Dentin Bonding Performance of Universal Adhesives in Primary Teeth In Vitro
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Krämer, N.; Frankenberger, R. Restorative therapy in deciduous teeth. Oralprophyl. Kinderzahnheilkd 2004, 26, 78–84. [Google Scholar]
- Meereis, C.T.W.; Münchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization shrinkage stress of resin-based dental materials: A systematic review and meta-analyses of composition strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.L.; Cavalheiro, C.P.; Gimenez, T.; Imparato, J.C.P.; Bussadori, S.K.; Lenzi, T.L. Bonding Performance of Universal and Contemporary Adhesives in Primary Teeth: A Systematic Review and Network Meta-Analysis of In Vitro Studies. Pediatr. Dent. 2021, 43, 170–177. [Google Scholar] [PubMed]
- Fröhlich, T.T.; Gindri, L.D.; Soares, F.Z.M.; de Oliveira Rocha, R. Does the etching strategy influence the bonding of universal adhesive systems to primary teeth? A systematic review and meta-analysis of in vitro studies. Eur. Arch. Paediatr. Dent. 2021, 22, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Nakajima, K.; Aoki-Tabei, N.; Arita, A.; Nishiyama, N. NMR study on the demineralization mechanism of the enamel and dentin surfaces in MDP-based all-in-one adhesive. Dent. Mater. J. 2018, 37, 693–701. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; van Landuyt, K.L.; Osaka, A.; Suzuki, K.; Minag, S.; et al. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Ogliari, A.O.; Yoshihara, K.; Zanchi, C.H.; Correr-Sobrinho, L.; Sinhoreti, M.A.; Correr, A.B.; Watson, T.F.; et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent. Mater. 2014, 30, e317–e323. [Google Scholar] [CrossRef]
- Inoue, S.; Koshiro, K.; Yoshida, Y.; de Munck, J.; Nagakane, K.; Suzuki, K.; Sano, H.; Van Meerbeek, B. Hydrolytic stability of self-etch adhesives bonded to dentin. J. Dent. Res. 2005, 84, 1160–1164. [Google Scholar] [CrossRef]
- Bücher, K.; Metz, I.; Pitchika, V.; Hickel, R.; Kühnisch, J. Survival characteristics of composite restorations in primary teeth. Clin. Oral Investig. 