Recent Advances and Outlook in 2D Nanomaterial-Based Flame-Retardant PLA Materials
Abstract
:1. Introduction
- ✓
- This review presents a complete discussion on 2D nanomaterials as flame-retardants in PLA systems.
- ✓
- Trends and progress in PLA/2D nanoparticle flame-retardant materials are presented here in a dedicated review paper.
- ✓
- The challenges researchers face in fabricating and utilizing PLA/2D nanoparticles flame-retardants in PLA systems are presented in a review article.
- ✓
- The future perspective converses herewith as a guide for emerging researchers in this niche(s).
1.1. PLA Feedstock
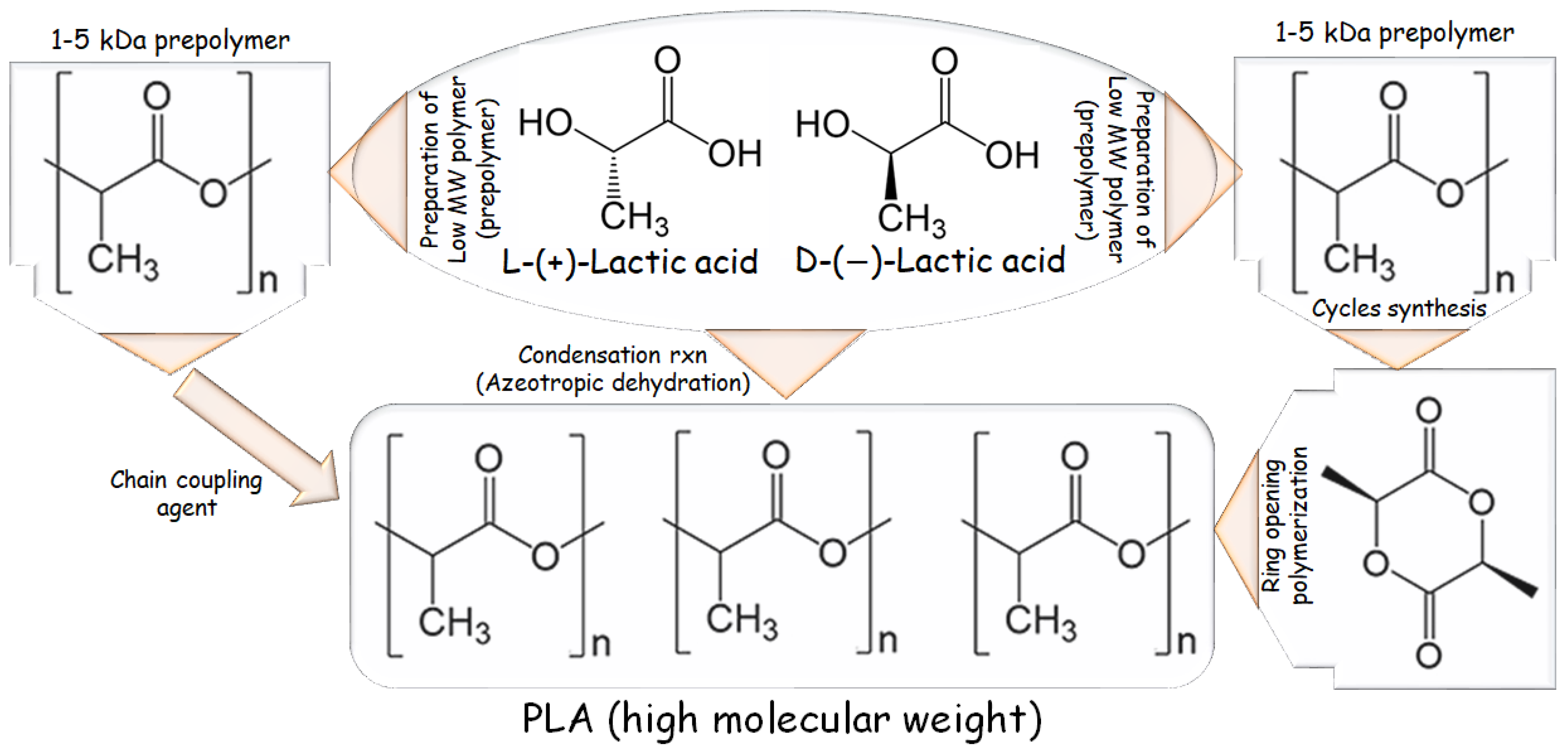
1.2. PLA Synthesis in Brief
1.3. Polymeric Materials Behavior in the Fire: PLA Exacts
1.3.1. Principle of Polymeric Materials That Resist Fire
Physical Action
Chemical Action
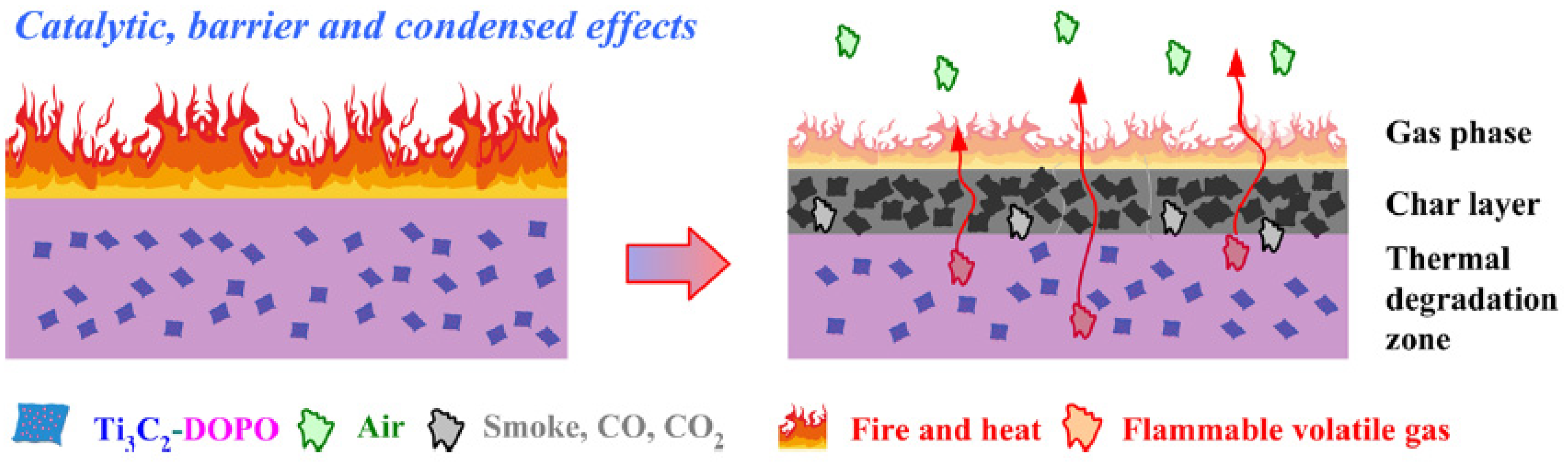
1.3.2. The Gas-Phase Mechanisms
1.3.3. The Condensed-Phase Mechanism
1.3.4. FR Mechanism of PLA-2D NPs NCP in Brief
2. Processing of PLA-2D NPs NCPs
