Methods to Increase or Decrease Resistance to Photodegradation and Biodegradation of Polyurethane/Polyisocyanurate (PU/PIR) Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Technical polymeric diisocyanate Purocyn B (supplied by Purinova, Bydgoszcz, Poland), whose main component was 4,4′-diphenyl-methane-diisocyanate (MDI). Density of Purocyn B at temperature of 25 °C was 1.23 g/cm3, viscosity was 200 mPas, and content of -NCO groups was 31.0%. Polyether and diisocyanate were characterized in accordance with appropriate standards like ASTM D 2849-69 and ASTM D 1638–70.
- -
- Rokopol RF-551—sorbitol oxyalkylation product (hydroxyl number—420 mg KOH/g, molecular weight—650 g/mol, functionality—4.5 (PCC Rokita S.A., Brzeg Dolny, Poland).
- -
- Stabilizer of foam structure—poly(oxyalkilene siloxane) surfactant Tegostab 8460 (Evonik, Essen, Germany).
- -
- Catalytic system: (a) trimerization catalyst—33% solution of anhydrous potassium acetate (Chempur, Poland) in diethylene glycol (Chempur, Poland); (b) polyurethane bond catalyst—33% solution of DABCO (1.4-diazabicyclo[2.2.2]octane (Alfa Aesar, Haverhill, MA, USA) in diethylene glycol (Chempur, Poland).
- -
- Blowing agent—carbon dioxide produced in situ in the reaction between water and isocyanate groups.
- -
- Flame retardant—Roflam P tris(2-chloro-1-methylethyl) phosphate (V) (PCC Rokita S.A., Henryka Sienkiewicza str. 4, 56-120 Brzeg Dolny, Poland).
- -
- Modifiers: (a) instant freeze-dried coffee Green Bellarom (Lidl, Bydgoszcz, Poland); (b) industrial food cocoa DecoMorreno (producent code 000136, Lidl Bydgoszcz, Poland) (Table 1).
2.2. Synthesis of the Rigid PU/PIR Foams
2.3. Methods
2.3.1. Analysis of Foaming Process
2.3.2. Thermostating in a Dryer
2.3.3. Aging in a Climate Chamber
2.3.4. Apparent Density
2.3.5. Compressive Strength
2.3.6. Foam Structure (SEM)
2.3.7. Chemical Structure (FTIR)
2.3.8. Measurement of Foam Color
2.3.9. Softening Point
2.3.10. Retention
2.3.11. Water Absorption and Absorbability
2.3.12. Closed Cell Content
2.3.13. Thermal Resistance
2.3.14. Standard Deviation from the Arithmetic Mean
3. Results and Discussion
3.1. Foaming Process and Density
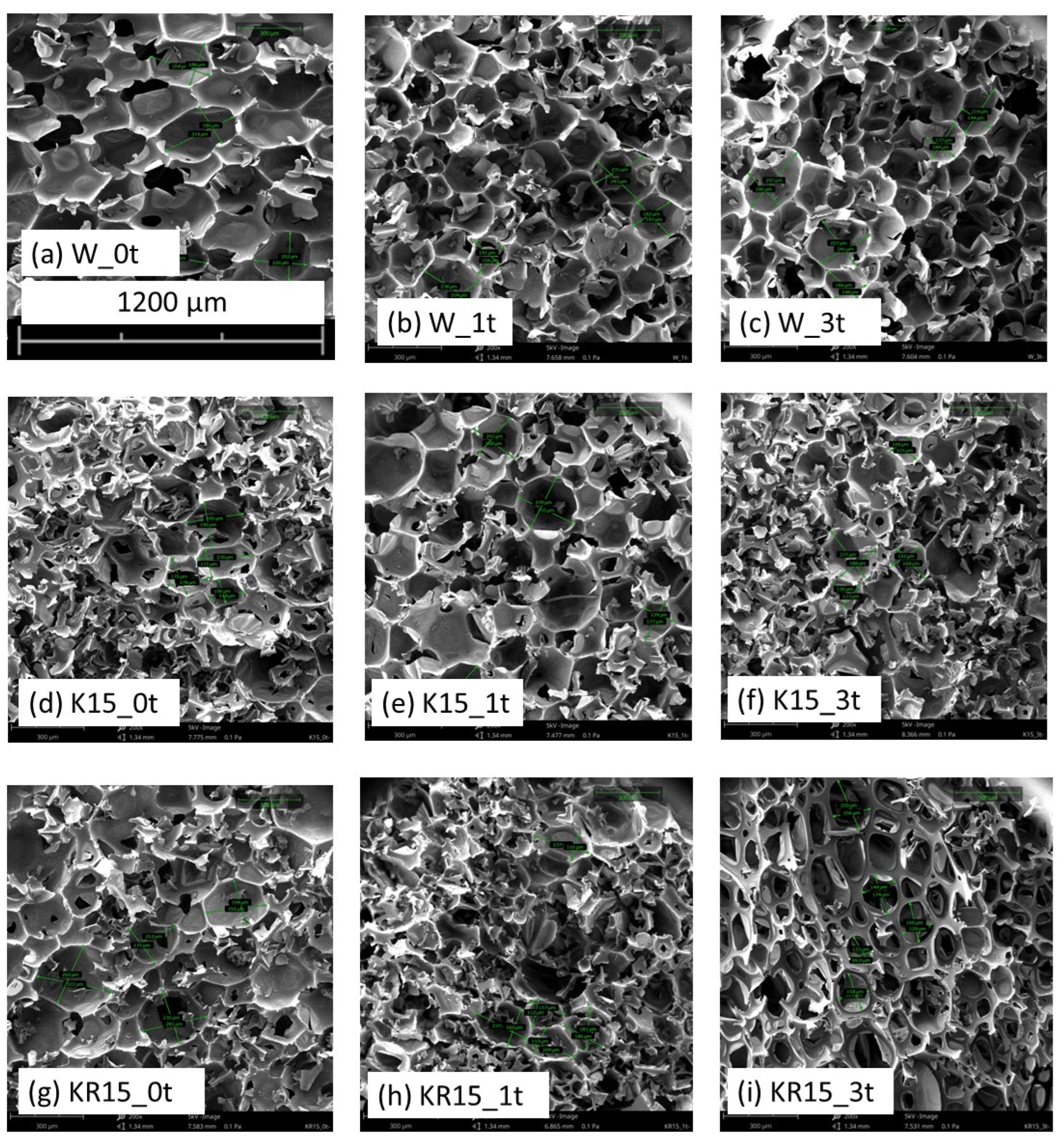
3.2. SEM
3.3. Aging Measurement
3.4. Retention, Fragility, OI
3.5. Conductivity versus Foam Density and Cell Size
