Transmission Electron Microscopy Peeled Surface Defect of Perovskite Quantum Dots to Improve Crystal Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CsPbBr3 QDs
2.3. Characterization of CsPbBr3 QDs
2.4. Carbon Coating
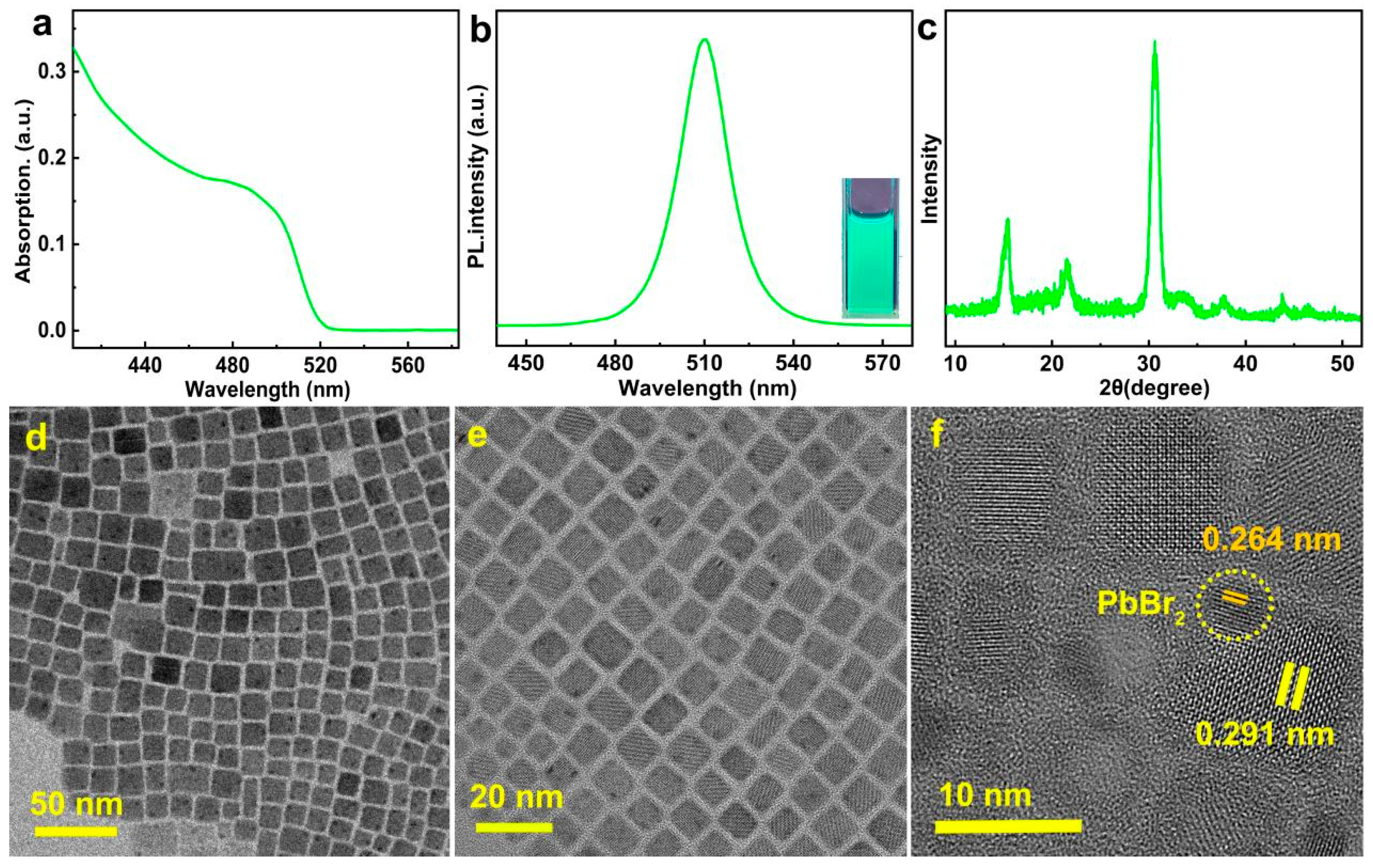
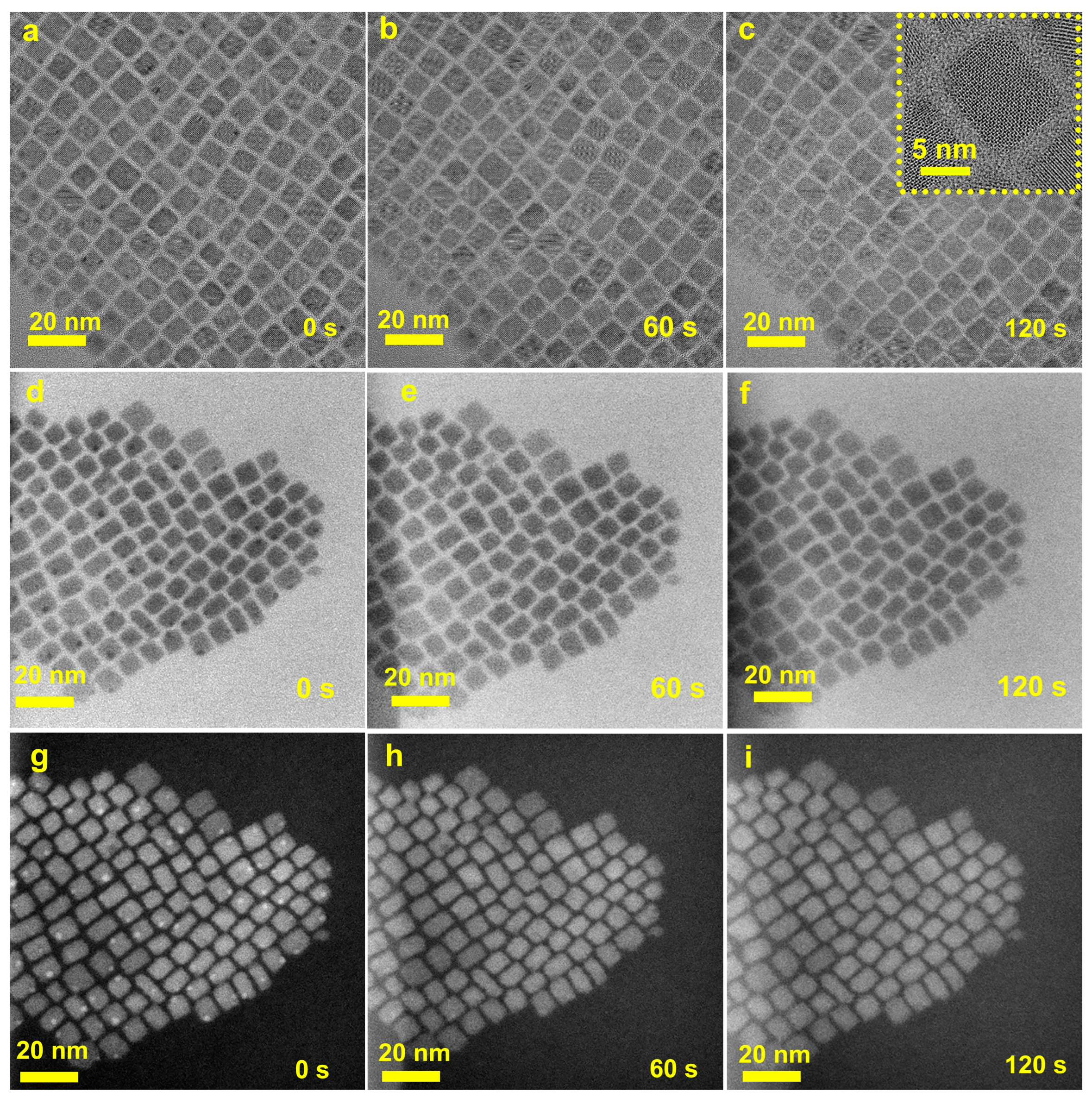
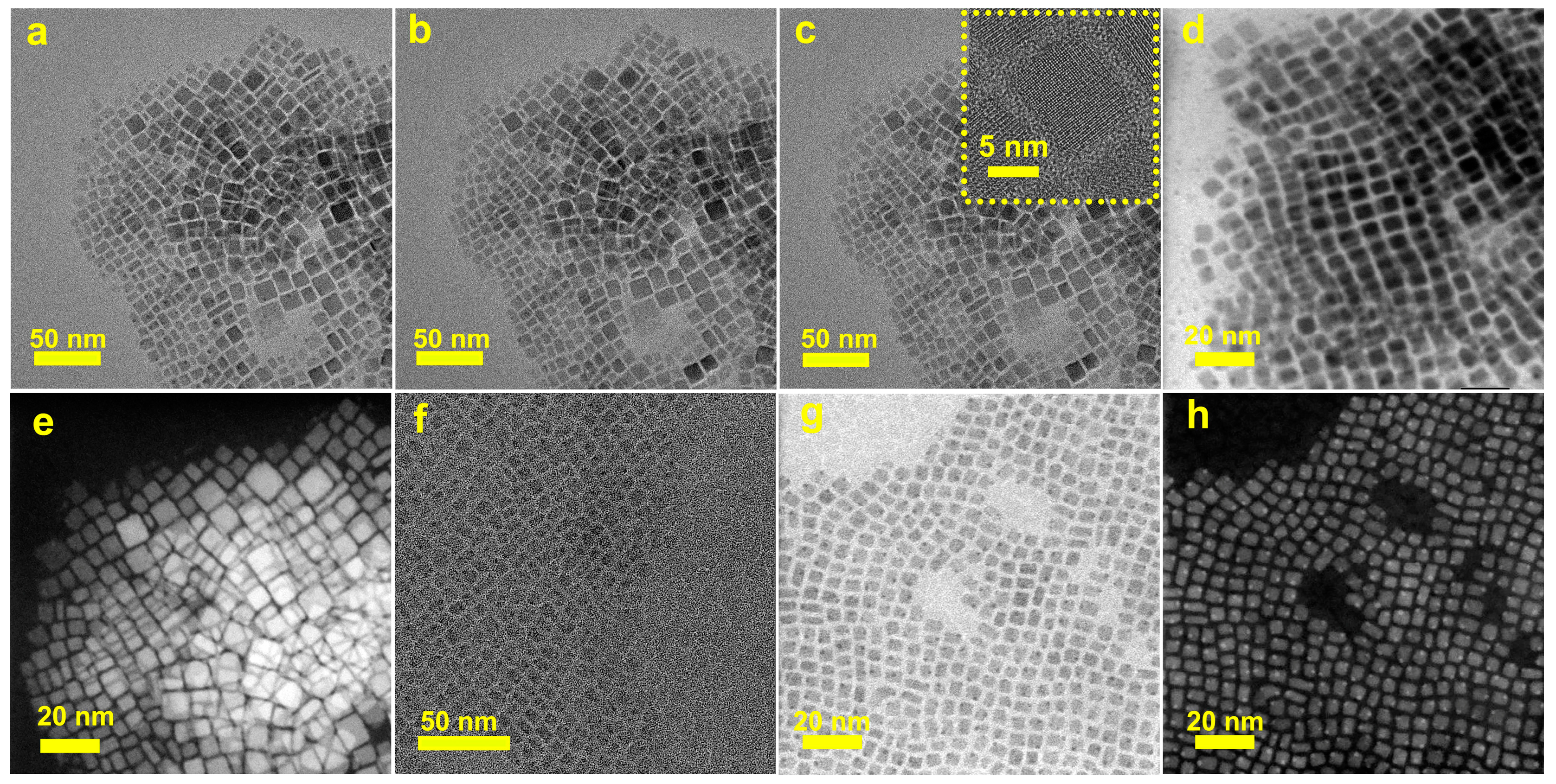
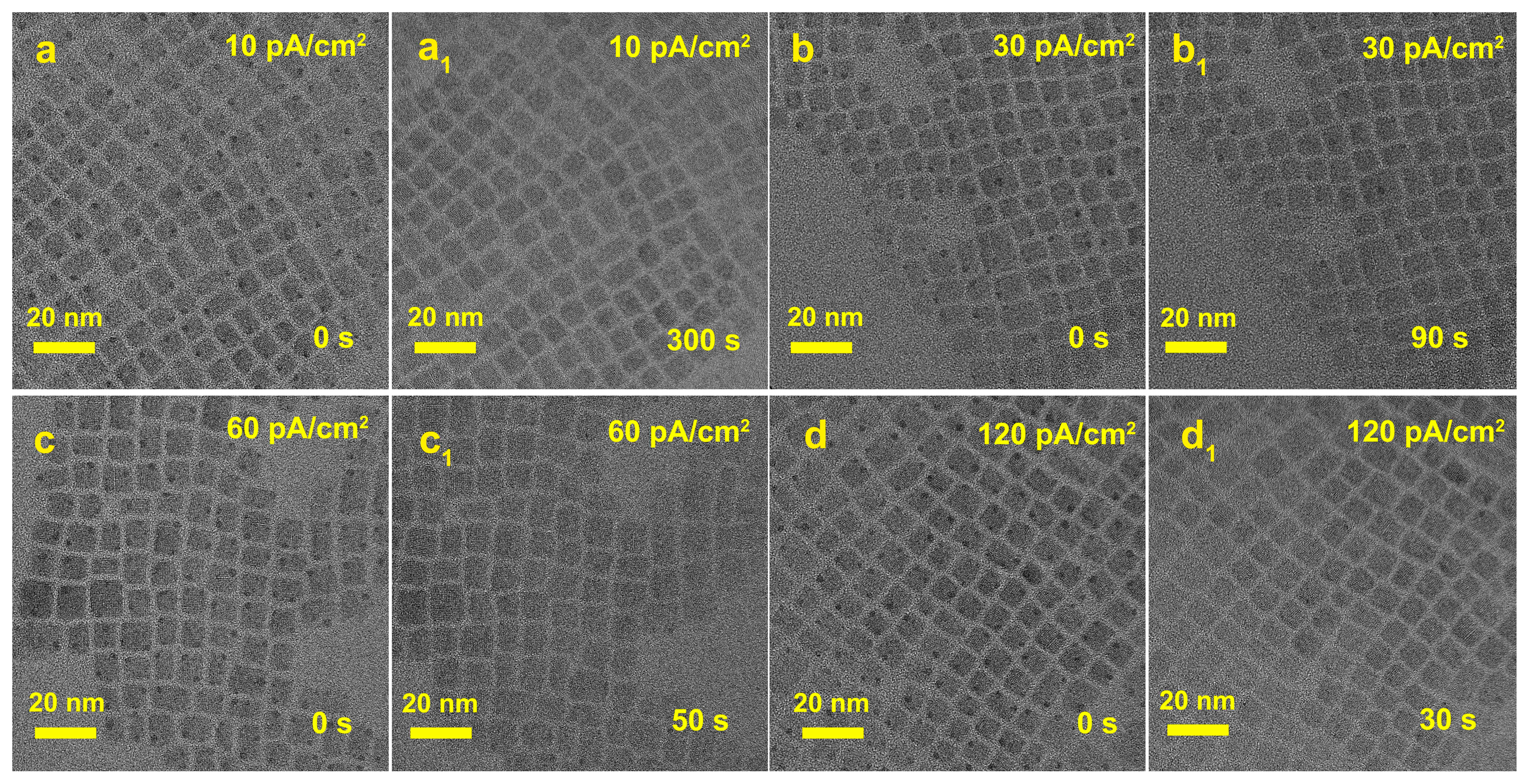
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 Perovskite Nanocrystals: Luminescence beyond Traditional Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 15424–15428. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X=Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; De Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.B.; Sadhanala, A.; Pazos-Outon, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Hoshi, K.; Pu, Y.-J.; Takeda, Y.; Hayashi, Y.; Ohisa, S.; Kawata, S.; Kido, J. High-Efficiency Perovskite Quantum-Dot Light-Emitting Devices by Effective Washing Process and Interfacial Energy Level Alignment. ACS Appl. Mater. Interfaces 2017, 9, 18054–18060. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Yang, K.; Li, F.; Liu, Y.; Xu, Z.; Li, Q.; Sun, K.; Qiu, L.; Zeng, Q.; Chen, Z.; Chen, W.; et al. All-Solution-Processed Perovskite Quantum Dots Light-Emitting Diodes Based on the Solvent Engineering Strategy. ACS Appl. Mater. Interfaces 2018, 10, 27374–27380. [Google Scholar] [CrossRef]
