Comparing the Healing Abilities of Fluorapatite and Hydroxyapatite Ceramics in Regenerating Bone Tissue: An In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Experimental Design and Surgical Procedure
- -
- Control: Rabbits that have undergone a “sham surgery”, with an empty hole in the bone left to heal (n = 10);
- -
- FAP: Rabbits that have implanted FAP granules (n = 18);
- -
- HAP: Rabbits that have implanted HAP granules (n = 18).
2.3. Qualitative Analysis of Bone Microstructure Changes
2.3.1. X-ray Imaging—Conventional Radiography
2.3.2. Histology
2.4. Quantitative Analysis of Bone Microstructure Changes
2.4.1. Dual-Energy X-ray Absorptiometry (DXA)
2.4.2. Peripheral Quantitative Computed Tomography (pQCT)
2.5. Statistical Analysis
3. Results
3.1. Qualitative Analysis of Bone Substitute Materials
3.2. Quantitative Analysis of Bone Substitute Materials
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Analysis of the Properties of Bone Cement with Respect to Its Manufacturing and Typical Service Lifetime Conditions. MATEC Web Conf. 2018, 244, 01004. [Google Scholar] [CrossRef]
- Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J. The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength. Materials 2021, 14, 2555. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jonak, J. Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity. Materials 2020, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Wilmes, P.; Schmitt, E.; Kelm, J.M. Elution of Gentamicin and Vancomycin from Polymethylmethacrylate Beads and Hip Spacers in Vivo. Acta Orthop. 2009, 80, 193–197. [Google Scholar] [CrossRef]
- Fang, C.-H.; Lin, Y.W.; Sun, J.-S.; Lin, F.-H. The Chitosan/Tri-Calcium Phosphate Bio-Composite Bone Cement Promotes Better Osteo-Integration: An in Vitro and in Vivo Study. J. Orthop. Surg. Res. 2019, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jojczuk, M.; Jonak, J.; Nogalski, A. Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements. Materials 2022, 15, 2197. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef]