3.6. Water Absorption, Absorbability, Closed Cell Content
3.7. Compressive Strength
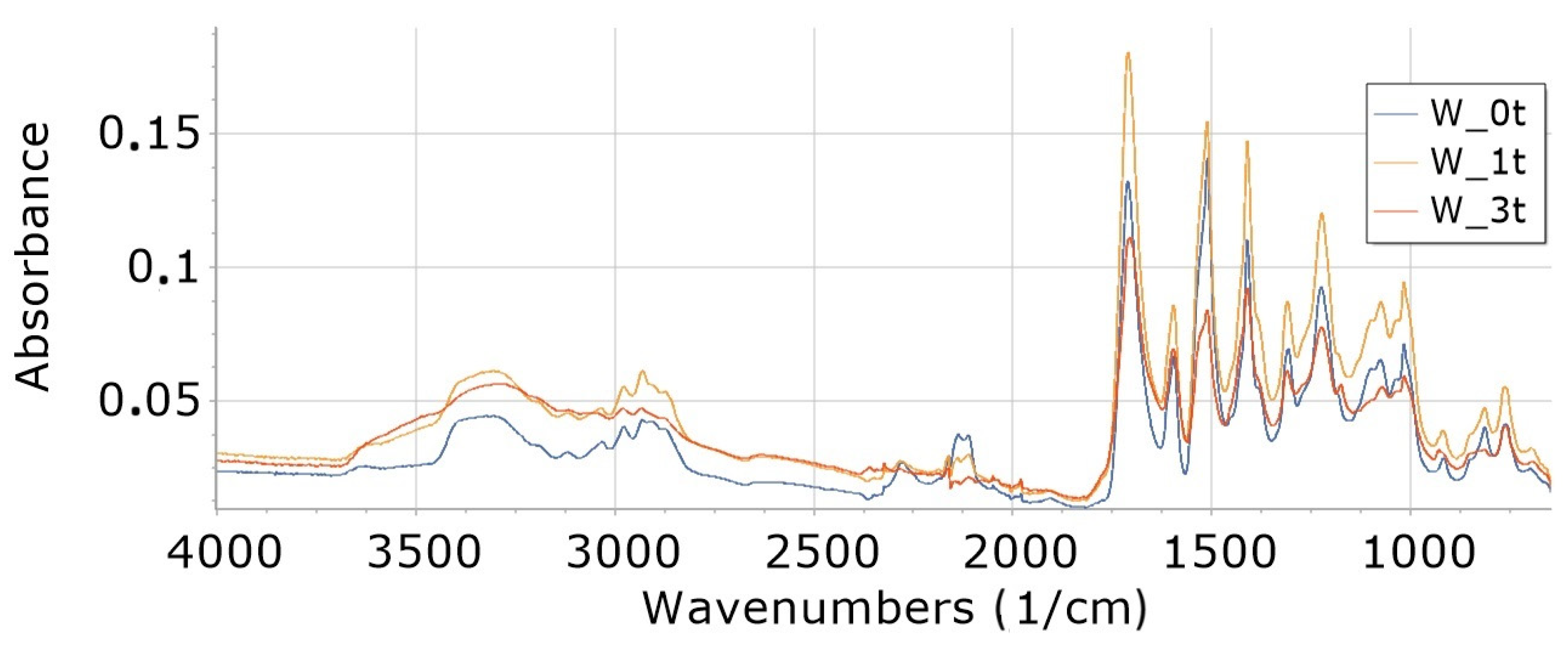
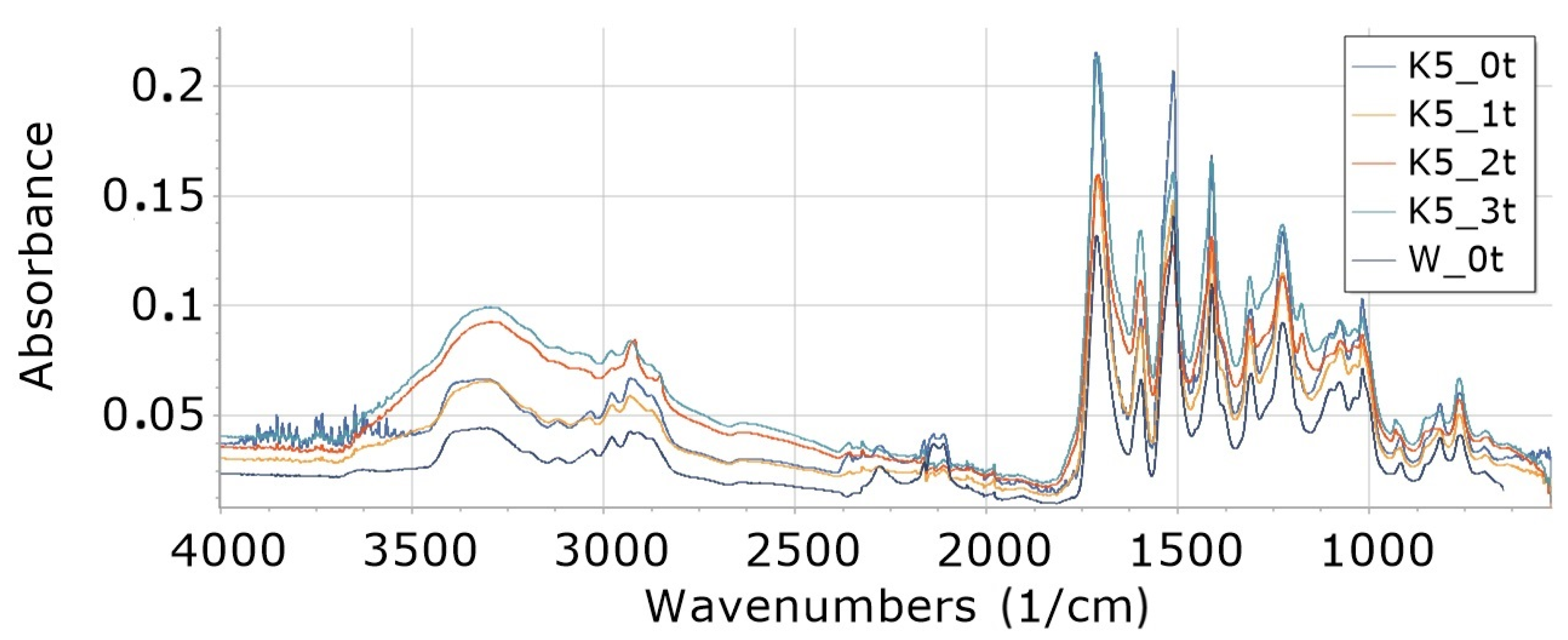
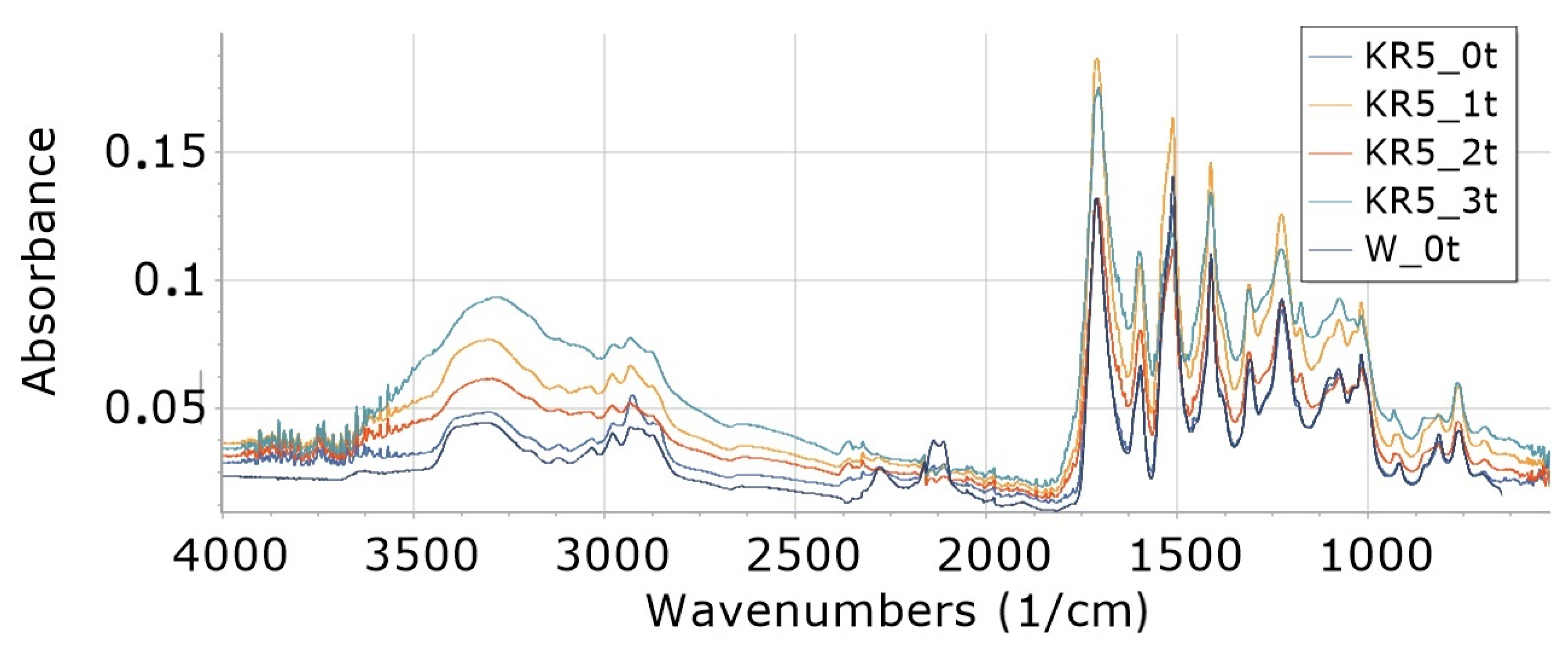
3.8. FTIR
3.8.1. Influence of Degradation Time on Absorbance of W, K5 and KR5
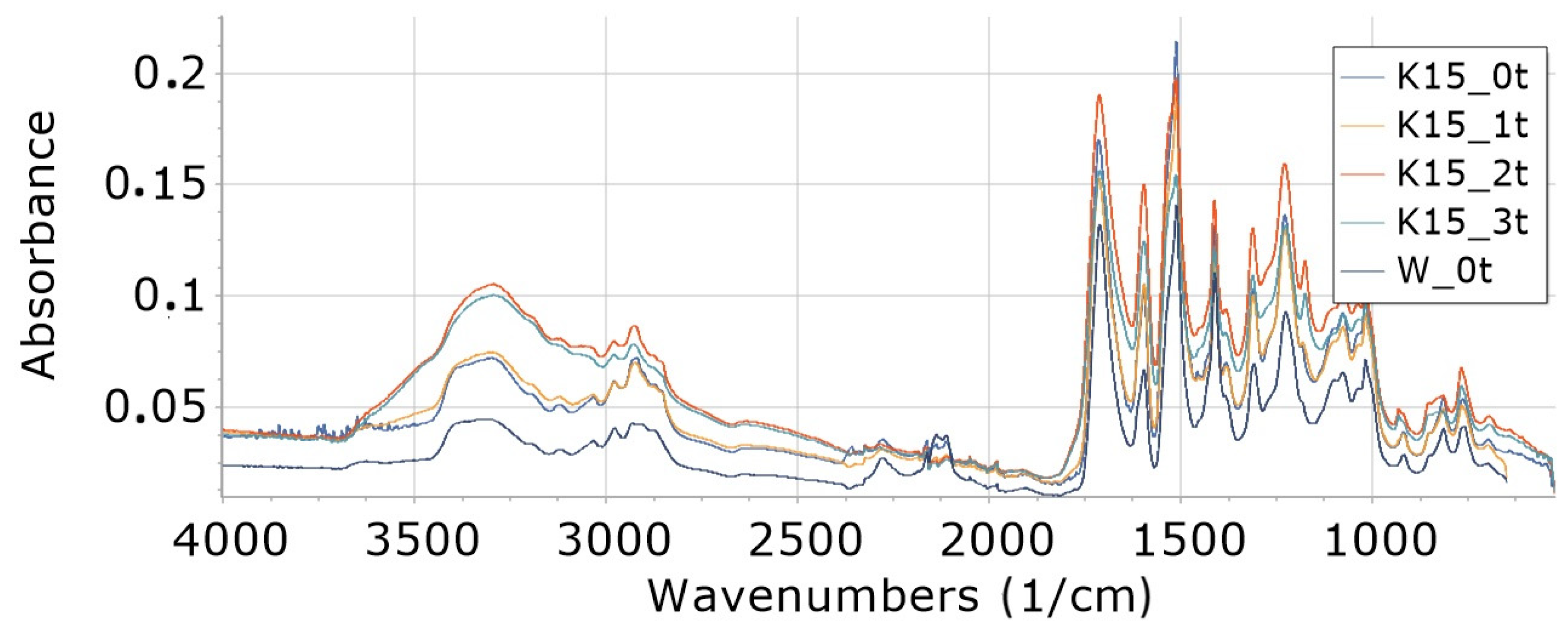
3.8.2. Influence of Degradation Time on Absorbance of K15 and KR15
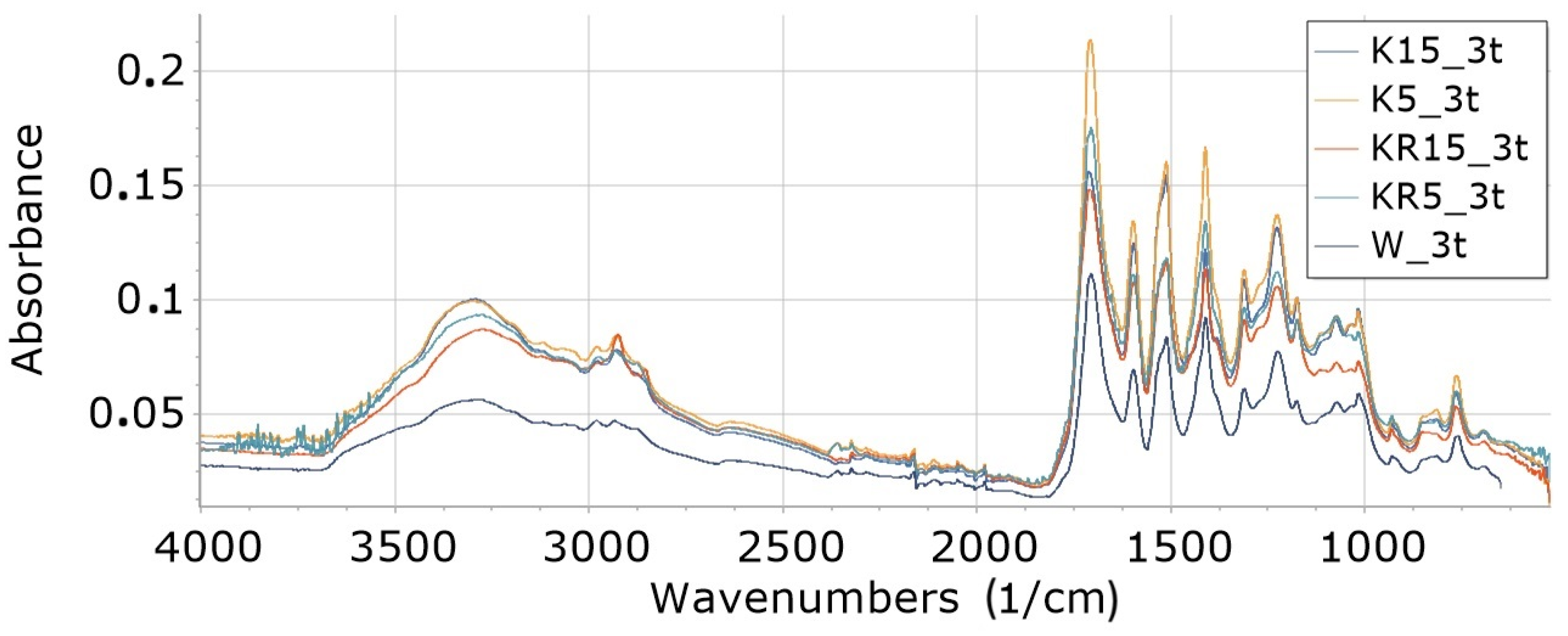
3.8.3. Influence of Type and Amount of Filler on Absorbance of Foam Degraded 3 Weeks (Foams 3t)
3.9. Color of Foams
3.10. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Bote, S.D.; Kiziltas, A.; Scheper, I.; Mielewski, D.; Narayan, R. Biobased Flexible Polyurethane Foams Manufactured from Lactide-based Polyester-ether Polyols for Automotive Applications. J. Appl. Polym. Sci. 2021, 138, 50690. [Google Scholar] [CrossRef]
- Ryszkowska, J.; Prociak, A.; Rokicki, G.; Ryszkowska, J. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2014. [Google Scholar]
- Swinarew, B. Poliuretany—Nowoczesne Wszechstronne Materiały. Część II—Pianki Poliuretanowe. Przetwórstwo Tworzyw 2015, 5, 428–434. [Google Scholar]
- Eaves, D. Handbook of Polymer Foams; Rapra Technology Ltd.: Shawbury, UK, 2004; p. 289. [Google Scholar]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic Materials: Strategic design and structure–function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Noorani, R. 3D Printing: Technology, Applications, and Selection; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315155494. [Google Scholar]
- Grzabka-Zasadzinska, A.; Bartczak, P.; Borysiak, S. Highly Insulative PEG-Grafted Cellulose Polyurethane Foams—From Synthesis to Application Properties. Materials 2021, 14, 6363. [Google Scholar] [CrossRef]
- Peyrton, J.; Avérous, L. Structure-Properties Relationships of Cellular Materials from Biobased Polyurethane Foams. Mater. Sci. Eng. 2021, R145, 100608. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Li, L. Flame Retardant Behavior of Ternary Synergistic Systems in Rigid Polyurethane Foams. Polymers 2019, 11, 207. [Google Scholar] [CrossRef]
- Ma, X.; Tu, R.; Cheng, X.D.; Zhu, S.G.; Ma, J.W.; Fang, T.Y. Experimental Study of Thermal Behavior of Insulation Material Rigid Polyurethane in Parallel, Symmetric, and Adjacent Building Facade Constructions. Polymers 2018, 10, 1104. [Google Scholar] [CrossRef]