- Lu, C.H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and ion substitution in colloidal metal halide perovskite nanocrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Zaidi, A.; Hussain, A.; Hassan, N.U.; Ali, J.; Ahmed, F.; Khan, M.U.; Iqbal, N.; Elnasr, T.A.S.; Helal, M.H. Single-and Multilayered Perovskite Thin Films for Photovoltaic Applictions. Nanomaterials 2022, 12, 3208. [Google Scholar] [CrossRef] [PubMed]
- Kuimalee, S.; Chairuangsri, T.; Pearce, J.T.; Edmonds, D.V.; Brown, A.P.; Brydson, R.M. Quantitative analysis of a complex metal carbide formed during furnace cooling of cast duplex stainless steel using EELS and EDS in the TEM. Micron 2010, 41, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, C.; Wang, H.; Wang, H.; Sun, J.; Zhang, Y.; Han, X.; Cao, Y.; Liu, S.; Sun, J. A Covalent P–C Bond Stabilizes Red Phosphorus in an Engineered Carbon Host for High-Performance Lithium-Ion Battery Anodes. ACS Nano 2021, 15, 3365–3375. [Google Scholar] [CrossRef] [PubMed]
- Susi, T.; Kotakoski, J.; Arenal, R.; Kurasch, S.; Jiang, H.; Skakalova, V.; Stephan, O.; Krasheninnikov, A.V.; Kauppinen, E.I.; Kaiser, U.; et al. Atomistic Description of Electron Beam Damage in Nitrogen-Doped Graphene and Single-Walled Carbon Nanotubes. ACS Nano 2012, 6, 8837–8846. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R. Mechanisms of radiation damage in beam-sensitive specimens, for TEM accelerating voltages between 10 and 300 kV. Microsc. Res. Tech. 2012, 75, 1550–1556. [Google Scholar] [CrossRef]
- Bell, D.C.; Mankin, M.; Day, R.W.; Erdman, N. Natasha Erdman, Successful application of Low Voltage Electron Microscopy to practical materials problems. Ultramicroscopy 2014, 145, 56–65. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Chuklina, N.G.; Sokolov, M.N.; Popov, A.I.; Gryaznov, D.V.; Kotomin, E.A.; Maier, J. Small radius electron and hole polarons in PbX2 (X=F, Cl, Br) crystals: A computational study. J. Mater. Chem. C 2021, 9, 16536–16544. [Google Scholar] [CrossRef]
- Reichling, M.; Wilson, R.; Bennewitz, R.; Williams, R.; Gogoll, S.; Stenzel, E.; Matthias, E. Surface colloid evolution during low-energy electron irradiation of CaF2(111). Surf. Sci. 1996, 366, 531–544. [Google Scholar] [CrossRef]
- Postawa, Z.; Kolodziej, J.; Baran, G.; Czuba, P.; Piatkowski, P.; Szymonski, M.; Plavina, I.; Popov, A. ESD of nonthermal halogen atoms from In-doped (001) KBr. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1995, 100, 228–231. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Y.; Liu, L.; Ying, X.; Hsiung, C.-E.; Sougrat, R.; Li, K.; Han, Y. Atomic-resolution transmission electron microscopy of electron beam–sensitive crystalline materials. Science 2018, 359, 675–679. [Google Scholar] [CrossRef]
