Recycling Local Waste Glass Bottles into Cement Paste: Effect on Hydration, Microstructure, and CO2 Emission
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.2. Mix Design and Specimen Preparation
2.3. Test Methods
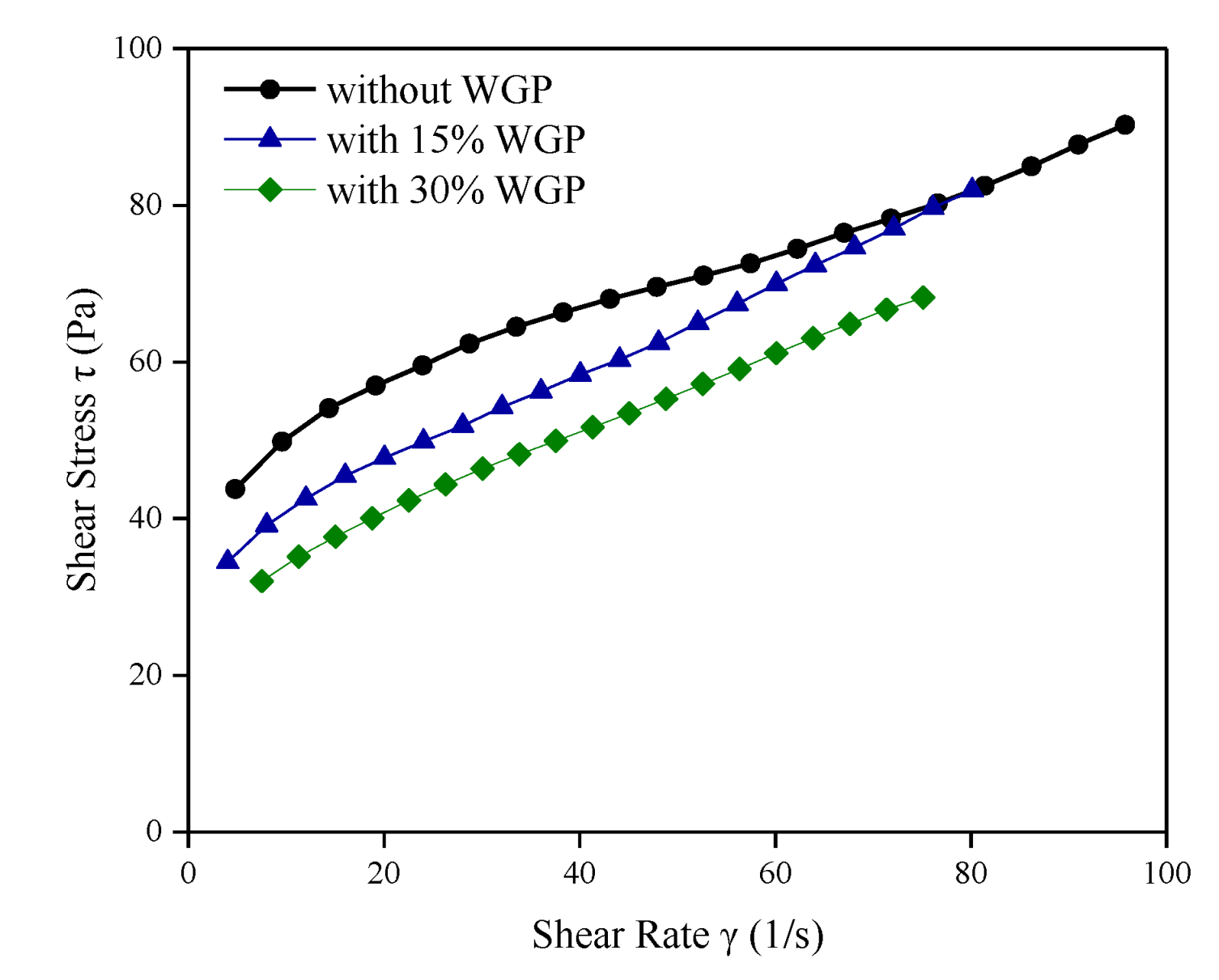
2.3.1. Rheology
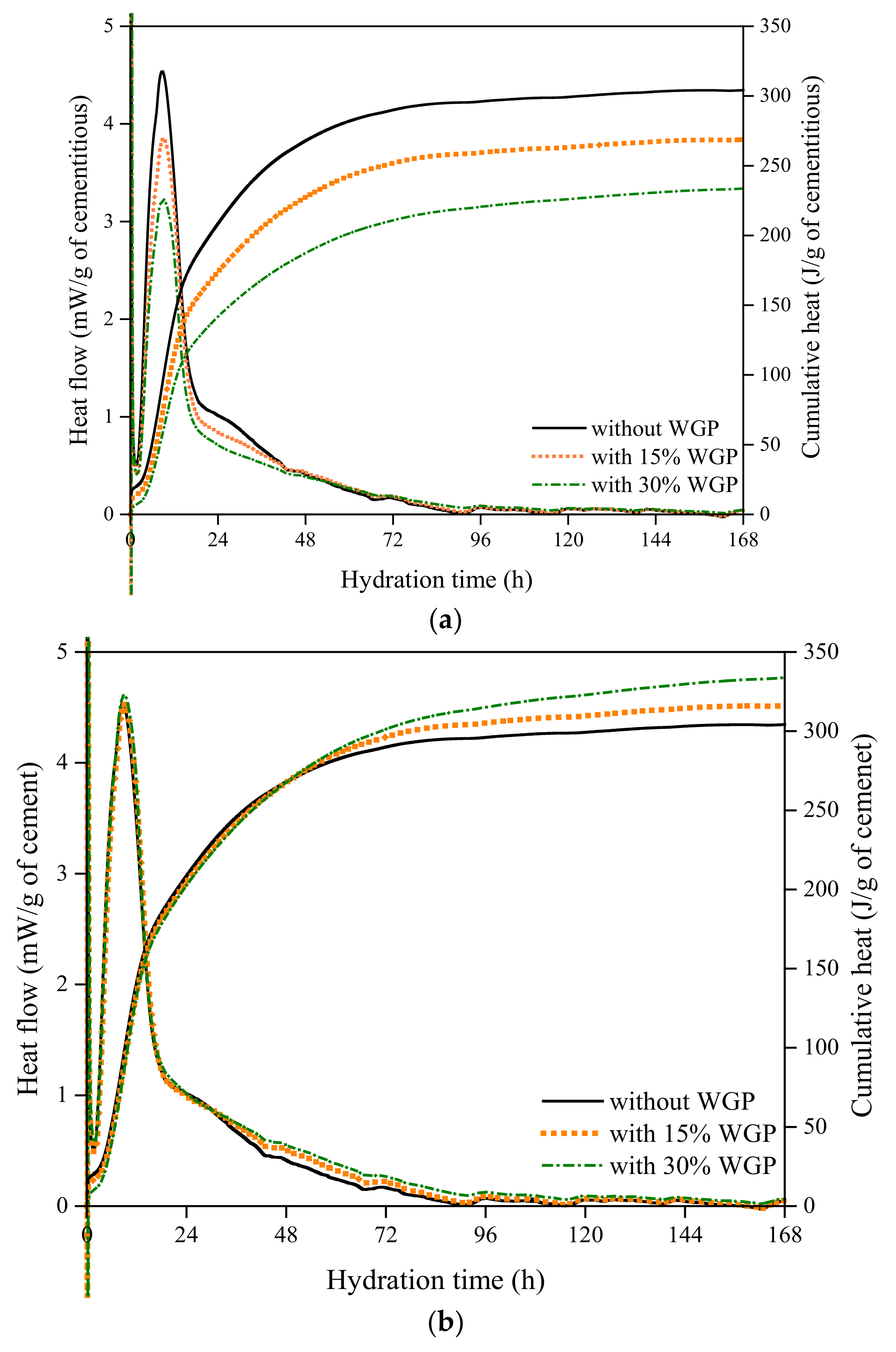
2.3.2. Heat of Hydration
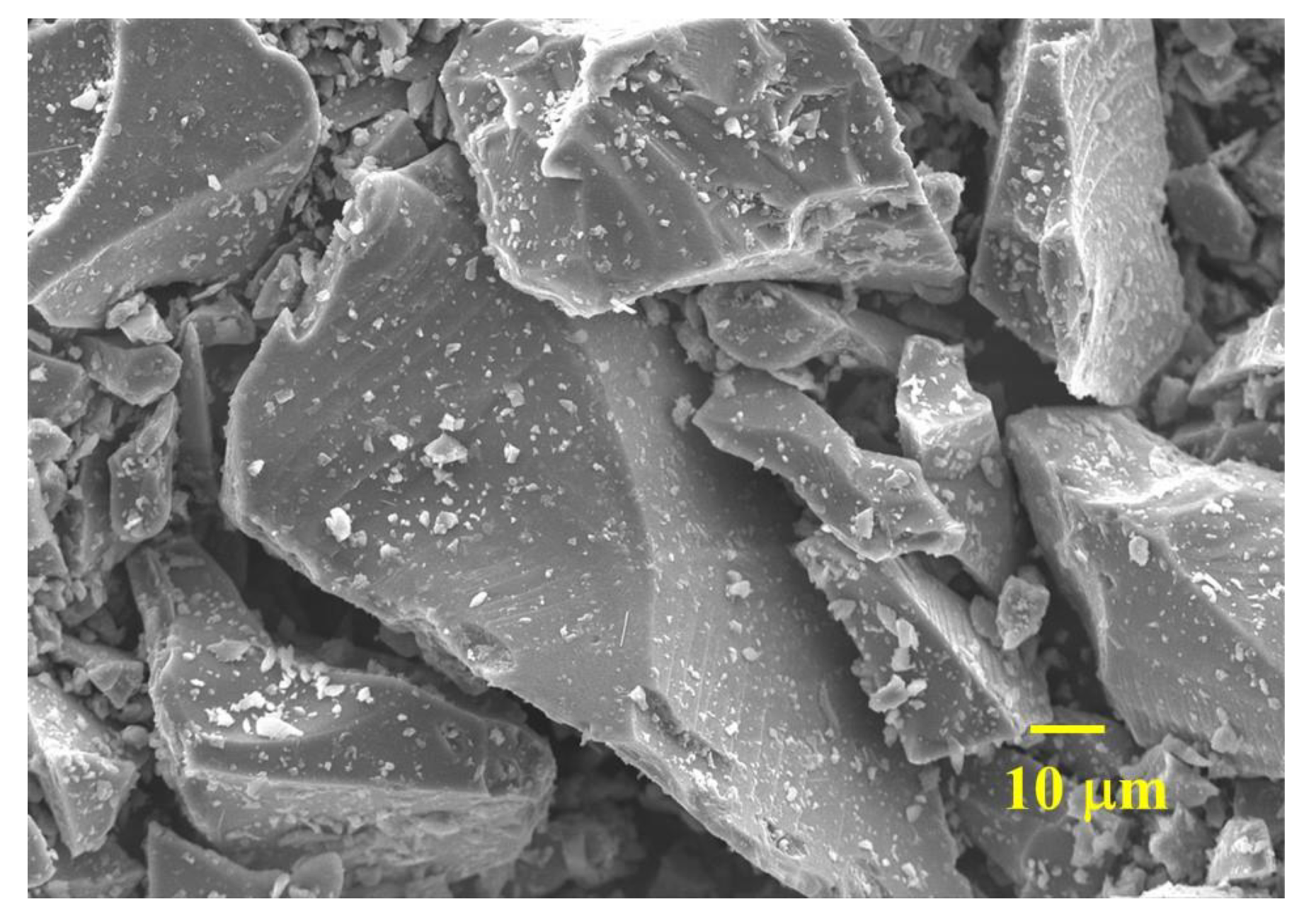
2.3.3. Microstructure Analysis
3. Results and Discussions
3.1. Rheological Properties
3.2. Hydration
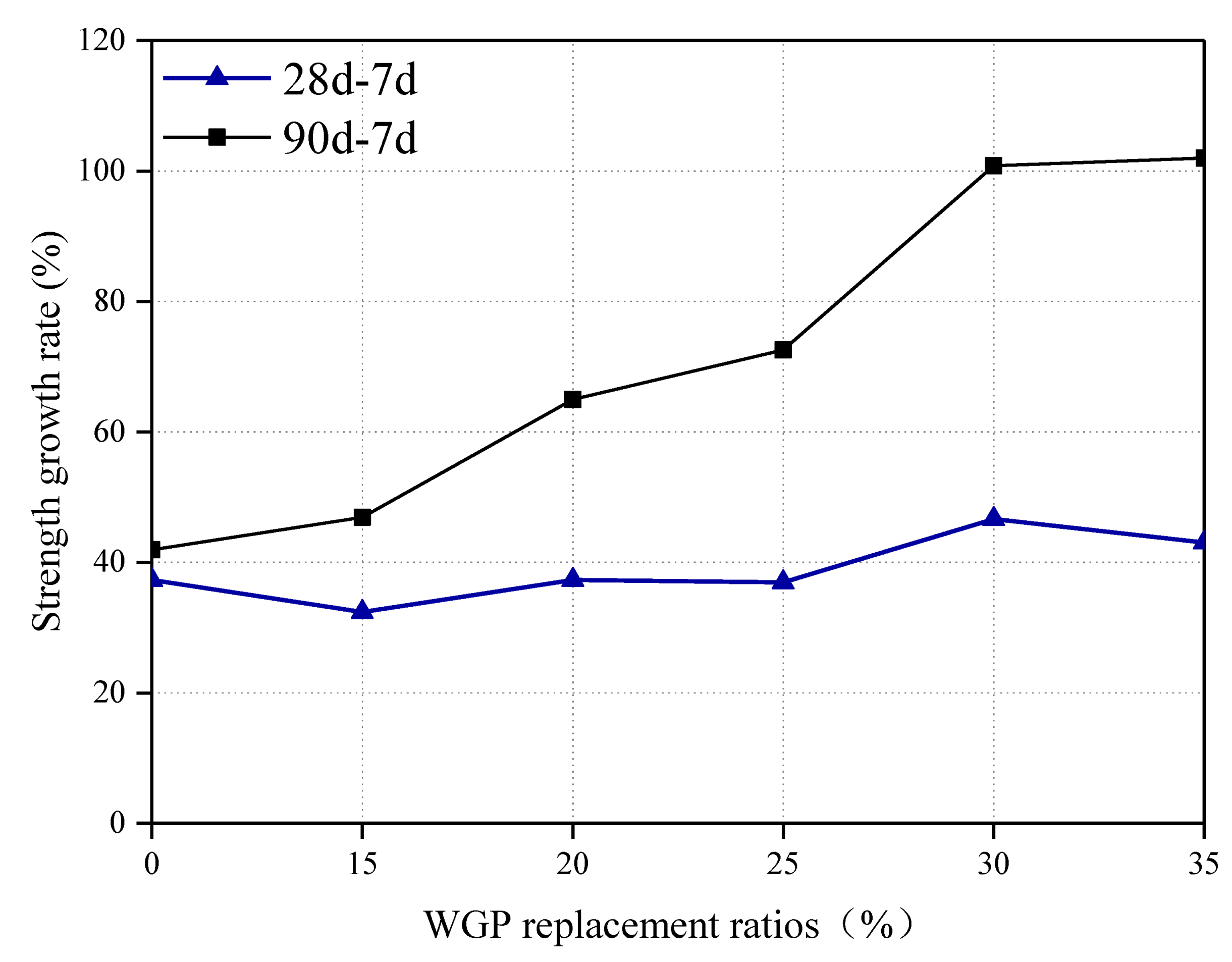
3.3. Strength Development
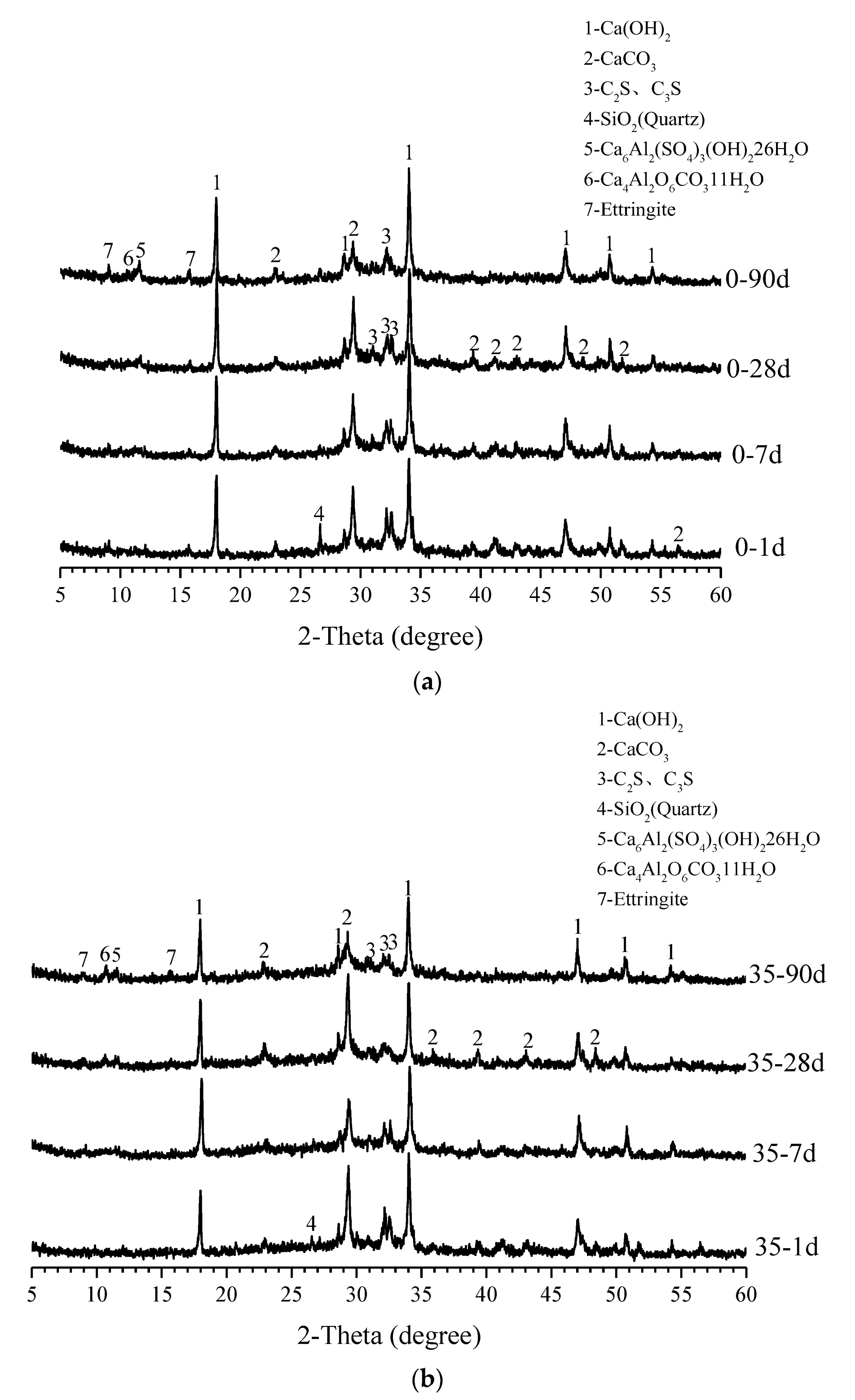
3.4. XRD Analysis
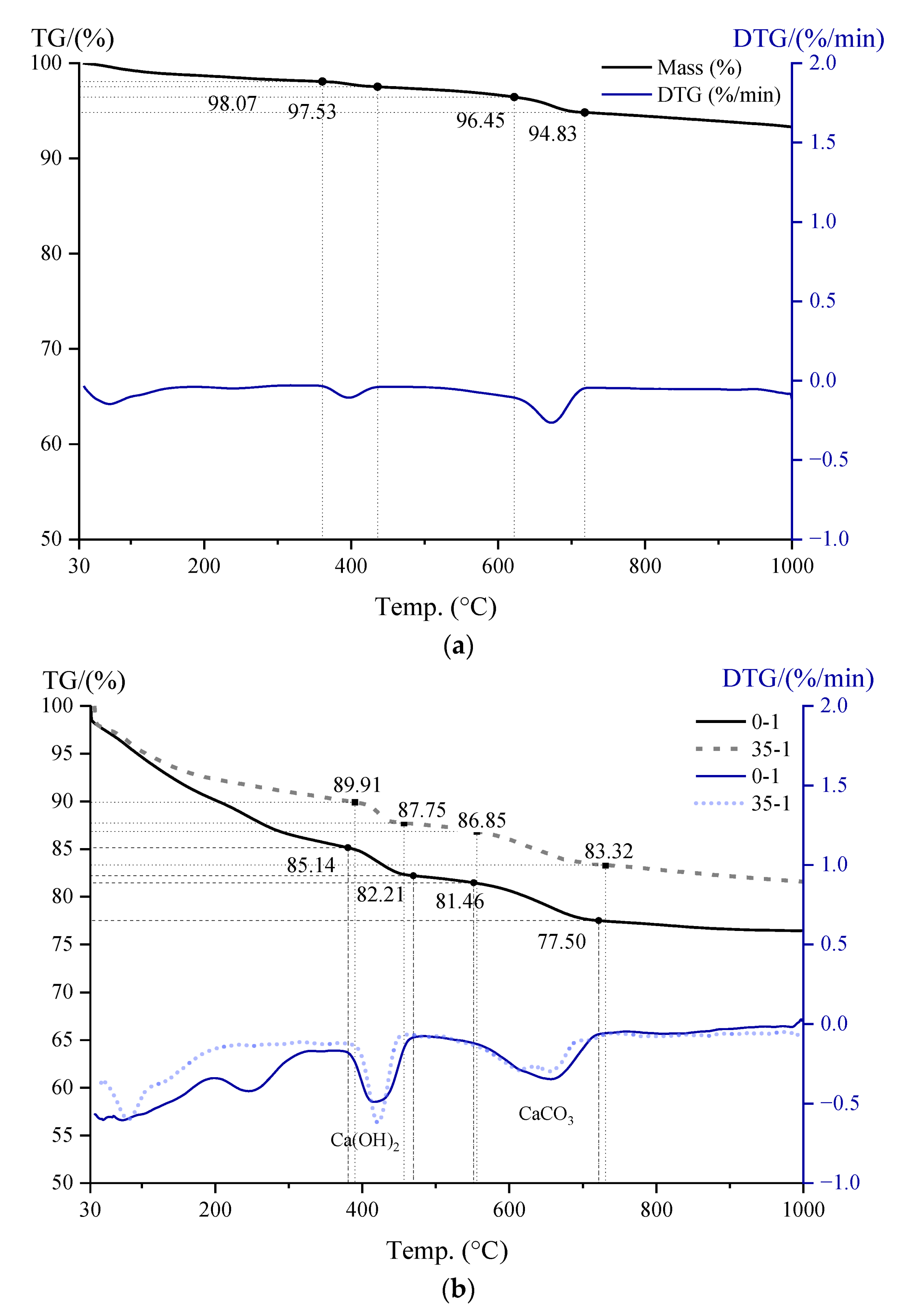
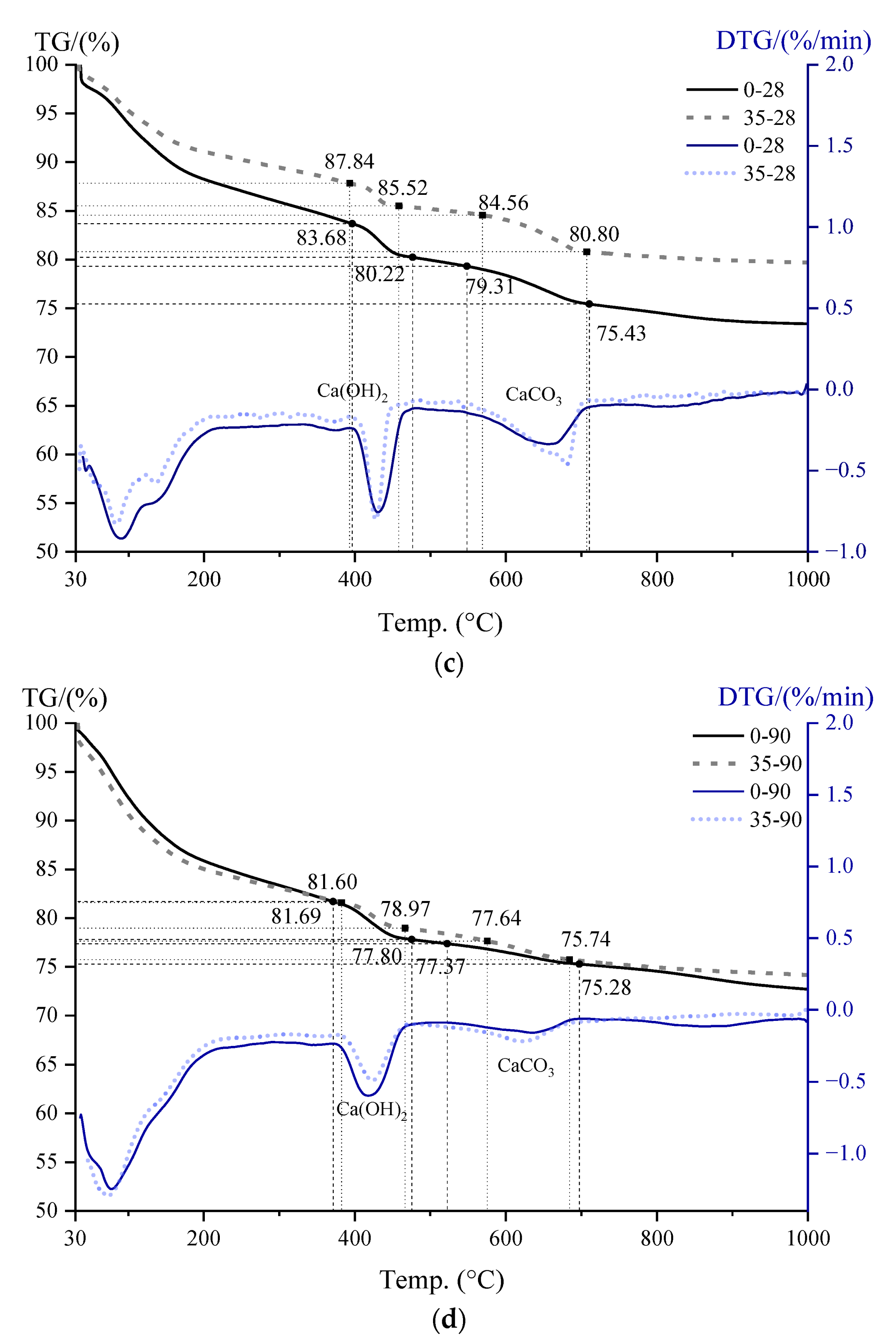
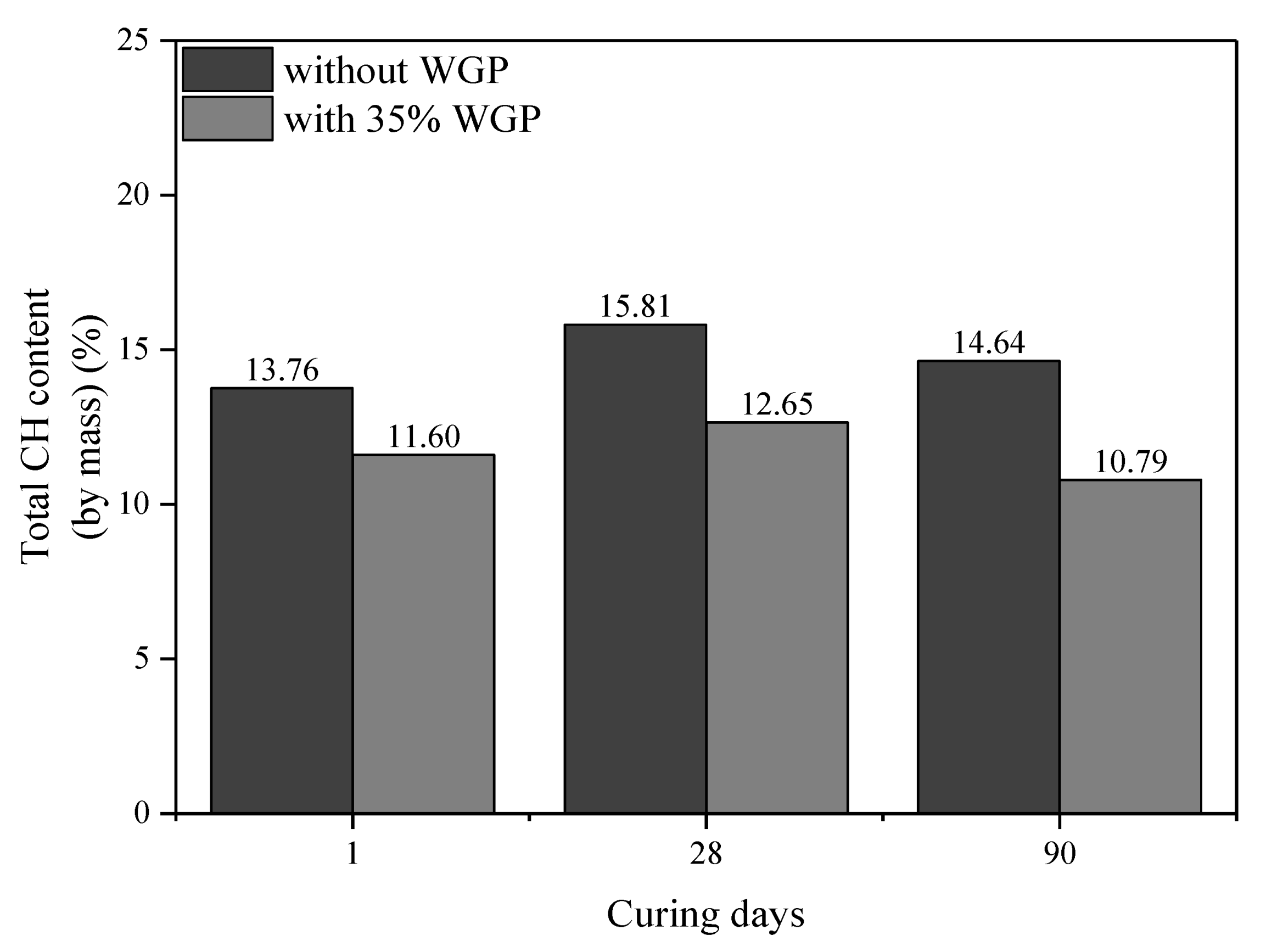
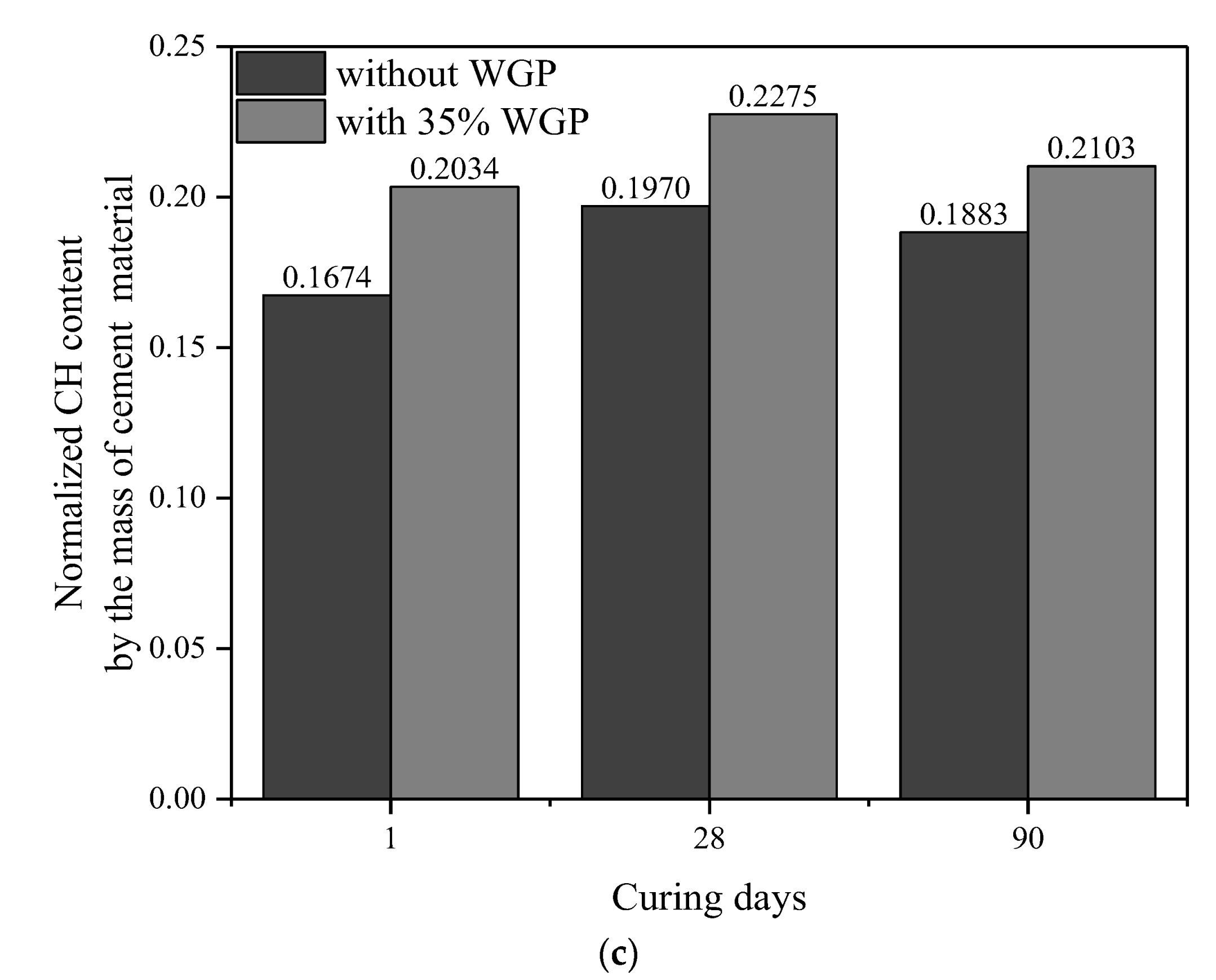
3.5. CH Content
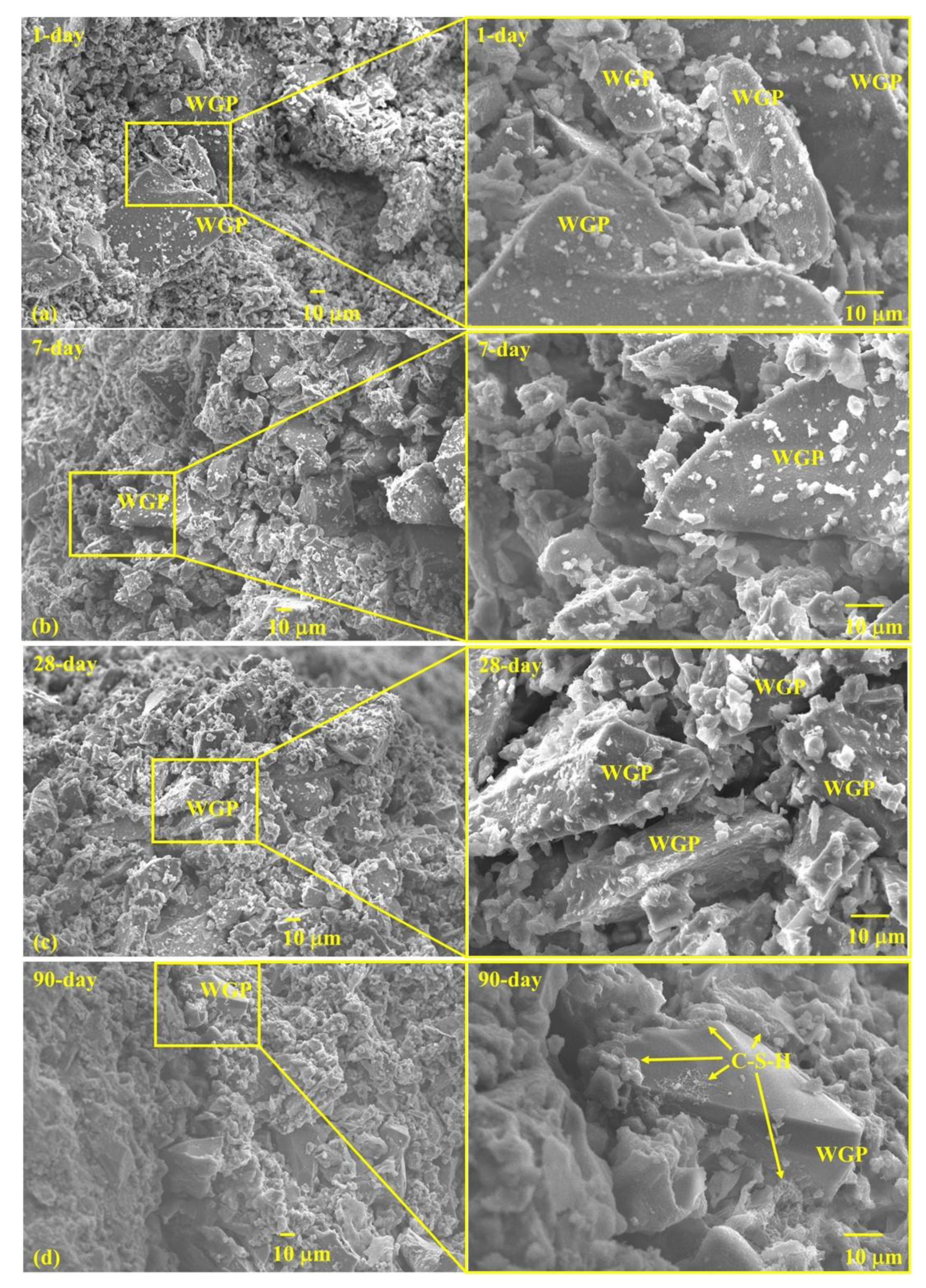
3.6. Microstructure
4. CO2 Emissions
5. Conclusions
- Recycling waste glass bottles into cement paste is technically feasible in terms of the mechanical properties required by relevant standards.
- WGP reduces the ecological impact of Portland cement by 34.9%, according to CO2 emission analysis via value engineering analysis.
- The addition of 35% WGP increased cement hydration by 21.53%, 15.48%, and 11.68% at curing ages of 1, 28, and 90 days, respectively, compared to without WGP.
- The pozzolanic reaction of WGP consumes CH in cementitious systems, as confirmed by XRD patterns and CH content analysis from TG-DTG curves. Compared with the samples without WGP, the normalized CH content per unit binder mass containing 35% WGP decreased by 21.01%, 24.94%, and 27.41% at the ages of 1, 28, and 90 days, respectively.