- Huang, G.B.; Yang, J.G.; Wang, X.; Gao, J.R.; Liang, H.D. A phosphorus-nitrogen containing dendrimer reduces the flammability of nanostructured polymers with embedded self-assembled gel networks. J. Mater. Chem. A 2013, 1, 1677–1687. [Google Scholar] [CrossRef]
- Li, Y.C.; Kim, Y.S.; Shields, J.; Davis, R. Controlling polyurethane foam flammability and mechanical behaviour by tailoring the composition of clay-based multilayer nanocoatings. J. Mater. Chem. A. 2013, 1, 12987–12997. [Google Scholar] [CrossRef]
- Jimenez, M.; Lesaffre, N.; Bellayer, S.; Dupretz, R.; Vandenbossche, M.; Duquesne, S.; Bourbigot, S. Novel flame retardant flexible polyurethane foam: Plasma induced graft-polymerization of phosphonates. RSC Adv. 2015, 5, 63853–63865. [Google Scholar] [CrossRef]
- Danihelová, A.; Sčensný, P.; Gerge, T.; Ondrejka, V.; Němec, M.; Igaz, R.; Štefko, J.; Mitterová, I. Influence of flame retardant impregnation on acoustic and ther mophysical properties of recycled technical textiles with the potential for use in wooden buildings. Polymers 2021, 13, 2598. [Google Scholar] [CrossRef]
- Sartori, I.; Hestnes, A.G. Energy use in the life cycle of conventional and low-energy buildings: A review article. Energy Build. 2007, 39, 249–257. [Google Scholar] [CrossRef]
- Borowicz, M.; Isbrandt, M.; Paciorek-Sadowska, J. Effect of New Eco-Polyols Based on PLAWaste on the Basic Properties of Rigid Polyurethane and Polyurethane/Polyisocyanurate Foams. Int. J. Mol. Sci. 2021, 22, 8981. [Google Scholar] [CrossRef]
- Pinto, A.; Fernandes, I.P.; Pinto, V.D.; Gomes, E.; Oliveira, C.F.; Pinto, P.C.R.C.R.; Mesquita, L.M.R.; Piloto, P.A.G.; Rodrigues, A.E.; Barreiro, M.-F. Valorization of lignin side-streams into polyols and rigid polyurethane foams-A contribution to the pulp and paper industry biorefinery. Energies 2021, 14, 3825. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of preparation process to produce polyurethane foam made by oilseed rape straw based polyol. Polym. Degrad. Stab. 2019, 166, 31–39. [Google Scholar] [CrossRef]
- Cao, H.; Liu, R.; Wu, B.; Li, Y.; Wang, K.; Yang, Y.; Li, A.; Zhuang, Y.; Cai, D.; Qin, P. Biobased Rigid Polyurethane Foam Using Gradient Acid Precipitated Lignin from the Black Liquor: Revealing the relationship between lignin structural features and polyurethane performances. Ind. Crops Prod. 2022, 177, 114480. [Google Scholar] [CrossRef]