- Liu, Y.; Ju, Z.; Zhang, B.; Wang, Y.; Nai, J.; Liu, T.; Tao, X. Visualizing the Sensitive Lithium with Atomic Precision: Cryogenic Electron Microscopy for Batteries. Accounts Chem. Res. 2021, 54, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Kotakoski, J.; Mangler, C. Atomic structure from large-area, low-dose exposures of materials: A new route to circum-vent radiation damage. Ultramicroscopy 2014, 145, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.L.; Lentzen, M.; Urban, K. Atomic-Resolution Imaging of Oxygen in Perovskite Ceramics. Science 2003, 299, 870–873. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, D.; Kisielowski, C.; Dou, L.; Kornienko, N.; Bekenstein, Y.; Wong, A.B.; Alivisatos, A.P.; Yang, P. Atomic Resolution Imaging of Halide Perovskites. Nano Lett. 2016, 16, 7530–7535. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, R.; Yang, H.; Ophus, C.; Ercius, P.; Bizarri, G.; Perrodin, D.; Shalapska, T.; Bourret, E.; Ciston, J.; Dahmen, U. Determination of the structural phase and octahedral rotation angle in halide perovskites. Appl. Phys. Lett. 2018, 112, 071901. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Li, Y.; Huang, W.; Zhang, Z.; Chen, G.; Wang, H.; Wu, G.