- WGP particles provide nucleation places for hydration products, facilitating subsequent growth of C-S-H and strength growth in late ages.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on concrete containing ground waste glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- 197-1 BE; Cement-Part 1: Composition, Specifications and Conformity Criteria for Common CementsCement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements. British Standard Institution: London, UK, 2011.
- Vereshchagina, T.; Kutikhina, E.; Solovyov, L.; Vereshchagin, S.; Mazurova, E.; Anshits, A. Hydrothermal Co-Processing of Coal Fly Ash Cenospheres and Soluble Sr(II) as Environmentally Sustainable Approach to Sr-90 Immobilization in a Mineral-like Form. Materials 2021, 14, 5586. [Google Scholar] [CrossRef]
- Zhai, Q.; Kurumisawa, K. Effect of accelerators on Ca(OH)2 activated ground granulated blast-furnace slag at low curing temperature. Cem. Concr. Compos. 2021, 124, 104272. [Google Scholar] [CrossRef]
- Gruber, K.A.; Ramlochan, T.; Boddy, A.; Hooton, R.D. Thomas MDA, Increasing concrete durability with high-reactivity metakaolin. Cem. Concr. Compos. 2001, 23, 479–484. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Liao, S.; Huang, Y. Grinding kinetics of waste glass powder and its composite effect as pozzolanic admixture in cement concrete. Constr. Build. Mater. 2020, 239, 117876. [Google Scholar] [CrossRef]
- Belouadah, M.; Rahmouni, Z.; Tebbal, N. Experimental characterization of ordinary concretes obtained by adding construction waste (glass, marble)—ScienceDirect. Procedia Comput. Sci. 2019, 158, 153–162. [Google Scholar] [CrossRef]