- Ryszkowska, J. Materiały Poliuretanowe Wytwarzane z Zastosowaniem Surowców Odnawialnych; OWPW: Warszawa, Poland, 2020. [Google Scholar]
- Liszkowska, J.; Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Czuprynski, B.; Moraczewski, K. Assessment of Photodegradation and Biodegradation of RPU/PIR Foams Modified by Natural Compounds of Plant Origin. Polymers 2020, 12, 33. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Stability and degradation of four agricultural wastes liquefied polyols based polyurethane foams. Appl. Polym. Sci. 2022, 139, e52893. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Effect of natural biomass fillers on the stability, degradability, and elasticity of crop straws liquefiedpolyols-based polyurethane foams. Appl. Polym. Sci. 2023, 140, e53324. [Google Scholar] [CrossRef]
- Yakushin, V.; Cabulis, U.; Fridrihsone, V.; Kravcheko, S.; Pauliks, R. Properties of polyurethane foam with 4th generation blowing agent. e-Polymers 2021, 21, 763–769. [Google Scholar] [CrossRef]
- Borowicz, M. Synthesis and Application of New Bio-Polyols Based on Vegetable Raw Materials for the Production of Bio-Composites in the Form of Rigid Polyurethane-Polyisocyanurate Foams. Ph.D. Thesis, West Pomeranian University of Technology, Szczecin, Poland, 2019. (In Polish). [Google Scholar]
- Szpiłyk, M.; Lubczak, R.; Lubczak, J. The biodegradable cellulose-derived polyol and polyurethane foam. Polym. Test. 2021, 100, 107250. [Google Scholar] [CrossRef]
- Boscolo, A.; Boscoletto, B.; Cellarosi, B. Effect of Structural Anisotropy on Thermal Ageing in Rigid PU Foams. J. Therm. Insul. 1992, 15, 294–310. [Google Scholar] [CrossRef]
- Guo, A.; Zhang, W.; Petrovic, Z.S. Structure-property relationships in polyurethanes derived from soybean oil. J. Mater. Sci. 2006, 41, 4914–4920. [Google Scholar] [CrossRef]
- Bomberg, M.T.; Kumaran, M.K.; Ascough, M.R.; Sylvester, R.G. Effect of time and temperature on R-value of rigid polyurethane foam insulation manufactured with alternative blowing agents. J. Therm. Insul. 1991, 14, 241–267. [Google Scholar] [CrossRef]
- Wilczyński, K. (Ed.) Przetwórstwo Tworzyw Polimerowych; OWPW: Warszawa, Poland, 2018. [Google Scholar]