-H.; Rolston, N.; Vila, R.; et al. Unravelling degradation mechanisms and atomic structure of organic-inorganic halide perovskites by cryo-EM. Joule 2019, 3, 2854–2866. [Google Scholar] [CrossRef]
- Cai, S.; Dai, J.; Shao, Z.; Rothmann, M.U.; Jia, Y.; Gao, C.; Hao, M.; Pang, S.; Wang, P.; Lau, S.P.; et al. Atomically resolved electrically active intragrain interfaces in perovskite semiconductors. J. Am. Chem. Soc. 2022, 144, 1910–1920. [Google Scholar] [CrossRef]
- Nann, T.; Skinner, W.M. Quantum Dots for Electro-Optic Devices. Acs Nano 2011, 5, 5291–5295. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.-J.; Zhu, T.-S.; Xuan, H.-C.; Li, M. Size Consideration on Shape Factor and Its Determination Role on the Thermodynamic Stability of Quantum Dots. J. Phys. Chem. C 2015, 119, 12002–12007. [Google Scholar] [CrossRef]
- Xing, J.; Yan, F.; Zhao, Y.; Chen, S.; Yu, H.; Zhang, Q.; Zeng, R.; Demir, H.V.; Sun, X.; Huan, A.; et al. High-Efficiency Light-Emitting Diodes of Organometal Halide Perovskite Amorphous Nano-particles. ACS Nano 2016, 10, 6623–6630. [Google Scholar] [CrossRef]
- Ju, S.; Mao, C.; Zheng, J.; Yang, K.; Lin, L.; Guo, T.; Hu, H.; Li, F. Perovskite Quantum Dot Light-Emitting Memcapacitor. ACS Appl. Nano Mater. 2023, 6, 9219–9225. [Google Scholar] [CrossRef]