2015, 19, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Amend, S.; Frankenberger, R.; Oschmann, T.; Lücker, S.; Winter, J.; Krämer, N. Long-term microtensile bond strength of self-etch adhesives and influence of 7-s phosphoric acid etching on adhesion of a 3-step etch-and-rinse adhesive to the dentine of primary teeth. Int. J. Paediatr. Dent. 2022, 32, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Chimeli, T.B.C.; D’Alpino, P.H.P.; Pereira, P.N.; Hilgert, L.A.; Di Hipólito, V.; Garcia, F.C.P. Effects of solvent evaporation on water sorption/solubility and nanoleakage of adhesive systems. J. Appl. Oral Sci. Rev. 2014, 22, 294–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mithiborwala, S.; Chaugule, V.; Munshi, A.K.; Patil, V. A comparison of the resin tag penetration of the total etch and the self-etch dentin bonding systems in the primary teeth: An in vitro study. Contemp. Clin. Dent. 2012, 3, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.G.; Kaaden, C.; Powers, J.M. Bond strength of self-etching primers to enamel and dentin of primary teeth. Pediatr. Dent. 2001, 23, 481–486. [Google Scholar] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; de Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Sumikawa, D.A.; Marshall, G.W.; Gee, L.; Marshall, S.J. Microstructure of primary tooth dentin. Pediatr. Dent. 1999, 21, 439–444. [Google Scholar]
- Krämer, N.; Tilch, D.; Lücker, S.; Frankenberger, R. Status of ten self-etch adhesives for bonding to dentin of primary teeth. Int. J. Paediatr. Dent. 2014, 24, 192–199. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Ruiz-Requena, M.E.; Toledano, M. MMPs activity and bond strength in deciduous dentine-resin bonded interfaces. J. Dent. 2013, 41, 549–555. [Google Scholar] [CrossRef]
- Tian, F.-C.; Wang, X.-Y.; Huang, Q.; Niu, L.-N.; Mitchell, J.; Zhang, Z.-Y.; Prananik, C.; Zhang, L.; Chen, J.-H.; Breshi, L.; et al. Effect of nanolayering of calcium salts of phosphoric acid ester monomers on the durability of resin-dentin bonds. Acta Biomater. 2016, 38, 190–200. [Google Scholar] [CrossRef]
- van Landuyt, K.L.; Yoshida, Y.; Hirata, I.; Snauwaert, J.; de Munck, J.; Okazaki, M.