.; Vashishth, D.; Linhardt, R.J. Extracellular Matrix Decorated Polycaprolactone Scaffolds for Improved Mesenchymal Stem/Stromal Cell Osteogenesis towards a Patient-tailored Bone Tissue Engineering Approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2153–2166. [Google Scholar] [CrossRef]
- Wu, Z.; Meng, Z.; Wu, Q.; Zeng, D.; Guo, Z.; Yao, J.; Bian, Y.; Gu, Y.; Cheng, S.; Peng, L.; et al. Biomimetic and Osteogenic 3D Silk Fibroin Composite Scaffolds with Nano MgO and Mineralized Hydroxyapatite for Bone Regeneration. J. Tissue Eng. 2020, 11, 204173142096779. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Jiezhang, W.; Chen, Z. Extracellular Vesicle-Functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-Angiogenic and Pro-Bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93 Pt B, 1354–1365. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Chang, P.H.; Chao, H.M.; Chern, E.; Hsu, S.H. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef]
- Șelaru, A.; Herman, H.; Vlăsceanu, G.M.; Dinescu, S.; Gharbia, S.; Baltă, C.; Roșu, M.; Mihali, C.V.; Ioniță, M.; Serafim, A.; et al. Graphene–Oxide Porous Biopolymer Hybrids Enhance In Vitro Osteogenic Differentiation and Promote Ectopic Osteogenesis In Vivo. Int. J. Mol. Sci. 2022, 23, 491. [Google Scholar] [CrossRef]
- Chiticaru, E.A.; Ionita, M. Graphene Toxicity and Future Perspectives in Healthcare and Biomedicine. FlatChem 2022, 35, 100417. [Google Scholar] [CrossRef]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Lovecchio, J.; Pannella, M.; Giardino, L.; Calzà, L.; Giordano, E. A Dynamic Culture Platform Enhances the Efficiency of the 3D HUVEC-based Tube Formation Assay. Biotechnol. Bioeng. 2019, 117, 789–797. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Marino, L.; Lovecchio, J.; Giordano, E.; Forsyth, N.R.; Selleri, C.; Maffulli, N.; Porta, G.D. Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Lamparelli, E.P.; Lovecchio, J.; Ciardulli, M.C.; Giudice, V.; Dale, T.P.; Selleri, C.; Forsyth, N.; Giordano, E.; Maffulli, N.; Della Porta, G. Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with HTGF-β1-Releasing PLGA Microcarrier. Pharmaceutics 2021, 13, 399. [Google Scholar] [CrossRef]
- Rauh, J.; Milan, F.; Günther, K.P.; Stiehler, M. Bioreactor systems for bone tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, G.N.; Sikavitsas, V.I.; Mikos, A.G. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003, 9, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.A.; Gomide, V.; Monteiro, F.J. The role of perfusion bioreactors in bone tissue engineering. Biomatter 2012, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Fisher, J.P. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef]
- Qi, P.; Ning, Z.; Zhang, X. Synergistic effects of 3D chitosan-based hybrid scaffolds and mesenchymal stem cells in orthopaedic tissue engineering. IET Nanobiotechnol. 2023, 17, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Pulieri, E.; Vozzi, G.; Ciardelli, G.; Ahluwalia, A.; Giusti, P. Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Pramanik, K.; Jabbari, E. Osteogenic differentiation of human mesenchymal stem cells in freeze-gelled chitosan/nano β-tricalcium phosphate porous scaffolds crosslinked with genipin. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Karageorgiou, V.; Fajardo, R.; Snyder, B.; Shinde-Patil, V.; Zichner, L.; Kaplan, D.; Langer, R.; Vunjak-Novakovic, G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004, 32, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J. Cell Biochem. 1994, 5, 283–294. [Google Scholar] [CrossRef]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal stem cells: Mechanisms and role in bone regeneration. Postgrad. Med. J. 2014, 90, 643–647. [Google Scholar] [CrossRef]
