Effect of Variable Synthesis Conditions on the Formation of Ye’elimite-Aluminate-Calcium (YAC) Cement and Its Hydration in the Presence of Portland Cement (OPC) and Several Accessory Additives
Abstract
:1. Introduction
- C4A3Ŝ + 2CŜH2 + 34H→C6AŜ3H32 + 2AH3;
- C4A3Ŝ + 18H→C4AŜH12 + 2AH3;
- C4A3Ŝ + 8CŜ + 6CH + 34H→C6AŜ3H32;
- βC2S + AH3 + 5H → C2ASH8.
2. Materials and Methods
2.1. Sulphate Raw Materials
- CaSO4·2H2O ↔ CaSO4·0.5H2O + 1.5H2O;
- CaSO4·0.5H2O ↔ CaSO4 + 0.5H2O;
- CaSO4 ↔ CaO + SO2 + 0.5O2.
2.1.1. Carbonate Raw Materials
2.1.2. Aluminous Raw Materials
2.2. Preparation of Experimental Variants
- -
- Anhydrite: 2.17% H2O;
- -
- Rea-gypsum: 20.02% H2O;
- -
- Calcium carbonate: 31.2% H2O;
- -
- Aluminium hydroxide: 33.56% H2O.
- CaCO3 + rea-gypsum (CaSO4·2H2O)+ Al(OH)3—(variant I);
- CaCO3 + natural anhydrite (CaSO4) + Al(OH)3—(variant II).
2.3. Synthesis of the Samples
2.4. Hydration of Samples
2.5. XRD Measurements
2.6. Shrinkage and Expansion Measurements
2.7. Compressive Strength of Cement Pastes
3. Results and Discussion
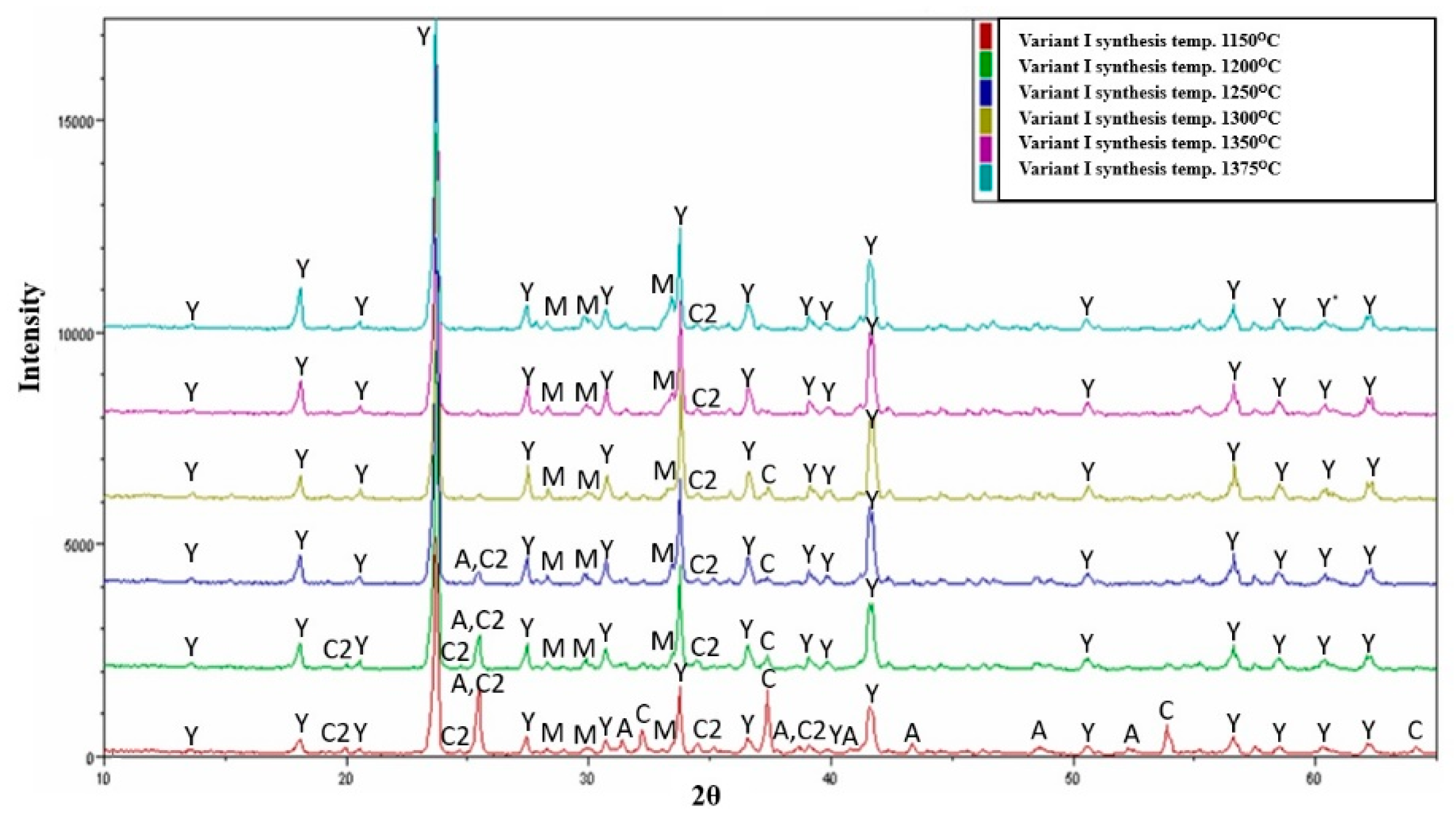
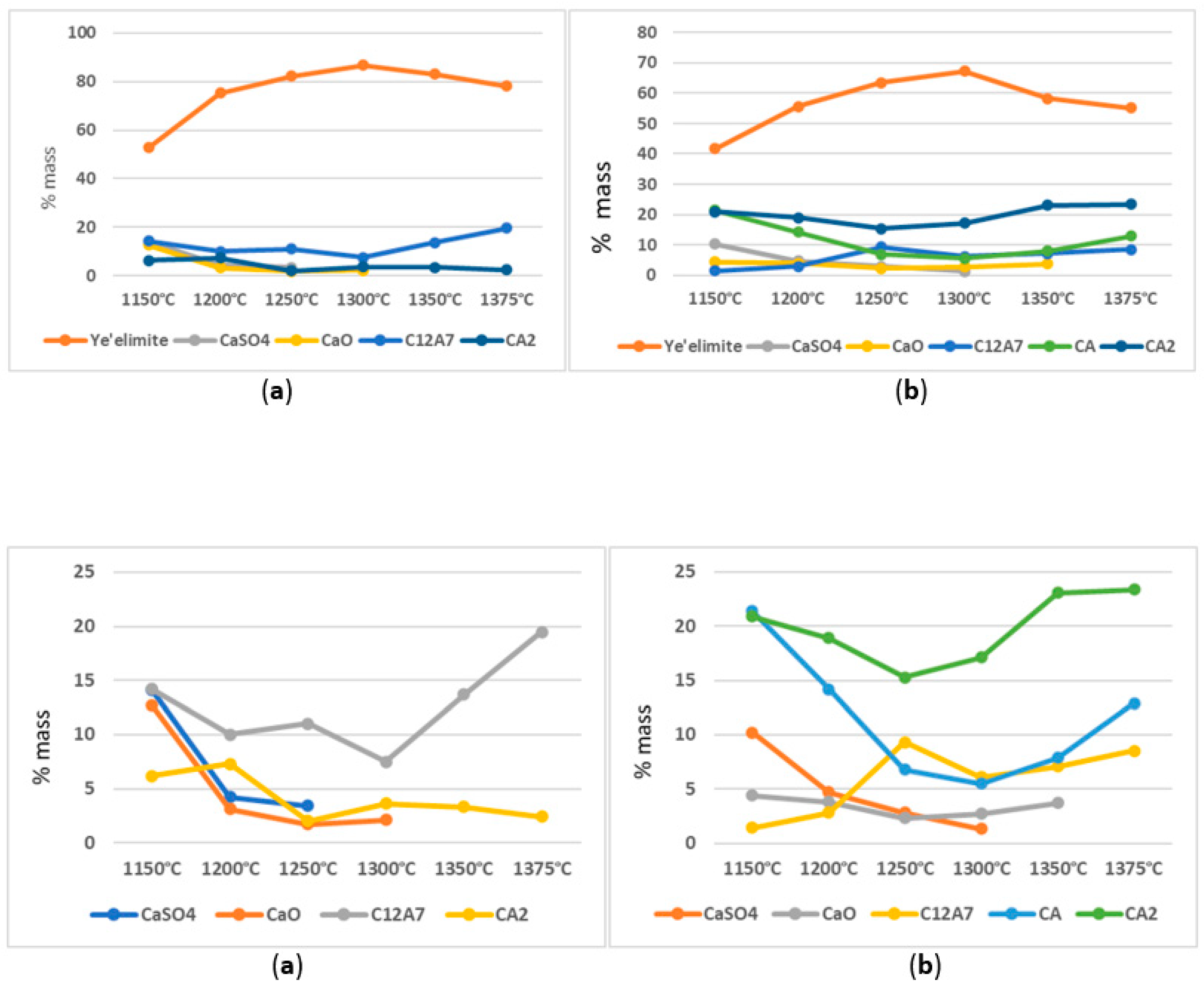
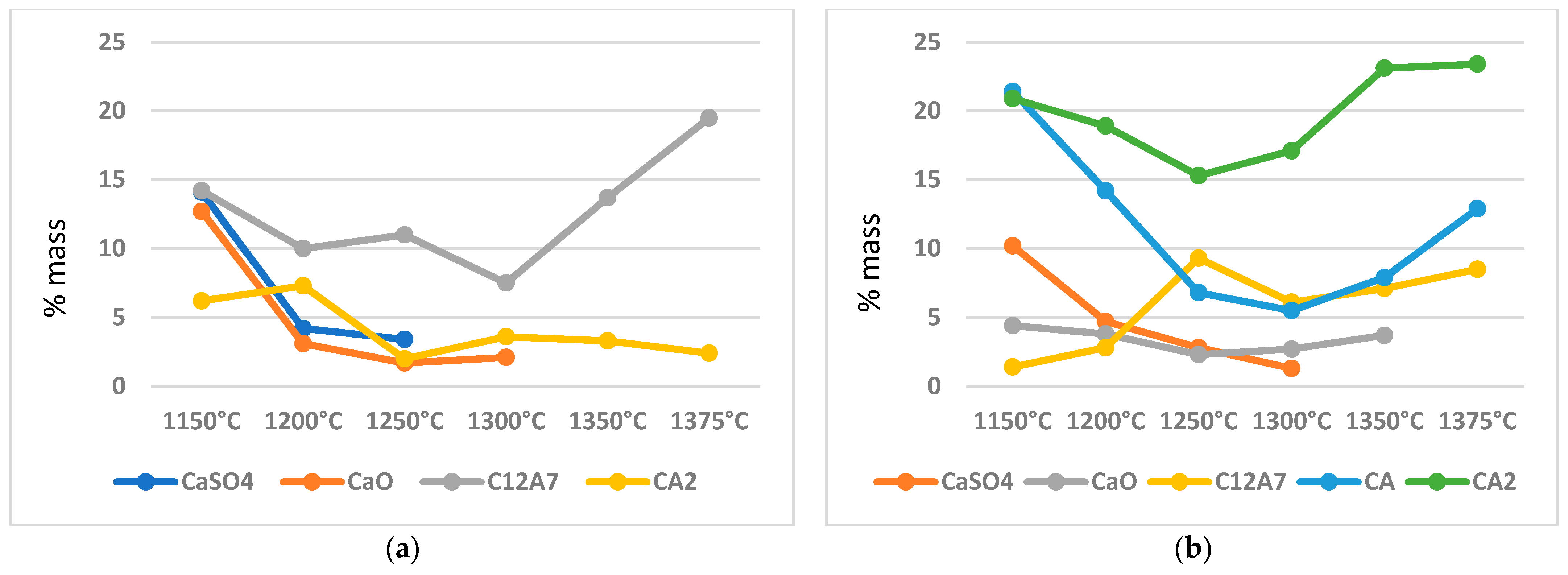