- Yin, W.; Li, X.; Sun, T.; Chen, Y.; Xu, F.; Yan, G.; Xu, M.; Tian, K. Utilization of waste glass powder as partial replacement of cement for the cementitious grouts with superplasticizer and viscosity modifying agent binary mixtures: Rheological and mechanical performances. Constr. Build. Mater. 2021, 286, 122953. [Google Scholar] [CrossRef]
- Pezeshkian, M.; Delnavaz, A.; Delnavaz, M. Development of UHPC mixtures using natural zeolite and glass sand as replacements of silica fume and quartz sand. Eur. J. Environ. Civ. Eng. 2019, 25, 2023–2038. [Google Scholar] [CrossRef]
- Vaitkevicius, V.; Serelis, E.; Hilbig, H. The effect of glass powder on the microstructure of ultra high performance concrete. Constr. Build. Mater. 2014, 68, 102–109. [Google Scholar] [CrossRef]
- Harbec, D.; Zidol, A.; Tagnit-Hamou, A.; Gitzhofer, F. Mechanical and durability properties of high performance glass fume concrete and mortars. Constr. Build. Mater. 2017, 134, 142–156. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cem. Concr. Res. 2006, 36, 457–468. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Pozzolanic properties of fine and coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Pereira-de-Oliveira, L.A.; Castro-Gomes, J.P.; Santos, P.M.S. The potential pozzolanic activity of glass and red-clay ceramic waste as cement mortars components. Constr. Build. Mater. 2012, 31, 197–203. [Google Scholar] [CrossRef]
- Lu, J.-X.; Zhan, B.-J.; Duan, Z.-H.; Poon, C.S. Using glass powder to improve the durability of architectural mortar prepared with glass aggregates. Mater. Des. 2017, 135, 102–111. [Google Scholar] [CrossRef]