- Janfada, A.; Asefnejad, A.; Khorasani, M.T.; Joupari, M.D. Reinforcement of Electrospun Polycaprolacton Scaffold Using KIT-6 to Improve Mechanical and Biological Performance. Polym. Test. 2020, 84, 106391. [Google Scholar] [CrossRef]
- Zhao, F.; Van Rietbergen, B.; Ito, K.; Hofmann, S. Flow rates in perfusion bioreactors to maximise mineralisation in bone tissue engineering in vitro. J. Biomech. 2018, 79, 232–237. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bhumiratana, S.; Cannizzaro, C.; Chao, P.H.; Lennon, D.P.; Caplan, A.I.; Vunjak-Novakovic, G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng. Part A 2008, 14, 1809–1820. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: Dynamic cell seeding and construct development. Biotechnol. Bioeng. 2005, 91, 482–493. [Google Scholar] [CrossRef]
- Pasini, A.; Lovecchio, J.; Ferretti, G.; Giordano, E. Medium Perfusion Flow Improves Osteogenic Commitment of Human Stromal Cells. Stem Cells Int. 2019, 2019, 1304194. [Google Scholar] [CrossRef]
- Lovecchio, J.; Gargiulo, P.; Vargas Luna, J.L.; Giordano, E.; Sigurjónsson, Ó.E. A Standalone Bioreactor System to Deliver Compressive Load under Perfusion Flow to HBMSC-Seeded 3D Chitosan-Graphene Templates. Sci. Rep. 2019, 9, 16854. [Google Scholar] [CrossRef]
- Fiorentini, E.; Granchi, D.; Leonardi, E.; Baldini, N.; Ciapetti, G. Effects of osteogenic differentiation inducers on in vitro expanded adult mesenchymal stromal cells. Int. J. Artif. Organs 2011, 34, 998–1011. [Google Scholar] [CrossRef]