- Kresakova, L.; Medvecky, L.; Vdoviakova, K.; Varga, M.; Danko, J.; Totkovic, R.; Spakovska, T.; Vrzgula, M.; Giretova, M.; Briancin, J.; et al. Long-Bone-Regeneration Process in a Sheep Animal Model, Using Hydroxyapatite Ceramics Prepared by Tape-Casting Method. Bioengineering 2023, 10, 291. [Google Scholar] [CrossRef]
- Russo, A.; Bianchi, M.; Sartori, M.; Boi, M.; Giavaresi, G.; Salter, D.; Jelic, M.; Maltarello, M.C.; Ortolani, A.; Sprio, S.; et al. Bone Regeneration in a Rabbit Critical Femoral Defect by Means of Magnetic Hydroxyapatite Macroporous Scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 546–554. [Google Scholar] [CrossRef]
- Lim, H.; Zhang, M.; Lee, J.P.; Jung, U.W.; Choi, S.-H. Effect of Different Hydroxyapatite:β-Tricalcium Phosphate Ratios on the Osteoconductivity of Biphasic Calcium Phosphate in the Rabbit Sinus Model. Int. J. Oral Maxillofac. Implant. 2015, 30, 65–72. [Google Scholar] [CrossRef]
- Lukaszewska-Kuska, M.; Krawczyk, P.; Martyła, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Effects of a Hydroxyapatite Coating on the Stability of Endosseous Implants in Rabbit Tibiae. Dent. Med. Probl. 2019, 56, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, S.; Wong, L.W.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of Rabbit Calvarial Defects Using Biphasic Calcium Phosphate and a Strontium Hydroxyapatite-Containing Collagen Membrane. Clin. Oral Implant. Res. 2015, 27, e206–e214. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, S.-T.; Lin, H.-C.; Tseng, T.-W.; Rao, Q.-L.; Chen, M. Expression of Osteopontin and Type I Collagen of HFOB 1.19 Cells on Sintered Fluoridated Hydroxyapatite Composite Bone Graft Materials. Implant Dent. 2010, 19, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, H.F.; Guidara, A.; Danlos, Y.; Bouaziz, J.; Coddet, C. Synthesis and Characterization of Alumina-Fluorapatite Coatings Deposited by Atmospheric Plasma Spraying. Mater. Lett. 2016, 185, 268–271. [Google Scholar] [CrossRef]
- Harrison, J.M.; Melville, A.J.; Forsythe, J.S.; Muddle, B.C.; Trounson, A.O.; Gross, K.A.; Mollard, R.A. Sintered Hydroxyfluorapatites—IV: The Effect of Fluoride Substitutions upon Colonisation of Hydroxyapatites by Mouse Embryonic Stem Cells. Biomaterials 2004, 25, 4977–4986. [Google Scholar] [CrossRef]