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Influence of the chemical structure of functional monomers on their adhesive performance. J. Dent. Res. 2008, 87, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Palosaari, H.; Wahlgren, J.; Larmas, M.; Sorsa, T.; Salo, T. Human odontoblast culture method: The expression of collagen and matrix metalloproteinases (MMPs). Adv. Dent. Res. 2001, 15, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Zhang, L.; Jiao, K.; Li, F.; Ding, Y.X.; Wang, D.Y.; Wang, M.Q.; Tay, F.R.; Chen, J.H. Localization of MMP-2; MMP-9; TIMP-1; and TIMP-2 in human coronal dentine. J. Dent. 2011, 39, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Hedenbjörk-Lager, A.; Hamberg, K.; Pääkkönen, V.; Tjäderhane, L.; Ericson, D. Collagen degradation and preservation of MMP-8 activity in human dentine matrix after demineralization. Arch. Oral Biol. 2016, 68, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Can-Karabulut, D.C.; Oz, F.T.; Karabulut, B.; Batmaz, I.; Ilk, O. Adhesion to primary and permanent dentin and a simple model approach. Eur. J. Dent. 2009, 3, 32–41. [Google Scholar] [CrossRef]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, 41–53. [Google Scholar] [CrossRef]
- Choi, A.-N.; Lee, J.-H.; Son, S.-A.; Jung, K.-H.; Kwon, Y.H.; Park, J.-K. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials 2017, 10, 1224. [Google Scholar] [CrossRef]
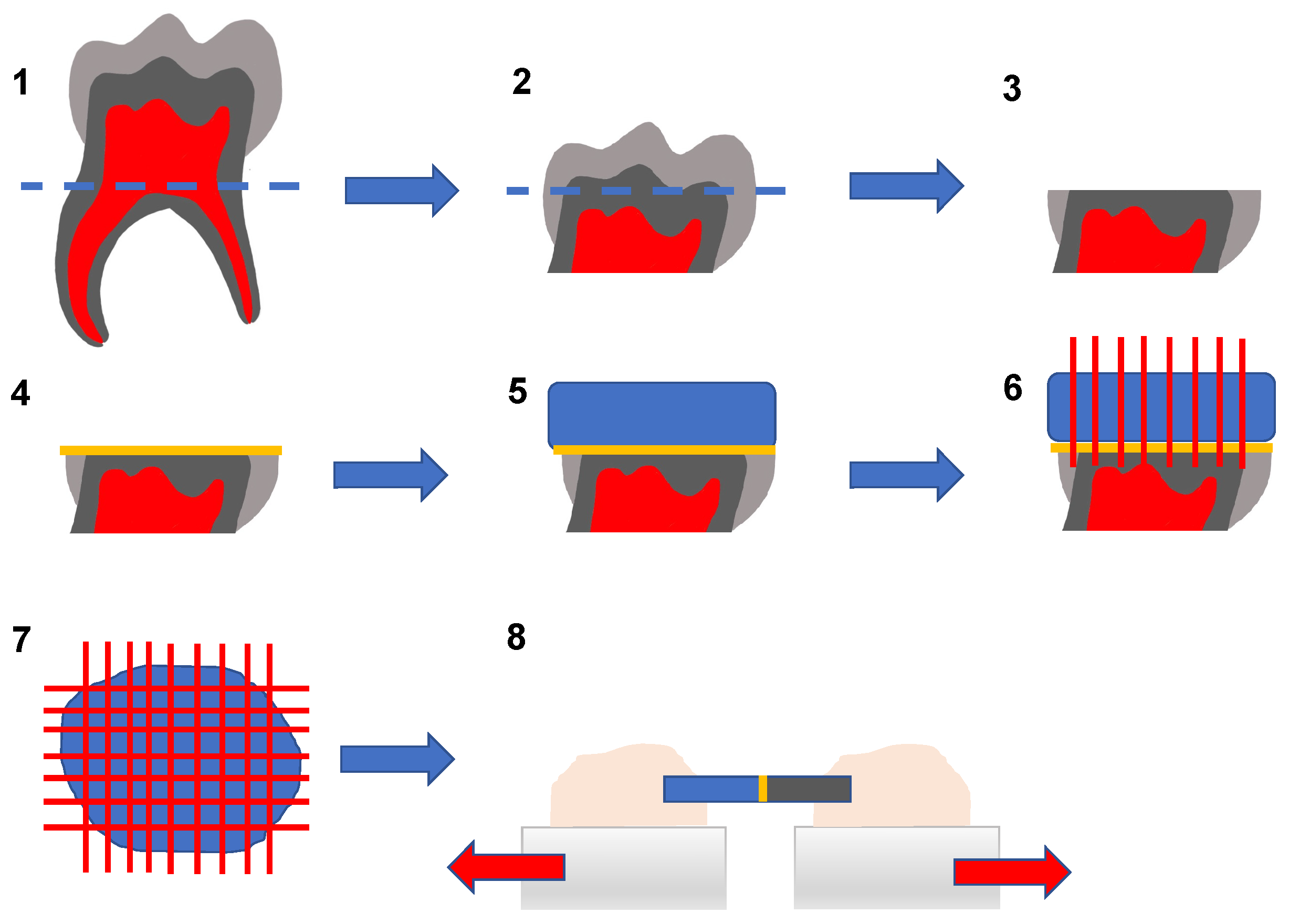





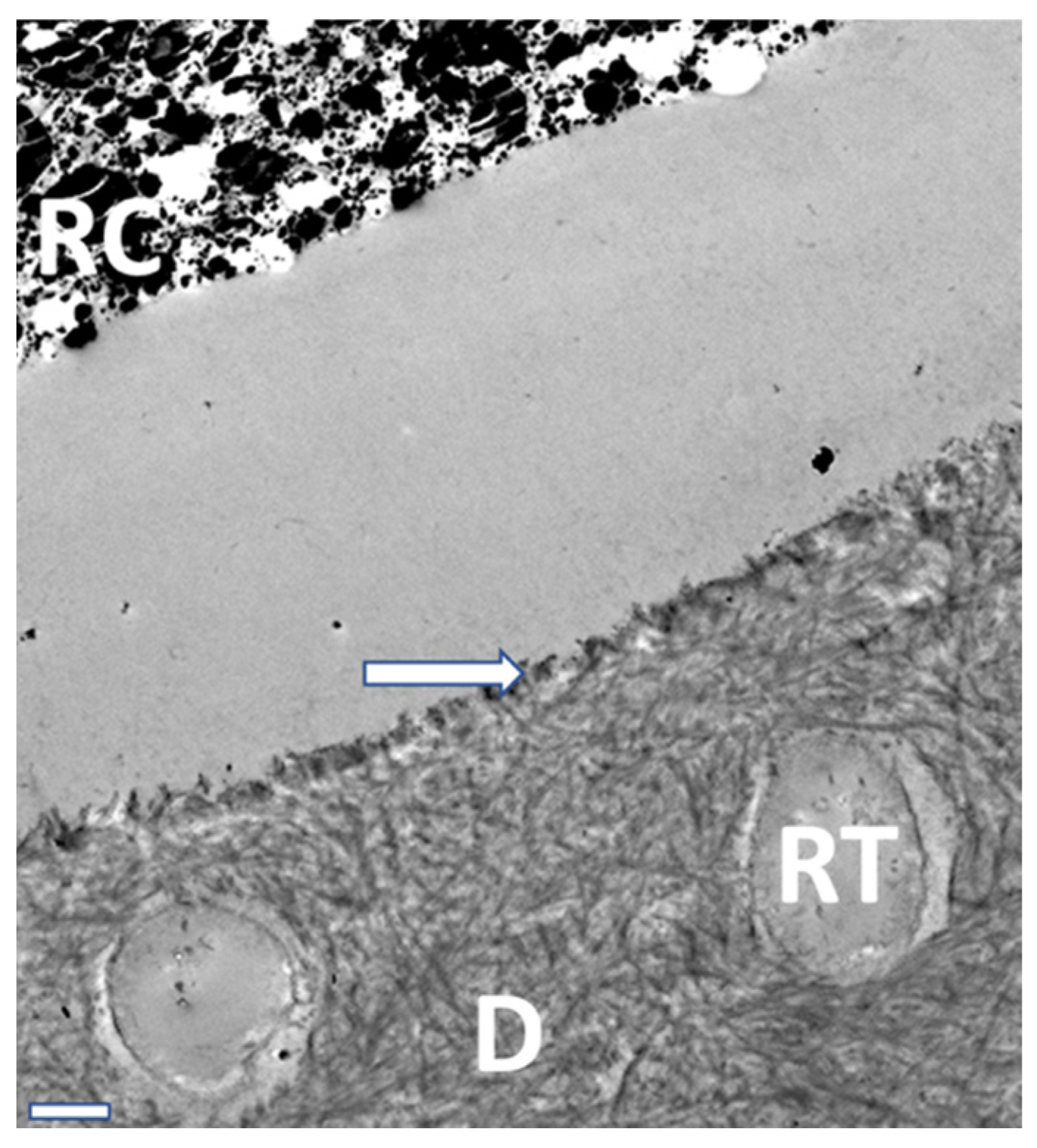
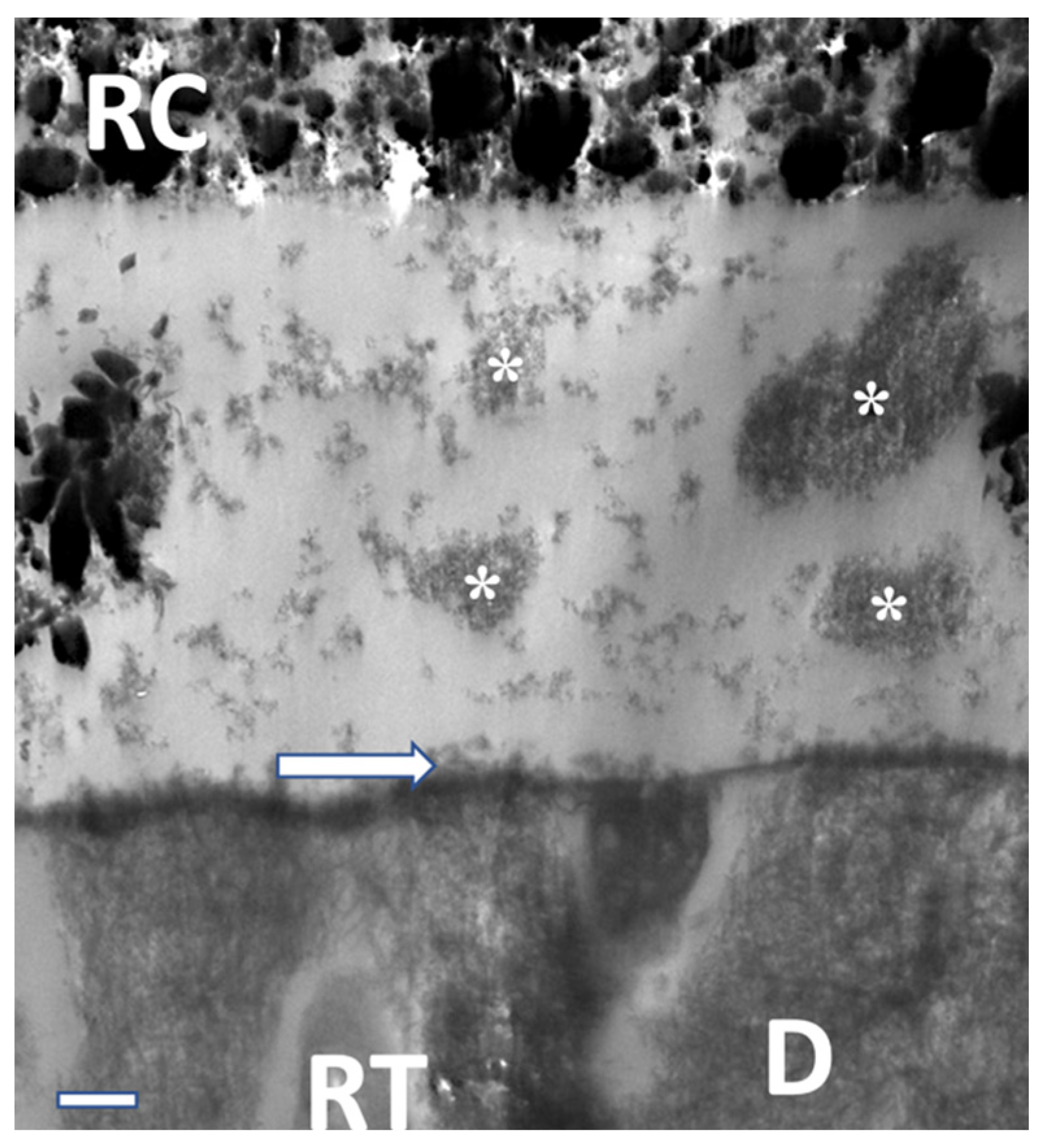
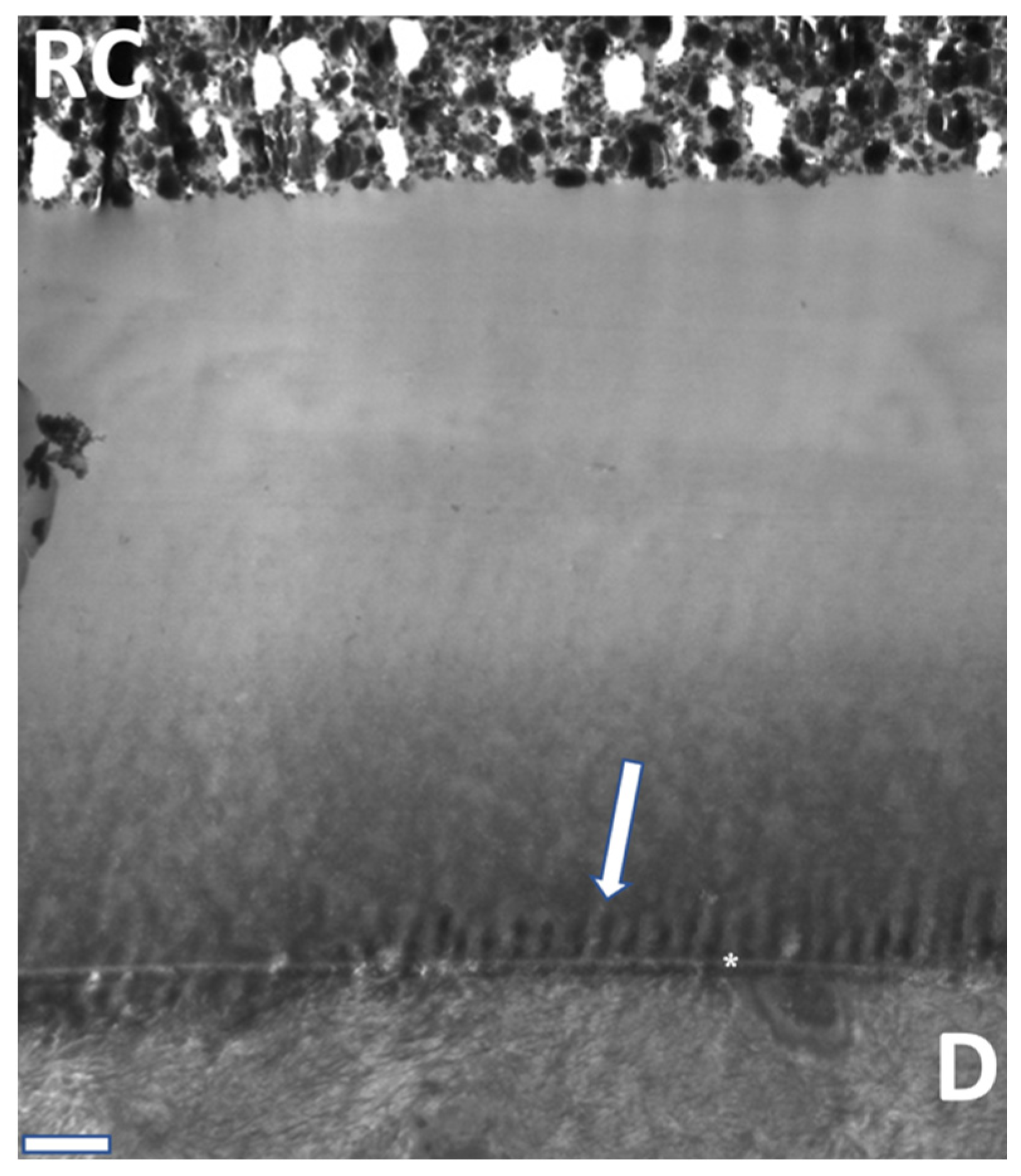
| Classification | pH | Demineralization |
|---|---|---|
| ultra-mild | >2.5 | ~300 nm |
| mild | ~2 | 1 µm |
| medium | 1–2 | 1–2 µm |
| strong | <1 | >2 µm |
| Adhesive | Functional Monomer | Solvent | pH Value | Application |
|---|---|---|---|---|
| Prime & Bond® NT (control) Dentsply Sirona GmbH | UDMA, PENTA | Acetone | 2.1 | (a), (b), (e), (g), (h) |
| iBond® Universal Kulzer Dental GmbH | 10-MDP | Acetone, water | 1.6–1.8 | (a), (b), (e), (f), (h) |
| G-Premio Bond, GC Europe N.V. | 10-MDP | Acetone, 2-Hydroxy-1,3-dimethacryl-oxypropan | 2.1 | (a), (b), (c), (i), (h) |
| Prime & Bond active™ Dentsply Sirona GmbH | 10-MDP | Isopropanol, water | 2.5 | (a), (b), (e), (g), (h) |
| Adhese® Universal, Ivoclar Vivadent | 10-MDP | Ethanol, water | 2.5–3.0 | (a), (b), (e), (h), (j) |