2.1. MCD/Intercalation
2.2. In Situ Polymerization
2.3. Solution Intercalation/Casting
2.4. Electrospinning
2.5. Melt Spinning
3. PLA/2D NPs FR Materials’ Trend and Advances
4. 2D Flame Retarding NPs as FRs in PLA Composite Systems
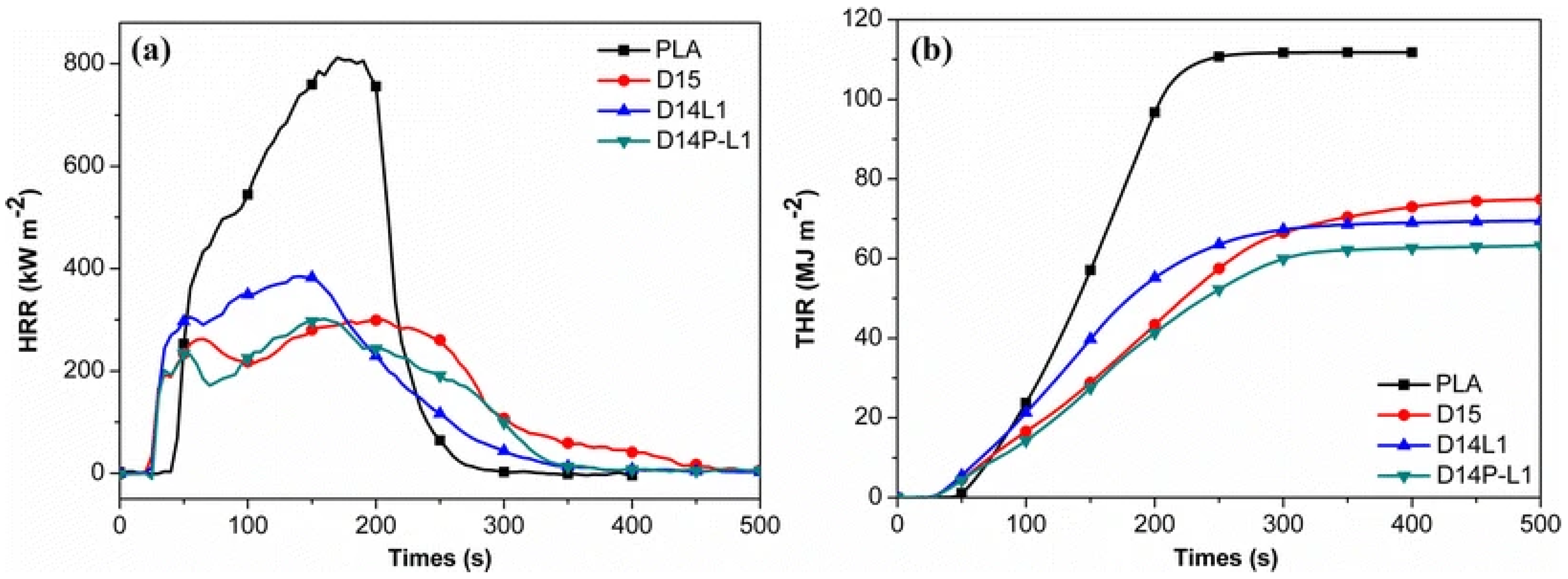
4.1. Nanoclays (NCs)
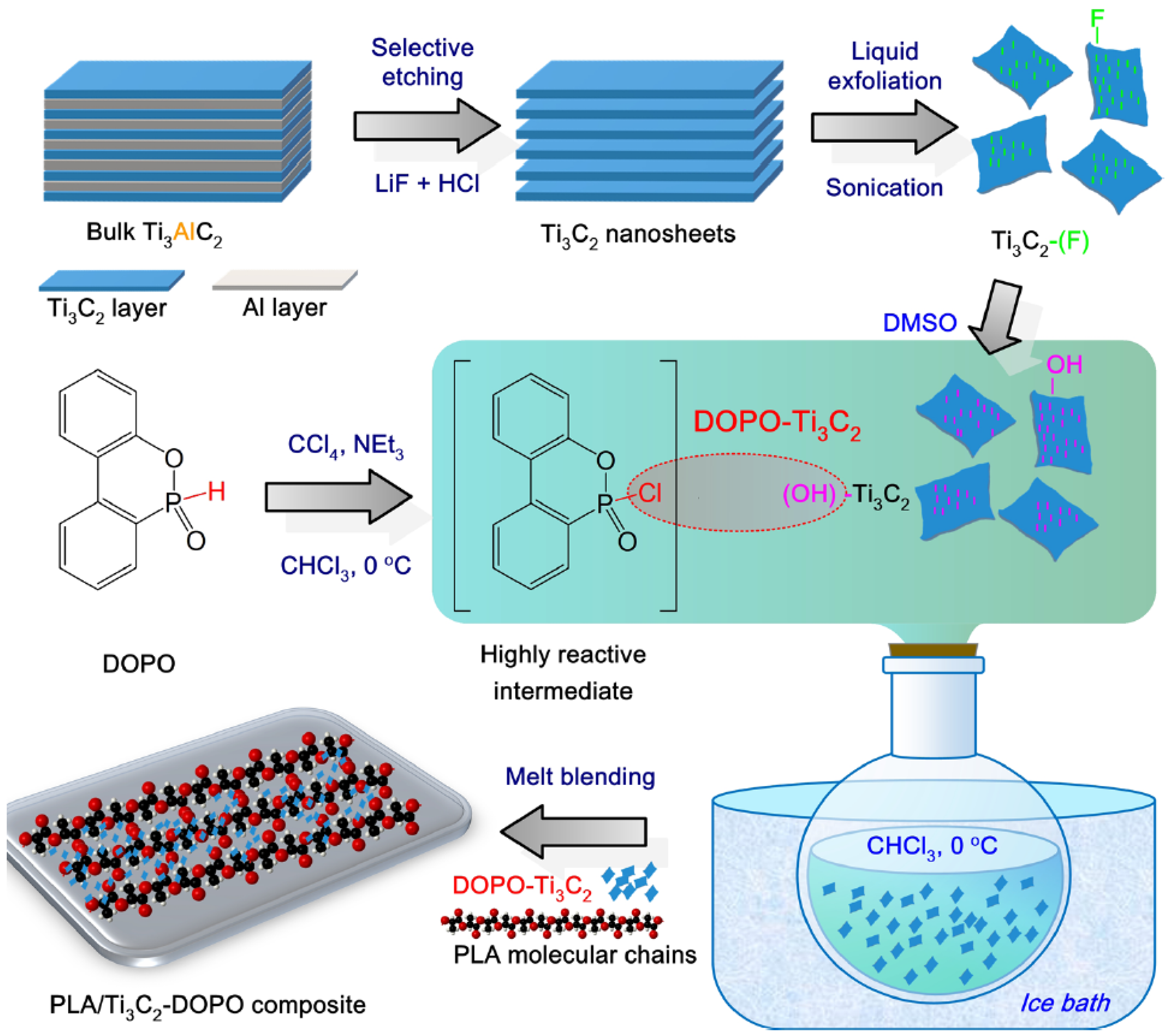
4.2. MXene
4.3. Graphene-Based NPs
4.3.1. Graphene
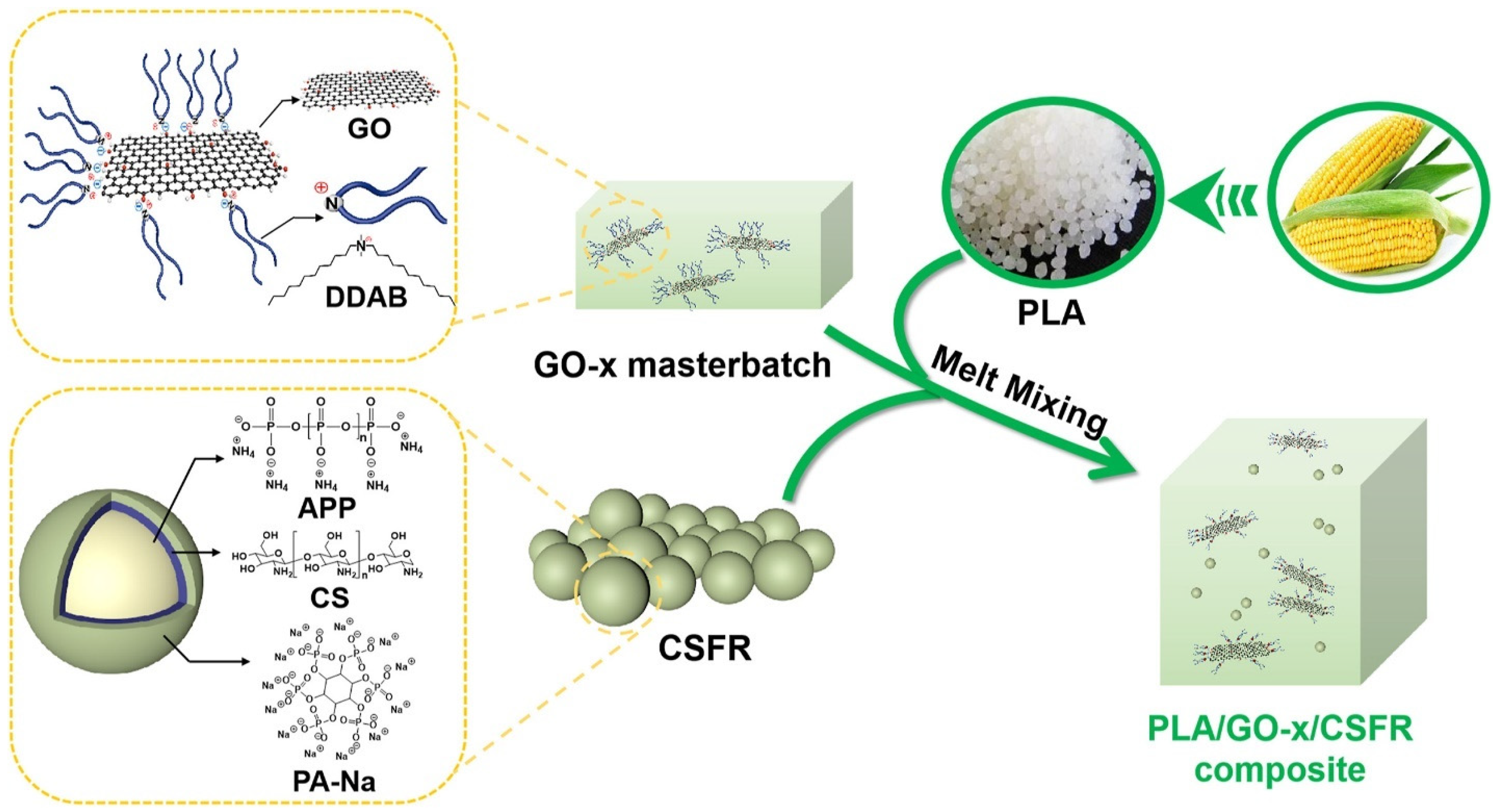
4.3.2. GO
4.3.3. rGO
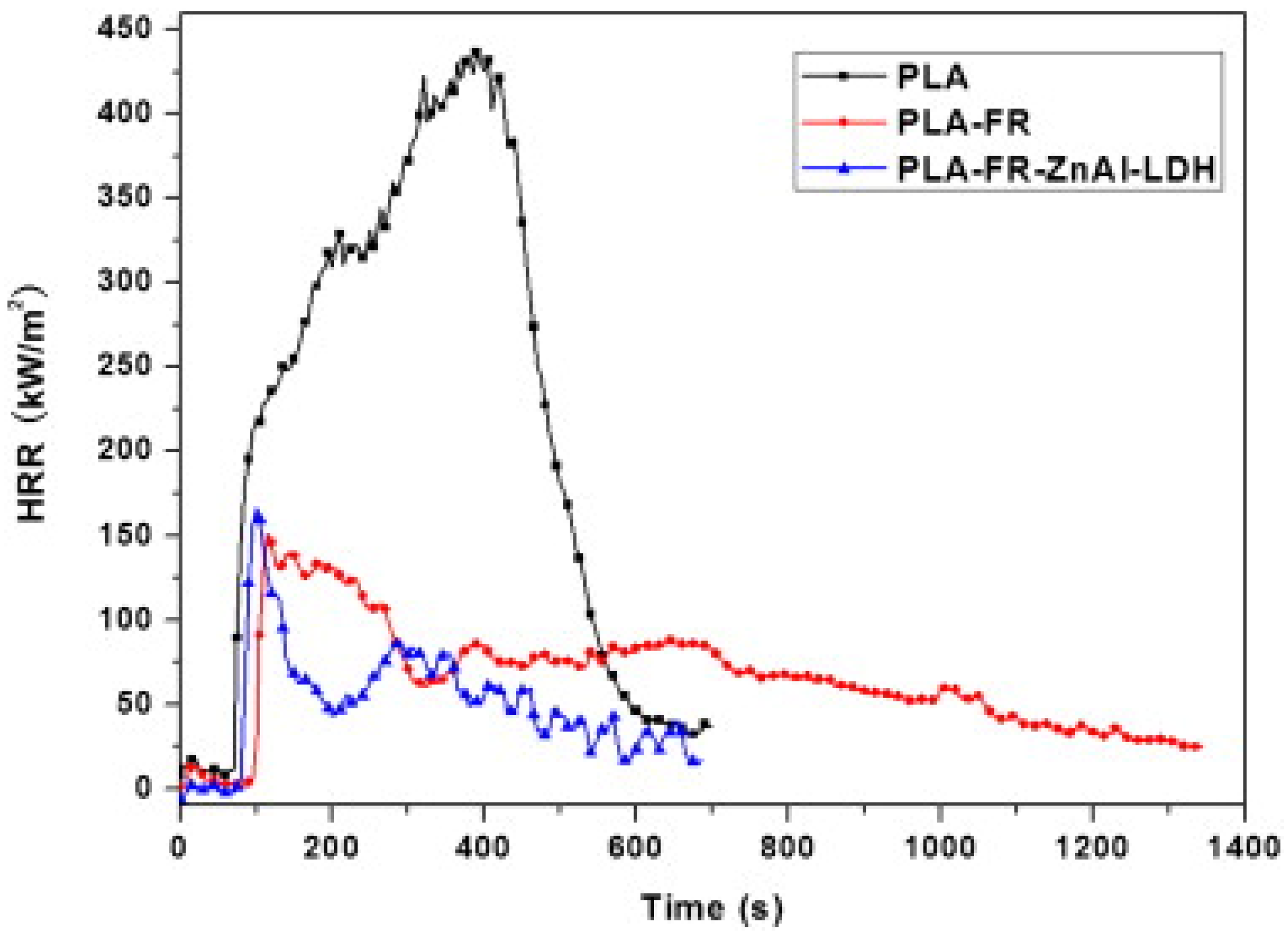
4.4. LDH
4.5. Others
5. Applications
6. Challenges
7. Conclusions and Prospect
7.1. Conclusions
7.2. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tawiah, B.; Yu, B.; Fei, B. Advances in Flame Retardant Poly(Lactic Acid). Polymers 2018, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- SpecialChem. Bio-Based Polymers Market to Grow at a CAGR of 14% by 2027. Available online: https://omnexus.specialchem.com/news/industry-news/bio-based-polymer-market-2027-000230145 (accessed on 19 June 2023).