3.1. Variable Temperature Synthesis and Quantitative Analysis—Variant I
3.2. Variable Temperature Synthesis and Quantitative Analysis—Variant II
3.3. Comparison of the Degree of Synthesis and the Amount of Crystalline Phases—Variants I and II
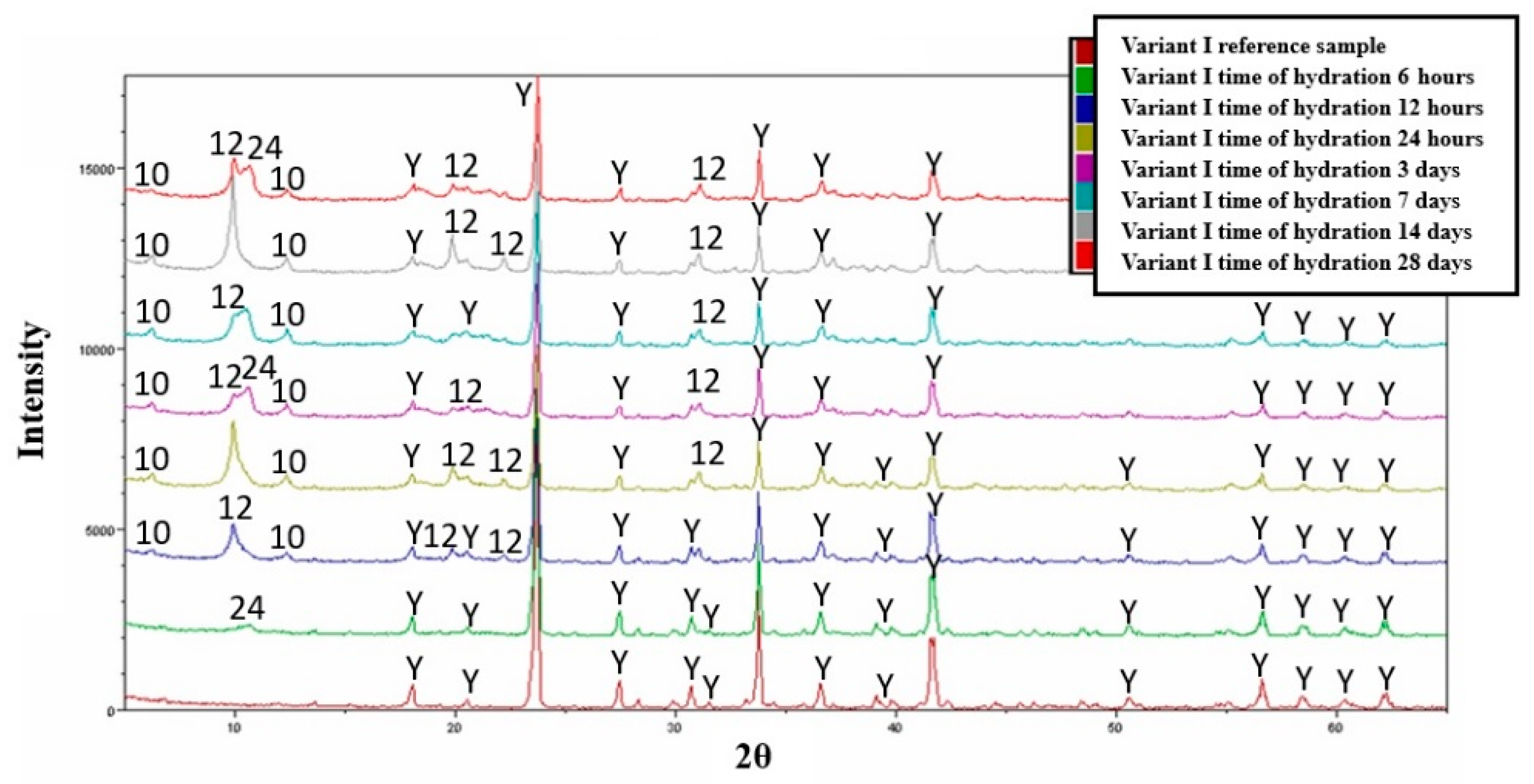
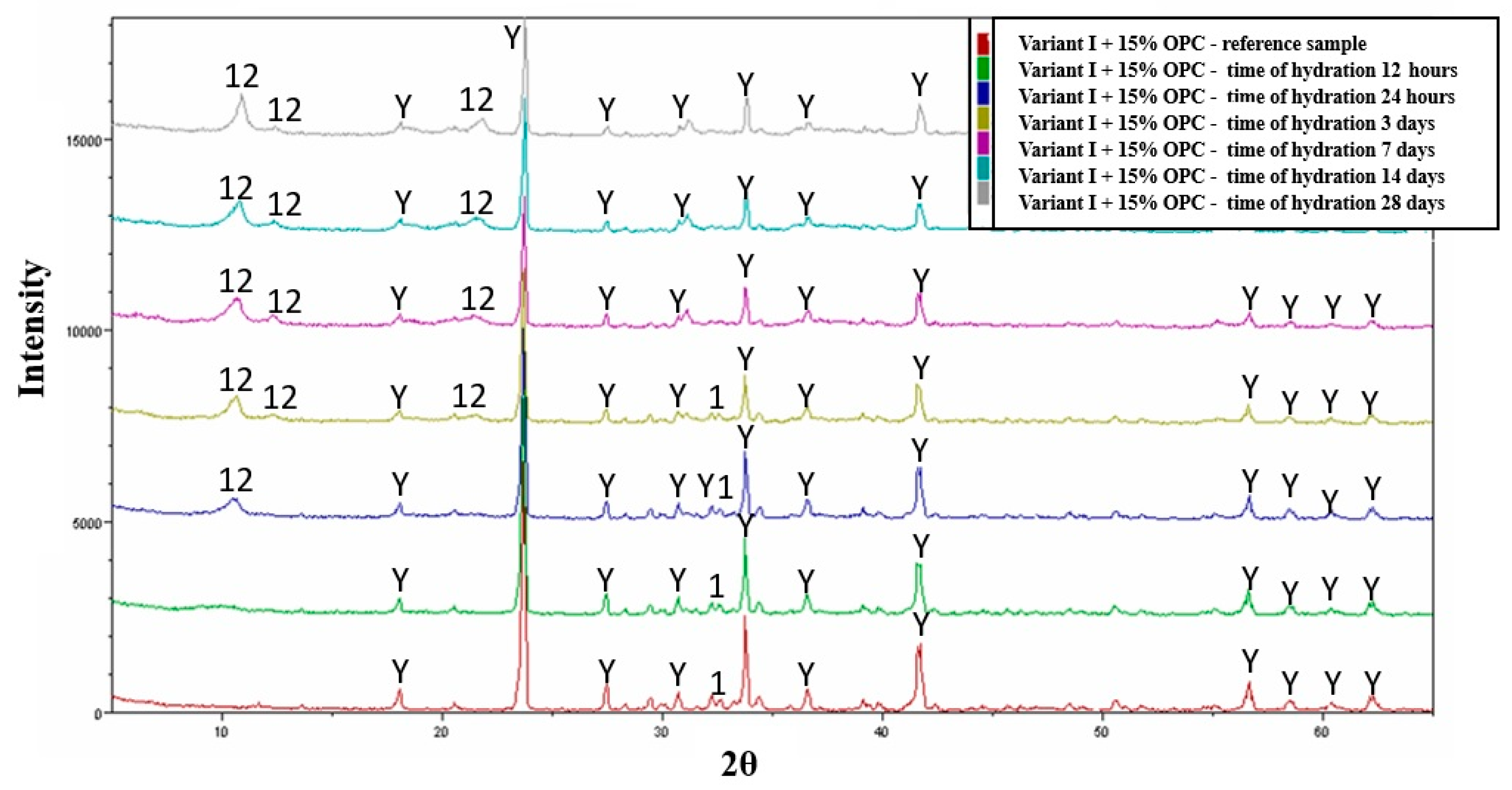
3.4. Hydration—Variant I
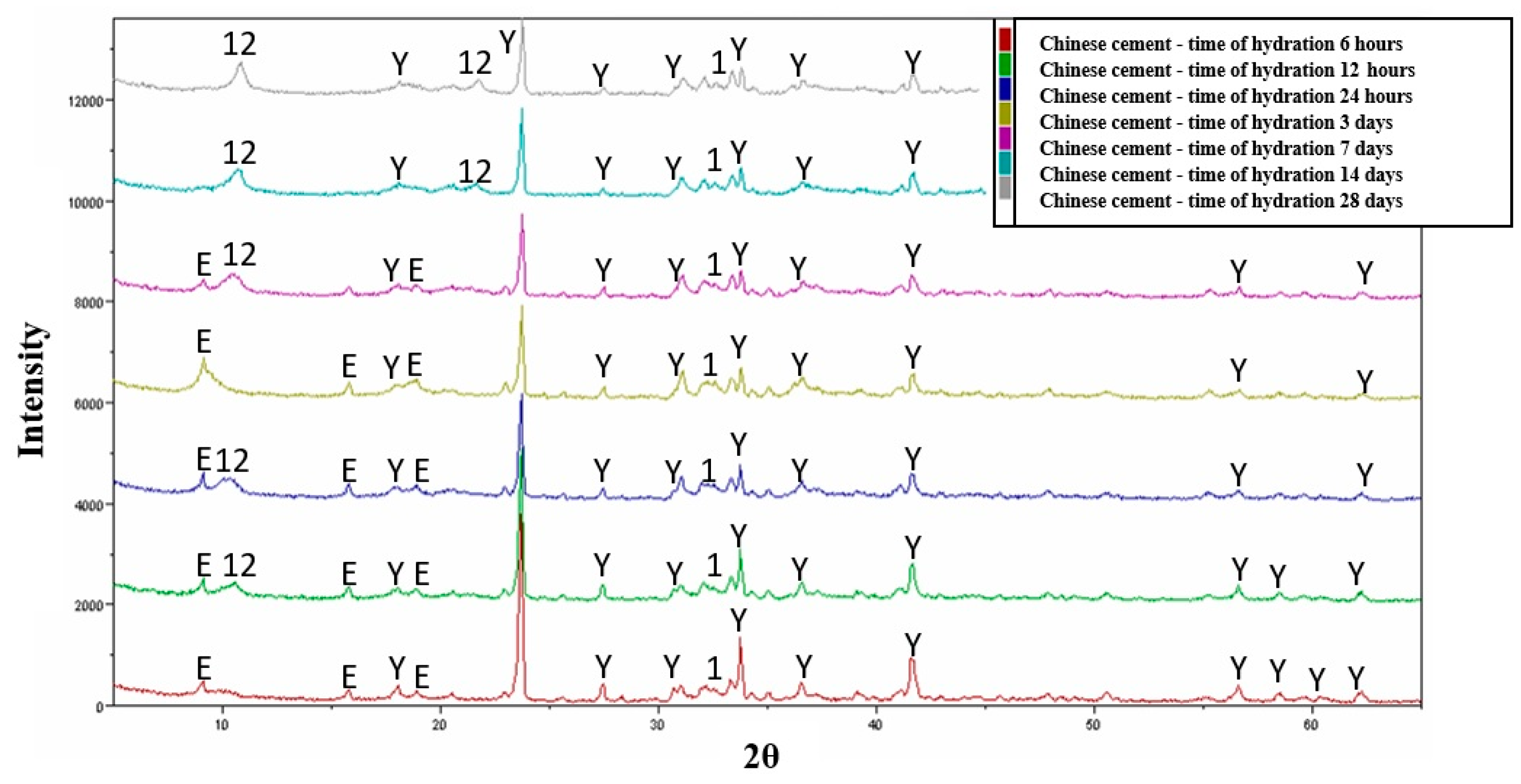
3.5. Hydration—Variant III and Chinese Cement
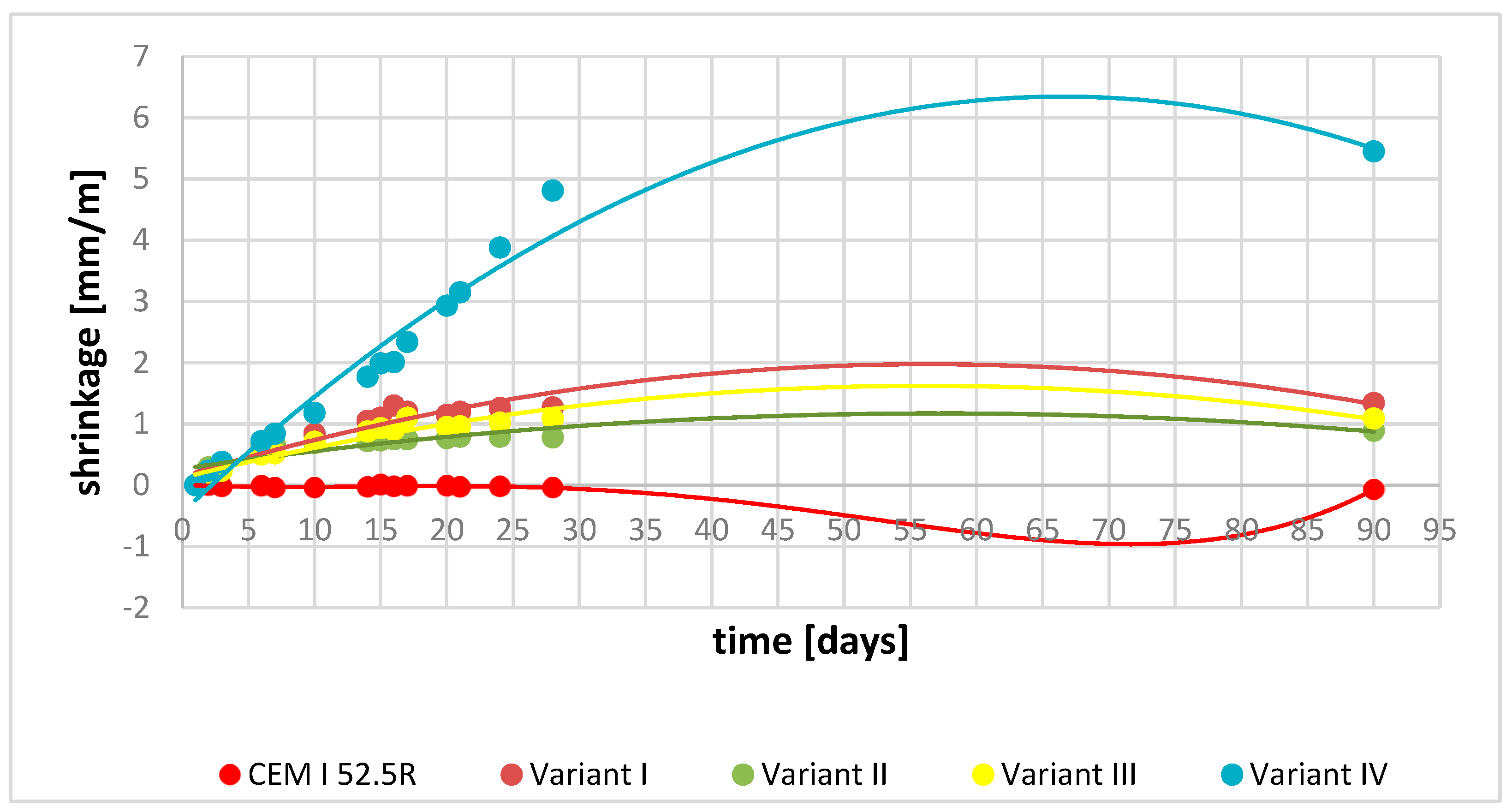
3.6. Shrinkage and Expansion Test Results
3.7. Compressive Strength Test Results
4. Conclusions