| All-Bond Universal®, Bisco Inc. | 10-MDP | Ethanol, water | 2.5–3.5 | (a), (d), (h), (j) |
| Adhesives/Fracture Mode | Control Group PBNT | IBU | GPB | PBa | AU | ABU | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Storage Period | 24 h | 6 mo | 12 mo | 24 h | 6 mo | 12 mo | 24 h | 6 mo | 12 mo | 24 h | 6 mo | 12 mo | 24 h | 6 mo | 12 mo | 24 h | 6 mo | 12 mo |
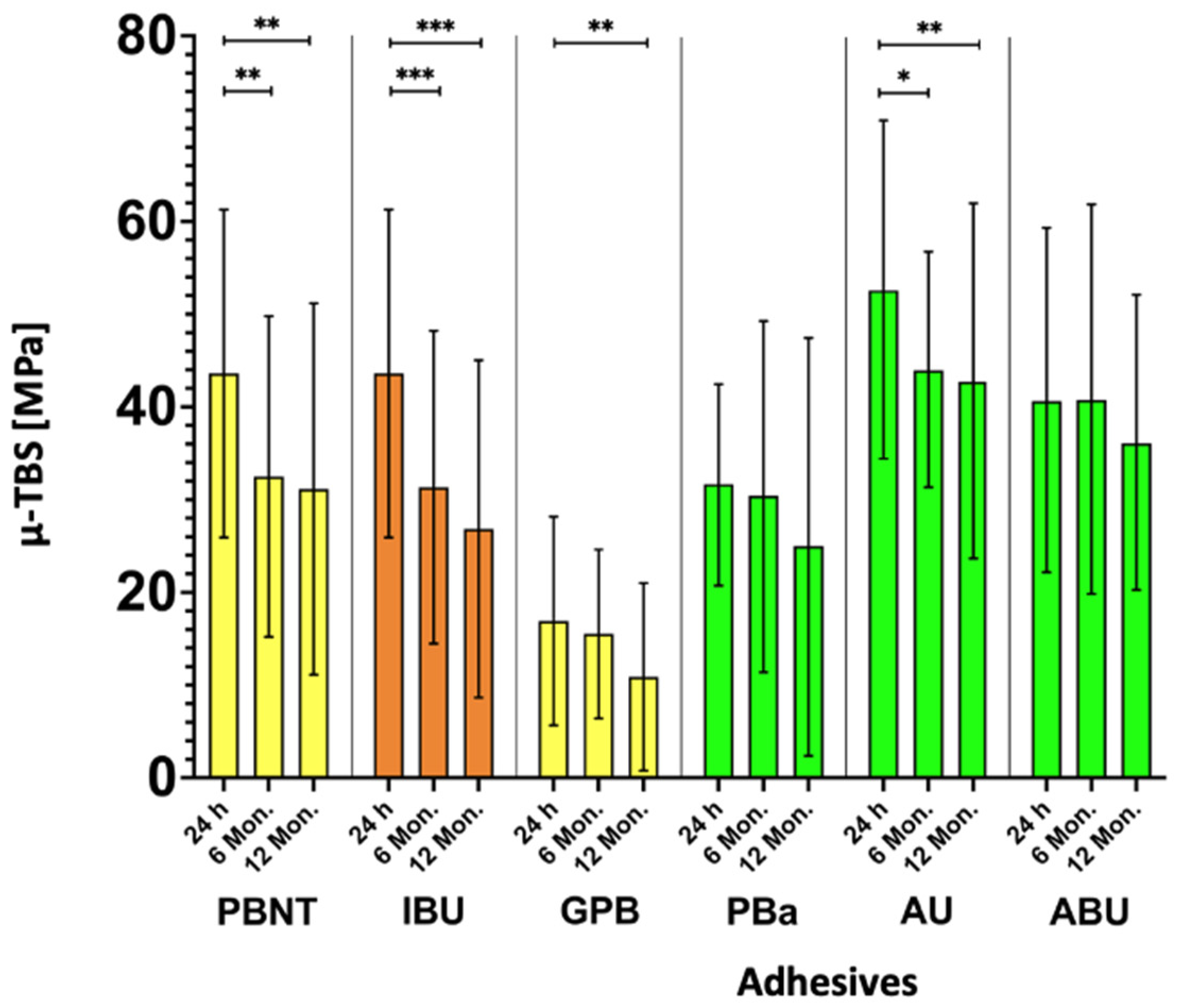
| µ-TBS (SD) [MPa] | 43.6 (17.7) | 32.5 (17.3) | 31.2 (20.0) | 44.5 (21.2) | 31.3 (16.9) | 26.9 (18.2) | 16.9 (11.3) | 15.5 (9.1) | 10.9 (10.1) | 31.7 (11.0) | 30.4 (19.0) | 25.0 (22.6) | 52.8 (18.3) | 44.1 (12.8) | 42.9 (19.3) | 40.8 (18.7) | 41.0 (21.1) | 36.3 (16.0) |
| Pre-test failures [%] | 0.0 | 0.0 | 14.6 | 0.0 | 0.0 | 0.0 | 0.0 | 4.9 | 12.2 | 0.0 | 3.4 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 4.4 | 0.0 |
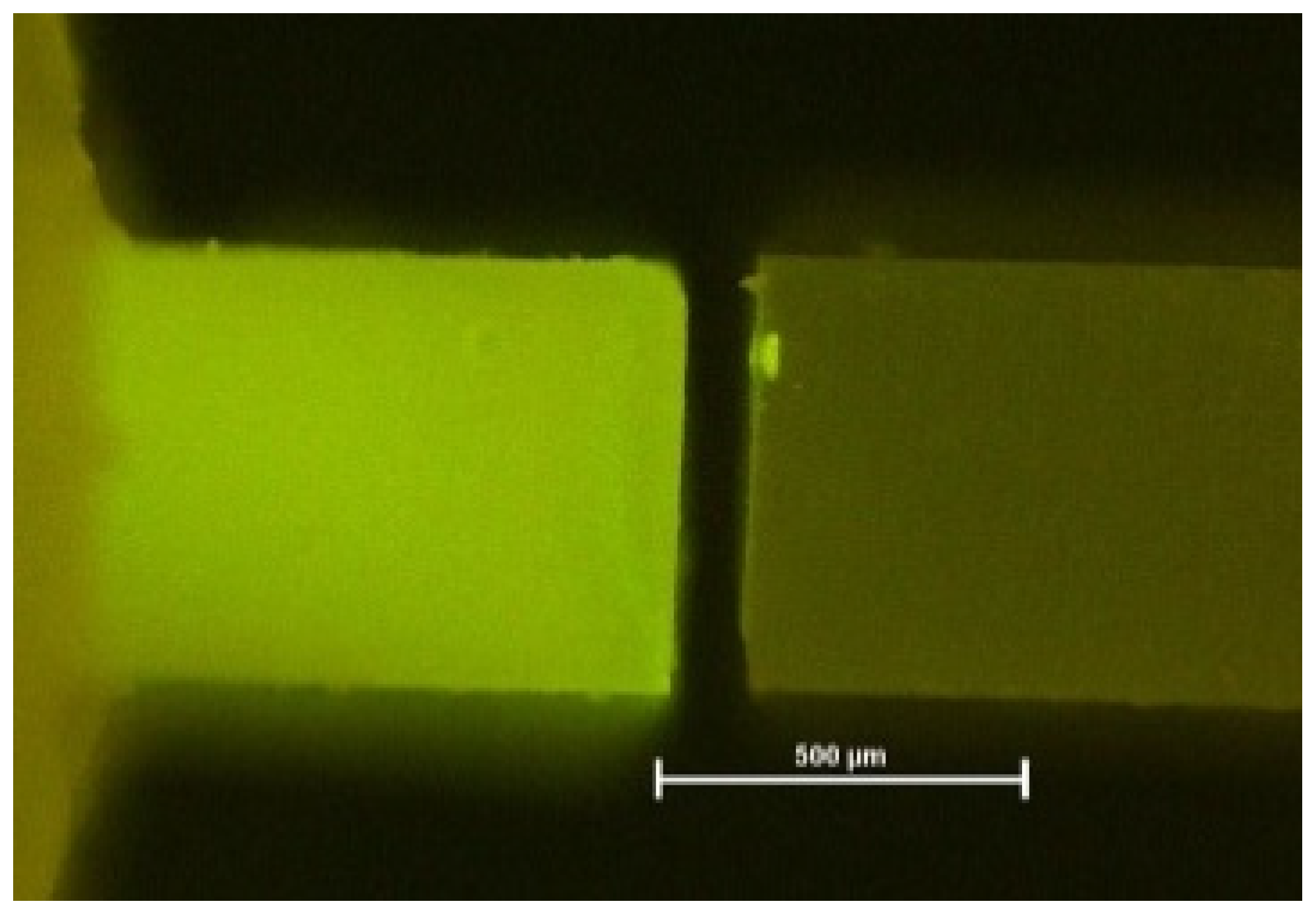
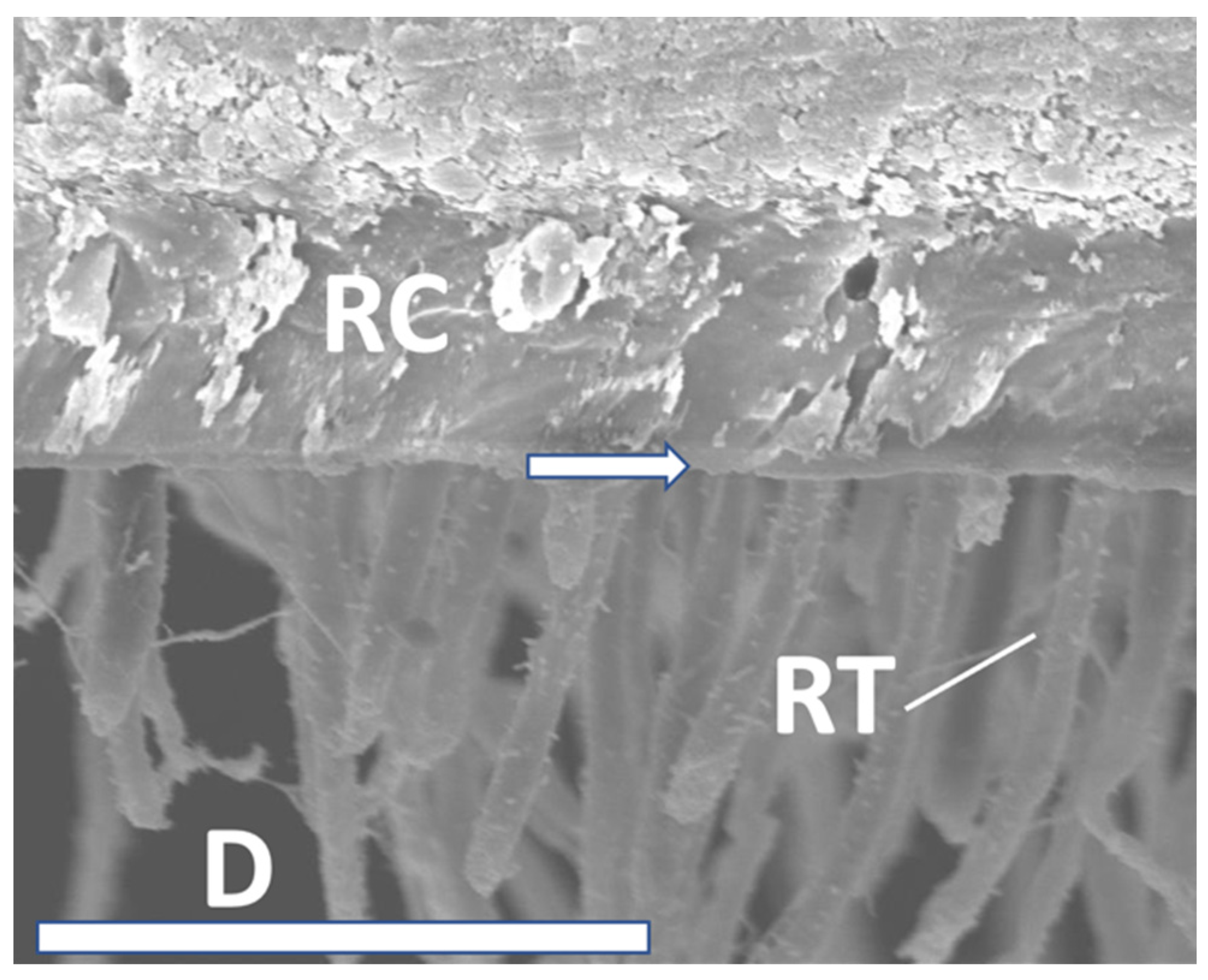
| Adhesive fractures [%] | 78.8 | 97.3 | 85.4 | 96.9 | 93.8 | 97.5 | 100.0 | 95.1 | 87.8 | 92.5 | 93.2 | 86.4 | 83.1 | 94.6 | 86.3 | 81.3 | 73.3 | 88.1 |