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silicate nanocomposites. 2. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer 2003, 44, 857–866. [Google Scholar]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. The effect of different organic modified montmorillonites (OMMTs) on the thermal properties and flammability of PLA/MCAPP/lignin systems. J. Appl. Polym. Sci. 2013, 127, 4967–4973. [Google Scholar] [CrossRef]
- Tong, X.-Z.; Song, F.; Li, M.-Q.; Wang, X.-L.; Chin, I.-J.; Wang, Y.-Z. Fabrication of graphene/polylactide nanocomposites with improved properties. Compos. Sci. Technol. 2013, 88, 33–38. [Google Scholar] [CrossRef]
- Malkappa, K.; Bandyopadhyay, J.; Ray, S.S. Thermal Degradation Characteristic and Flame Retardancy of Polylactide-Based Nanobiocomposites. Molecules 2018, 23, 2648. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, J.; Wu, P. Polypropylene-grafted graphene oxide sheets as multifunctional compatibilizers for polyolefin-based polymer blends. J. Mater. Chem. 2012, 22, 14997–15005. [Google Scholar] [CrossRef]
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649. [Google Scholar] [CrossRef]
- Bioplastics, E. Continued Growth: Global Production Capacities of Bioplastics 2022–2027. Available online: https://docs.european-bioplastics.org/publications/market_data/2022/Report_Bioplastics_Market_Data_2022_short_version.pdf (accessed on 19 June 2023).
- Liu, P.; Zheng, Z.; Xu, Q.; Qian, Z.; Liu, J.; Ouyang, J. Valorization of dairy waste for enhanced D-lactic acid production at low cost. Process Biochem. 2018, 71, 18–22. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Klupsaite, D. Application of acid tolerant Pedioccocus strains for increasing the sustainability of lactic acid production from cheese whey. LWT-Food Sci. Technol. 2016, 72, 399–406. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.-S.; Nguyen, T.N.; Kim, S.K.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.-C. Production of L-and D-lactic acid from waste Curcuma longa biomass through simultaneous saccharification and cofermentation. Bioresour. Technol. 2013, 146, 35–43. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, I.; Ladero, M.; Santos, V. Production of d-lactic acid by Lactobacillus delbrueckii ssp. delbrueckii from orange peel waste: Techno-economical assessment of nitrogen sources. Appl. Microbiol. Biotechnol. 2018, 102, 10511–10521. [Google Scholar] [CrossRef]
- Pleissner, D.; Neu, A.-K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, R.; Schneider, R.; Hoss Lunelli, B.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. A simple biorefinery concept to produce 2G-lactic acid from sugar beet pulp (SBP): A high-value target approach to valorize a waste stream. Molecules 2020, 25, 2113. [Google Scholar] [CrossRef]
- Helmes, R.J.; López-Contreras, A.M.; Benoit, M.; Abreu, H.; Maguire, J.; Moejes, F.; van den Burg, S.W. Environmental impacts of experimental production of lactic acid for bioplastics from Ulva spp. Sustainability 2018, 10, 2462. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Sukumara, S.; Laurent, A.; Fantke, P. Environmental hotspots of lactic acid production systems. GCB Bioenergy 2020, 12, 19–38. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Storti, G.; Lattuada, M. Synthesis of bioresorbable polymers for medical applications. In Bioresorbable Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 153–179. [Google Scholar]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Selatile, M.K.; Ojijo, V.; Sadiku, R.; Ray, S.S. Development of bacterial-resistant electrospun polylactide membrane for air filtration application: Effects of reduction methods and their loadings. Polym. Degrad. Stab. 2020, 178, 109205. [Google Scholar] [CrossRef]
- Solarski, S.; Ferreira, M.; Devaux, E.; Fontaine, G.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Da Silva Ferreira, A. Designing polylactide/clay nanocomposites for textile applications: Effect of processing conditions, spinning, and characterization. J. Appl. Polym. Sci. 2008, 109, 841–851. [Google Scholar] [CrossRef]
- Ghosh, A.; Orasugh, J.T.; Ray, S.S.; Chattopadhyay, D. Integration of 3D Printing–Coelectrospinning: Concept Shifting in Biomedical Applications. ACS Omega 2023, 8, 28002–28025. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Macskásy, H.; Palyi, G. Plastics: Their Behaviour in Fires; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire Retard. Mater. 2001, 1, 31–68. [Google Scholar]
- Shan, X.; Hu, Y.; Chen, H.; Yuan, X. Study on thermal property, flame retardancy and mechanism of poly (lactic acid)/intumescent flame retardant/NiAl layer double hydroxide nanocomposites. Sci. Adv. Mater. 2015, 7, 1848–1857. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.; Dai, X.; Huo, C.; Xie, J.; Li, X.; Wang, X. Improving the crystallization and fire resistance of poly (lactic acid) with nano-ZIF-8@ GO. J. Mater. Sci. 2018, 53, 7083–7093. [Google Scholar] [CrossRef]
- Chow, W.S.; Teoh, E.L. Flexible and flame resistant poly(lactic acid)/organomontmorillonite nanocomposites. J. Appl. Polym. Sci. 2015, 132, 41253. [Google Scholar] [CrossRef]
- Fukushima, K.; Murariu, M.; Camino, G.; Dubois, P. Effect of expanded graphite/layered-silicate clay on thermal, mechanical and fire retardant properties of poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1063–1076. [Google Scholar] [CrossRef]
- Homa, P.; Wenelska, K.; Mijowska, E. Enhanced thermal properties of poly (lactic acid)/MoS2/carbon nanotubes composites. Sci. Rep. 2020, 10, 740. [Google Scholar] [CrossRef]
- Lewin, M.; Zhang, J.; Pearce, E.; Zammarano, M. Polyamide 6 treated with pentabromobenzyl acrylate and layered silicates. Polym. Adv. Technol. 2010, 21, 825–834. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, Y.; Tawiah, B.; Sun, J.; Yuen, R.K.K.; Fei, B. DOPO-Decorated Two-Dimensional MXene Nanosheets for Flame-Retardant, Ultraviolet-Protective, and Reinforced Polylactide Composites. ACS Appl. Mater. Interfaces 2021, 13, 21876–21887. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, C.; Zhang, H.; Zhang, Y.; Fang, Z.; Song, R.; Lin, Z.; Feng, J.; Song, P. Combination effects of a bio-based fire retardant and functionalized graphene oxide on a fire retardant and mechanical properties of polylactide. Mater. Today Chem. 2023, 30, 101565. [Google Scholar] [CrossRef]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The history of the science and technology of electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7, 155892501200702S155892501200710. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Ray, S.S. Graphene-Based Electrospun Fibrous Materials with Enhanced EMI Shielding: Recent Developments and Future Perspectives. ACS Omega 2022, 7, 33699–33718. [Google Scholar] [CrossRef] [PubMed]
- Vaia, R.A.; Giannelis, E.P. Lattice model of polymer melt intercalation in organically-modified layered silicates. Macromolecules 1997, 30, 7990–7999. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Ray, S.S.; Scriba, M.; Wesley-Smith, J. A combined experimental and theoretical approach to establish the relationship between shear force and clay platelet delamination in melt-processed polypropylene nanocomposites. Polymer 2014, 55, 2233–2245. [Google Scholar] [CrossRef]
- Treece, M.A.; Zhang, W.; Moffitt, R.D.; Oberhauser, J.P. Twin-screw extrusion of polypropylene-clay nanocomposites: Influence of masterbatch processing, screw rotation mode, and sequence. Polym. Eng. Sci. 2007, 47, 898–911. [Google Scholar] [CrossRef]
- Zhu, L.; Xanthos, M. Effects of process conditions and mixing protocols on structure of extruded polypropylene nanocomposites. J. Appl. Polym. Sci. 2004, 93, 1891–1899. [Google Scholar] [CrossRef]
- Poulesquen, A.; Vergnes, B. A study of residence time distribution in co-rotating twin-screw extruders. Part I: Theoretical modeling. Polym. Eng. Sci. 2003, 43, 1841–1848. [Google Scholar] [CrossRef]
- Bigio, D.; Pappas, W.; Brown II, H.; Debebe, B.; Dunham, W. SPE-ANTEC Tech. Papers. 2011. Available online: https://www.tib.eu/en/search/id/TIBKAT%3A666073457/69th-annual-technical-conference-of-the-Society/ (accessed on 1 July 2023).