- Wang, S.; Baxter, L.; Fonseca, F. Biomass fly ash in concrete: SEM, EDX and ESEM analysis. Fuel 2008, 87, 372–379. [Google Scholar] [CrossRef]
- Schwarz, N.; Neithalath, N. Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cem. Concr. Res. 2008, 38, 429–436. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Al-Sodani, K.A.A.; AlAteah, A.H.; Al-Tholaia, M.M.H.; Adewumi, A.A.; Bakare, A.O.; Usman, A.K.; Momohjimoh, I. Performances of the Synergy of Silica Fume andWaste Glass Powder in Ternary Blended Concrete. Appl. Sci. 2022, 12, 6637. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Al-Sodani, K.A.A.; Adewumi, A.A.; Alateah, A.H.; Al-Tholaia, M.M.H.; Shamsah, S.M.I.; Qazi, U.Y.; Alanazi, G.D.S. Microstructural Characteristics, Modeling ofMechanical Strength and Thermal Performance of Industrial Waste Glass Blended Concrete. Appl. Sci. 2022, 12, 8600. [Google Scholar] [CrossRef]
- Mohammadyan-Yasouj, S.E.; Ghaderi, A. Experimental investigation of waste glass powder, basalt fibre, and carbon nanotube on the mechanical properties of concrete. Constr. Build. Mater. 2020, 252, 119115. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Pugazhmani, G.; Sripragadeesh, R.; Muthu, D.; Venkatasubramanian, C. Experimental study on the mechanical and durability properties of concrete with waste glass powder and ground granulated blast furnace slag as supplementary cementitious materials. Constr. Build. Mater. 2017, 156, 739–749. [Google Scholar] [CrossRef]
- GB175; Common Portland Cement, in Common Portland Cement. Ministry of Construction of China: Beijing, China, 2020.