- Gentleman, E.; Stevens, M.M.; Hill, R.E.; Brauer, D.S. Surface Properties and Ion Release from Fluoride-Containing Bioactive Glasses Promote Osteoblast Differentiation and Mineralization in Vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef]
- Li, Z.; Huang, B.; Mai, S.; Wu, X.; Zhang, H.; Qiao, W.; Luo, X.; Chen, Z. Effects of Fluoridation of Porcine Hydroxyapatite on Osteoblastic Activity of Human MG63 Cells. Sci. Technol. Adv. Mater. 2015, 16, 035006. [Google Scholar] [CrossRef]
- Shah, F.A.; Brauer, D.S.; Wilson, R.P.; Hill, R.A.; Hing, K.A. Influence of Cell Culture Medium Composition on In Vitro Dissolution Behavior of a Fluoride-Containing Bioactive Glass. J. Biomed. Mater. Res. Part A 2013, 102, 647–654. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Pałka, K.; Jozefaciuk, G.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Ginalska, G. Fluorapatite Ceramics for Bone Tissue Regeneration: Synthesis, Characterization and Assessment of Biomedical Potential. Mater. Sci. Eng. C 2020, 116, 111211. [Google Scholar] [CrossRef]
- Pajchel, L.; Borkowski, L. Solid-State NMR and Raman Spectroscopic Investigation of Fluoride-Substituted Apatites Obtained in Various Thermal Conditions. Materials 2021, 14, 6936. [Google Scholar] [CrossRef]
- Osorio, C.C.; Escobar, L.M.; González, M.C.; Gamboa, L.F.; Chambrone, L. Evaluation of Density, Volume, Height and Rate of Bone Resorption of Substitutes of Autologous Bone Grafts for the Repair of Alveolar Clefts in Humans: A Systematic Review. Heliyon 2020, 6, e04646. [Google Scholar] [CrossRef] [PubMed]
- Dayashankara Rao, J.K.; Bhatnagar, A.; Pandey, R.; Arya, V.; Arora, G.; Kumar, J.; Bootwala, F.; Devi, W.N. A comparative evaluation of iliac crest bone graft with and without injectable and advanced platelet rich fibrin in secondary alveolar bone grafting for cleft alveolus in unilateral cleft lip and palate patients: A randomized prospective study. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.Y.; Liu, C. The Effectiveness of Using Platelet-Rich Concentrate with Iliac Bone Graft in the Repair of Alveolar Cleft: A Meta-Analysis of Randomized Controlled Trials. Int. J. Oral Maxillofac. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Langley-Hobbs, S. Biology and radiological assessment of fracture healing. Practice 2003, 25, 26–35. [Google Scholar] [CrossRef]
- Cummings, S.R.; Bates, D.W.; Black, D.M. Clinical Use of Bone Densitometry. JAMA 2002, 288, 1889. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Hart, C.; Halbritter, S.A.; Morton, D.; Buser, D. Early Loading of Nonsubmerged Titanium Implants with a Chemically Modified Sand-Blasted and Acid-Etched Surface: 6-Month Results of a Prospective Case Series Study in the Posterior Mandible Focusing on Peri-Implant Crestal Bone Changes and Implant Stability Quotient (ISQ) Values. Clin. Implant Dent. Relat. Res. 2009, 11, 338–347. [Google Scholar] [CrossRef]
- Ono, H.; Sase, T.; Tanaka, Y.; Takasuna, H. Histological Assessment of Porous Custom-Made Hydroxyapatite Implants 6 Months and 2.5 Years after Cranioplasty. Surg. Neurol. Int. 2017, 8, 8. [Google Scholar] [CrossRef]
- Pelto-Vasenius, K.; Hirvensalo, E.; Vasenius, J.; Rokkanen, P. Osteolytic Changes After Polyglycolide Pin Fixation in Chevron Osteotomy. Foot Ankle Int. 1997, 18, 21–25. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Adverse Tissue Reactions to Bioabsorbable Fixation Devices. Clin. Orthop. Relat. Res. 2000, 371, 216–227. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, L.; Cao, Z.; Liu, X.; Pan, J. Periosteal Skeletal Stem Cells and Their Response to Bone Injury. Front. Cell Dev. Biol. 2022, 10, 812094. [Google Scholar] [CrossRef]
- Rana, R.; Wu, J.S.; Eisenberg, R.L. Periosteal Reaction. Am. J. Roentgenol. 2009, 193, W259–W272. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.