- Urbanczyk, L.; Ngoundjo, F.; Alexandre, M.; Jérôme, C.; Detrembleur, C.; Calberg, C. Synthesis of polylactide/clay nanocomposites by in situ intercalative polymerization in supercritical carbon dioxide. Eur. Polym. J. 2009, 45, 643–648. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, H.X.; Chen, Z.K. Microstructure and thermal behavior of polylactide/clay nanocomposites melt compounded under supercritical CO2. Adv. Polym. Technol. 2011, 30, 174–182. [Google Scholar] [CrossRef]
- Sinha, R.S. Clay-Containing Polymer Nanocomposites: From Fundamentals to Real Applications; Newnes: Newton, MA, USA, 2013. [Google Scholar]
- Guo, Y.; Chang, C.-C.; Halada, G.; Cuiffo, M.A.; Xue, Y.; Zuo, X.; Pack, S.; Zhang, L.; He, S.; Weil, E.; et al. Engineering flame retardant biodegradable polymer nanocomposites and their application in 3D printing. Polym. Degrad. Stab. 2017, 137, 205–215. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Lu, H.; Hu, Y. Cobalt oxide/graphene composite for highly efficient CO oxidation and its application in reducing the fire hazards of aliphatic polyesters. J. Mater. Chem. 2012, 22, 3426–3431. [Google Scholar] [CrossRef]
- Ye, L.; Ren, J.; Cai, S.-y.; Wang, Z.-g.; Li, J.-b. Poly(lactic acid) nanocomposites with improved flame retardancy and impact strength by combining of phosphinates and organoclay. Chin. J. Polym. Sci. 2016, 34, 785–796. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Leuteritz, A.; Wang, Y.-Z.; Wagenknecht, U.; Heinrich, G. Preparation and burning behaviors of flame retarding biodegradable poly(lactic acid) nanocomposite based on zinc aluminum layered double hydroxide. Polym. Degrad. Stab. 2010, 95, 2474–2480. [Google Scholar] [CrossRef]
- Isitman, N.A.; Dogan, M.; Bayramli, E.; Kaynak, C. The role of nanoparticle geometry in flame retardancy of polylactide nanocomposites containing aluminium phosphinate. Polym. Degrad. Stab. 2012, 97, 1285–1296. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Zhao, X.; Li, X.; Gu, X.; Li, Y. Flame-retarding nanoparticles as the compatibilizers for immiscible polymer blends: Simultaneously enhanced mechanical performance and flame retardancy. J. Mater. Chem. A 2019, 7, 4903–4912. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Solarski, S.; Mahjoubi, F.; Ferreira, M.; Devaux, E.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Da silva Ferreira, A.; et al. (Plasticized) Polylactide/clay nanocomposite textile: Thermal, mechanical, shrinkage and fire properties. J. Mater. Sci. 2007, 42, 5105–5117. [Google Scholar] [CrossRef]
- Pluta, M. Morphology and properties of polylactide modified by thermal treatment, filling with layered silicates and plasticization. Polymer 2004, 45, 8239–8251. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Yu, T.; Yuan, W.; Ren, J. Flame-retardancy and anti-dripping effects of intumescent flame retardant incorporating montmorillonite on poly (lactic acid). Polym. Adv. Technol. 2009, 20, 1114–1120. [Google Scholar] [CrossRef]
- Long, L.; Yin, J.; He, W.; Xiang, Y.; Qin, S.; Yu, J. Synergistic effect of different nanoparticles on flame retardant poly(lactic acid) with bridged DOPO derivative. Polym. Compos. 2019, 40, 1043–1052. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Liu, C.; Wang, H.; Guo, J.; Fu, L.; Feng, Y.; Wang, L.; Yang, F.; Liu, M. Engineering titanium carbide ultra-thin nanosheets for enhanced fire safety of intumescent flame retardant polylactic acid. Compos. Part B Eng. 2022, 236, 109792. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Orasugh, J.T.; Temane, L.T.; Ray, S.S. Application of MXenes in Water Purification, CO2 Capture and Conversion. In Two-Dimensional Materials for Environmental Applications; Kumar, N., Gusain, R., Sinha Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 17–74. [Google Scholar]
