Real-Time Nondestructive Viscosity Measurement of Soft Tissue Based on Viscoelastic Response Optical Coherence Elastography
Abstract
1. Introduction
2. Materials and Methods
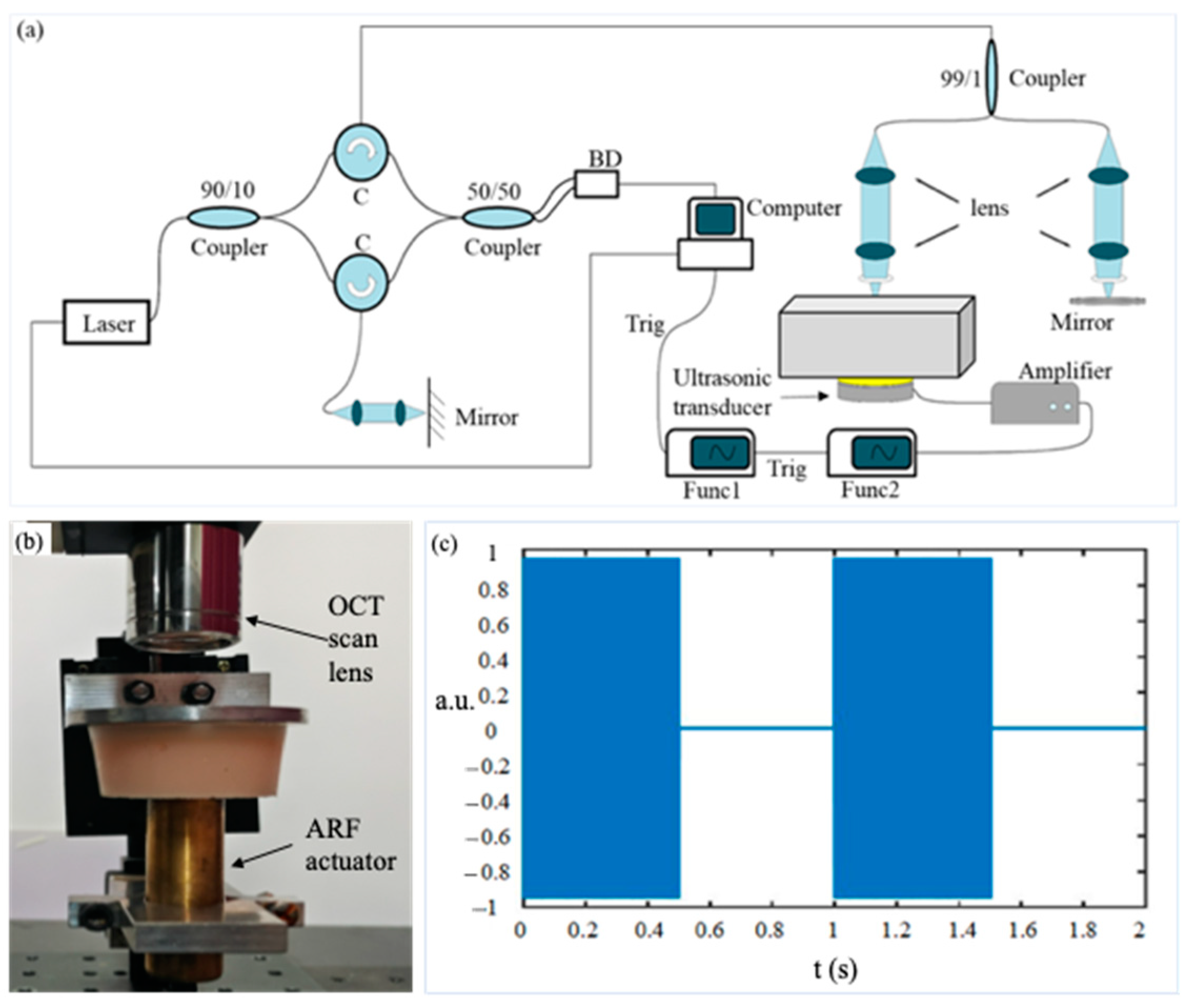
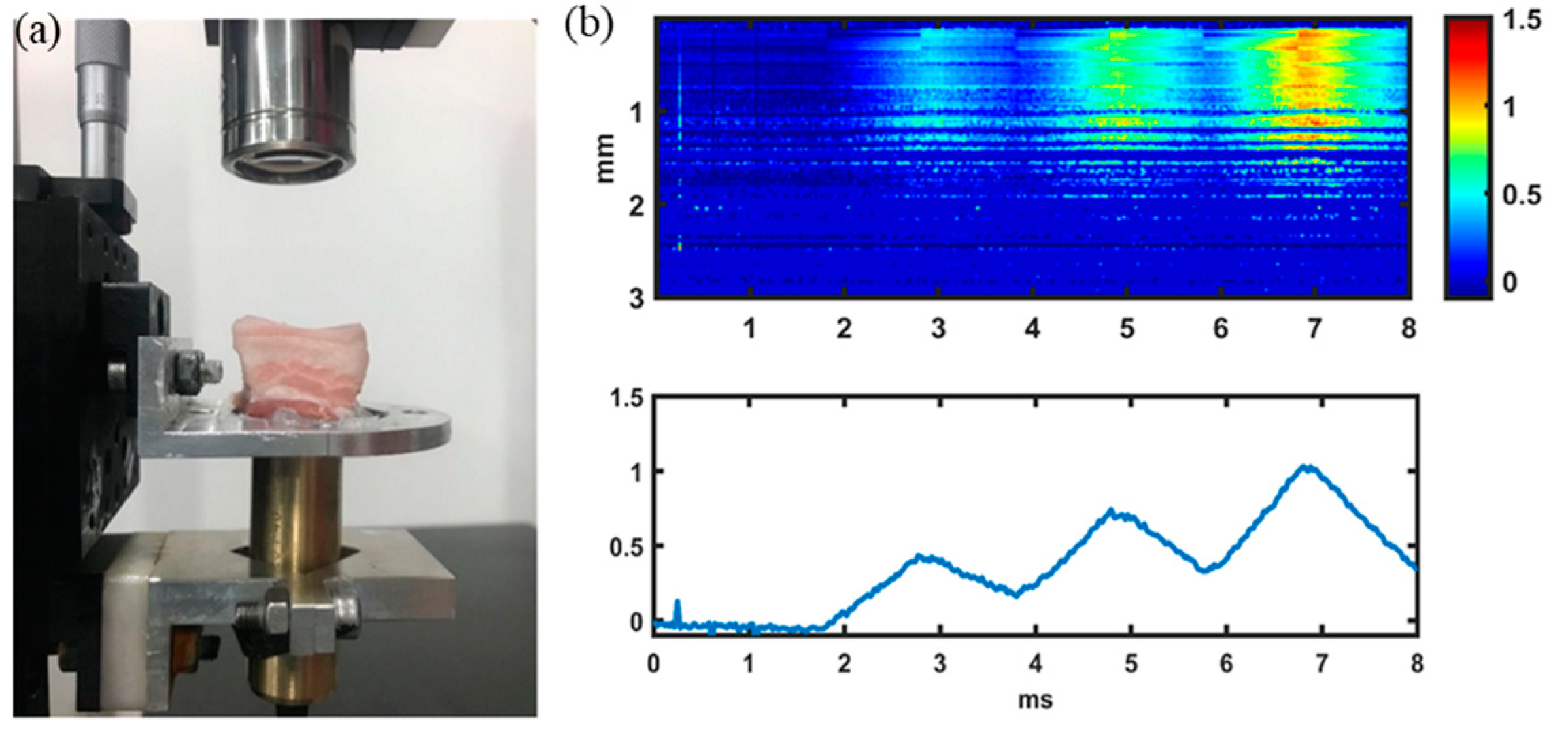
2.1. VisR-OCE Measurement System
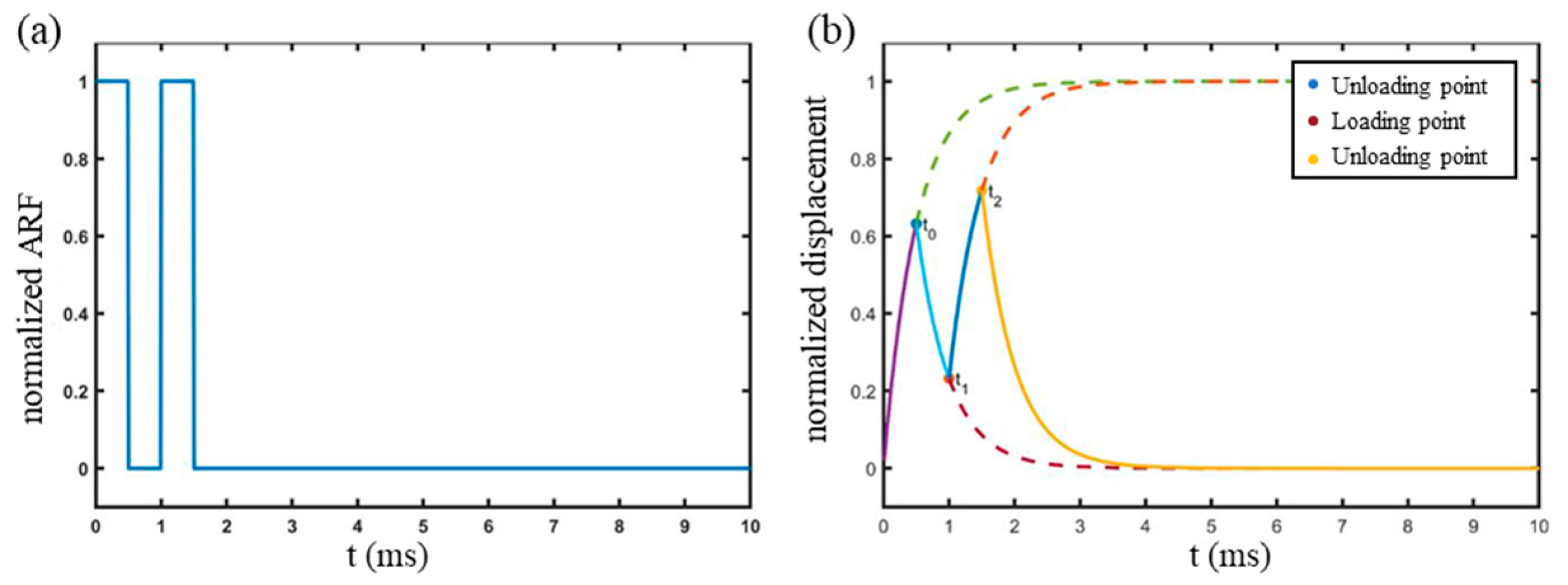
2.2. Relaxation Time Constant Measurement
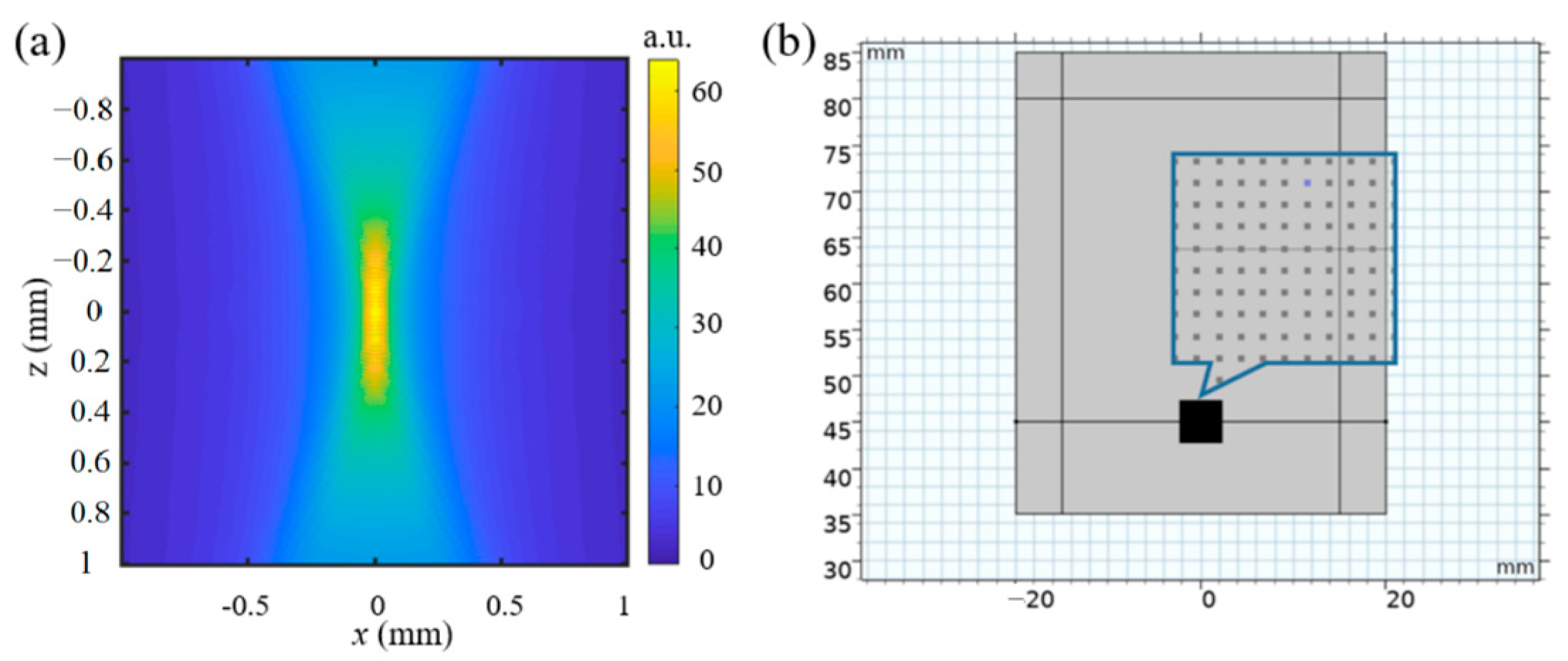
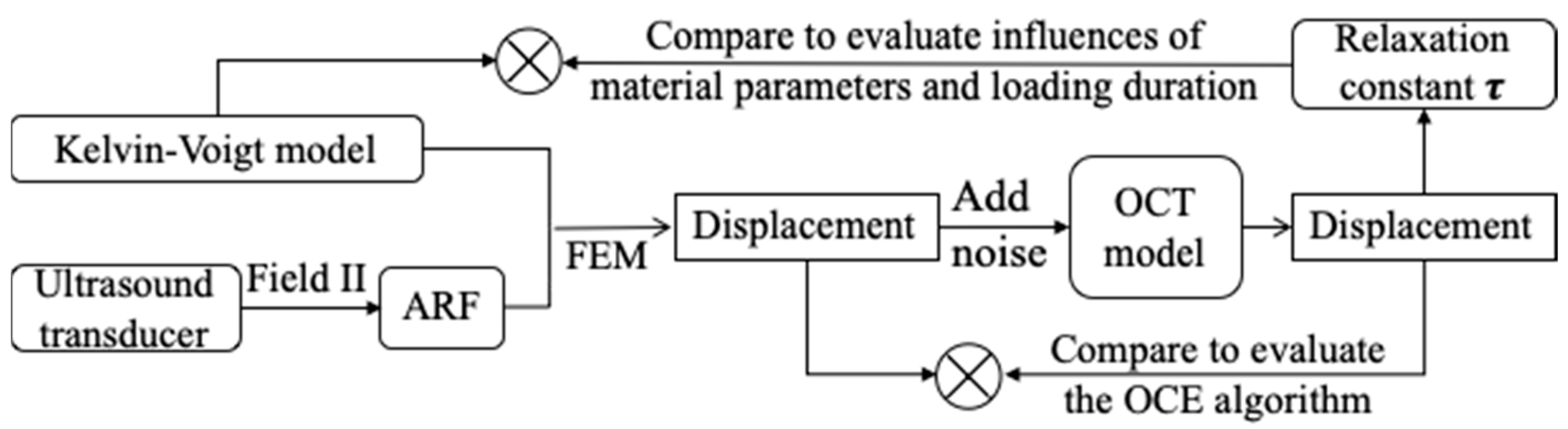
2.3. Virtual Experiment by Numerical Modeling
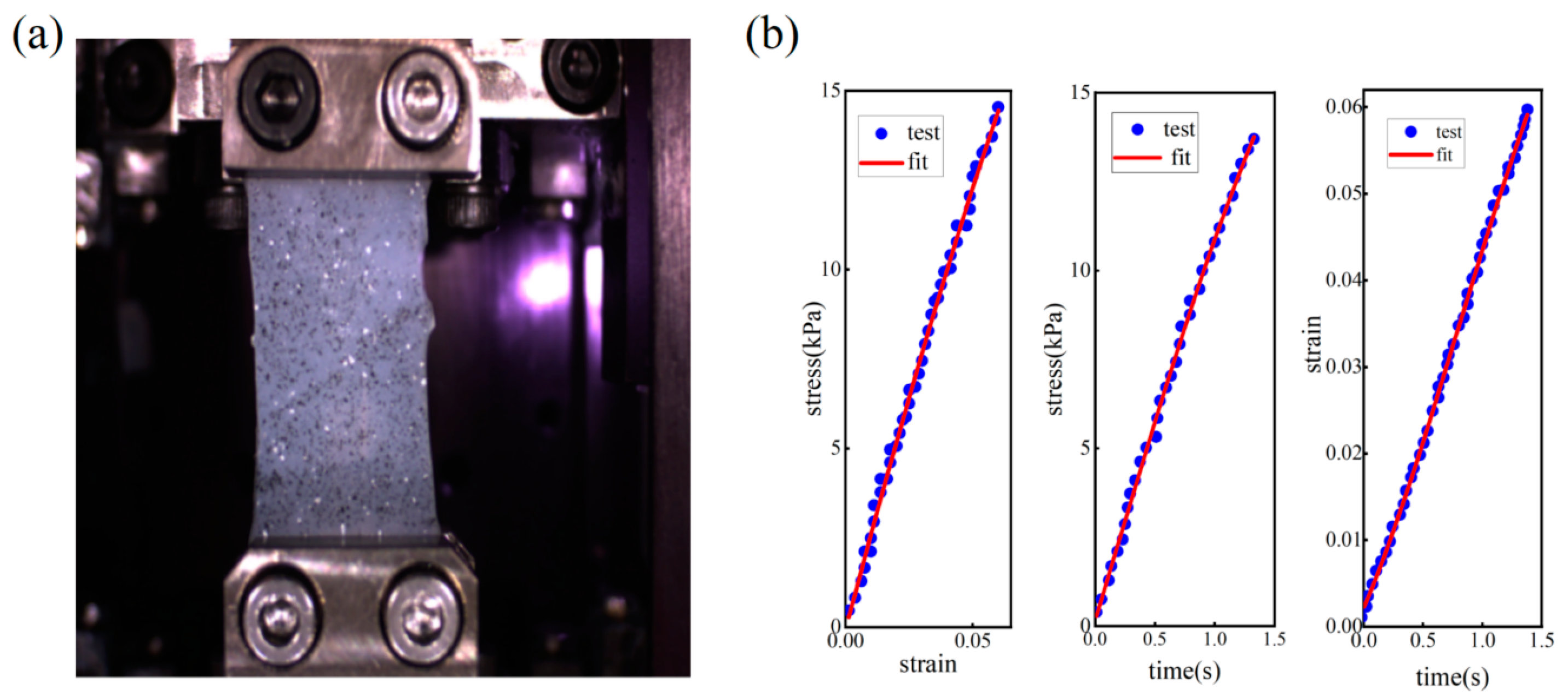
2.4. Soft Tissue Phantom Fabrication and Relaxation Time Constant Measurement
3. Results
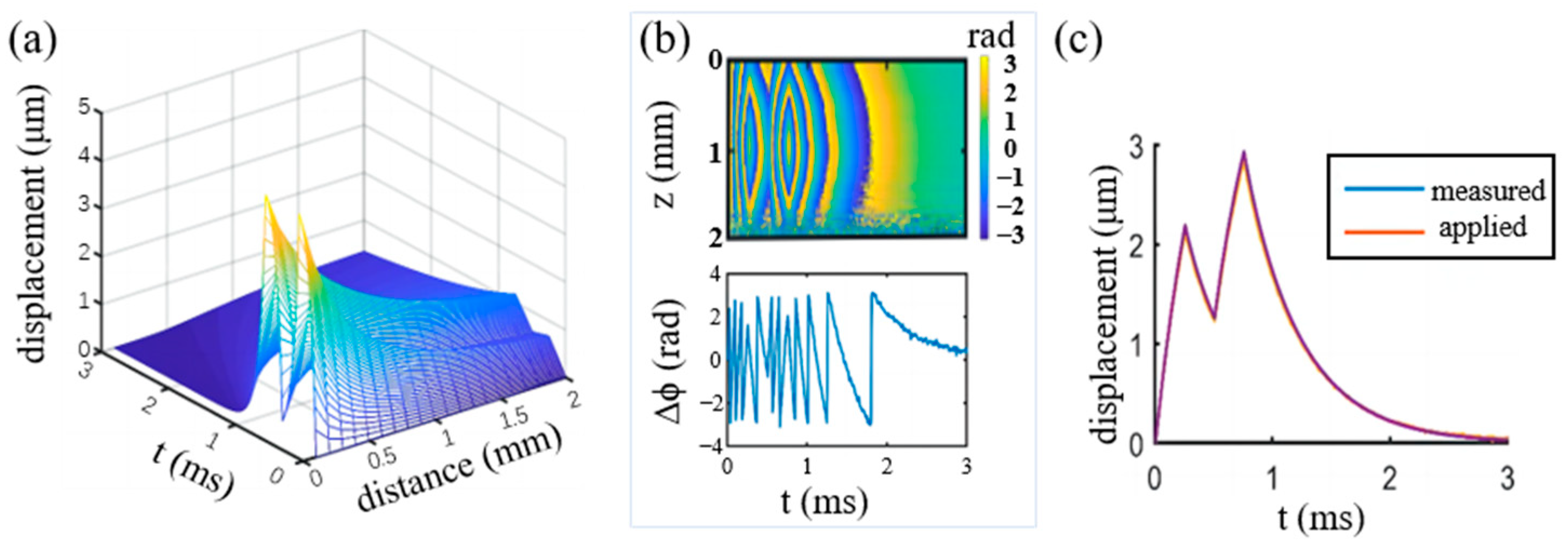
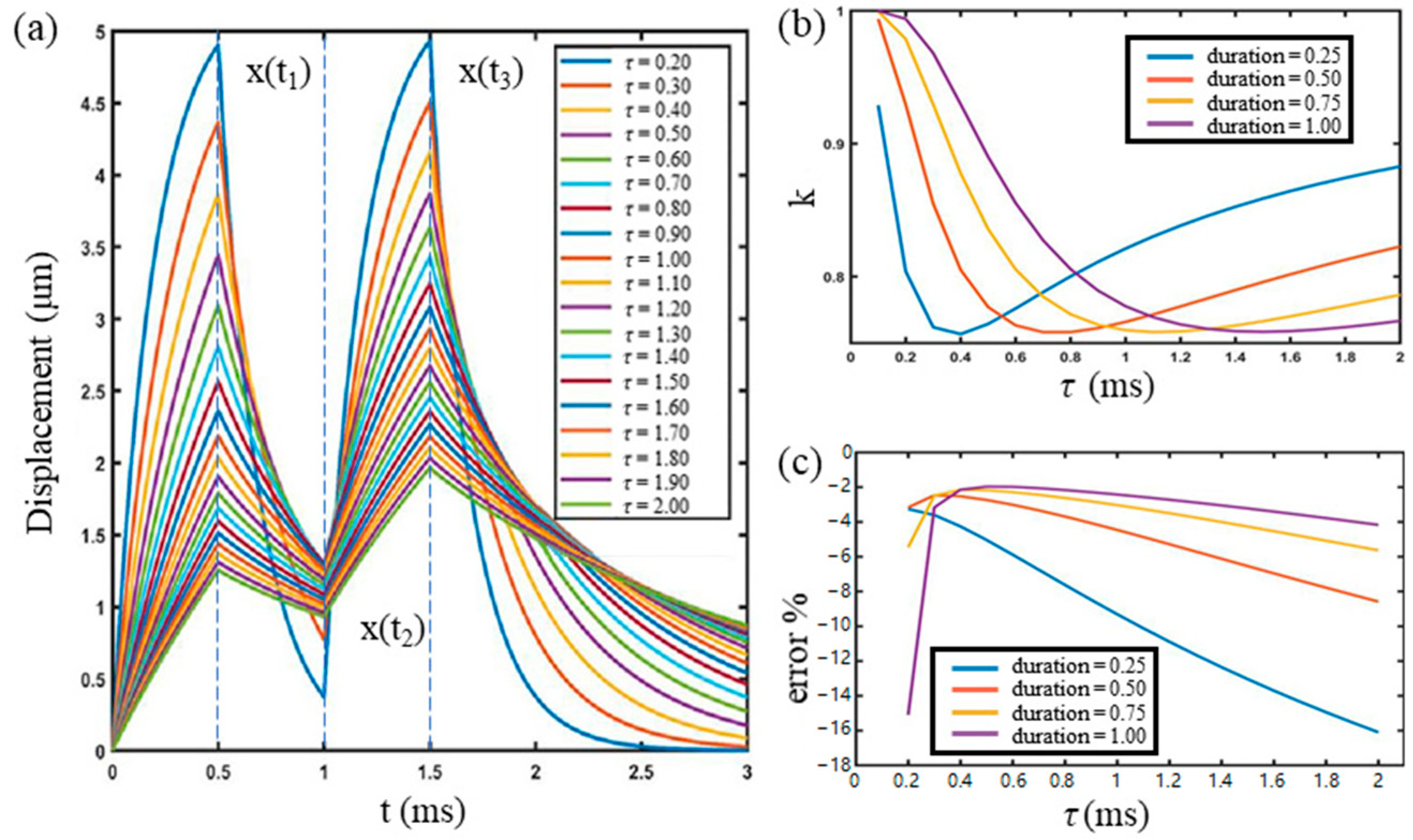
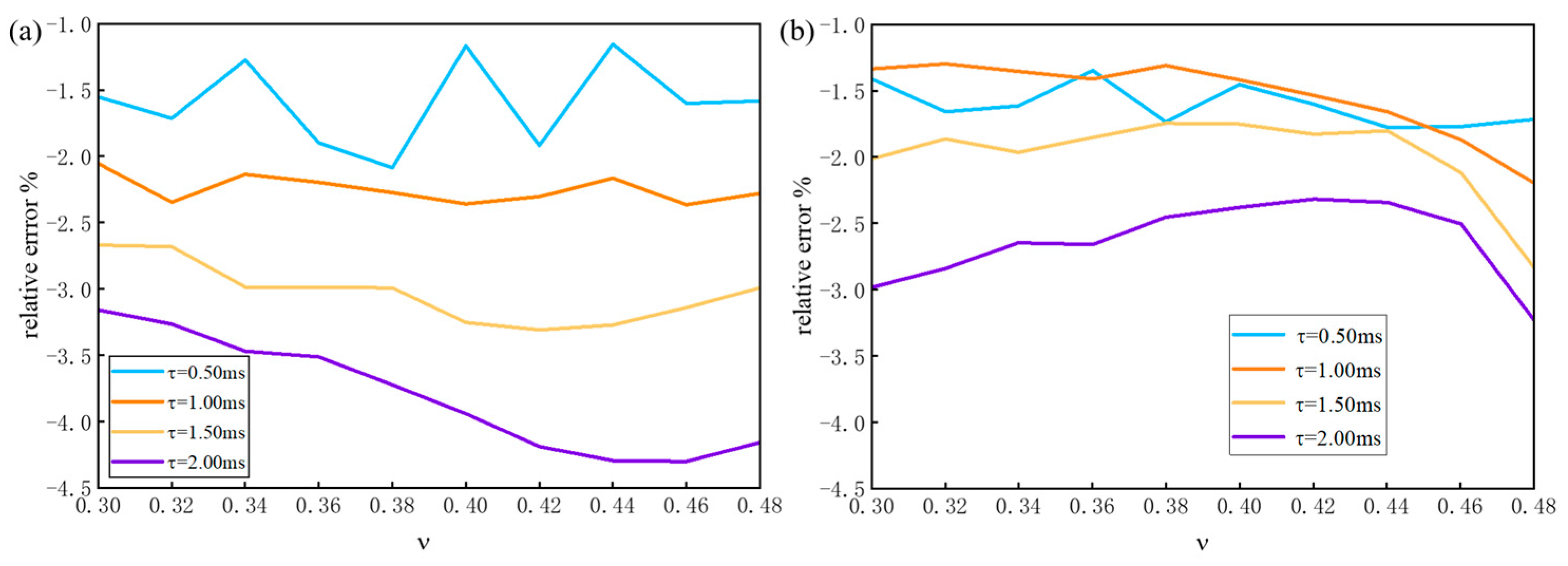
3.1. Virtual Experiment by Numerical Modeling
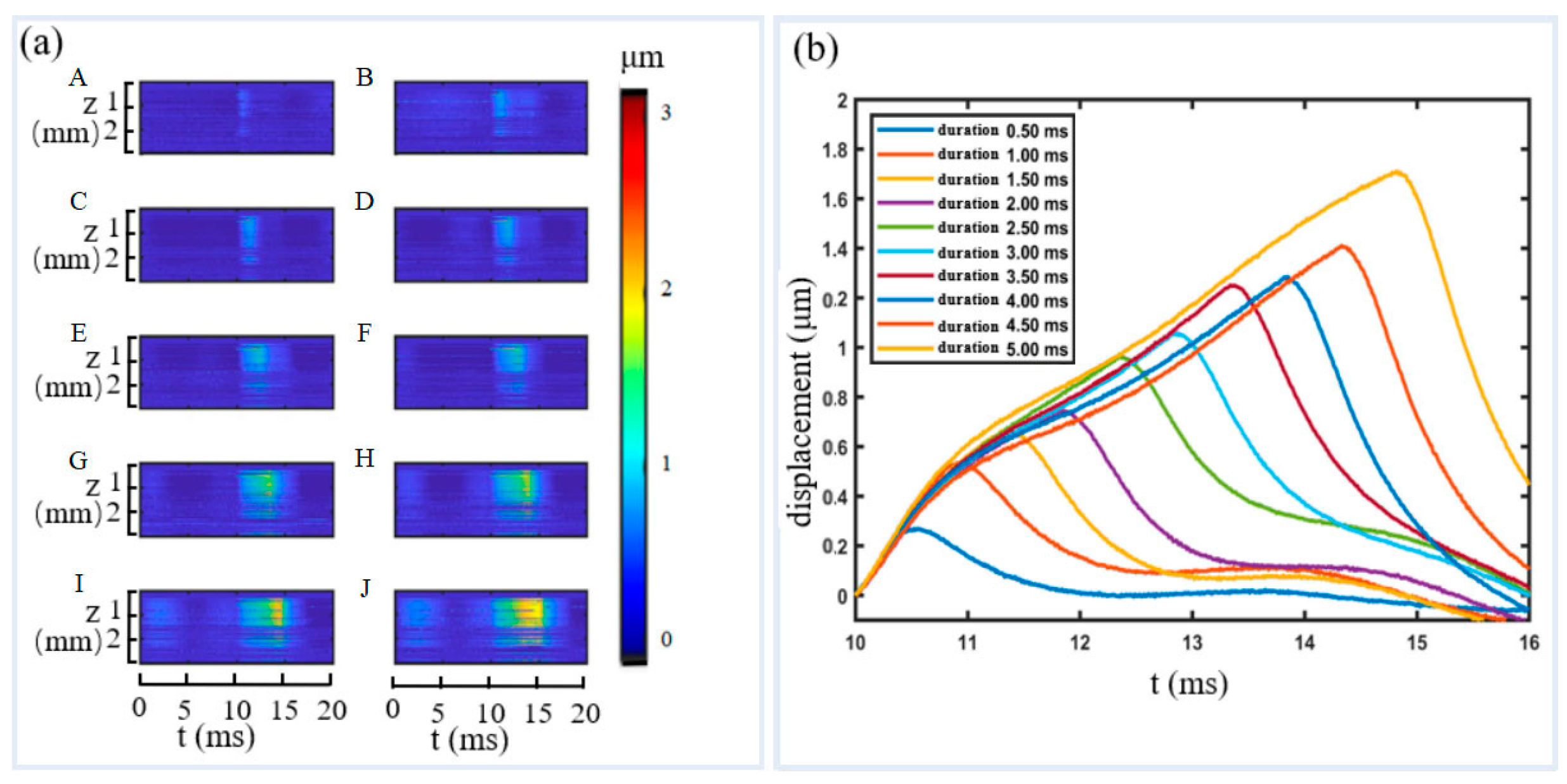
3.2. Soft Tissue Phantom Measurement
3.3. Pork Meat Measurement
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Fle, G.; Bhatt, M.; Qu, Z.; Ghazavi, S.; Yazdani, L.; Bosio, G.; Rafati, I.; Cloutier, G. Viscoelasticity Imaging of Biological Tissues and Single Cells Using Shear Wave Propagation. Front. Phys. 2021, 9, 666192. [Google Scholar] [CrossRef]