| Cohesive fractures [%] | 21.2 | 2.7 | 0.0 | 3.1 | 6.2 | 2.5 | 0.0 | 0.0 | 0.0 | 7.5 | 3.4 | 7.4 | 16.9 | 5.4 | 13.7 | 18.7 | 22.2 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danevitch, N.; Frankenberger, R.; Lücker, S.; Gärtner, U.; Krämer, N. Dentin Bonding Performance of Universal Adhesives in Primary Teeth In Vitro. Materials 2023, 16, 5948. https://doi.org/10.3390/ma16175948
Danevitch N, Frankenberger R, Lücker S, Gärtner U, Krämer N. Dentin Bonding Performance of Universal Adhesives in Primary Teeth In Vitro. Materials. 2023; 16(17):5948. https://doi.org/10.3390/ma16175948
Chicago/Turabian StyleDanevitch, Nina, Roland Frankenberger, Susanne Lücker, Ulrich Gärtner, and Norbert Krämer. 2023. "Dentin Bonding Performance of Universal Adhesives in Primary Teeth In Vitro" Materials 16, no. 17: 5948. https://doi.org/10.3390/ma16175948
APA StyleDanevitch, N., Frankenberger, R., Lücker, S., Gärtner, U., & Krämer, N. (2023). Dentin Bonding Performance of Universal Adhesives in Primary Teeth In Vitro. Materials, 16(17), 5948. https://doi.org/10.3390/ma16175948








