Effect of an Early-Age Exposure on the Degradation Mechanisms of Cement Paste under External Sulfate Attack
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulations and Exposure Conditions
2.2. Accelerated Test for ESA
2.3. Experimental Techniques for Investigation
3. Results
3.1. Expansion Measurements
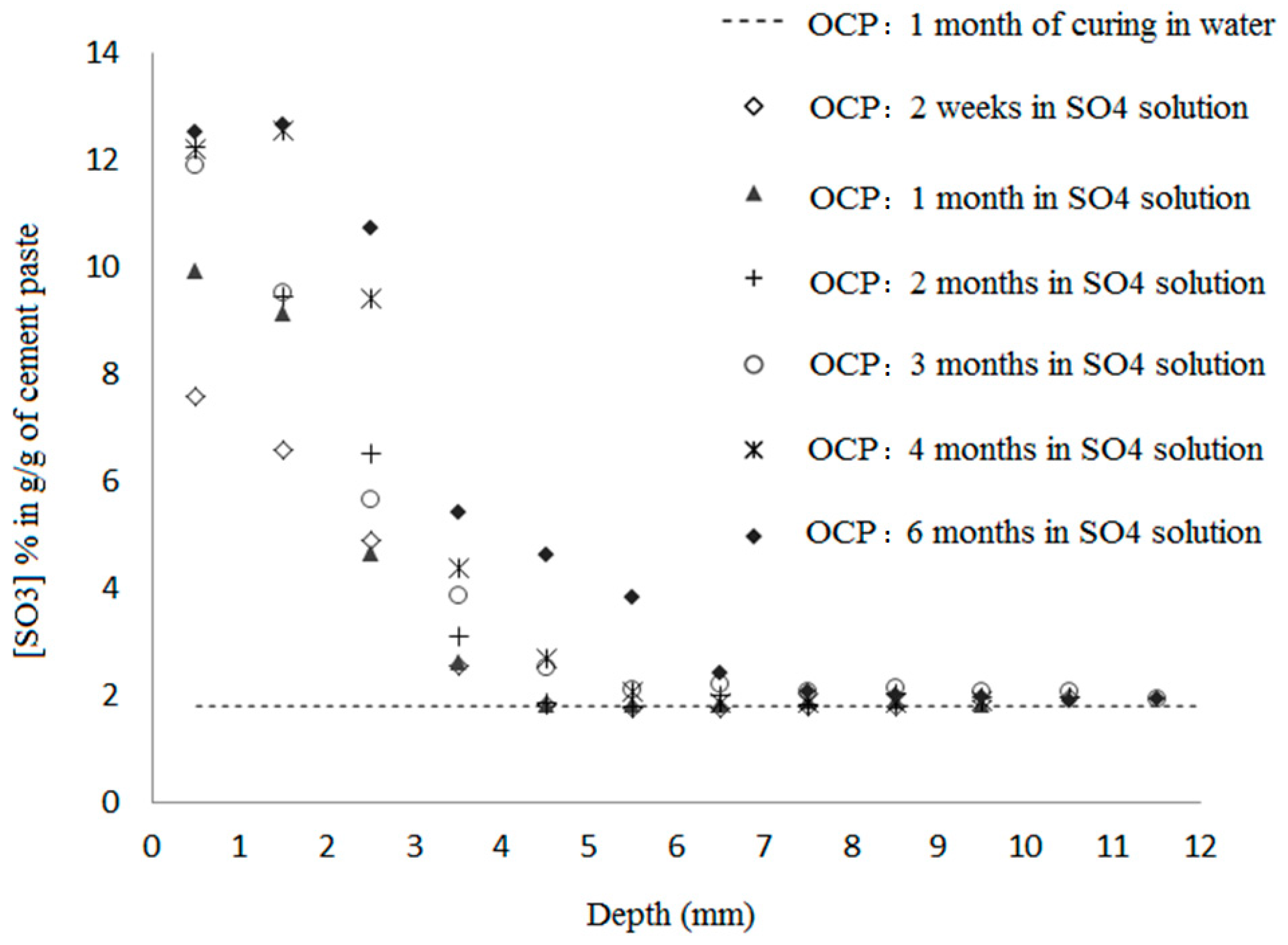
3.2. Sulfates Profiles
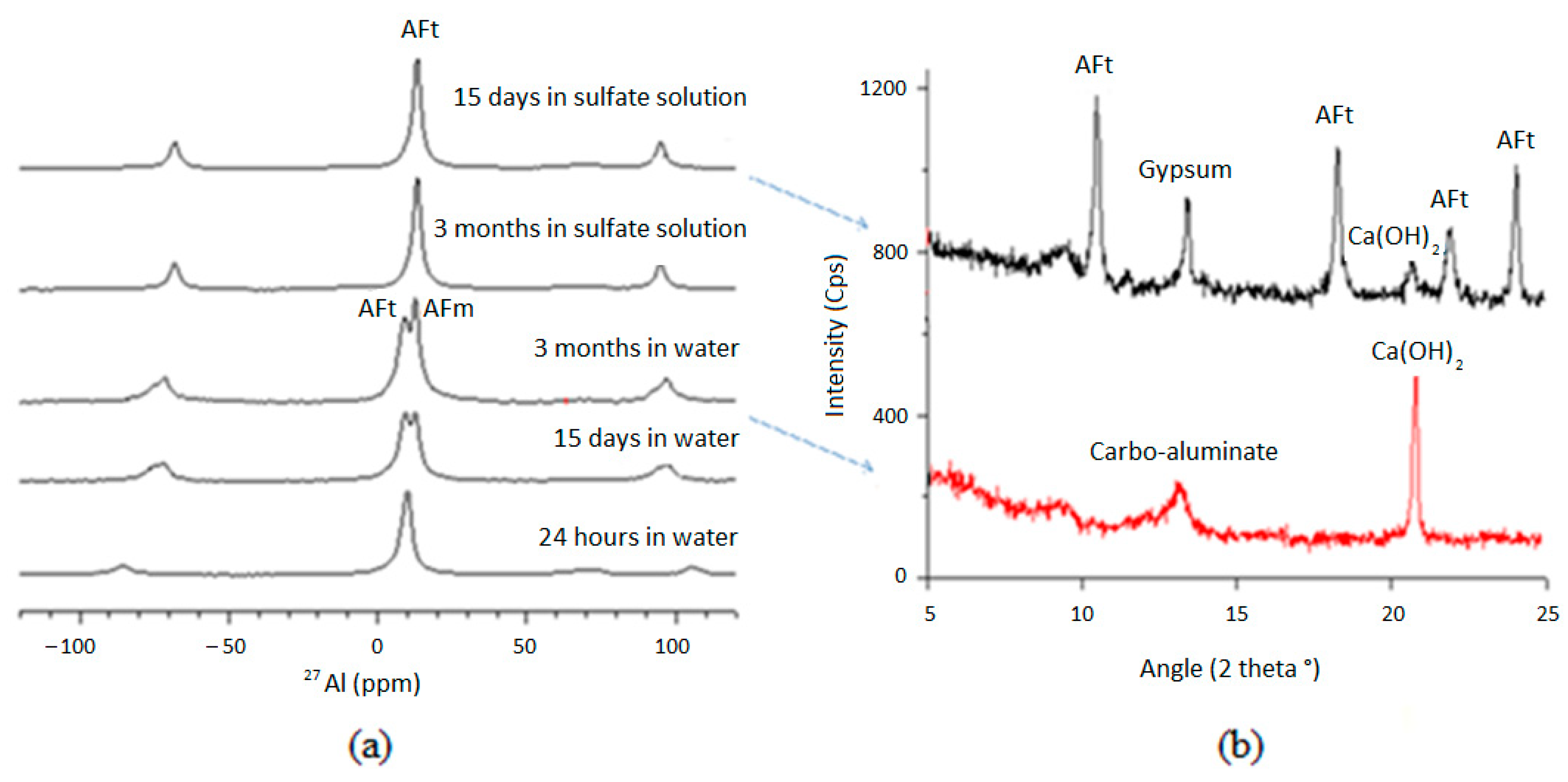
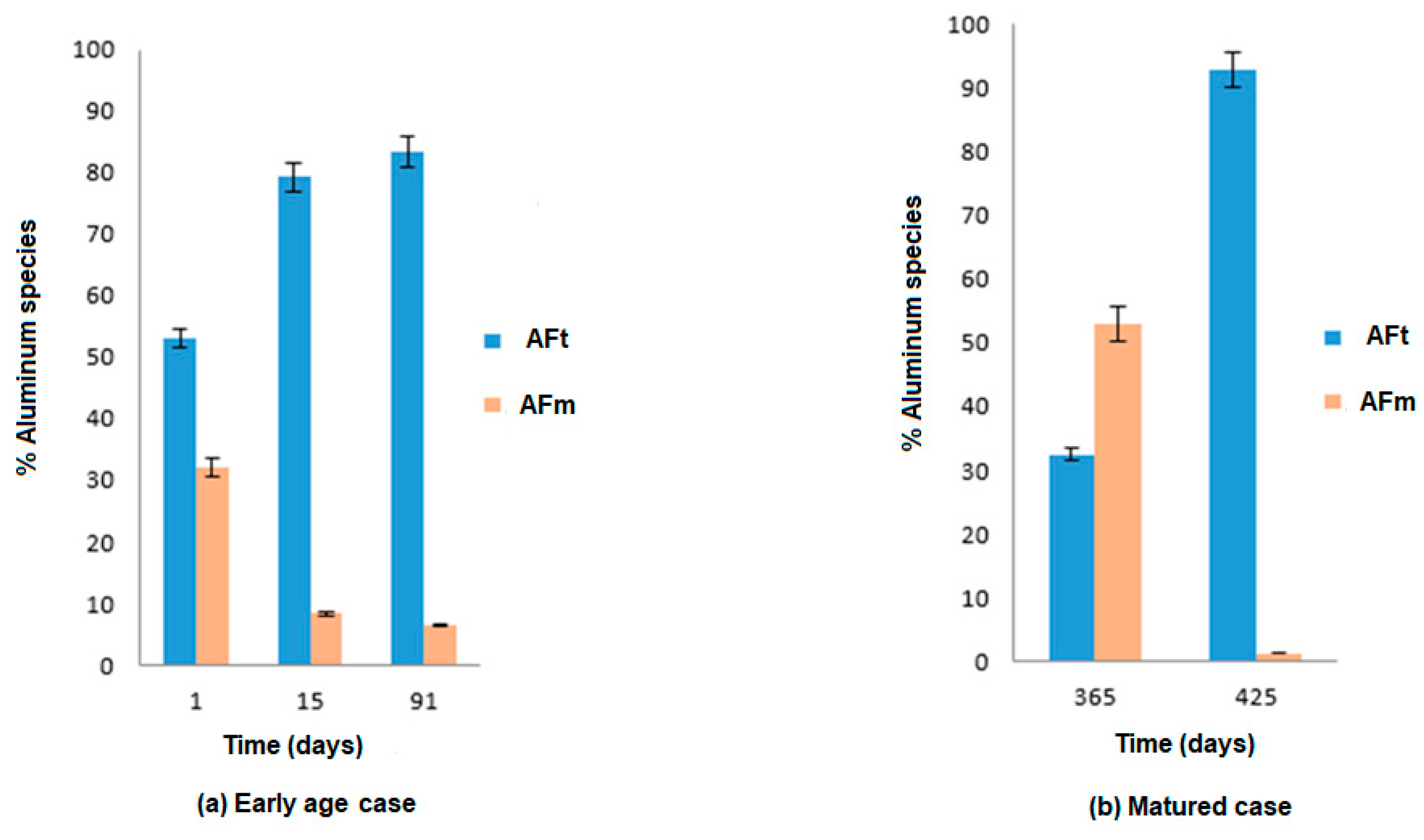
3.3. Chemical Mechanisms