- Huang, H.; Dong, D.; Li, W.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Synergistic effect of MXene on the flame retardancy and thermal degradation of intumescent flame retardant biodegradable poly (lactic acid) composites. Chin. J. Chem. Eng. 2020, 28, 1981–1993. [Google Scholar] [CrossRef]
- Temane, L.T.; Orasugh, J.T.; Ray, S.S. Adsorptive Removal of Pollutants Using Graphene-based Materials for Water Purification. In Two-Dimensional Materials for Environmental Applications; Kumar, N., Gusain, R., Sinha Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 179–244. [Google Scholar]
- Tawiah, B.; Yu, B.; Yuen, R.K.K.; Hu, Y.; Wei, R.; Xin, J.H.; Fei, B. Highly efficient flame retardant and smoke suppression mechanism of boron modified graphene Oxide/Poly(Lactic acid) nanocomposites. Carbon 2019, 150, 8–20. [Google Scholar] [CrossRef]
- Khalili, P.; Tshai, K.; Kong, I. Natural fiber reinforced expandable graphite filled composites: Evaluation of the flame retardancy, thermal and mechanical performances. Compos. Part A Appl. Sci. Manuf. 2017, 100, 194–205. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Cai, W.; Qiu, S.; Hu, Y.; Liew, K.M. Studies on Synthesis of Electrochemically Exfoliated Functionalized Graphene and Polylactic Acid/Ferric Phytate Functionalized Graphene Nanocomposites as New Fire Hazard Suppression Materials. ACS Appl. Mater. Interfaces 2016, 8, 25552–25562. [Google Scholar] [CrossRef]
- Wei, Z.; Cai, C.; Huang, Y.; Wang, P.; Song, J.; Deng, L.; Fu, Y. Eco-friendly strategy to a dual-2D graphene-derived complex for poly (lactic acid) with exceptional smoke suppression and low CO2 production. J. Clean. Prod. 2021, 280, 124433. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, X.; Zhan, Y.; Wang, Y.; Wang, X. Improving the flame retardancy of poly(lactic acid) using an efficient ternary hybrid flame retardant by dual modification of graphene oxide with phenylphosphinic acid and nano MOFs. J. Hazard. Mater. 2020, 384, 121260. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Tang, X.; Li, X.; Peng, M.; Fang, Z. Combination of a bio-based polyphosphonate and modified graphene oxide toward superior flame retardant polylactic acid. RSC Adv. 2018, 8, 4304–4313. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Fang, Z.-P.; Wang, D.-Y. Core-shell flame retardant/graphene oxide hybrid: A self-assembly strategy towards reducing fire hazard and improving toughness of polylactic acid. Compos. Sci. Technol. 2018, 165, 161–167. [Google Scholar] [CrossRef]
- Ran, S.; Fang, F.; Guo, Z.; Song, P.; Cai, Y.; Fang, Z.; Wang, H. Synthesis of decorated graphene with P, N-containing compounds and its flame retardancy and smoke suppression effects on polylactic acid. Compos. Part B Eng. 2019, 170, 41–50. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Hedayati, K.; Khaleghi, M.; Khonakdar, H.A. New ternary PLA/organoclay-hydrogel nanocomposites: Design, preparation and study on thermal, combustion and mechanical properties. Mater. Des. 2016, 110, 811–820. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Shih, Y.-F. Flame-retardant recycled bamboo chopstick fiber-reinforced poly(lactic acid) green composites via multifunctional additive system. J. Taiwan Inst. Chem. Eng. 2016, 65, 452–458. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Yu, C.-B.; Guo, W.; Wang, S.-F.; Chuang, T.-H.; Lin, Y.-H. Thermal properties and flammability of polylactide nanocomposites with aluminum trihydrate and organoclay. Carbohydr. Polym. 2012, 87, 1119–1123. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T. Multiwalled carbon nanotubes and sepiolite nanoclays as flame retardants for polylactide and its natural fibre reinforced composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 954–963. [Google Scholar] [CrossRef]