- Soares, B.; Gama, N.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Netoa, C.P.; Ferreiraa, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Liszkowska, J.; Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Isbrandt, M.; Moraczewski, K. Stabilization of rigid PUR-PIR foam. Polym. Sci. 2019, 5, 12. [Google Scholar]
- Liszkowska, J.; Moraczewski, K.; Borowicz, M.; Paciorek-Sadowska, J.; Czupryński, B.; Isbrandt, M. The Effect of Accelerated Aging Conditions on the Properties of Rigid Polyurethane-Polyisocyanurate Foams Modified by Cinnamon Extract. Appl. Sci. 2019, 9, 2663. [Google Scholar] [CrossRef]
- Liszkowska, J. The effect of ground coffee on the mechanical and application properties of rigid PUR-PIR foams. Polimery 2018, 63, 305–310. [Google Scholar] [CrossRef]
- Danielewska, A.; Maciąg, K. (Eds.) Selected Plant Properties—The Latest Reports; WN TYGIEL sp. z o.o.: Lublin, Poland, 2019. [Google Scholar]
- Agrawal, A.; Kaura, R.; Walia, R.S. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Chu, Y.-F. (Ed.) Coffee: Emerging Health Effects and Disease Prevention; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Czaplińska, M.; Czepas, J.; Gwoździński, K. Structure, antioxidant and anti-cancer properties flavonoid bike. Adv. Biochem. 2012, 58, 235–244. [Google Scholar]
- Gichimu, B.M.; Gichuru, E.K.; Mamati, G.E.; Nyende, A.B. Novel Neuroprotective Formulations Based on St. John’s Wort Extract. J. Food Res. 2014, 3, 3. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P. Coffee fermentation and flavor—An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef]
- Spătaru, N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Anodic Voltammetry of Xanthine, Theophylline, Theobromine and Caffeine at Conductive Diamond Electrodes and Its Analytical Application. Electroanalysis 2002, 14, 721–728. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of Coffee Roasting on the Incorporation of Phenolic Compounds into Melanoidins and Their Relationship with Antioxidant Activity of the Brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Shim, J.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Cocoa phytochemicals: Recent advances in molecular mechanisms on health. Crit. Rev. Food Sci. Nutr. 2014, 54, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Technical Card of Cocoa; MASPEX-GMW Sp. z o.o.: Wadowice, Poland.