- Shayan, A.; Xu, A.M. Value-added utilisation of waste glass in concrete. Cem. Concr. Res. 2004, 34, 81–89. [Google Scholar] [CrossRef]
- ASTM C1866; Standard Specification for Ground-Glass Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for USE in concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- 196-1 BE; Methods of Testing Cement-Part 1: Determination of Strength. British Standard Institution: London, UK, 2016.
- ASTM C311; Standard Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- GB/T 12957; Test Method for Activity of Industrial Waste Slag Used As Addition to Cement. Ministry of Construction of China: Beijing, China, 2005.
- BS EN 450-1; Fly Ash for Concrete-Part 1: Definition, Specifications and Conformity Criteria. British Standard Institution: London, UK, 2012.
- GB/T 2847; Pozzolanic Materials Used for Cement Production. Ministry of Construction of China: Beijing, China, 2005.
- GB17671; Method of testing cements-Determination of strength. Ministry of Construction of China: Beijing, China, 2020.
- Lai, M.H.; Binhowimal, S.A.M.; Hanzic, L.; Wang, Q.; Ho, J.C.M. Cause and mitigation of dilatancy in cement powder paste. Constr. Build. Mater. 2020, 236, 117595. [Google Scholar] [CrossRef]
- Wallevik, O.H.; Feys, D.; Wallevik, J.E.; Khayat, K.H. Avoiding inaccurate interpretations of rheological measurements for cement-based materials. Cem. Concr. Res. 2015, 78, 100–109. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Mo, K.H.; Shi, C. A critical review of waste glass powder—Multiple roles of utilization in cement-based materials and construction products. J. Environ. Manag. 2019, 242, 440–449. [Google Scholar] [CrossRef]