- Anselme, K.; Broux, O.; Noel, B.; Bouxin, B.; Bascoulergue, G.; Dudermel, A.F.; Bianchi, F.; Jeanfils, J.; Hardouin, P. In Vitro control of human bone marrow stromal cells for bone tissue engineering. Tissue Eng. 2002, 8, 941–953. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Vlasceanu, G.M.; Șelaru, A.; Dinescu, S.; Balta, C.; Herman, H.; Gharbia, S.; Hermenean, A.; Ionita, M.; Costache, M. Comprehensive Appraisal of Graphene–Oxide Ratio in Porous Biopolymer Hybrids Targeting Bone-Tissue Regeneration. Nanomaterials 2020, 10, 1444. [Google Scholar] [CrossRef]
- Kronstadt, S.M.; Patel, D.B.; Born, L.J.; Levy, D.; Lerman, M.J.; Mahadik, B.; McLoughlin, S.T.; Fasuyi, A.; Fowlkes, L.; Van Heyningen, L.H.; et al. Mesenchymal Stem Cell Culture within Perfusion Bioreactors Incorporating 3D-Printed Scaffolds Enables Improved Extracellular Vesicle Yield with Preserved Bioactivity. Adv. Healthc. Mater. 2023, 2023, 2300584. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Reseland, J.E.; Haugen, H.J. Osteoblasts in a Perfusion Flow Bioreactor—Tissue Engineered Constructs of TiO2 Scaffolds and Cells for Improved Clinical Performance. Cells 2022, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yassin, M.A.; Schwarz, T.; Mustafa, K.; Hansmann, J. Optimization and Validation of a Custom-Designed Perfusion Bioreactor for Bone Tissue Engineering: Flow Assessment and Optimal Culture Environmental Conditions. Front. Bioeng. Biotechnol. 2022, 10, 811942. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, C.; Mohan, R.R.; Vacun, G.; Schwarz, T.; Haller, B.; Sun, Y.; Kahlig, A.; Kluger, P.; Finne-Wistrand, A.; Walles, H.; et al. A Perfusion Bioreactor System Efficiently Generates Cell-loaded Bone Substitute Materials for Addressing Critical Size Bone Defects. Biotechnol. J. 2015, 10, 1727–1738. [Google Scholar] [CrossRef]
- Lovecchio, J.; Jónsdóttir-Buch, S.M.; Einarsdóttir, G.K.; Gíslason, M.K.; Örlygsson, G.; Sigurjónsson, Ó.E.; Gargiulo, P. Assessment of a Perfusion Bioreactors System using μCT Technology and 3D Modeling Methods. Biomed. Eng./Biomed. Tech. 2014, 59, S302–S305. [Google Scholar] [CrossRef][Green Version]