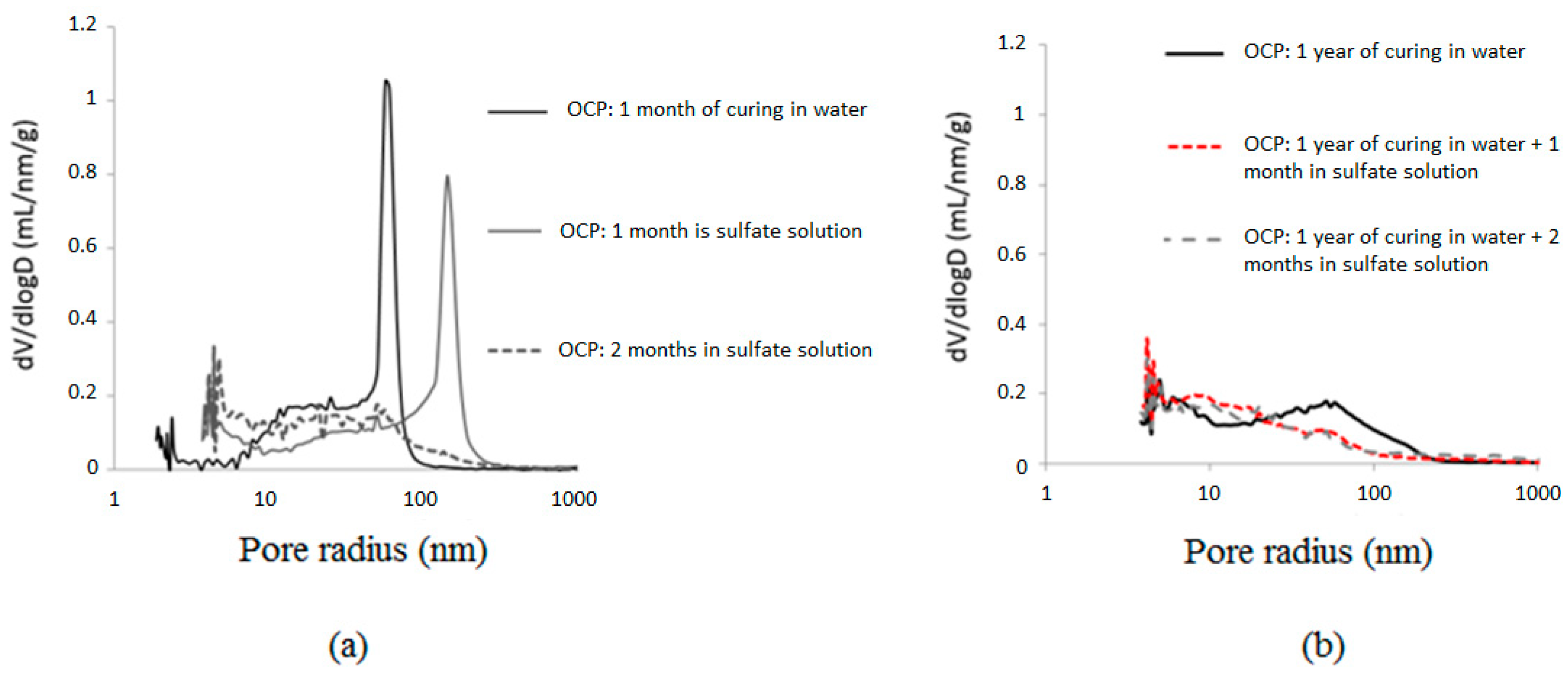
3.4. Microstructure Changes
4. Discussions
5. Conclusions
- -
- The sulfate profiles in the two curing cases are similar, with a slight difference in the maximum sulfate content. There is a more significant effect in the first three millimeters when the exposure occurs at an early age. During this early curing, the material is highly porous and permeable. Chemical interactions with the cement matrix delay sulfate ingress.