- Ren, J.; Meijerink, A.; Zhou, X.; Wu, J.; Zhang, G.; Wang, Y. In Situ Embedding Synthesis of CsP-bBr3@Ce-MOF@SiO2 Nanocomposites for High Efficiency Light-Emitting Diodes: Suppressing Reabsorption Losses through the Waveguiding Effect. ACS Appl. Mater. Interfaces 2022, 14, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Zhou, T.; Jin, F.; Liang, G.; Liao, Y.; Zhao, A.; Yan, W. Transmission Electron Microscopy Peeled Surface Defect of Perovskite Quantum Dots to Improve Crystal Structure. Materials 2023, 16, 6010. https://doi.org/10.3390/ma16176010
Yuan L, Zhou T, Jin F, Liang G, Liao Y, Zhao A, Yan W. Transmission Electron Microscopy Peeled Surface Defect of Perovskite Quantum Dots to Improve Crystal Structure. Materials. 2023; 16(17):6010. https://doi.org/10.3390/ma16176010
Chicago/Turabian StyleYuan, Longfei, Taixin Zhou, Fengmin Jin, Guohong Liang, Yuxiang Liao, Aijuan Zhao, and Wenbo Yan. 2023. "Transmission Electron Microscopy Peeled Surface Defect of Perovskite Quantum Dots to Improve Crystal Structure" Materials 16, no. 17: 6010. https://doi.org/10.3390/ma16176010
APA StyleYuan, L., Zhou, T., Jin, F., Liang, G., Liao, Y., Zhao, A., & Yan, W. (2023). Transmission Electron Microscopy Peeled Surface Defect of Perovskite Quantum Dots to Improve Crystal Structure. Materials, 16(17), 6010. https://doi.org/10.3390/ma16176010