- Catheline, S.; Gennisson, L.J.; Delon, G.; Fink, M.; Sinkus, R.; Abouelkaram, S.; Culioli, J. Measurement of viscoelastic properties of homogeneous soft solid using transient elastography: An inverse problem approach. J. Acoust. Soc. Am. 2004, 116, 3734. [Google Scholar] [CrossRef] [PubMed]
- Shergold, O.A.; Fleck, N.A.; Radford, D. The uniaxial stress versus strain response of pig skin and silicone rubber at low and high strain rates. Int. J. Impact Eng. 2006, 32, 1384–1402. [Google Scholar] [CrossRef]
- Clancy, N.T.; Nilsson, G.E.; Anderson, C.D.; Leahy, M.J. A new device for assessing changes in skin viscoelasticity using indentation and optical measurement. Skin Res. Technol. 2010, 16, 210–228. [Google Scholar] [CrossRef]
- Gubarkova, E.V.; Sovetsky, A.A.; Vorontsov, D.A.; Buday, P.A.; Sirotkina, M.A.; Plekhanov, A.A.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Gamayunov, S.V.; et al. Compression optical coherence elastography versus strain ultrasound elastography for breast cancer detection and differentiation: Pilot study. Biomed. Opt. Express 2022, 13, 2859–2880. [Google Scholar] [CrossRef]
- Gubarkova, E.V.; Sovetsky, A.A.; Matveev, L.A.; Matveyev, A.L.; Vorontsov, D.A.; Plekhanov, A.A.; Kuznetsov, S.S.; Gamayunov, S.V.; Vorontsov, A.Y.; Sirotkina, M.A.; et al. Nonlinear Elasticity Assessment with Optical Coherence Elastography for High-Selectivity Differentiation of Breast Cancer Tissues. Materials 2022, 15, 3308. [Google Scholar] [CrossRef]