- Lu, J.-X.; Duan, Z.-H.; Poon, C.S. Combined use of waste glass powder and cullet in architectural mortar. Cem. Concr. Compos. 2017, 82, 34–44. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Utilizing Waste Recycled Glass as Sand/Cement Replacement in Concrete. J. Mater. Civ. Eng. 2009, 21, 709–721. [Google Scholar] [CrossRef]
- Liu, G.; Florea, M.V.A.; Brouwers, H.J.H. Performance evaluation of sustainable high strength mortars incorporating high volume waste glass as binder. Constr. Build. Mater. 2019, 202, 574–588. [Google Scholar] [CrossRef]
- Lu, J.-X.; Duan, Z.-H.; Poon, C.S. Fresh properties of cement pastes or mortars incorporating waste glass powder and cullet. Constr. Build. Mater. 2017, 131, 793–799. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of curing temperature and glass type on the pozzolanic reactivity of glass powder. Cem. Concr. Res. 2014, 58, 103–111. [Google Scholar] [CrossRef]
- Maraghechi, H.; Maraghechi, M.; Rajabipour, F.; Pantano, C.G. Pozzolanic reactivity of recycled glass powder at elevated temperatures: Reaction stoichiometry, reaction products and effect of alkali activation. Cem. Concr. Compos. 2014, 53, 105–114. [Google Scholar] [CrossRef]
- Huang, L.; Yan, P. Effect of alkali content in cement on its hydration kinetics and mechanical properties. Constr. Build. Mater. 2019, 228, 116833. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Waste Glass Powder as Cement Replacement in Concrete. J. Adv. Concr. Technol. 2014, 12, 468–477. [Google Scholar] [CrossRef]