- -
- The physical and chemical interactions with cement paste hydrates appear to have faster kinetics than diffusion through concentration gradients and capillary adsorption. This finding confirms that ESA is characterized by both the diffusion and binding of sulfate ions in the cement matrix, with mainly a chemical fixation.
- -
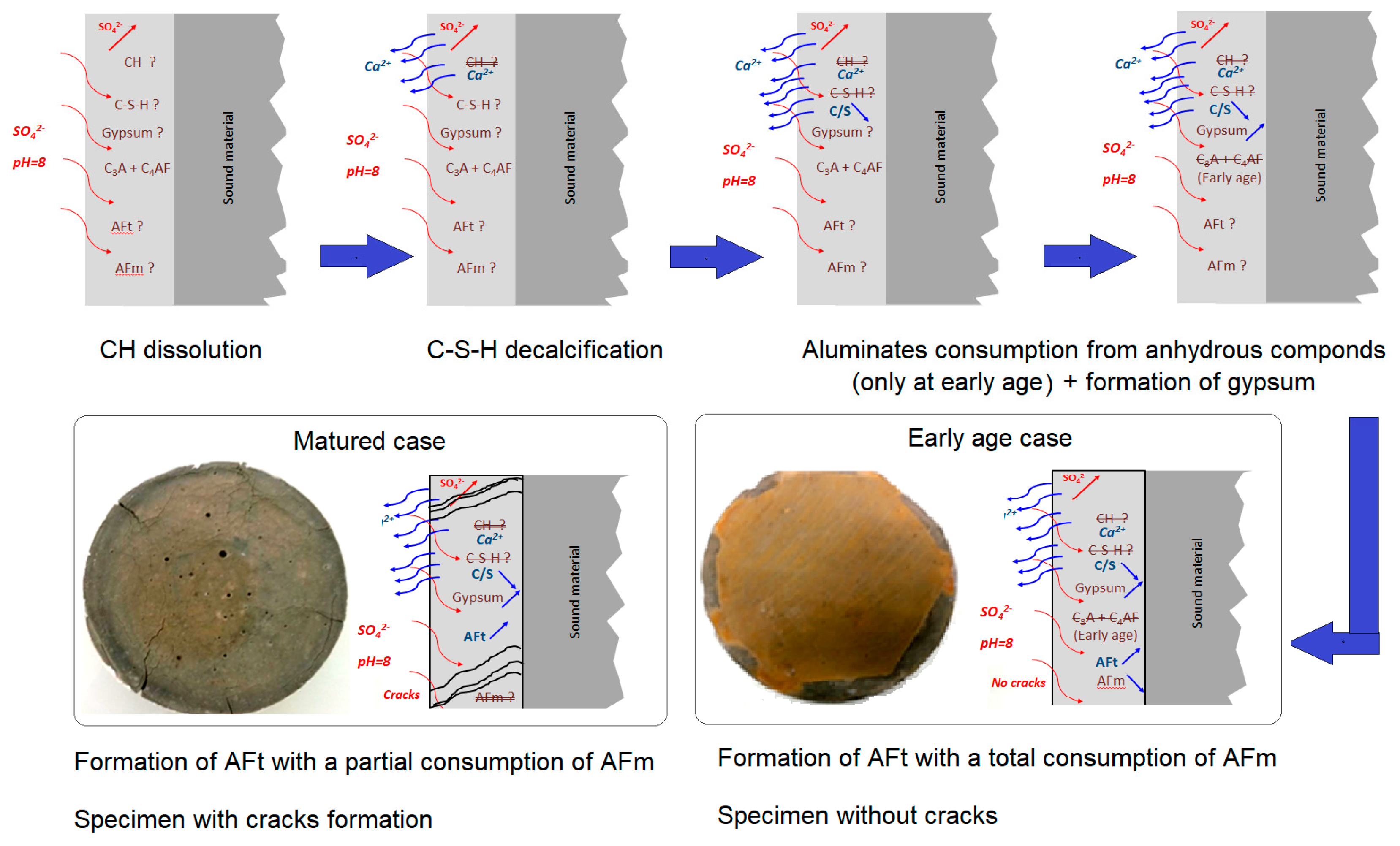
- Matured Portland cement paste showed rapid degradation. This is due to the presence of a significant quantity of compressed ettringite and gypsum, as highlighted by SEM analysis. On the other hand, Portland cement pastes that were exposed to sulfate solution early in the process did not develop cracks or spalls after one year of exposure.