- Carretier, V.; Delcroix, J.; Pucci, M.F.; Rublon, P.; Lopez-Cuesta, J.-M. Influence of Sepiolite and Lignin as Potential Synergists on Flame Retardant Systems in Polylactide (PLA) and Polyurethane Elastomer (PUE). Materials 2020, 13, 2450. [Google Scholar] [CrossRef]
- Rabelo, L.H.; Munhoz, R.A.; Marini, J.; Maestrelli, S.C. Development and Characterization of PLA Composites with High Contents of a Brazilian Refractory Clay and Improved Fire Performance. Mater. Res. 2021, 25, e20210444. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Shi, Y.; Liu, M.; Yao, A.; Feng, Y.; Fu, L.; Lv, Y.; Yang, F.; Yu, B. Supramolecular engineered ultrathin MXene towards fire safe polylactic acid composites. Compos. Commun. 2023, 37, 101405. [Google Scholar] [CrossRef]
- Shan, X.; Song, L.; Xing, W.; Hu, Y.; Lo, S. Effect of Nickel-Containing Layered Double Hydroxides and Cyclophosphazene Compound on the Thermal Stability and Flame Retardancy of Poly(lactic acid). Ind. Eng. Chem. Res. 2012, 51, 13037–13045. [Google Scholar] [CrossRef]
- Jin, X.; Gu, X.; Chen, C.; Tang, W.; Li, H.; Liu, X.; Bourbigot, S.; Zhang, Z.; Sun, J.; Zhang, S. The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 12235–12250. [Google Scholar] [CrossRef]
- Ding, P.; Kang, B.; Zhang, J.; Yang, J.; Song, N.; Tang, S.; Shi, L. Phosphorus-containing flame retardant modified layered double hydroxides and their applications on polylactide film with good transparency. J. Colloid Interface Sci. 2015, 440, 46–52. [Google Scholar] [CrossRef]
- Das, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Nanocomposites based on chloroprene rubber: Effect of chemical nature and organic modification of nanoclay on the vulcanizate properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar] [CrossRef]
- Cai, G.; Dasari, A.; Yu, Z.-Z.; Du, X.; Dai, S.; Mai, Y.-W.; Wang, J. Fire response of polyamide 6 with layered and fibrillar nanofillers. Polym. Degrad. Stab. 2010, 95, 845–851. [Google Scholar] [CrossRef]



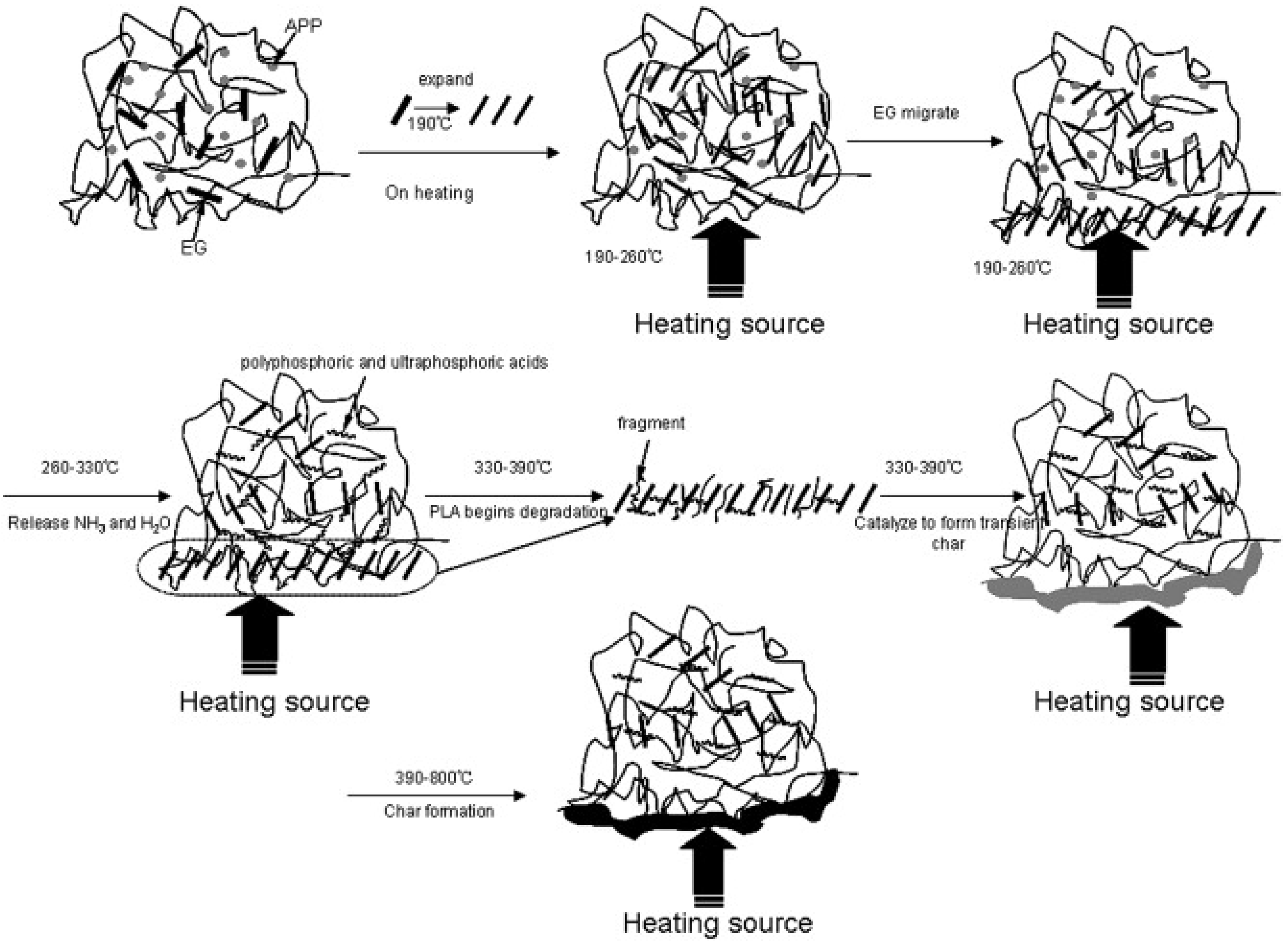
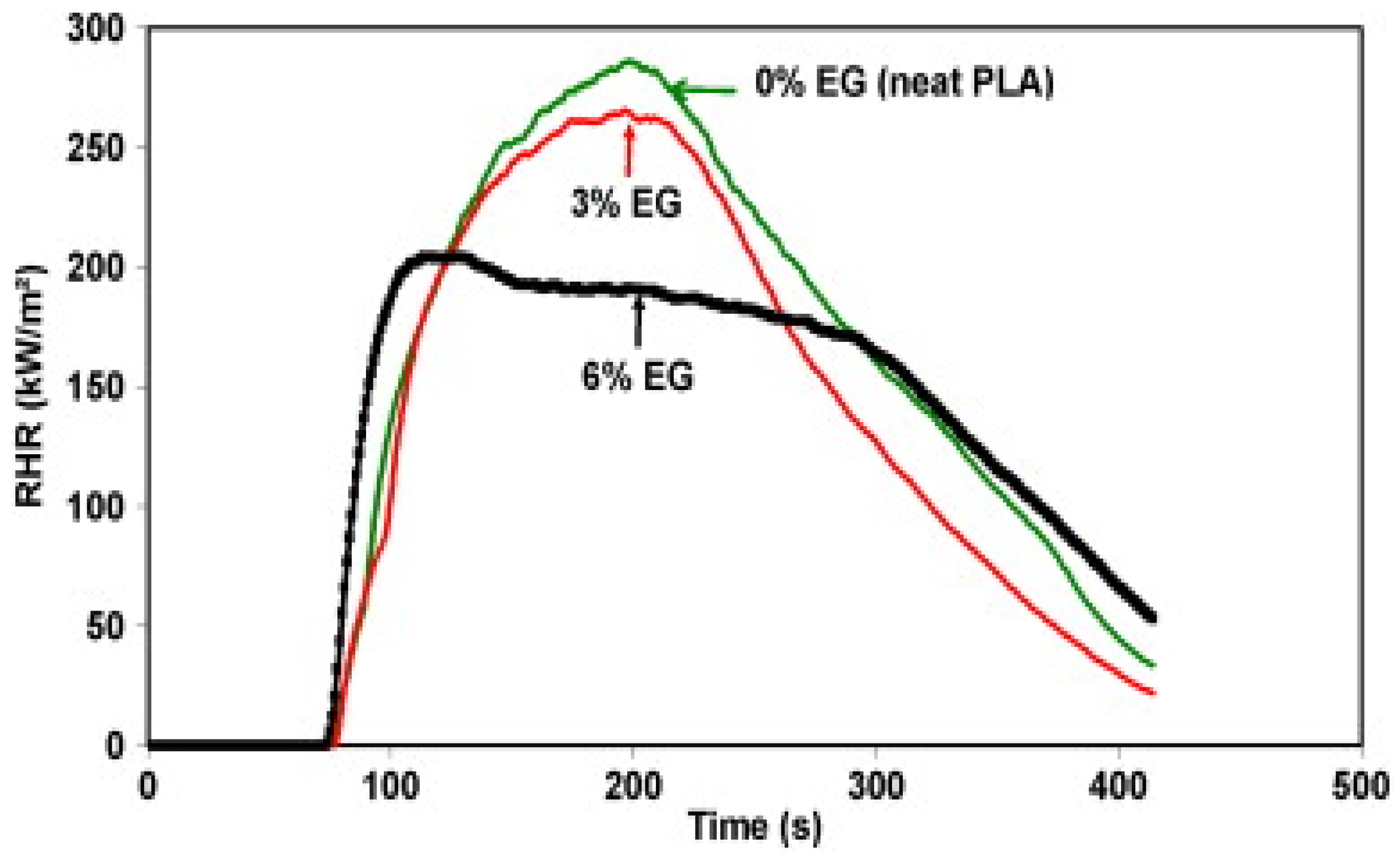

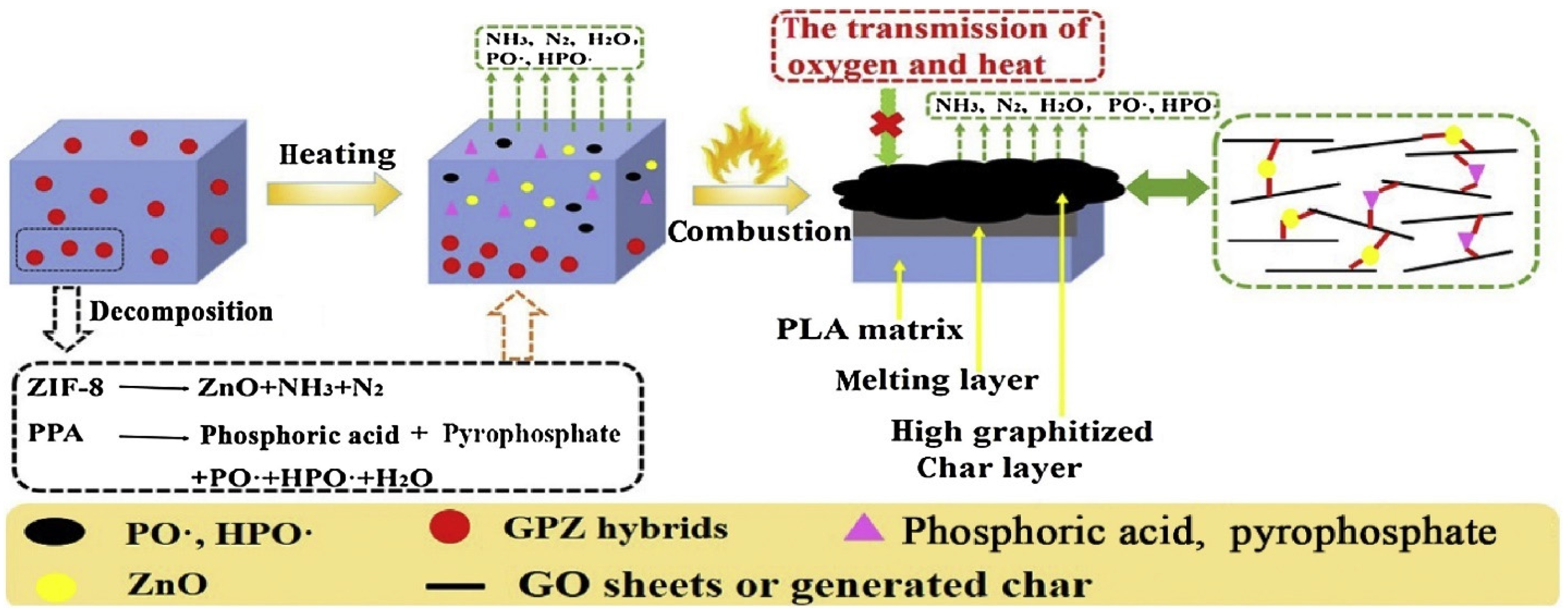

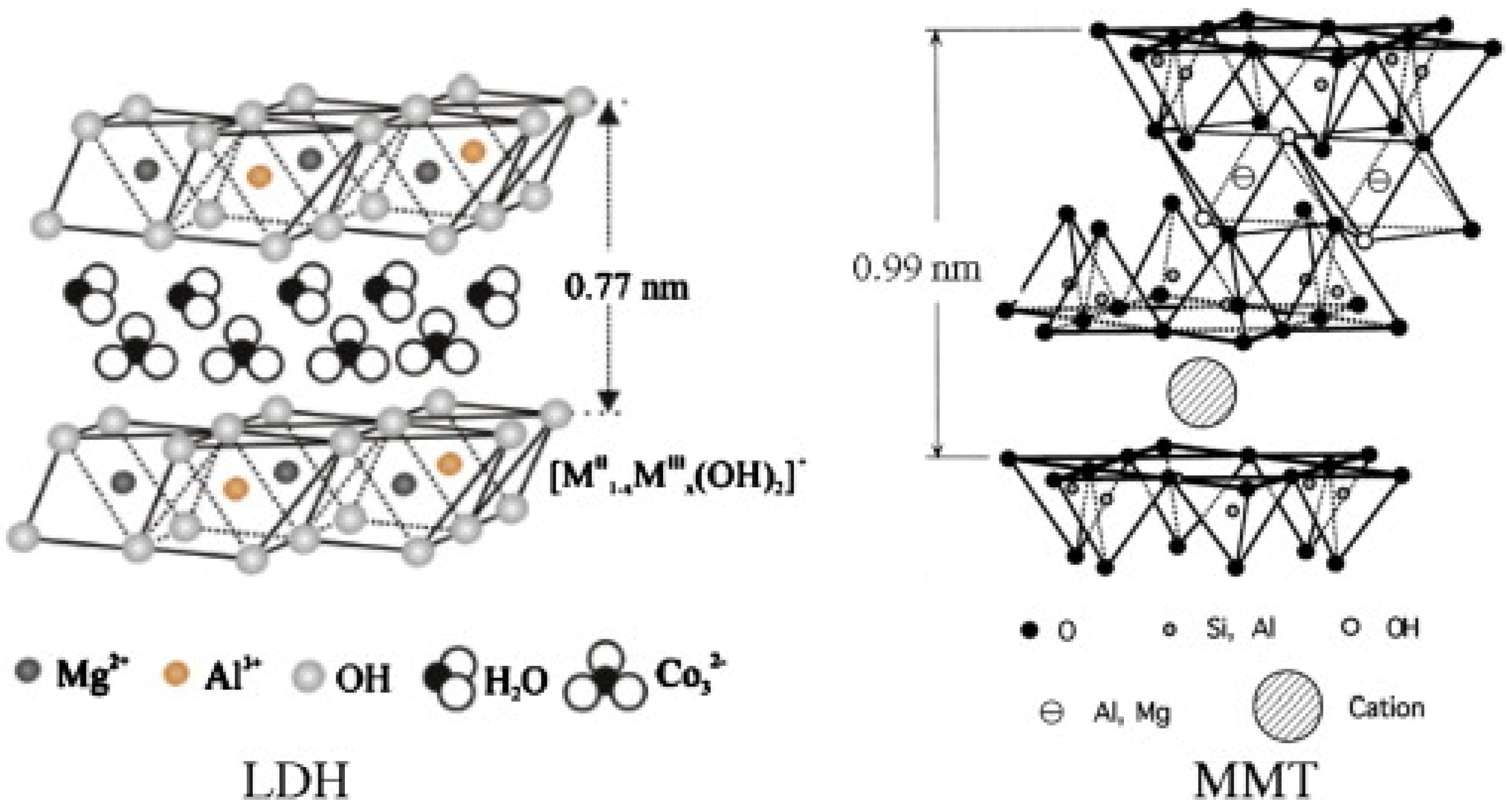

| Feedstock Class | Feedstock Source |
|---|---|
| 1st generation | Potatoes, rice, wheat, sugar cane, corn, and sugar beet. |
| 2nd generation | Switch grass, corn straw, palm fruit branches, sugar cane bagasse, wheat straw, and wood. |
| 3rd generation | Algae-based biomass, food industry by-products, industrial waste, municipal waste. |
| Sample | Flame to Holding Clamp | Flame Dripping | Ignited Cotton | Residual Mass (%) | Observations |
|---|---|---|---|---|---|
| PLA | No | Yes | Yes | 64 (±4) | Intense dripping |
| PLA-3C30B | Yes | Yes | Yes | 26 (±1) | Burning with drips, charring |
| PLA-3EG | Yes | Yes | Yes | 31 (±5) | Burning with drips, charring |
| PLA-6EG | Yes | Yes | Yes | 31 (±5) | |
| PLA-9EG | Yes | Yes | Yes | 35 (±5) | |
| PLA–3C30B–3EG | Yes | No | Yes | 40 (±2) | Compact char a |
| PLA–3C30B–6EG | Yes | No | No | 55 (±1) | Compact char |
| PLA–3C30B–9EG | Yes | No | No | 58 (±5) | Compact char |
| PLA Formulation | Processing Method | 2D NMTs-Based FR | 2D FR wt.% | 2D FR Aspect Ratio | LOI % | UL94 | Calorimetry Parameters (CCP) | TGA Data | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pHRR Reduction % | OIT (°C) | T5% (°C) | Residue %/Max. T (°C) | ||||||||
| GR/graphene | |||||||||||