-H.; Seitz, H.; Warnke, F.; Becker, S.; Sivananthan, S.; Sherry, E.; Liu, Q.; Wiltfang, J.; Douglas, T.E.L. Ceramic Scaffolds Produced by Computer-Assisted 3D Printing and Sintering: Characterization and Biocompatibility Investigations. J. Biomed. Mater. Res. Part B 2010, 93, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Hanifi, A.; Mortazavi, V. Preparation and Bioactivity Evaluation of Bone-like Hydroxyapatite Nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Meskinfam, M.; Sadjadi, M.S.; Jazdarreh, H.; Zare, K. Biocompatibility Evaluation of Nano Hydroxyapatite-Starch Biocomposites. J. Biomed. Nanotechnol. 2011, 7, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Benito-Garzón, L.; Sanz, S.; Garzon-Gutierrez, A.; Carrodeguas, R.G.; Rodriguez, M.; De Castro, A.G.; Perez, M.C. Novel Osteoinductive and Osteogenic Scaffolds of Monetite, Amorphous Calcium Phosphate, Hydroxyapatite, and Silica Gel: Influence of the Hydroxyapatite/Monetite Ratio on Their In Vivo Behavior and on Their Physical and Chemical Properties. ACS Biomater. Sci. Eng. 2020, 6, 3440–3453. [Google Scholar] [CrossRef]
- Tredwin, C.; Young, A.B.; Georgiou, G.; Shin, S.-H.; Kim, H.-W.; Knowles, J.C. Hydroxyapatite, Fluor-Hydroxyapatite and Fluorapatite Produced via the Sol–Gel Method. Optimisation, Characterisation and Rheology. Dent. Mater. 2013, 29, 166–173. [Google Scholar] [CrossRef]
- Minoshima, M.; Kikuta, J.; Omori, Y.; Seno, S.; Suehara, R.; Maeda, H.; Matsuda, H.; Ishii, M.; Kikuchi, K. In Vivo Multicolor Imaging with Fluorescent Probes Revealed the Dynamics and Function of Osteoclast Proton Pumps. ACS Cent. Sci. 2019, 5, 1059–1066. [Google Scholar] [CrossRef]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.W.; Gluck, S.L. Osteoclastic Bone Resorption by a Polarized Vacuolar Proton Pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.G.; Hu, J.C.-C. How Fluoride Protects Dental Enamel from Demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134. [Google Scholar] [CrossRef]
- Krupski, W.; Kruk-Bachonko, J.; Tatara, M.R. Computed Tomography Diagnostic of Uncommon Case of Osteopetrosis in 80-Year-Old Man—Case Report. Med. Lith. 2020, 56, 518. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kojima, T.; Iijima, T.; Yokokura, S.; Kawano, H.; Yamamoto, A.; Matsuda, K. Resorption of Synthetic Porous Hydroxyapatite and Replacement by Newly Formed Bone. J. Orthop. Sci. 2001, 6, 444–447. [Google Scholar] [CrossRef]