- Zykov, A.A.; Matveyev, A.L.; Matveev, L.A.; Shabanov, D.V.; Zaitsev, V.Y. Novel Elastography-Inspired Approach to Angiographic Visualization in Optical Coherence Tomography. Photonics 2022, 9, 401. [Google Scholar] [CrossRef]
- Shah, R.; Pierce, M.C.; Silver, F.H. A method for nondestructive mechanical testing of tissues and implants. J. Biomed. Mater. Res. Part A 2017, 105, 15–22. [Google Scholar] [CrossRef]
- Nightingale, K.; McAleavey, S.; Trahey, G. Shear-wave generation using acoustic radiation force: In vivo and ex vivo results. Ultrasound Med. Biol. 2003, 29, 1715–1723. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Nightingale, K.R. Acoustic radiation force-based elasticity imaging methods. Interface Focus 2011, 1, 553–564. [Google Scholar] [CrossRef]
- Zhu, J.; He, X.; Chen, Z. Acoustic radiation force optical coherence elastography for elasticity assessment of soft tissues. Appl. Spectrosc. Rev. 2019, 54, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Song, S.; Arnal, B.; Wong, E.Y.; Huang, Z.; Wang, R.K.; O’Donnell, M. Shear wave pulse compression for dynamic elastography using phase-sensitive optical coherence tomography. J. Biomed. Opt. 2014, 19, 16013. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ma, T.; He, Y.; Zhu, J.; Dai, C.; Yu, M.; Huang, S.; Lu, F.; Shung, K.K.; Zhou, Q.; et al. Acoustic Radiation Force Optical Coherence Elastography of Corneal Tissue. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Li, C.; Ling, Y.; Yang, Y.; Vorstius, J.B.; Keatch, R.P.; Wang, R.K.; Huang, Z. Quantitative evaluation of degenerated tendon model using combined optical coherence elastography and acoustic radiation force method. J. Biomed. Opt. 2013, 18, 111417. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Singh, M.; Aglyamov, S.R.; Liu, C.H.; Nair, A.; Raghunathan, R.; Wu, C.; Li, J.; Larin, K.V. Quantifying tissue viscoelasticity using optical coherence elastography and the Rayleigh wave model. J. Biomed. Opt. 2016, 21, 90504. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Singh, M.; Wu, C.; Liu, C.H.; Raghunathan, R.; Aglyamov, S.R.; Vantipalli, S.; Twa, M.D.; Larin, K.V. Optical coherence elastography assessment of corneal viscoelasticity with a modified Rayleigh-Lamb wave model. J. Mech. Behav. Biomed. Mater. 2017, 66, 87–94. [Google Scholar] [CrossRef]
- Wang, Y.; Shemonski, N.D.; Adie, S.G.; Boppart, S.A.; Insana, M.F. Dynamic method of optical coherence elastography in determining viscoelasticity of polymers and tissues. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Zhou, K.; Li, C.; Chen, S.; Nabi, G.; Huang, Z. Feasibility study of using the dispersion of surface acoustic wave impulse for viscoelasticity characterization in tissue mimicking phantoms. J. Biophotonics 2019, 12, e201800177. [Google Scholar] [CrossRef]
- Shigao, C.; Urban, M.W.; Cristina, P.; Randall, K.; Yi, Z.; Aiping, Y.; Greenleaf, J.F. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 55–62. [Google Scholar] [CrossRef]
- Nenadic, I.; Urban, M.W.; Mitchell, S.A.; Greenleaf, J.F. Lamb Wave Shearwave Dispersion Ultrasound Vibrometry (SDUV) Validation Study. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 45–48. [Google Scholar] [CrossRef]
- Mauldin, F.W.; Haider, M.A.; Loboa, E.G.; Behler, R.H.; Euliss, L.E.; Pfeiler, T.W.; Gallippi, C.M. Monitored steady-state excitation and recovery (MSSR) radiation force imaging using viscoelastic models. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1597–1610. [Google Scholar] [CrossRef]
- Wijesinghe, P.; McLaughlin, R.A.; Sampson, D.D.; Kennedy, B.F. Parametric imaging of viscoelasticity using optical coherence elastography. Phys. Med. Biol. 2015, 60, 2293–2307. [Google Scholar] [CrossRef]