- -
- Although AFt formation is known to cause the degradation of cementitious materials when they are exposed to sulfate ions, the chemical mechanism varies with curing duration. At an early age, the main reagents are external sulfates and aluminates from the anhydrous cement and part of AFm. In the mature exposition case, these reagents are external sulfates, calcium derived from the dissolution of CH and decalcification of C-S-H, and aluminates derived from the total dissolution of AFm.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, G.; Shi, M.; Guo, M.; Fan, H. Degradation Mechanism of Concrete Subjected to External Sulfate Attack: Comparison of Different Curing Conditions. Materials 2020, 13, 3179. [Google Scholar] [CrossRef]
- Wagner, M.; Heisig, A.; Machner, A.; Beddoe, R.; Heinz, D. External Sulfate Attack on Cementitious Binders: Limitations and Effects of Sample Geometry on the Quantification of Expansion Stress. Materials 2022, 15, 3677. [Google Scholar] [CrossRef]
- Liu, G.; Tang, Y.; Wang, J. Effects of carbonation degree of semi-dry carbonated converter steel slag on the performance of blended cement mortar—Reactivity, hydration, and strength. J. Build. Eng. 2023, 63, 105529. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco-material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Planel, D.; Sercombe, J.; Le Bescop, P.; Adenot, F.; Torrenti, J.-M. Long-term performance of cement paste during combined calcium leaching–sulfate attack: Kinetics and size effect. Cem. Concr. Res. 2006, 36, 137–143. [Google Scholar] [CrossRef]
- Kaddah, F.; Ranaivomanana, H.; Amiri, O.; Rozière, E. Accelerated carbonation of recycled concrete aggregates: Investigation on the microstructure and transport properties at cement paste and mortar scales. J. CO2 Util. 2022, 57, 101885. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, G. Understanding Chloride Diffusion Coefficient in Cementitious Materials. Materials 2023, 16, 3464. [Google Scholar] [CrossRef]
- Martin, R.-P.; Omikrine-Metalssi, O.; Toutlemonde, F. Importance of considering the coupling between transfer properties, alkali leaching and expansion in the modelling of concrete beams affected by Internal Swelling Reactions. Constr. Build. Mater. 2013, 49, 23–30. [Google Scholar] [CrossRef]
- Omikrine-Metalssi, O.; Kchakech, B.; Lavaud, S.; Godart, B. A new model for the analysis of the structural/mechanical performance of concrete structures affected by DEF—Case study of an existing viaduct. Struct. Concr. 2016, 17, 1104–1113. [Google Scholar] [CrossRef]
- Al Shamaa, M.; Lavaud, S.; Divet, L.; Nahas, G.; Torrenti, J.-M. Influence of relative humidity on delayed ettringite formation. Cem. Concr. Compos. 2015, 58, 14–22. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Effects of gypsum formation on the performance of cement mortars during external sulfate attack. Cem. Concr. Res. 2003, 33, 325–332. [Google Scholar] [CrossRef]
- Chen, X.; Gu, X.; Xia, X.; Li, X.; Zhang, Q. A Chemical-Transport-Mechanics Numerical Model for Concrete under Sulfate Attack. Materials 2021, 14, 7710. [Google Scholar] [CrossRef]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. Multi-criteria analysis of the mechanism of degradation of Portland cement based mortars exposed to external sulphate attack. Cem. Concr. Res. 2012, 42, 1327–1335. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Chen, W.; Huang, B.; Yuan, Y.; Deng, M. Deterioration Process of Concrete Exposed to Internal Sulfate Attack. Materials 2020, 13, 1336. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Romer, M.; Neuenschwander, J.; Scrivener, K. Physical and microstructural aspects of sulfate attack on ordinary and limestone blended Portland cements. Cem. Concr. Res. 2009, 39, 1111–1121. [Google Scholar] [CrossRef]