- Regardless of the type of sulphate ion carrier used (natural anhydrite and rea-gypsum), the optimal temperature for the synthesis of variants I and II is 1300 °C. Its application leads to the highest content of crystalline ye’elimite.
- In addition to crystalline ye’elimite, the phase composition of the blends also includes calcium aluminates such as C12A7, CA, and CA2.
- The main hydration products of variants I and II are hydrated calcium aluminates and dodeca-calcium-glycosulphate.
- The hydration products of variant III, which combines variant I with 15% OPC (Ordinary Portland Cement), include dodeca-calcium-glycosulphate, hydrated silicates, and hydrated calcium aluminates.
- In the case of Chinese cement hydration, the primary hydration product is crystalline ettringite, followed by dodeca-calcium-glycosulphate. Additionally, the phase composition includes hydrated calcium silicates of the CSH type derived from the β-C2S phase and pseudo-crystalline Al(OH)3.
- The obtained YAC cements and their mixtures showed no shrinkage or slight expansion properties with stable results of compressive strength tests.
- The sinters obtained from variants I and II constitute a special ye’elimite-calcium-aluminate (YAC) binder, which, due to its phase composition after hydration, is predisposed for use in the construction of reactors for biogas production in eco-energetics applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, D.; Cui, Y.; Li, H.; Yang, K.; Xu, W.; Chen, Y. On the future of Chinese cement industry. Cem. Concr. Res. 2015, 78, 2–13. [Google Scholar] [CrossRef]
- CemBR. 2020–2021 Performance, and Supply and Demand to 2025. Global Cement Magazine, 2022; 8–15. Available online: https://www.globalcement.com/pdf/eGCMarch2022ns.pdf(accessed on 21 February 2022).