- Okuda, T.; Ioku, K.; Yonezawa, I.; Minagi, H.; Gonda, Y.; Kawachi, G.; Kamitakahara, M.; Shibata, Y.; Murayama, H.; Kurosawa, H.; et al. The Slow Resorption with Replacement by Bone of a Hydrothermally Synthesized Pure Calcium-Deficient Hydroxyapatite. Biomaterials 2008, 29, 2719–2728. [Google Scholar] [CrossRef]
- Ganguly, S. Preparation/Processing of Polymer-Graphene Composites by Different Techniques. In Elsevier eBooks; Woodhead Publishing: Cambridge, UK, 2022; pp. 45–74. [Google Scholar]
- Zheng, H.; Zhang, W.; Li, B.; Zhu, J.-J.; Wang, C.; Gao, S.; Wu, G.; Yang, X.; Huang, Y.; Ma, L. Recent Advances of Interphases in Carbon Fiber-Reinforced Polymer Composites: A Review. Compos. Part B-Eng. 2022, 233, 109639. [Google Scholar] [CrossRef]
- Maia, M.T.; Luz, E.P.C.G.; Andrade, F.K.; De Freitas Rosa, M.; de Fatima Borges, M.; Arcanjo, M.R.A.; Vieira, R.S. Advances in Bacterial Cellulose/Strontium Apatite Composites for Bone Applications. Polym. Rev. 2021, 61, 736–764. [Google Scholar] [CrossRef]
- Bailey, E.; Winey, K.I. Dynamics of Polymer Segments, Polymer Chains, and Nanoparticles in Polymer Nanocomposite Melts: A Review. Prog. Polym. Sci. 2020, 105, 101242. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Kim, H.W.; Lee, J.Y.; Bae, C.J.; Koh, Y.H.; Kong, Y.M.; Kim, H.E. Stability and Cellular Responses to Fluorapatite–Collagen Composites. Biomaterials 2005, 26, 2957–2963. [Google Scholar] [CrossRef]
- Busch, S.; Dolhaine, H.; DuChesne, A.; Heinz, S.; Hochrein, O.; Laeri, F.; Podebrad, O.; Vietze, U.; Weiland, T.; Kniep, R. Biomimetic Morphogenesis of Fluorapatite-Gelatin Composites: Fractal Growth, the Question of Intrinsic Electric Fields, Core/Shell Assemblies, Hollow Spheres and Reorganization of Denatured Collagen. Eur. J. Inorg. Chem. 1999, 1999, 1643–1653. [Google Scholar] [CrossRef]

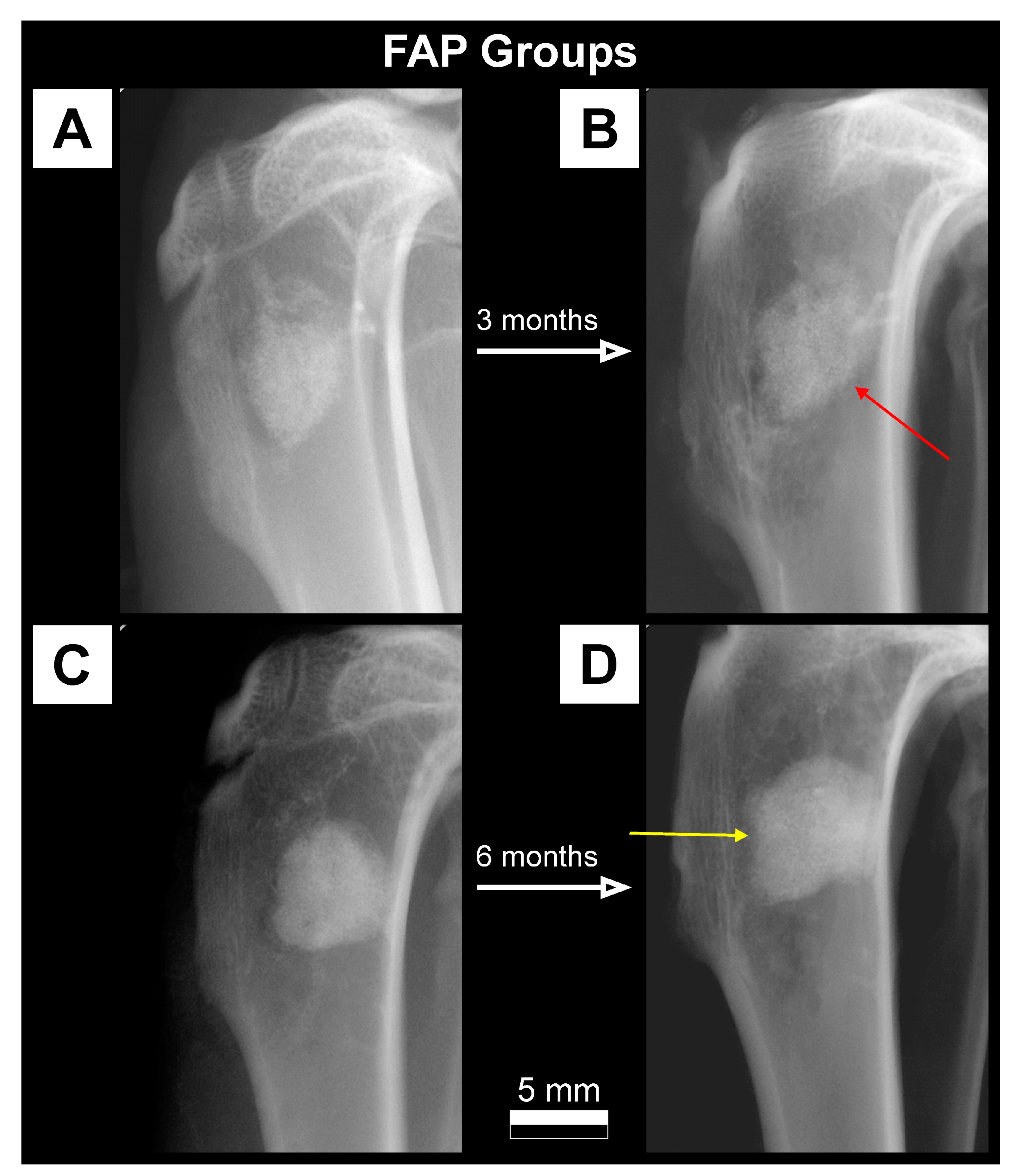

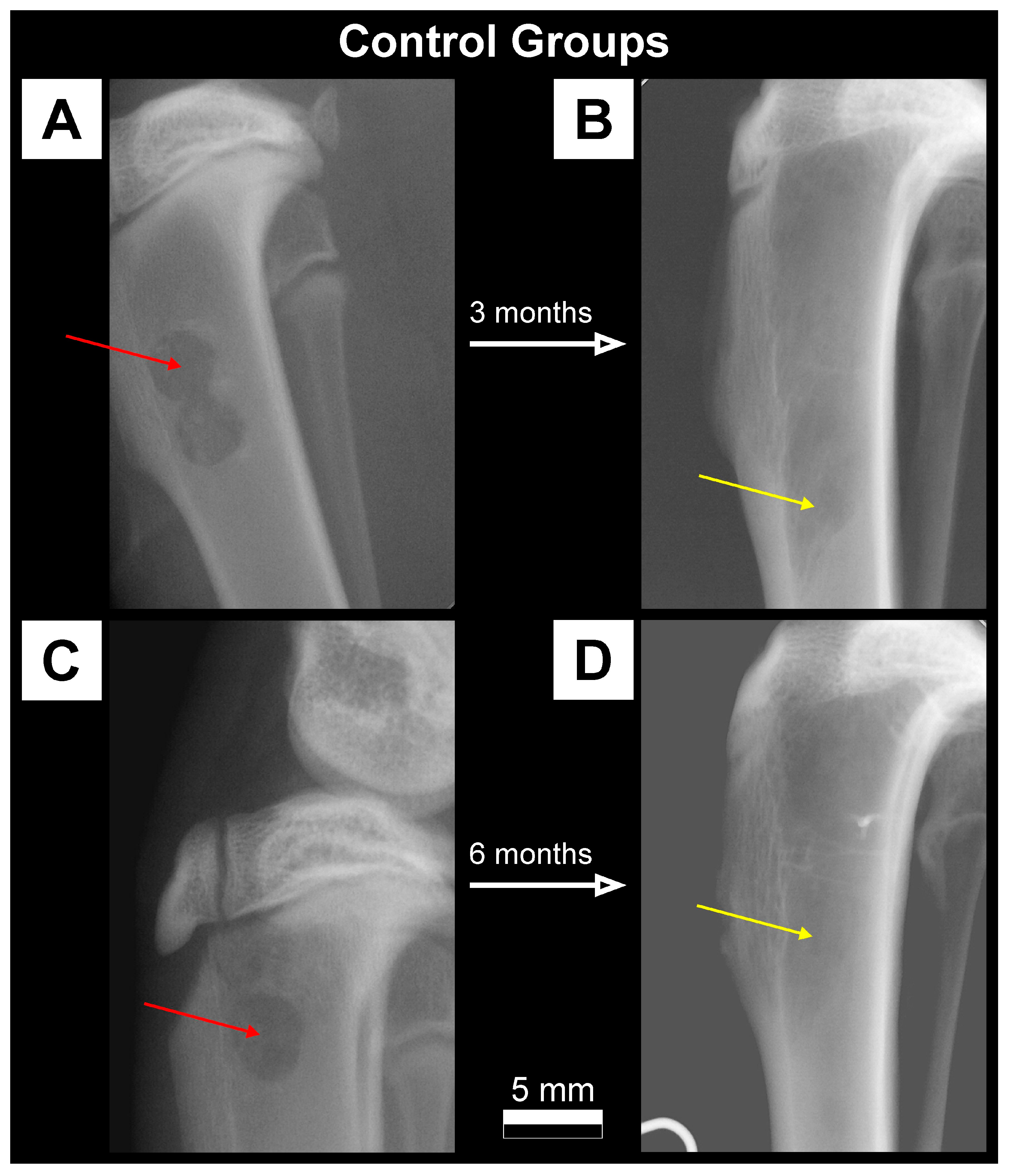




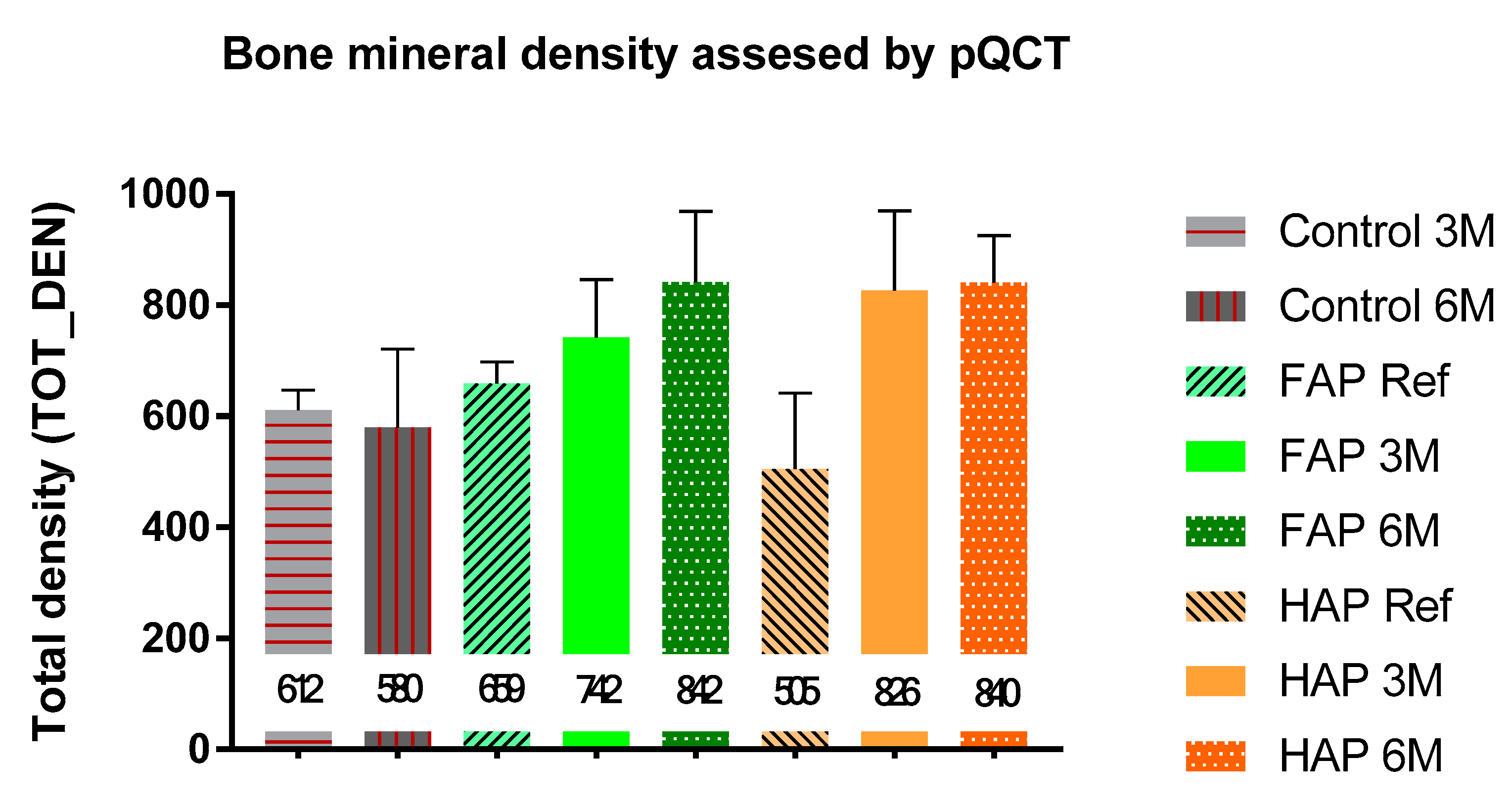