- Jabbour, M.; Quiertant, M.; Baroghel-Bouny, V. A Critical Review of Existing Test-Methods for External Sulfate Attack. Materials 2022, 15, 7554. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Wagner, M.; Decker, M.; Kunther, W.; Machner, A.; Beddoe, R.E.; Heisig, A.; Heinz, D. Gypsum formation mechanisms and their contribution to crystallisation pressure in sulfate resistant hardened cement pastes during early external sulfate attack at low sulfate concentrations. Cem. Concr. Res. 2023, 168, 107138. [Google Scholar] [CrossRef]
- Yu, C.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solution. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Zuo, X.-B.; Zheng, Z.-K.; Li, X.-N.; Zou, Y.-X.; Li, L. Mesoscale numerical simulation on the deterioration of cement-based materials under external sulfate attack. Eng. Fail. Anal. 2023, 151, 107419. [Google Scholar] [CrossRef]
- Ran, B.; Omikrine-Metalssi, O.; Fen-Chong, T.; Dangla, P.; Li, K. Pore crystallization and expansion of cement pastes in sulfate solutions with and without chlorides. Cem. Concr. Res. 2023, 166, 107099. [Google Scholar] [CrossRef]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.-M.; Roussel, N.; Divet, L.; de Lacaillerie, E. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Metalssi, O.O.; Touhami, R.R.; Barberon, F.; de Lacaillerie, E.; Roussel, N.; Divet, L.; Torrenti, J.-M. Understanding the degradation mechanisms of cement-based systems in combined chloride-sulfate attack. Cem. Concr. Res. 2023, 164, 107065. [Google Scholar] [CrossRef]
- Shao, W.; Li, Q.; Zhang, W.; Shi, D.; Li, H. Numerical modeling of chloride diffusion in cement-based materials considering calcium leaching and external sulfate attack. Constr. Build. Mater. 2023, 401, 132913. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Chu, H.; Jin, M.; Zhang, Y. Deterioration of pastes exposed to leaching, external sulfate attack and the dual actions. Constr. Build. Mater. 2016, 116, 52–62. [Google Scholar] [CrossRef]
- Rozière, E.; Loukili, A.; El Hachem, R.; Grondin, F. Durability of concrete exposed to leaching and external sulphate attacks. Cem. Concr. Res. 2009, 39, 1188–1198. [Google Scholar] [CrossRef]
- Bary, B.; Leterrier, N.; Deville, E.; Le Bescop, P. Coupled chemo-transport-mechanical modelling and numerical simulation of external sulfate attack in mortar. Cem. Concr. Compos. 2014, 49, 70–83. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Q.; Huang, X. Mechanical Properties and Damage Evolution of Concrete Materials Considering Sulfate Attack. Materials 2021, 14, 2343. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mo, R.; Zhou, X.; Xu, J.; Jin, Z.; Zhao, T. A chemo-thermo-damage-transport model for concrete subjected to combined chloride-sulfate attack considering the effect of calcium leaching. Constr. Build. Mater. 2021, 306, 124918. [Google Scholar] [CrossRef]
- Feng, P.; Garboczi, E.J.; Bullard, C.M.J.W. Microstructural origins of cement paste degradation by external sulfate attack. Constr. Build. Mater. 2015, 96, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Biczok, I. Concrete Corrosion Concrete Protection; Chemical Publishing: New York, NY, USA, 1967. [Google Scholar]
- Kanaan, D.; Soliman, A.M.; Suleiman, A.R. Zero-Cement Concrete Resistance to External Sulfate Attack: A Critical Review and Future Needs. Sustainability 2022, 14, 2078. [Google Scholar] [CrossRef]
- Brown, P.W. An evaluation of sulfate resistance of cements in a controlled environment. Cem. Concr. Res. 1981, 11, 137–143. [Google Scholar] [CrossRef]
- ASTM International C1012-04; Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2004.