- Rivaz, H.; Boctor, E.M.; Choti, M.A.; Hager, G.D. Real-time regularized ultrasound elastography. IEEE Trans. Med. Imaging 2011, 30, 928. [Google Scholar] [CrossRef] [PubMed]
- Selzo, M.R.; Gallippi, C.M. Viscoelastic Response (VisR) Imaging for Assessment of Viscoelasticity in Voigt Materials. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2488–2500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selzo, M.R.; Moore, C.J.; Hossain, M.M.; Palmeri, M.L.; Gallippi, C.M. On the Quantitative Potential of Viscoelastic Response (VisR) Ultrasound Using the One-Dimensional Mass-Spring-Damper Model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Gallippi, C.M. Viscoelastic Response Ultrasound Derived Relative Elasticity and Relative Viscosity Reflect True Elasticity and Viscosity: In Silico and Experimental Demonstration. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Lin, X.; Liu, H.; Sun, C. Optical coherence elastography of bilayer soft tissue based on harmonic surface wave spectroscopy. Opt. Lasers Eng. 2021, 145, 106667. [Google Scholar] [CrossRef]
- Findley, W.N.; Lai, J.S.; Onaran, K. Creep and Relaxation of Non-Linear Viscoelastic Materials; Courier Corporation: North Chelmsford, MA, USA, 1989. [Google Scholar]