| PLA/Ferric Phytate-graphene | MCD + CM | PLA/graphene | 3 | High | - | - | 40 | - | - | ~5 (600 °C), N2 | [69] |
| PLA/EG | MCD + CM | PLA/EG | 12 | High | - | HB | 30 | - | 347 | -(600 °C), air | [67] |
| PLA/APP/EG | MCD + CM | PLA/EG | 11.25 | - | 36.5 | V-0 | 38.3 | - | 322.9 | 9.3 (800 °C), N2 | [68] |
| PLA/f-graphene | MCD + CM | PLA/graphene | 3 | High | - | - | 40 | - | - | ~2 (600 °C), N2 | [69] |
| GO/GIO | |||||||||||
| PLA/GO-3/4%CSFR | MCD + IM | PLA/GO | 6.6 | - | 29.7 | V-0 | 14.8 | - | 329.6 | 3.4 (800 °C), N2 | [37] |
| PLA/polyphosphonate (BPPT) and polyethyleneimine-GO (M-GO) (PLA/2.4BPPT/0.6M-GO) | Solution mixing (SM)/casting + MC | PLA/GO | 0.6 | - | 36 | V-0 | 2.3 | - | 327 | 1.9 (600 °C), N2 | [72] |
| PLA-4 | SM/intercalation & film casting (FCT) | PLA/GPZ | 2 of GPZ | - | 27 | V-2 | 39.4 | - | - | 3.5 (600 °C), N2 | [71] |
| PLA/Co3O4/graphene | MCD | PLA/graphene | 0.5 | - | - | - | 40 | - | - | - | [50] |
| PLA/ZIF-8@GO, 0.5 wt.% (PLA-2) | SM/intercalation & film casting (FCT) | PLA/GO | 0.5 | High | 24.0 | V-2 | - | - | - | - | [31] |
| PLA/graphene-hybrid (PLA/15%GOH) | PLA/graphene | 15 | - | - | V-0 | 47 | - | - | - | [73] | |
| rGO | |||||||||||
| rGO-AZOB (azo-boron (AZOB))/SMB (sodium metaborate (SMB))/PLA | SM + CM | PLA/rGO | 0.5 | - | 31.2 | V-0 | 76.5 | - | 265.3 | 9.81 (700 °C), N2 | [65] |
| TRG/PLA | SM + CM | PLA/TrGO | 10 | - | - | - | - | 197.9 | 274.0 | 44 (700 °C), N2 | [5] |
| TRG/PLA/Py-PLA-OH | SM + CM | PLA/TrGO | 10 | - | - | - | - | 204.2 | 304.7 | 39 (700 °C), N2 | [5] |
| PLA-CO3O4/rGO | MCD | PLA/rGO | 0.5–1 | - | - | - | 40 | - | 300 and 450 | - | [50] |
| PLA/rGO | MCD + CM | PLA/rGO | 2 | - | - | - | 14.9 | - | 41.7 (750 °C), N2 | [74] | |
| PLA/LDH-rGO (PLA-PPC/5d2D-Gc) | SM/casting + CM | PLA/rGO | 2.5 | - | - | - | 50.24 | - | - | - | [70] |
| PLA-PBS/5d2D-Gc | SM/casting + CM | PLA:PBS/rGO | 2.5 | - | - | - | 29 | - | - | - | [70] |
| Clays | |||||||||||
| PLA/aluminium diethylphosphinate (AlPi) O-MMT | MCD + CM | PLA/O-MMT | 5 | - | 28 | V-0 | 26.2 | - | 328 | 4.87 (700 °C) N2 | [51] |
| PLA/O-MMT/FR (PLA/O-MMT/FR30) | MCD + CM | PLA/O-MMT | 5 | - | - | V-0 | - | - | 258.4 | 4.75 (700 °C) N2 | [32] |
| PLA/O-MMT | SM + FCT | PLA/O-MMT | 3 | - | - | - | - | - | 325 | 3.66 (800 °C) N2 | [75] |
| PLA/O-MMT/bamboo fibres | MCD + CM | PLA/NC/recycles bamboo fibres | 6 | - | - | V-0 | - | - | 377.8 | 21.8 (850 °C) N2 | [76] |
| PLA/C30B | MCD | PLA-Methyl-tallow-bis(2-hydroxyethyl)-ammonium treated MMT (2 wt.%) | 4 | - | - | - | 38 | - | - | - | [56] |
| PLA/O-MMT/Aluminum trihydrate | MCD | PLA/O-MMT | 5 | - | 42 | V-0 | 65 | - | - | 320 (650 °C), air | [77] |
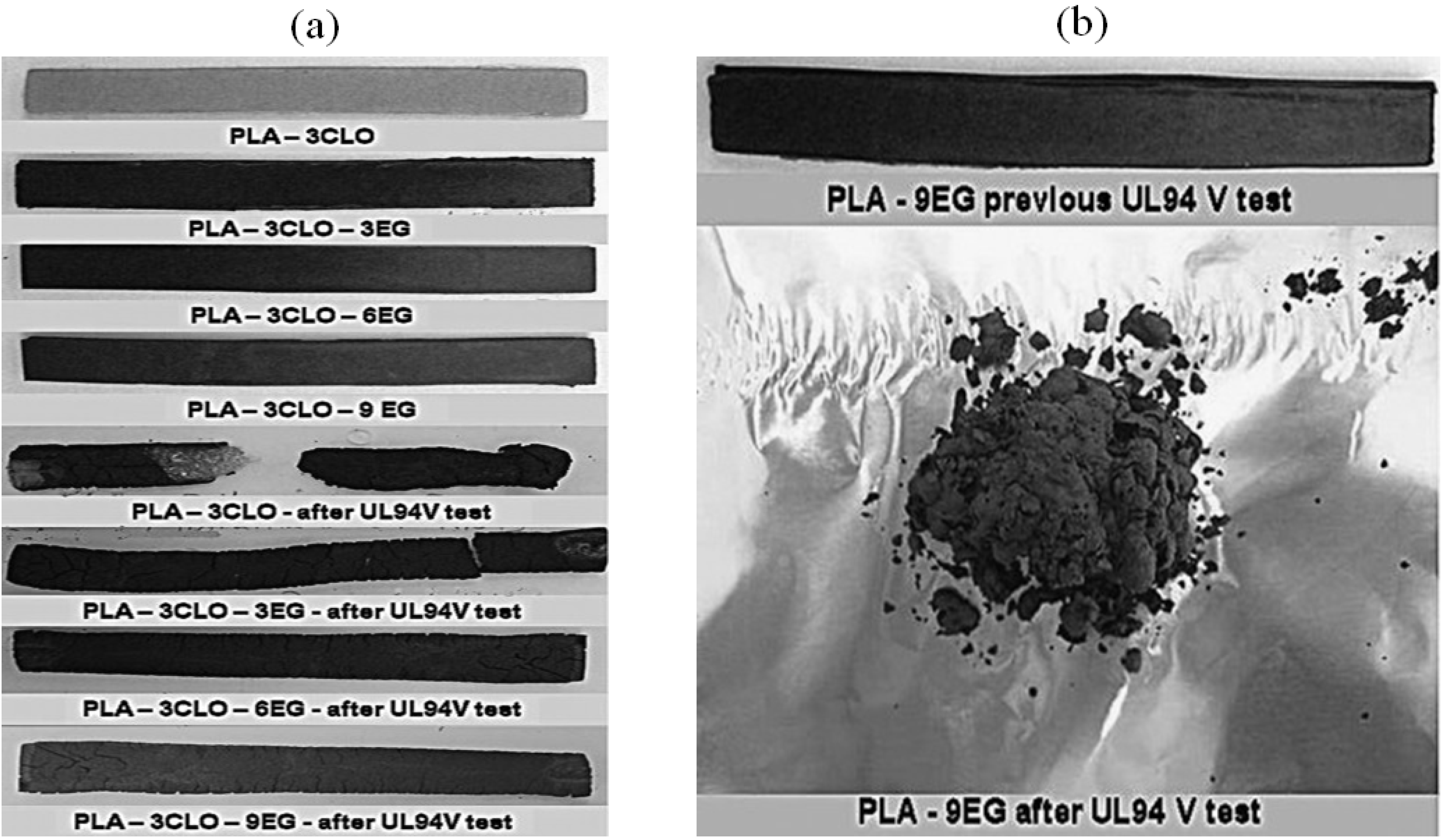
| PLA/3C30B | MCD | PLA/CL | 3 CL | 200–1500 | Limit | 355 | ≥0.5 (500 °C), N2 | [33] | |||
| PLA/3C30B/3EG | MCD | PLA/CL/EG | 3 CL & EG | 200–1500 | HB | 355 | <10 (500 °C), N2 | [33] | |||
| PLA/3C30B/6EG | MCD | PLA/CL/EG | 3 CL & 6 EG | 200–1500 | HB | 354 | ~10 (500 °C), N2 | [33] | |||
| PLA/3C30B/9EG | MCD | PLA/CL/EG | 3 CL & 9 EG | 200–1500 | HB | 353 | >11 (500 °C), N2 | [33] | |||
| PLA/4 wt.% B104 | MCD + melt spinning + knitting | PLA/CL | 4 | 100–500 | - | - | 46 | - | - | - | [24] |
| PLA/MWCNT-sepiolite NC (Sep) | MCD | PLA/MWCNT/Sep | 2 & 10 (MWCNT & Sep) | 100–300 | - | - | 45 | - | 334 | 11 (600 °C) | [78] |
| PLA/MWCNT-sepiolite NC-hemp fibres | MCD + CM | PLA/MWCNT/Sep-hemp fibres | 20 (MWCNT & Sep) + 30 vol.% hemp | 100–300 | - | - | 58 | - | 336 | 13 (600 °C), air | [78] |
| PLA/NC-Al diethylphosphinate (AlPi) | MCD + CM | PLA/NC | 20 | High | 27.5 | BC | 36 | - | 333 for T10% | 4.3 (600 °C) | [53] |
| PLA/NC | MCD + CM | PLA/NC | 20 | High | 23 | BC | 51 | - | 333 for T10% | 4.3 (600 °C) | [53] |