| Parameter [Units] | Type of Ceramics | Statistically Significant Difference * | |
|---|---|---|---|
| FAP | HAP | ||
| Fluorine content [atomic %] | 4.12 (0.3) | 0 | yes |
| Solid phase density, SPD [g/cm3] | 3.02 (0.03) | 2.91 (0.02) | yes |
| Bulk density, BD [g/cm3] | 0.75 (0.01) | 0.55 (0.01) | yes |
| Porosity, P [%] | 75 (0.19) | 81 (0.15) | yes |
| Total intrusion volume, V [cm3/g] | 1.01 (0.02) | 1.47 (0.02) | yes |
| Group of Animals | Healing Period | |
|---|---|---|
| 3 Months | 6 Months | |
| Control | Control 3M | Control 6M |
| (n = 5) | (n = 5) | |
| FAP * | FAP 3M | FAP 6M |
| (n = 9) | (n = 9) | |
| HAP * | HAP 3M | HAP 6M |
| (n = 9) | (n = 9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkowski, L.; Jojczuk, M.; Belcarz, A.; Pawlowska-Olszewska, M.; Kruk-Bachonko, J.; Radzki, R.; Bienko, M.; Slowik, T.; Lübek, T.; Nogalski, A.; et al. Comparing the Healing Abilities of Fluorapatite and Hydroxyapatite Ceramics in Regenerating Bone Tissue: An In Vivo Study. Materials 2023, 16, 5992. https://doi.org/10.3390/ma16175992
Borkowski L, Jojczuk M, Belcarz A, Pawlowska-Olszewska M, Kruk-Bachonko J, Radzki R, Bienko M, Slowik T, Lübek T, Nogalski A, et al. Comparing the Healing Abilities of Fluorapatite and Hydroxyapatite Ceramics in Regenerating Bone Tissue: An In Vivo Study. Materials. 2023; 16(17):5992. https://doi.org/10.3390/ma16175992
Chicago/Turabian StyleBorkowski, Leszek, Mariusz Jojczuk, Anna Belcarz, Marta Pawlowska-Olszewska, Joanna Kruk-Bachonko, Radoslaw Radzki, Marek Bienko, Tymoteusz Slowik, Tomasz Lübek, Adam Nogalski, and et al. 2023. "Comparing the Healing Abilities of Fluorapatite and Hydroxyapatite Ceramics in Regenerating Bone Tissue: An In Vivo Study" Materials 16, no. 17: 5992. https://doi.org/10.3390/ma16175992
APA StyleBorkowski, L., Jojczuk, M., Belcarz, A., Pawlowska-Olszewska, M., Kruk-Bachonko, J., Radzki, R., Bienko, M., Slowik, T., Lübek, T., Nogalski, A., & Ginalska, G. (2023). Comparing the Healing Abilities of Fluorapatite and Hydroxyapatite Ceramics in Regenerating Bone Tissue: An In Vivo Study. Materials, 16(17), 5992. https://doi.org/10.3390/ma16175992