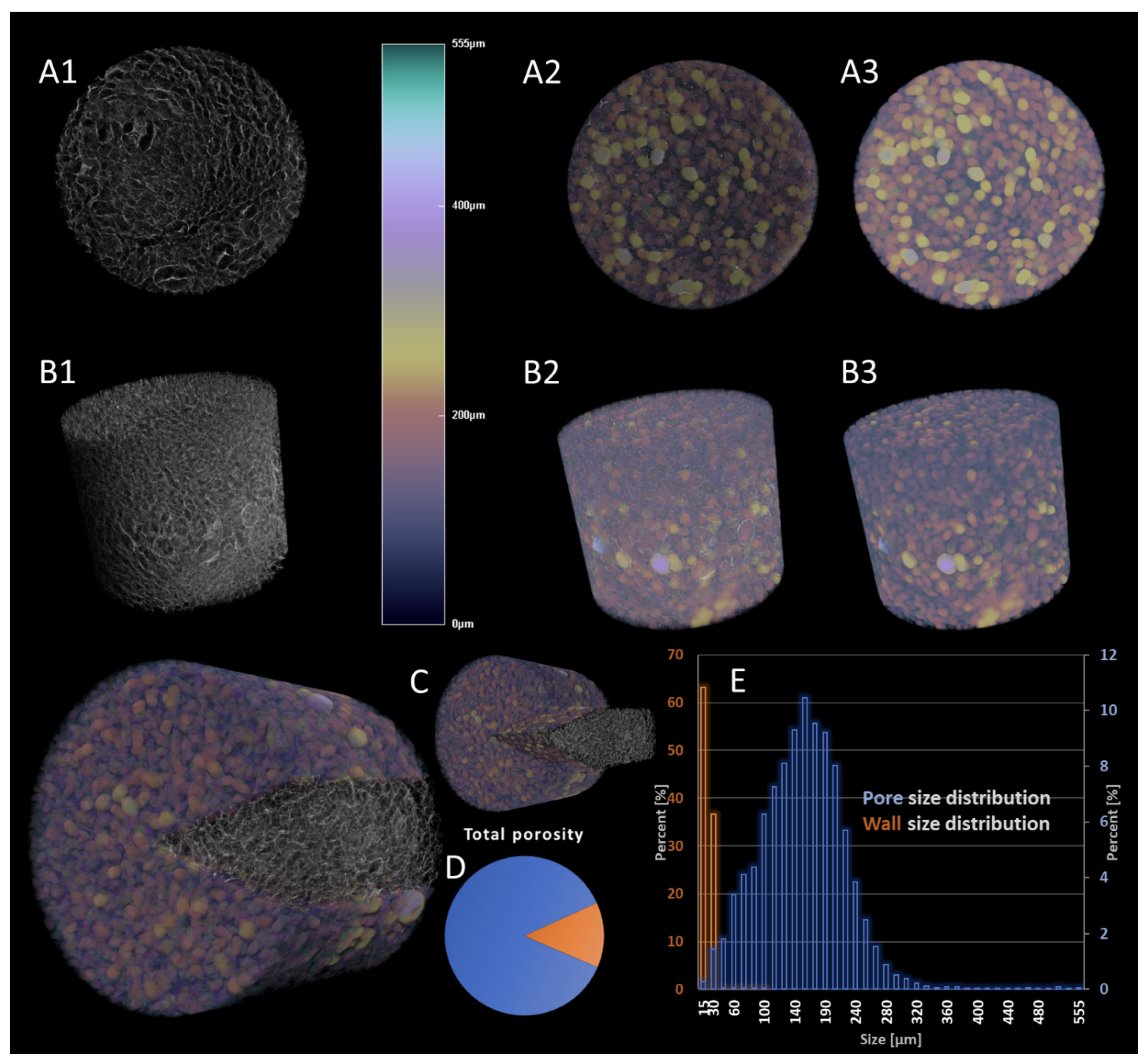
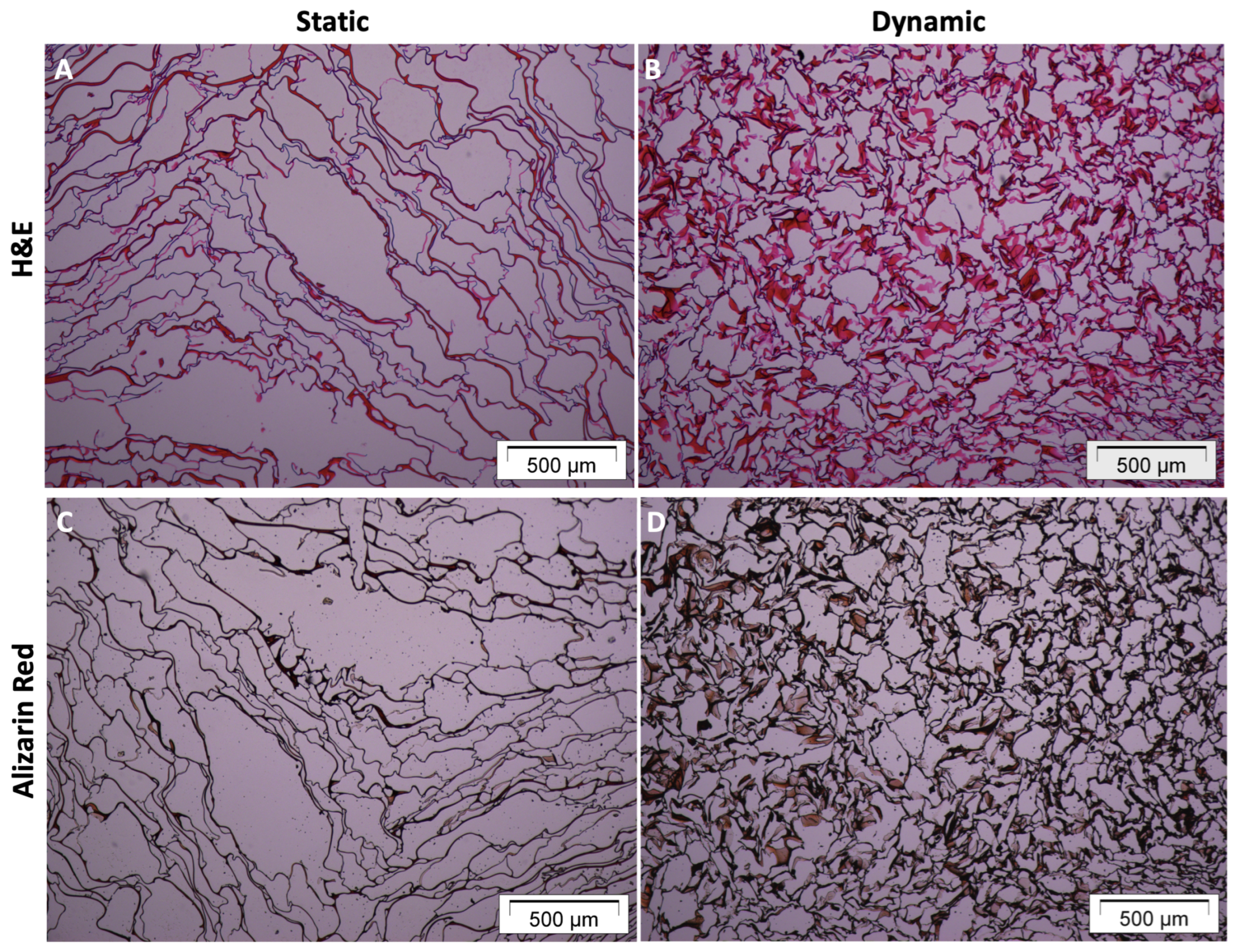
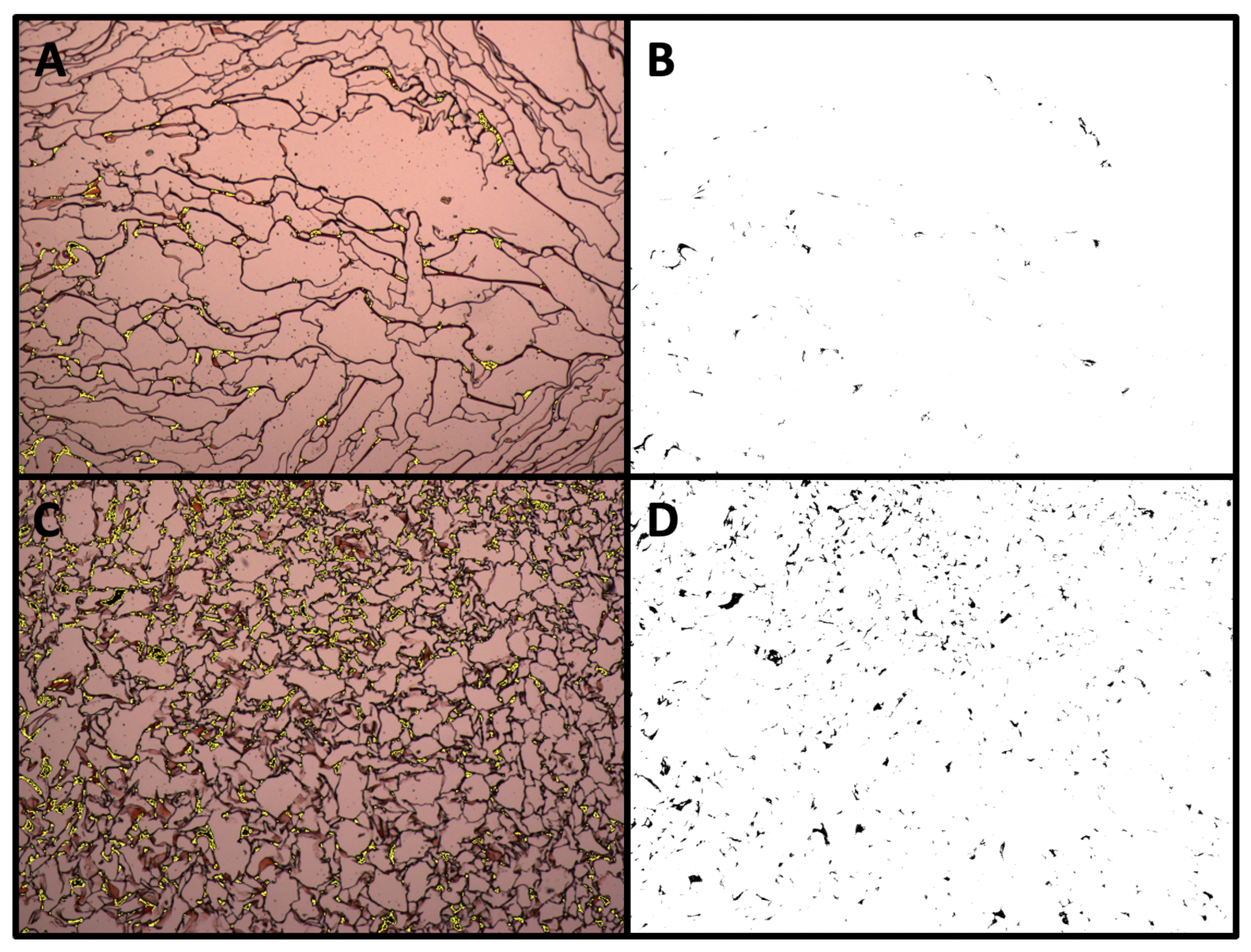
| Slice | Count | Total Area | Average Size |
|---|---|---|---|
| ARS Static | 892 | 0.610 | 6.837 × 10 |
| ARS Dynamic | 4754 | 6.730 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boretti, G.; Giordano, E.; Ionita, M.; Vlasceanu, G.M.; Sigurjónsson, Ó.E.; Gargiulo, P.; Lovecchio, J. Human Bone-Marrow-Derived Stem-Cell-Seeded 3D Chitosan–Gelatin–Genipin Scaffolds Show Enhanced Extracellular Matrix Mineralization When Cultured under a Perfusion Flow in Osteogenic Medium. Materials 2023, 16, 5898. https://doi.org/10.3390/ma16175898
Boretti G, Giordano E, Ionita M, Vlasceanu GM, Sigurjónsson ÓE, Gargiulo P, Lovecchio J. Human Bone-Marrow-Derived Stem-Cell-Seeded 3D Chitosan–Gelatin–Genipin Scaffolds Show Enhanced Extracellular Matrix Mineralization When Cultured under a Perfusion Flow in Osteogenic Medium. Materials. 2023; 16(17):5898. https://doi.org/10.3390/ma16175898
Chicago/Turabian StyleBoretti, Gabriele, Emanuele Giordano, Mariana Ionita, George Mihail Vlasceanu, Ólafur Eysteinn Sigurjónsson, Paolo Gargiulo, and Joseph Lovecchio. 2023. "Human Bone-Marrow-Derived Stem-Cell-Seeded 3D Chitosan–Gelatin–Genipin Scaffolds Show Enhanced Extracellular Matrix Mineralization When Cultured under a Perfusion Flow in Osteogenic Medium" Materials 16, no. 17: 5898. https://doi.org/10.3390/ma16175898
APA StyleBoretti, G., Giordano, E., Ionita, M., Vlasceanu, G. M., Sigurjónsson, Ó. E., Gargiulo, P., & Lovecchio, J. (2023). Human Bone-Marrow-Derived Stem-Cell-Seeded 3D Chitosan–Gelatin–Genipin Scaffolds Show Enhanced Extracellular Matrix Mineralization When Cultured under a Perfusion Flow in Osteogenic Medium. Materials, 16(17), 5898. https://doi.org/10.3390/ma16175898