- Kurang, R.Y.; Kamengon, R.Y. Phytochemical and antioxidant activities of Robusta coffee leaves extracts from Alor Island, East Nusa Tenggara. AIP Conf. Proc. 2021, 2349, 020028. [Google Scholar] [CrossRef]
- Liszkowska, J.; Czupryński, B.; Paciorek-Sadowska, J. Thermal properties of polyurethane-polyisocyanurate (PUR-PIR) foams modified with tris(5-hydroxypentyl)-2-hydroxypropane-1,2,3-tricarboxylate. J. Adv. Chem. Eng. 2016, 6, 148–154. [Google Scholar] [CrossRef]
- Czupryński, B.; Liszkowska, J.; Paciorek-Sadowska, J. Modification of rigid polyurethane-polyisocyanurate foam selected powder fillers. Polimery 2008, 3, 48–52. [Google Scholar]
- ASTM Standard D7487-13e1; Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test. ASTM International: West Conshohocken, PA, USA, 2016.
- Broniewski, T.; Kapko, J.; Płaczek, W.; Thomalla, J. Test Methods and Evaluation of Plastic Properties; WNT: Warsaw, Poland, 2000. [Google Scholar]
- Sobków, M.; Czaja, K. Influence of accelered ageing conditons on the process of polyolefines degradation. Polimery 2003, 48, 627–632. [Google Scholar] [CrossRef]
- Es-tor. Operating Instructions for the Climate Chamber Model CCK-40/300 NG.
- Bajda, M.; Pogoda, M. Study of the effect of conveyor belt accelerated heat aging on its strength. Transport 2016, 1, 16–22. [Google Scholar]
- Szczepaniak, W. Instrumental Methods in Chemical Analysis; PWN: Warsaw, Poland, 2011. [Google Scholar]
- Czech-Polak, J.; Oliwa, R.; Oleksy, M.; Budzik, G. Rigid polyurethane foams with improved flame resistance. Polimery 2018, 63, 115–124. [Google Scholar] [CrossRef]
- Smykowska, M. Pianka poliuretanowa w opakowaniach-stworzona, by chronić. Opakowanie 2015, 03, 1. [Google Scholar]
- Akdogan, E.; Erdem, M.; Erdem, U.M.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2019, 137, 47611. [Google Scholar] [CrossRef]
- Buahom, P.; Thongmongkol, K.; Alshrah, K.; Gong, P.; Park, C.B. Effects of Cell Anisotropy on Conductive and Radiative Thermal Transport in Polymeric Foam Insulation. Energy 2023, 275, 127473. [Google Scholar] [CrossRef]
- Glicksman, L.R. Heat transfer in foams. In Low Density Cellular Plastics; Hilyard, N.C., Cunningham, A., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 104–152. [Google Scholar] [CrossRef]
- Fang, W.; Tang, Y.; Zhang, H.; Tao, W. Numerical predictions of the effective thermal conductivity of the rigid polyurethane foam. J. Wuhan Univ. Technol. 2017, 32, 703–708. [Google Scholar] [CrossRef]
- Wu, J.W.; Sung, W.F.; Chu, H.S. Thermal conductivity of polyurethane foams. Int. J. Heat Mass Tran. 1999, 42, 2211–2217. [Google Scholar] [CrossRef]
- Lim, H.; Kim, S.H.; Kim, B.K. Effects of silicon surfactant in rigid polyurethane foams. Express Polym. Lett. 2008, 2, 194–200. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, C.; Liu, H.; Huang, Q. Fabrication and characterization of novel foaming polyurethane hollow fiber membrane. Chin. J. Chem. Eng. 2019, 27, 935–943. [Google Scholar] [CrossRef]
- Radziszewska-Zielina, E. Analiza porównawcza parametrów materiałów termoizolacyjnych mających zastosowanie jako izolacja ścian zewnętrznych. Przegląd Budowlany 2009, 4, 32. [Google Scholar]
- Kocjan, R. Chemia Analityczna. Podręcznik dla Studentów. Analiza Instrumentalna; Tom 2; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2002. [Google Scholar]
- Wirpsza, Z. Polyurethanes: Chemistry, Technology, Application; WNT: Warsaw, Poland, 1991. (In Polish) [Google Scholar]
- Gómez, E.F.; Luo, X.; Li, C.; Michel, F.C.; Li, Y. Biodegradability of crude glycerol-based polyurethane foams during composting, anaerobic digestion and soil incubation. Polym. Degrad. Stab. 2014, 102, 195–203. [Google Scholar] [CrossRef]
- Wendels, S.; Heinrich, B.; Donnio, B.; Avérous, L. Green and controlled synthesis of short diol oligomers from polyhydroxyalkanoate to develop fully biobased thermoplastics. Eur. Polym. J. 2021, 153, 110531. [Google Scholar] [CrossRef]
- Uram, K.; Kuranska, M.; Andrzejewski, J.; Prociak, A. Rigid polyurethane foams modified with biochar. Materials 2021, 14, 5616. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, A.; Haddar, N.; Elleuch, K.; Bienvenu, Y. Influence of thermal aging on tensile and creep behavior of thermoplastic polyurethane. C. R. Mécanique 2011, 339, 666–673. [Google Scholar] [CrossRef]