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Hanein, T.; Galvez-Martos, J.L.; Bannerman, M.N. Carbon footprint of calcium sulfoaluminate clinker production. J. Clean Prod. 2018, 172, 2278–2287. [Google Scholar] [CrossRef]
- Nabila Bouha, F.; Kacimi, L.; De la Torre, A. Manufacture of rich-sulfoaluminate belite cement at low temperature from waste mixture by dry and hydrothermal processes. Constr. Build. Mater. 2022, 314, 125641. [Google Scholar] [CrossRef]
- ben Haha, M.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res. 2019, 123, 105778. [Google Scholar] [CrossRef]
- Koga, G.Y.; Albert, B.; Nogueira, R.P. On the hydration of Belite-Ye’elimite-Ferrite (BYF) cement pastes: Effect of the water-to-cement ratio and presence of fly ash. Cem. Concr. Res. 2020, 137, 106215. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; de la Torre, A.G. Sulfoaluminate cement. In Eco-Efficient Concrete; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 488–522. [Google Scholar] [CrossRef]
- da Costa, E.B.; Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Gobbo, L.A.; Kirchheim, A.P. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr. Build. Mater. 2016, 122, 373–383. [Google Scholar] [CrossRef]
- Berger, S.; Coumes, C.C.D.; Le Bescop, P.; Damidot, D. Influence of a thermal cycle at early age on the hydration of calcium sulphoaluminate cements with variable gypsum contents. Cement Concr. Res. 2011, 41, 149–160. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Boehm-Courjault, E.; Zajac, M.; Ben Haha, M.; Scrivener, K. Hydration reactions and stages of clinker composed mainly of stoichiometric ye’elimite. Cem. Concr. Res. 2019, 116, 120–133. [Google Scholar] [CrossRef]
- Gartner, E.M.; MacPhee, D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011, 41, 736–749. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Hu, W.; Wu, K. Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cem. Concr. Compos. 2014, 46, 26–31. [Google Scholar] [CrossRef]
- Gomes, S.; Francois, M.; Pellissier, C.; Evrard, O. Characterization and comparative study of coal combustion residues from a primary and additional flue gas secondary desulfurisation process. Cem. Concr. Res. 1998, 28, 1605–1619. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J Clean Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Tayeh, A.; Akeed, M.H.; Qaidi, S.; Abu Bakar, B.H. Ultra-high-performance concrete: Impacts of steel fibre shape and content on flowability, compressive strength and modulus of rupture. Case Stud. Constr. Mater. 2022, 17, e01615. [Google Scholar] [CrossRef]
- Abd, S.M.; Mhaimeed, I.S.; Tayeh, B.A.; Najm, H.M.; Qaidi, S. Investigation of the use of textile carbon yarns as sustainable shear reinforcement in concrete beams. Case Stud. Constr. Mater. 2023, 18, e01765. [Google Scholar] [CrossRef]
- Taylor, H.F.W. The Chemistry of Cements; Thomas Telford: London, UK, 1997. [Google Scholar]
- Akerman, K. Gips i anhydryt. In Występowanie i Zastosowania w Przemyśle i w Budownictwie; PWN: Warszawa, Poland, 1964. [Google Scholar]
- Stark, J.; Wicht, B. Zement und Kalk. In Der Baustoff als Werkstoff; Birkhäuser Verlag: Basel, Switzerland, 2000. [Google Scholar]
- Pyzalski, M.; Brylewski, T.; Sujak, A.; Durczak, K. Changes in the Phase Composition of Calcium Aluminoferrites Based on the Synthesis Condition and Al2O3/Fe2O3 Molar Ratio. Materials 2023, 16, 4234. [Google Scholar] [CrossRef]
- PN-EN 196-1: 2016-07; Cement Test Methods-Part 1: Determination of Strength. Polski Komitet Normalizacyjny: Warsaw, Poland, 2016.
- X’PertHighScore Plus Software, Philips X Pert High Score Plus 3.0—Free Download Suggestions. Available online: https://www.informer.com/ (accessed on 1 November 2004).
- Home ICSD (fiz-karlsruhe.de). Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 21 August 2023).
- ASTM C 845–96; Standard Specification for Expansive Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 1996.
- Pyzalski, M.; Dąbek, J.; Adamczyk, A.; Brylewski, T. Physicochemical Study of the Self-Disintegration of Calcium Orthosilicate (β→γ) in the Presence of the C12A7 Aluminate Phase. Materials 2021, 14, 6459. [Google Scholar] [CrossRef]
- Wolf, J.J.; Jansen, D.; Goetz-Neunhoeffer, F.; Neubauer, J. Application of thermodynamic modeling to predict the stable hydrate phase assemblages in ternary CSA-OPC-anhydrite systems and quantitative verification by QXRD. Cem. Concr. Res. 2020, 128, 105956. [Google Scholar] [CrossRef]
- Barnes, P. Structure and Performance of Cements; Applied Science Publishers: London, UK; New York, NY, USA, 1983. [Google Scholar]