- ASTM International C452-06; Standard Test Method for potential Expansion of Portland-Cement Mortars Exposed to Sulphate. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2006.
- ASTM International C150-09; Standard Specification for Portland Cement. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2009.
- Massiot, D.; King, I.; Calvé, S.L.; Alonso, B.; Durand, J.O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one and two-dimensional solid-state NMR spectra, Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- de Lacaillerie, J.-B.D.; Fretigny, C.; Massiot, D. MAS NMR Spectra of Quadrupolar Nuclei in Disordered Solids: The Czjzek Model. J. Magn. Reson. 2008, 192, 244–251. [Google Scholar] [CrossRef]
- Richardson, I.G. Model structures for C-(A)-S-H(I), Acta Crystallographica Section B Structural Science. Cryst. Eng. Mater. 2014, 70, 903–923. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Skibsted, J. Influence of the Ca/Si ratio of the C-S-H phase on the interaction with sulfate ions and its impact on the Ettringite crystallization pressure. Cem. Concr. Res. 2015, 69, 37–49. [Google Scholar] [CrossRef]








| Chemical Composition (%) | CEM I |
|---|---|
| CaO | 62.81 |
| SiO2 | 20.22 |
| Al2O3 | 4.85 |
| Fe2O3 | 2.92 |
| CaO (free) | 1.58 |
| MgO | 0.84 |
| SO3 | 2.88 |
| S | 0 |
| K2O | 0.77 |
| Na2O | 0.34 |
| Ignition Loss | 2.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metalssi, O.O.; Ragoug, R.; Barberon, F.; Lacaillerie, J.-B.d.d.; Roussel, N.; Divet, L.; Torrenti, J.-M. Effect of an Early-Age Exposure on the Degradation Mechanisms of Cement Paste under External Sulfate Attack. Materials 2023, 16, 6013. https://doi.org/10.3390/ma16176013
Metalssi OO, Ragoug R, Barberon F, Lacaillerie J-Bdd, Roussel N, Divet L, Torrenti J-M. Effect of an Early-Age Exposure on the Degradation Mechanisms of Cement Paste under External Sulfate Attack. Materials. 2023; 16(17):6013. https://doi.org/10.3390/ma16176013
Chicago/Turabian StyleMetalssi, Othman Omikrine, Rim Ragoug, Fabien Barberon, Jean-Baptiste d’Espinose de Lacaillerie, Nicolas Roussel, Loïc Divet, and Jean-Michel Torrenti. 2023. "Effect of an Early-Age Exposure on the Degradation Mechanisms of Cement Paste under External Sulfate Attack" Materials 16, no. 17: 6013. https://doi.org/10.3390/ma16176013
APA StyleMetalssi, O. O., Ragoug, R., Barberon, F., Lacaillerie, J.-B. d. d., Roussel, N., Divet, L., & Torrenti, J.-M. (2023). Effect of an Early-Age Exposure on the Degradation Mechanisms of Cement Paste under External Sulfate Attack. Materials, 16(17), 6013. https://doi.org/10.3390/ma16176013