- Birta, N.; Doca, N.; Vlase, G.; Vlase, T. Kinetic of sorbitol decomposition under nonisothermal conditions. J. Therm. Anal. Calorim. 2008, 92, 635–638. [Google Scholar] [CrossRef]
- Lendvai, L.; Karger-Kocsis, J.; Kmetty, Á.; Drakopoulos, S.X. Production and characterization of microfibrillated cellulose-reinforced thermoplastic starch composites. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.-T.; Vázquez, A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2008, 73, 55–63. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J.; Stumborg, M. Properties of biodegradable citric acidmodified granular starch/thermoplastic pea starch composites. Carbohydr. Polym. 2009, 75, 1–8. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Katsikas, L.; Popović, I.; Jovanović, S.; Jeremić, K. Thermal stability of starch benzoate. Polym. Degrad. Stab. 2005, 87, 177–182. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]


| Compound | Content In KR Foam | Content in K Foam |
|---|---|---|
| Polyphenols (e.g., flavonoids, phenol acids, chlorogenic acid) | 45.0% | 0.0% |
| Minerals | 4.4% | <0.3 ppm |
| Carrier to active substances (e.g., lignin, cellulose, polysaccharides) Fat Carbohydrates | About 50.6% - 41% | About 94.9% 10.5% 13.0% |
| Filler (wt.%) | ||
|---|---|---|
| Foam | Instant Coffee | Cocoa |
| W_0t | 0 | 0 |
| KR5_0t | 5 | 0 |
| KR10_0t | 10 | 0 |
| KR15_0t | 15 | 0 |
| K5_0t | 0 | 5 |
| K10_0t | 0 | 10 |
| K15_0t | 0 | 15 |
| Foam | Cream Time (s) | String Gel Time (s) | Tack Free Time (s) | Free Rise Time (s) | Tmax (°C) | Apparent Density (Kg/m3) |
|---|---|---|---|---|---|---|
| W_0t | 10 | 21 | 23 | 40 | 142 | 38.44 ± 3.6 |
| KR5_0t | 10 | 27 | 30 | 41 | 160 | 37.67 ± 3.6 |
| KR10_0t | 10 | 33 | 35 | 45 | 140 | 39.36 ± 3.6 |
| KR15_0t | 10 | 37 | 40 | 55 | 149 | 41.46 ± 3.6 |
| K5_0t | 10 | 27 | 29 | 40 | 153 | 35.12 ± 3.6 |
| K10_0t | 10 | 28 | 31 | 43 | 146 | 36.52 ± 3.6 |
| K15_0t | 10 | 29 | 35 | 48 | 139 | 30.09 ± 3.6 |
| Foam | H (μm) | W (μm) | AC (−) | SA (mm2) |
|---|---|---|---|---|
| W_0t | 231 ± 29 | 203 ± 30 | 1.14 ± 0.27 | 0.047 ± 0.01 |
| W_1t | 208 ± 29 | 193 ± 30 | 1.08 ± 0.27 | 0.040 ± 0.01 |
| W_3t | 217 ± 29 | 170 ± 30 | 1.30 ± 0.27 | 0.037 ± 0.01 |
| K15_0t | 200 ± 29 | 149 ± 30 | 1.34 ± 0.27 | 0.030 ± 0.01 |
| K15_1t | 217 ± 29 | 188 ± 30 | 1.15 ± 0.27 | 0.041 ± 0.01 |
| K15_3t | 164 ± 29 | 138 ± 30 | 1.19 ± 0.27 | 0.023 ± 0.01 |
| KR15_0t | 268 ± 29 | 215 ± 30 | 1.24 ± 0.27 | 0.058 ± 0.01 |
| KR15_1t | 205 ± 29 | 138 ± 30 | 1.49 ± 0.27 | 0.028 ± 0.01 |
| KR15_3t | 168 ± 29 | 131 ± 30 | 1.28 ± 0.27 | 0.022 ± 0.01 |
| Foam | ∆lz (%) | ∆lp (%) | ∆V (%) | ∆m (%) |
|---|---|---|---|---|
| W_48 | +0.39 ± 0.01 | +0.39 ± 0.01 | −1.77 ± 0.01 | +3.27 ± 0.01 |
| K5_48 | +1.38 ± 0.01 | +0.98 ± 0.01 | −2.93 ± 0.01 | +4.27 ± 0.01 |
| K10_48 | −1.39 ± 0.01 | +1.12 ± 0.01 | −3.08 ± 0.01 | +4.56 ± 0.01 |
| K15_48 | +1.40 ± 0.01 | +2.94 ± 0.01 | −4.68 ± 0.01 | +5.63 ± 0.01 |
| KR5_48 | +0.16 ± 0.01 | +0.10 ± 0.01 | −0.44 ± 0.01 | +0.60 ± 0.01 |
| KR10_48 | +0.16 ± 0.01 | +0.12 ± 0.01 | −0.34 ± 0.01 | +1.10 ± 0.01 |
| KR15_48 | +0.16 ± 0.01 | +0.14 ± 0.01 | −0.42 ± 0.01 | +0.71 ± 0.01 |
| Foam | K (%) | Λ (kW/mK) ±0.00006 | OI (%vol. of O2) | R (%) |
|---|---|---|---|---|
| W_0t | 16.42 ± 6.25 | 0.0289 | 24.7 ± 0.52 | 83.44 ± 2 |
| KR5_0t | 18.96 ± 6.25 | 0.0350 | 24.4 ± 0.52 | 84.96 ± 2 |
| KR10_0t | 13.97 ± 6.25 | 0.0350 | 24.3 ± 0.52 | 84.68 ± 2 |
| KR15_0t | 13.79 ± 6.25 | 0.0350 | 24.2 ± 0.52 | 82.62 ± 2 |
| K5_0t | 17.82 ± 1.85 | 0.0350 | 24.2 ± 0.52 | 84.54 ± 2 |
| K10_0t | 13.14 ± 1.85 | 0.0350 | 23.8 ± 0.52 | 81.71 ± 2 |
| K15_0t | 14.09 ± 1.85 | 0.0350 | 23.0 ± 0.52 | 78.17 ± 2 |
| Foam | N (%) | Ch (%) | Z (%) | Sp (°C) |
|---|---|---|---|---|
| W_0t | 12.33 ± 13 | 5.15 ± 4.8 | 87.0 ± 24 | 184 ± 6 |
| KR5_0t | 23.51 ± 13 | 2.72 ± 4.8 | 44.1 ± 24 | 178 ± 6 |
| KR10_0t | 29.98 ± 13 | 2.86 ± 4.8 | 32.7 ± 24 | 180 ± 6 |
| KR15_0t | 53.37 ± 13 | 16.16 ± 4.8 | 9.6 ± 24 | 184 ± 6 |
| K5_0t | 19.45 ± 13 | 0.84 ± 4.8 | 41.1 ± 24 | 192 ± 6 |
| K10_0t | 24.55 ± 13 | 2.14 ± 4.8 | 30.2 ± 24 | 183 ± 6 |
| K15_0t | 43.81 ± 13 | 4.62 ± 4.8 | 9.0 ± 24 | 171 ± 6 |
| Foam | CSa_0t (kPas) | CSb_0t (kPas) | CSb_2t (kPas) | CSb_3t (kPas) | CV1 (−) | CV2 (−) |