- Durczak, K.; Pyzalski, M.; Pilarski, K.; Brylewski, T.; Sujak, A. The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes. Materials 2021, 14, 1707. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Pyzalski, M.; Durczak, K.; Brylewski, T.; Murzyn, P.; Pilarski, K. Studies on Cement Pastes Exposed to Water and Solutions of Biological Waste. Materials 2022, 15, 1931. [Google Scholar] [CrossRef] [PubMed]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Peukert, A. Cementy Powszechnego Użytku i Specjalne; Polski Cement: Kraków, Poland, 2000. [Google Scholar]
- Chang, J.; Zhang, Y.; Shang, X.; Zhao, J.; Yu, X. Effects of amorphous AH3 phase on mechanical properties and hydration process of C4A3S-CSH2-CH-H2O system. Constr. Build. Mater. 2017, 133, 314–322. [Google Scholar] [CrossRef]
- Kurdowski, W. Chemia Cementu i Betonu; Stowarzyszenie Producentów Cementu: Kraków, Poland, 2010. [Google Scholar]



| Component | Fraction [%] | Fraction in Terms of Calcinated Substance |
|---|---|---|
| % H2O hygroscopic at 50 °C | 0.11 | - |
| % H2O crystallic at 350 °C | 0.94 | - |
| % of roasting losses at 900 °C | 217 | - |
| % CaO | 40.45 | 40.87 |
| % MgO | 0.00 | 0.00 |
| % SO3 | 57.79 | 58.40 |
| % Al2O3 | 0.07 | 0.08 |
| % Fe2O3 | 0.00 | 0.00 |
| % SiO2 + insoluble parts | 0.64 | 0.65 |
| % CaSO4 | 94.69 | - |
| % CaSO4·H2O | 4.49 | - |
| % MgSO4 | 0.00 | - |
| % CaCO3 | 0.00 | - |
| Total | 99.89 | 100.00 |
| Component | Fraction [%] | Fraction in Terms of Calcinated Substance |
|---|---|---|
| Humidity | 6.0 | - |
| Crystallization water | 20.02 | - |
| SO3 | 45.70 | 57.14 |
| CaO | 32.90 | 41.15 |
| MgO | 0.12 | 0.16 |
| Na2O | 0.09 | 0.12 |
| K2O | 0.03 | 0.05 |
| SiO2 | 0.78 | 1.0 |
| Cl- | 0.01 | 0.02 |
| Al2O3 | 0.09 | 0.12 |
| Fe2O3 | 0.26 | 0.33 |
| Total | 100.18 | 100 |
| No. | Variant I | No. | Variant II |
|---|---|---|---|
| 1. | CaCO3 * | 1. | CaCO3 |
| 2. | Rea-gypsum (CaSO4·2H2O) | 2. | Natural anhydrite (CaSO4) |
| 3. | Al(OH)3 | 3. | Al(OH)3 |
| Sample | Flow [mm] | W/C | Linear Changes [mm/m] [days] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | 7 | 10 | 14 | 15 | 16 | 17 | 20 | 21 | 24 | 28 | 90 | |||
| 52.5R | 172 | 0.45 | 0.00 | −0.01 | −0.02 | −0.01 | −0.04 | −0.04 | −0.03 | −0.01 | −0.02 | −0.01 | −0.01 | −0.03 | −0.02 | −0.04 | −0.07 |
| V I | 160 | 0.50 | 0.00 | 0.19 | 0.29 | 0.59 | 0.62 | 0.84 | 1.05 | 1.10 | 1.30 | 1.20 | 1.15 | 1.20 | 1.26 | 1.27 | 1.34 |
| V II | 165 | 0.45 | 0.00 | 0.29 | 0.28 | 0.64 | 0.66 | 0.69 | 0.72 | 0.73 | 0.75 | 0.75 | 0.77 | 0.79 | 0.79 | 0.78 | 0.89 |
| V III | 160 | 0.50 | 0.00 | 0.16 | 0.24 | 0.50 | 0.52 | 0.71 | 0.88 | 0.93 | 0.90 | 1.10 | 0.95 | 0.96 | 1.02 | 1.09 | 1.09 |
| V IV | 160 | 0.50 | 0.00 | 0.24 | 0.38 | 0.72 | 0.84 | 1.18 | 1.77 | 1.99 | 2.01 | 2.34 | 2.93 | 3.15 | 3.88 | 4.81 | 5.45 |
| Sample | Compressive Strength [MPa] [days] | ||||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 28 | 90 | |
| CEM I 52.5R | 9.5 | 28.1 | 45.0 | 55.9 | 64.3 |
| Variant I | 29 | 33.0 | 43 | 50.3 | 55.5 |
| Variant II | 32.1 | 37 | 44 | 52 | 60.2 |
| Variant III | 27 | 24.4 | 38.6 | 49.1 | 51.1 |
| Variant IV | 12.3 | 19.8 | 27.1 | 43.6 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durczak, K.; Pyzalski, M.; Brylewski, T.; Sujak, A. Effect of Variable Synthesis Conditions on the Formation of Ye’elimite-Aluminate-Calcium (YAC) Cement and Its Hydration in the Presence of Portland Cement (OPC) and Several Accessory Additives. Materials 2023, 16, 6052. https://doi.org/10.3390/ma16176052
Durczak K, Pyzalski M, Brylewski T, Sujak A. Effect of Variable Synthesis Conditions on the Formation of Ye’elimite-Aluminate-Calcium (YAC) Cement and Its Hydration in the Presence of Portland Cement (OPC) and Several Accessory Additives. Materials. 2023; 16(17):6052. https://doi.org/10.3390/ma16176052
Chicago/Turabian StyleDurczak, Karol, Michał Pyzalski, Tomasz Brylewski, and Agnieszka Sujak. 2023. "Effect of Variable Synthesis Conditions on the Formation of Ye’elimite-Aluminate-Calcium (YAC) Cement and Its Hydration in the Presence of Portland Cement (OPC) and Several Accessory Additives" Materials 16, no. 17: 6052. https://doi.org/10.3390/ma16176052
APA StyleDurczak, K., Pyzalski, M., Brylewski, T., & Sujak, A. (2023). Effect of Variable Synthesis Conditions on the Formation of Ye’elimite-Aluminate-Calcium (YAC) Cement and Its Hydration in the Presence of Portland Cement (OPC) and Several Accessory Additives. Materials, 16(17), 6052. https://doi.org/10.3390/ma16176052