- Khmiri, A.; Chaabouni, M.; Samet, B. Chemical behaviour of ground waste glass when used as partial cement replacement in mortars. Constr. Build. Mater. 2013, 44, 74–80. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Liu, G.Y.; Fan, L.; Jin, D.Z.; Lu, R.Y.; Li, L.J. Progress in Stimulating Techniques and Mechanism of Activity of Glass Powder. Sci. Technol. Eng. 2016, 16, 152–194. [Google Scholar]
- Shi, C.; Yuan, Q. Methods for Testing and Analysis of Cement-Based Materials; China Construction Industry Press: Beijing, China, 2018. [Google Scholar]
- Mehta, P.K.; Monteiro, P. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Lian, H.Z.; Tong, L.; Chen, E.Y. Phase Research Foundation of Building Materials; Tsinghua University Press: Beijing, China, 1995. [Google Scholar]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.A.; Ye, G.; van Breugel, K. Understanding the adhesion mechanisms between C S H and fillers. Cem. Concr. Res. 2017, 100, 275–283. [Google Scholar] [CrossRef]
- Mahoutian, M.; Shao, Y. Production of cement-free construction blocks from industry wastes. J. Clean. Prod. 2016, 137, 1339–1346. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Gao, P.-F.; Li, D.-C.; Yu, X.-L. Economic analysis of glass powder concrete based on value engineering analysis. Concrete 2020, 6, 163–165+174. [Google Scholar]
- Yu, H.; Yang, H.; Zhang, H.; Jie, Z. Research of Carbon Emission of Cement in Lifecycle. Sichuan Build. Mater. 2017, 43, 3. [Google Scholar]
- Cabrera, J.G.; Woolley, G.R. Life cycle benefits of calcium silicate replacements. Waste Manag. 1996, 16, 215–220. [Google Scholar] [CrossRef]

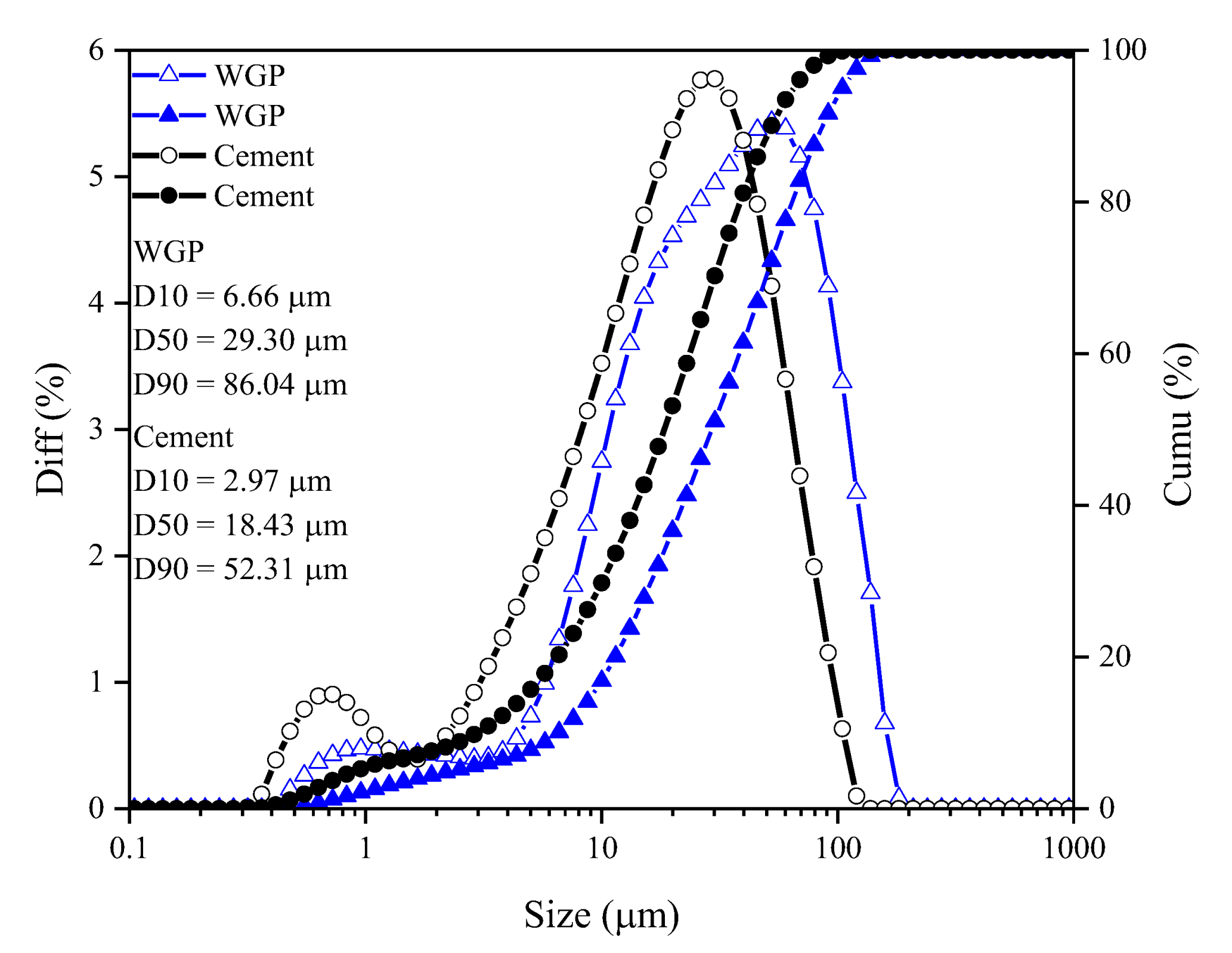