- Schlaikjer, M.; Torp-Pedersen, S.; Jensen, J.A. Simulation of RF data with tissue motion for optimizing stationary echo canceling filters. Ultrasonics 2004, 41, 415–419. [Google Scholar] [CrossRef]
- Jachowicz, J.; McMullen, R.; Prettypaul, D. Indentometric analysis of in vivo skin and comparison with artificial skin models. Skin Res. Technol. 2007, 13, 299–309. [Google Scholar] [CrossRef]
- Marques, S.P.C.; Creus, G.J. Computational Viscoelasticity; Springerbriefs in Applied Sciences & Technology; Springer: Berlin/Heidelberg, Germany, 2012; pp. iv–v. [Google Scholar]
- Gai, S.; Zhang, Z.; You, J.; Zou, Y.; Liu, D. Qualitative and quantitative detection of water-injected pork by low-field nuclear magnetic resonance technique combined with chemometrics. Food Sci. 2020, 41, 243–247. [Google Scholar]
- Yasar, T.K.; Royston, T.J.; Magin, R.L. Wideband MR elastography for viscoelasticity model identification. Magn. Reson. Med. 2013, 70, 479–489. [Google Scholar] [CrossRef]
- Kearney, S.P.; Khan, A.; Dai, Z.J.; Royston, T.J. Dynamic viscoelastic models of human skin using optical elastography. Phys. Med. Biol. 2015, 60, 6975–6990. [Google Scholar] [CrossRef]


| Voigt Model | (kPa) | (ms) | |
|---|---|---|---|