| PLA/Sepiolite/lignin (PLA-2) | MCD + IM | PLA/CL | 3 | High | - | - | 37 | - | 310 | 16.4 (900 °C), N2 | [79] |
| PLA/NC (PLA/15RC) | MCD + IM | PLA/NC | 15 | - | - | V-2 | - | - | 326 | 12.7 (750 °C), N2 | [80] |
| P17M1C | 3D printing | PLA/CL | 1 | - | 27 | V-0 | 42 | - | - | 5–10 (800 °C), N2 | [49] |
| MXenes | |||||||||||
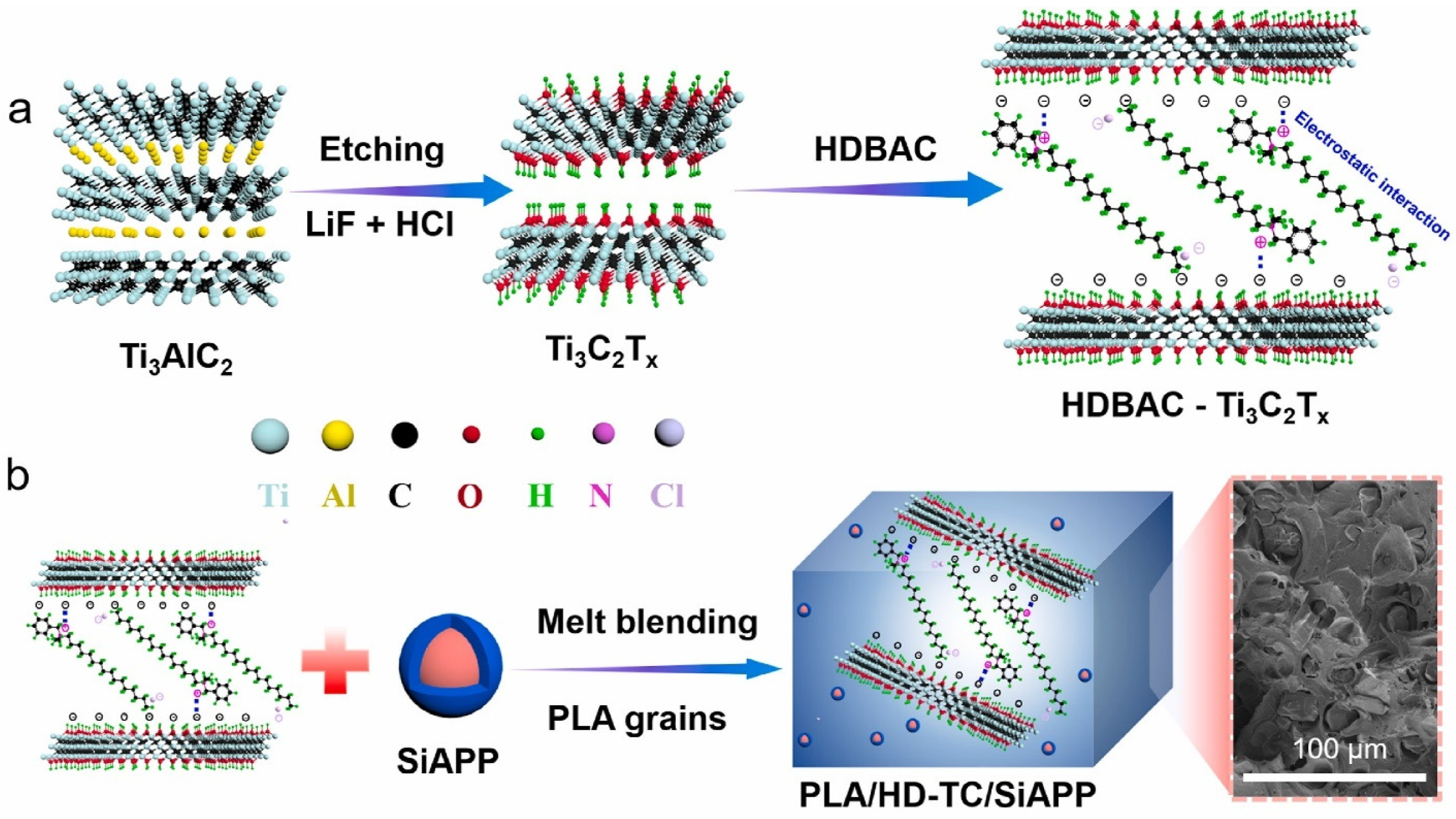
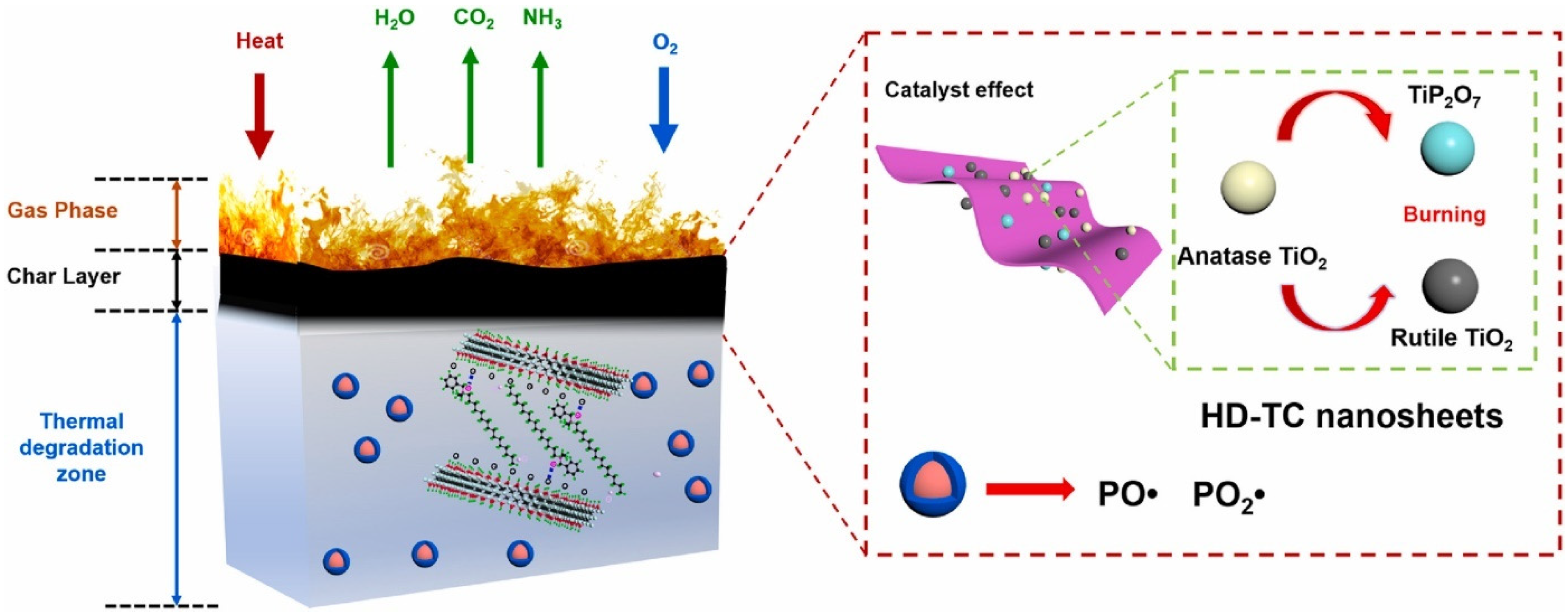
| PLA/MHCSN-Ti3C2Tx (MHCSN-TC)/SiAPP | MCD | PLA/MXene | 1 | - | 32.7 | V-0 | 64.1 | - | - | - | [81] |
| PLA/benzyldimethylhexadecylammonium chloride (HDBAC) modified Ti3C2Tx | MCD + CM | PLA/MXene | 15 | - | 33.3 | V-0 | 49.8%, 31.9%, 60.3% and 52.7% | - | 327 | 10.33 (700 °C) | [60] |
| PLA/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO)- MXene (Ti3C2) | MCD + CM | PLA/MXene | 3 | High | 33.7 | V-0 | - | - | - | - | [36] |
| PLA/IFR/MXene | MCD + CM | PLA/MXene | 0.5 | - | 33.0 | V-0 | 64.6 | - | - | - | [63] |
| LDH | |||||||||||
| PLA | MCD | PLA | - | - | - | - | (pHRR: 480 W/g) | - | - | 1.7 (700 °C) | [52] |
| PLA/FR-MgAl-LDH | MCD | PLA/LDH | 2 | - | - | - | 46 (pHRR: 199 W/g) | - | - | 1.7 (700 °C) | [52] |
| PLA/FR-ZnAl-LDH | MCD | PLA/LDH | 2 | - | - | - | 58.5 (pHRR: 259 W/g) | - | - | 58.5 (700 °C) | [52] |
| PLA/HPCP/LDH-SDS | MCD | PLA/LDH-SDS | 2NiFe, 2NiAl, & 2NiCr | - | 29 | V-0 | - | - | - | 4.1, 6.3, & 7.2 (700 °C) | [82] |
| PLA/LDH-15% DT-M (PLA/DT-M/PA–LDH) | MCD + CM | PLA/LDH | 1 | - | 38.9 | V-0 | 63 | - | 350 | 10.6 (700 °C) | [83] |
| PLA/LDH (LCP-10) | Solution exfoliation + FCT | PLA/LDH | 10 | - | - | - | 10 | - | 331.6 | <10 (600 °C) | [84] |
| PLA/NiAl LDH/Silane coated APP, pentaerythritol phosphate (PEPA) | MCD | PLA/NiAl LDH | 2.5 | - | 30 | V-0 | 21 | - | 315 | 17.3 (700 °C) | [30] |
| MoS2 | |||||||||||
| PLA/MoS2/M xOy/CNTs | MCD | PLA/MoS2 | ~0.67 | 0.02–0.16 | - | - | - | - | ~323 | 5–10 (800 °C), N2 | [34] |
| FR PLA-2D Material System | Electrical/Electronic | Automobile (AT)/Aerospace (AER) | Biomedical Materials | Furniture (FT)/Packaging (PK)/Building (BD) & Construction (CT) | Ref. |
|---|---|---|---|---|---|
| Graphene-based | |||||
| PLA/M-GO | ✓ | - | ✓ | ✓ | [72] |
| PLA/GO (PLA/GO-3/4%CSFR) | - | - | - | ✓ | [37] |
| PLA-GPZ | ✓ | - | - | - | [71] |
| PLA–C30B–EG | ✓ | ✓ | ✓ | ✓ | [33] |
| PLA/GO-3/4%CSFR | - | - | - | ✓ | [37] |
| PLA/15%GOH | - | - | ✓ | ✓ | [73] |
| Clay-based | |||||
| PLA/O-MMT | ✓ | - | - | - | [77] |
| P17M1C (PLA/MMT) | ✓ | ✓ | - | - | [49] |
| PLA/O-MMT | ✓ | ✓ | ✓ | ✓ | [53] |
| MXene-based | |||||
| PLA/MXene | ✓ | ✓ | ✓ | ✓ | [36] |
| PLA/MXene | ✓ | ✓ | ✓ | ✓ | [60] |
| LDH-based | |||||
| PLA/LDH | ✓ | ✓ | ✓ | ✓ | [52] |
| PLA/LDH | ✓ | ✓ | ✓ | ✓ | [84] |
| PLA/LDH | ✓ | ✓ | ✓ | ✓ | [82] |
| MoS2 | |||||
| PLA/MoS2/M xOy/CNTs | ✓ | ✓ | - | - | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temane, L.T.; Orasugh, J.T.; Ray, S.S. Recent Advances and Outlook in 2D Nanomaterial-Based Flame-Retardant PLA Materials. Materials 2023, 16, 6046. https://doi.org/10.3390/ma16176046
Temane LT, Orasugh JT, Ray SS. Recent Advances and Outlook in 2D Nanomaterial-Based Flame-Retardant PLA Materials. Materials. 2023; 16(17):6046. https://doi.org/10.3390/ma16176046
Chicago/Turabian StyleTemane, Lesego Tabea, Jonathan Tersur Orasugh, and Suprakas Sinha Ray. 2023. "Recent Advances and Outlook in 2D Nanomaterial-Based Flame-Retardant PLA Materials" Materials 16, no. 17: 6046. https://doi.org/10.3390/ma16176046
APA StyleTemane, L. T., Orasugh, J. T., & Ray, S. S. (2023). Recent Advances and Outlook in 2D Nanomaterial-Based Flame-Retardant PLA Materials. Materials, 16(17), 6046. https://doi.org/10.3390/ma16176046