|---|---|---|---|---|---|---|
| W_0t | 128.21 ± 19 | 251.63 ± 42 | 151.80 ± 18 | 140.60 ± 21 | 60.3 ± 9.6 | 55.9 ± 9 |
| KR5_0t | 101.87 ± 19 | 167.82 ± 42 | 134.00 ± 18 | 90.95 ± 21 | 79.8 ± 9.6 | 76.9 ± 9 |
| KR10_0t | 129.79 ± 19 | 199.14 ± 42 | 99.98 ± 18 | 89.86 ± 21 | 50.2 ± 9.6 | 54.2 ± 9 |
| KR15_0t | 83.96 ± 19 | 131.40 ± 42 | 88.19 ± 18 | 67.45 ± 21 | 67.6 ± 9.6 | 51.3 ± 9 |
| K5_0t | 111.07 ± 19 | 188.12 ± 42 | 124.20 ± 18 | 115.71 ± 21 | 66.0 ± 9.6 | 61.5 ± 9 |
| K10_0t | 94.45 ± 19 | 135.81 ± 42 | 108.55 ± 18 | 97.14 ± 21 | 66.0 ± 9.6 | 71.5 ± 9 |
| K15_0t | 76.15 ± 19 | 126.69 ± 42 | 99.81 ± 18 | 88.19 ± 21 | 78.8 ± 9.6 | 69.6 ± 9 |
| Sample | L* (−) | a* (−) | b* (−) | ΔE (−) |
|---|---|---|---|---|
| Foam 0t | ||||
| W_0t | 82.07 | −0.59 | 23.74 | 85.43665 |
| K5_0t | 78.96 | 2.46 | 17.32 | 80.87469 |
| K15_0t | 71.89 | 5.4 | 16.47 | 73.94994 |
| KR5_0t | 83.29 | −0.93 | 15.85 | 84.78981 |
| Foam 1t | ||||
| W_1t | 73.01 | 4.82 | 37.64 | 82.28282 |
| K5_1t | 63.65 | 15.17 | 38.75 | 76.04613 |
| K15_1t | 62.41 | 16.72 | 37.93 | 74.92164 |
| KR5_1t | 61.72 | 17.94 | 43.81 | 77.78508 |
| KR10_1t | 61.49 | 14.75 | 40.93 | 75.32495 |
| KR15_1t | 60.35 | 11.83 | 37.02 | 71.78128 |
| Foam 2t | ||||
| W_2t | 62.73 | 4.94 | 31.58 | 70.40421 |
| K5_2t | 53.53 | 20.90 | 44.57 | 72.72383 |
| K15_2t | 51.03 | 21.22 | 42.81 | 69.90741 |
| KR5_2t | 51.53 | 21.69 | 43.99 | 71.14012 |
| KR15_2t | 45.64 | 21.82 | 41.70 | 65.55923 |
| Foam 3t | ||||
| W_3t | 53.93 | 16.97 | 38.54 | 68.42337 |
| K5_3t | 47.32 | 22.77 | 41.71 | 67.06250 |
| K15_3t | 49.02 | 21.74 | 40.42 | 67.15180 |
| KR5_3t | 49.10 | 23.49 | 47.65 | 72.34026 |
| KR10_3t | 50.74 | 21.55 | 42.71 | 69.73589 |
| KR15_3t | 43.45 | 22.35 | 41.55 | 64.13913 |
| Foam | Stage 1 | Stage 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Start of the Change of Mass | Start of the Change of Mass | ||||||||
| T1 (°C) ±0.1 | Weight Loss (%) ±0.1 | T2 (°C) ±0.4 | Weight Loss (%) ±0.1 | Tmax (°C) ±0.1 | T5 (°C) ±0.4 | T20 (°C) ±0.4 | T50 (°C) ±0.4 | Residue at 900 °C (%) ± 0.1 | |
| W_0t | 45.0 | 1.0 | 227.0 | 30.0 | 317.0 | 207.0 | 292.0 | 340.0 | 2.0 |
| W_1t | 50.0 | 0.0 | 25.02 | 15.0 | 318.7 | 210.9 | 285.0 | 331.5 | 10.0 |
| W_2t | - | - | - | - | - | - | - | - | - |
| W_3t | - | - | - | - | - | - | - | - | - |
| KR5_0t | 33.0 | 5.0 | 233.0 | 73.0 | 319.0 | 198.0 | 319.0 | 353.0 | 5.5 |
| KR5_1t | 38.8 | 34.0 | 228.2 | 89.0 | 309.9 | 188.8 | 287.4 | 371.1 | 2.7 |
| KR5_2t | 46.0 | 36.0 | 247.5 | 72.0 | 318.0 | 185.0 | 276.0 | 465.7 | 1.7 |
| KR5_3t | 57.0 | 9.9 | 250.5 | 76.0 | 308.0 | 186.8 | 278.6 | 470.9 | 18.0 |
| KR15_0t | 27.0 | 5.0 | 230.0 | 26.0 | 317.0 | 96.0 | 233.0 | 344.0 | 5.5 |
| KR15_1t | 53.5 | 8.0 | 231.3 | 42.0 | 314.5 | 193.4 | 276.1 | 346.6 | 8.8 |
| KR15_2t | 46.2 | 7.6 | 243.7 | 52.3 | 314.8 | 188.6 | 272.8 | 399.6 | 13.5 |
| KR15_3t | - | - | - | - | - | - | - | - | - |
| K5_0t | 42.0 | 33.0 | 224.7 | 58.1 | 312.3 | 197.1 | 282.4 | 365.5 | 0.8 |
| K5_1t | 38.0 | 5.0 | 225.1 | 35.9 | 365.3 | 168.2 | 268.7 | 365.3 | 2.2 |
| K5_2t | 50.4 | 12.1 | 270.0 | 63.0 | 316.0 | 182.6 | 271.6 | 387.9 | 19.4 |
| K5_3t | 46.6 | 10.0 | 234.3 | 60.0 | 314.1 | 184.6 | 268.8 | 414.7 | 14.7 |
| K15_0t | 51.0 | 2.5 | 215.6 | 31.6 | 311.9 | 187.7 | 274.9 | 327.1 | 13.0 |
| K15_1t | - | - | - | - | - | - | - | - | - |
| K15_2t | 49.0 | 36.5 | 229.1 | 37.8 | 308.8 | 173.3 | 263.3 | 369.3 | 2.7 |
| K15_3t | 53.9 | 8.3 | 231.4 | 38.4 | 308.5 | 75.3 | 68.2 | 367.5 | 15.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liszkowska, J.; Gozdecka, G.; Sitarz, M. Methods to Increase or Decrease Resistance to Photodegradation and Biodegradation of Polyurethane/Polyisocyanurate (PU/PIR) Foams. Materials 2023, 16, 5930. https://doi.org/10.3390/ma16175930
Liszkowska J, Gozdecka G, Sitarz M. Methods to Increase or Decrease Resistance to Photodegradation and Biodegradation of Polyurethane/Polyisocyanurate (PU/PIR) Foams. Materials. 2023; 16(17):5930. https://doi.org/10.3390/ma16175930
Chicago/Turabian StyleLiszkowska, Joanna, Grażyna Gozdecka, and Magdalena Sitarz. 2023. "Methods to Increase or Decrease Resistance to Photodegradation and Biodegradation of Polyurethane/Polyisocyanurate (PU/PIR) Foams" Materials 16, no. 17: 5930. https://doi.org/10.3390/ma16175930