| 13 | Range (0.1, 0.1, 2) | Range (0.3, 0.02, 0.48) | |
| 130 | Range (0.1, 0.1, 2) | Range (0.3, 0.02, 0.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, W.; Chen, Q.; Hu, Y.; Li, Y.; Zheng, X.; Fang, D.; Liu, H.; Sun, C. Real-Time Nondestructive Viscosity Measurement of Soft Tissue Based on Viscoelastic Response Optical Coherence Elastography. Materials 2023, 16, 6019. https://doi.org/10.3390/ma16176019
Liu Z, Liu W, Chen Q, Hu Y, Li Y, Zheng X, Fang D, Liu H, Sun C. Real-Time Nondestructive Viscosity Measurement of Soft Tissue Based on Viscoelastic Response Optical Coherence Elastography. Materials. 2023; 16(17):6019. https://doi.org/10.3390/ma16176019
Chicago/Turabian StyleLiu, Zhixin, Weidong Liu, Qi Chen, Yongzheng Hu, Yurun Li, Xiaoya Zheng, Dian Fang, Hai Liu, and Cuiru Sun. 2023. "Real-Time Nondestructive Viscosity Measurement of Soft Tissue Based on Viscoelastic Response Optical Coherence Elastography" Materials 16, no. 17: 6019. https://doi.org/10.3390/ma16176019
APA StyleLiu, Z., Liu, W., Chen, Q., Hu, Y., Li, Y., Zheng, X., Fang, D., Liu, H., & Sun, C. (2023). Real-Time Nondestructive Viscosity Measurement of Soft Tissue Based on Viscoelastic Response Optical Coherence Elastography. Materials, 16(17), 6019. https://doi.org/10.3390/ma16176019