| Types | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO2 | SrO | MnO | Na2O | P2O5 | Cl | ZnO | Rb2O | ZrO2 | Density (g/cm3) | D50 (μm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 68.52 | 14.03 | 5.98 | 3.80 | 3.08 | 2.55 | 0.91 | 0.58 | 0.16 | 0.14 | 0.13 | 0.06 | 0.02 | 0.02 | 0.01 | - | 3.10 | 18.43 |
| WGP | 18.62 | 64.90 | 3.17 | 0.26 | 0.16 | 1.54 | 0.88 | 0.12 | 0.10 | - | 10.06 | 0.04 | 0.05 | - | 0.04 | 0.08 | 2.51 | 29.29 |
| Groups | Content | ||
|---|---|---|---|
| Cement | WGP | Water | |
| Plain paste | 1367.00 | 0.00 | 546.80 |
| 15% WGP | 1161.95 | 205.05 | 546.80 |
| 20% WGP | 1093.60 | 273.40 | 546.80 |
| 25% WGP | 1025.25 | 341.75 | 546.80 |
| 30% WGP | 956.90 | 410.10 | 546.80 |
| 35% WGP | 888.55 | 478.45 | 546.80 |
| Mixture | w/b | Water(g) | Cement (g) | WGP (g) | Hydration Peaks | Intensity of Hydration Peaks | ||
|---|---|---|---|---|---|---|---|---|
| Age (h) | Relative Value (%) | Intensity (mW/g) | Relative Value (%) | |||||
| Plain paste | 0.4 | 1.8827 | 4.7069 | 0.0000 | 8.69 | 1.000 | 4.54 | 1.000 |
| 15% GP | 0.4 | 1.8827 | 4.0009 | 0.7060 | 8.99 | 1.035 | 3.85 | 0.848 |
| 30% GP | 0.4 | 1.8827 | 3.2948 | 1.4121 | 9.01 | 1.037 | 3.23 | 0.711 |
| Type | Age (Days) | Original Content | Carbonation Reaction Content | Total CH (%) | Normalized CH | |||
|---|---|---|---|---|---|---|---|---|
| CH (%) | CaCO3 (%) | CaCO3 (%) | CH (%) | Per 1 g Binder | Per 1 g Cement | |||
| Cement | - | 2.22 | 3.68 | - | - | - | - | - |
| Plain paste | 1 | 12.05 | 9.00 | 5.32 | 3.94 | 13.76 | 0.1674 | 0.1674 |
| 35% WGP | 1 | 8.88 | 8.02 | 5.63 | 4.17 | 11.60 | 0.1322 | 0.2034 |
| Plain paste | 28 | 14.22 | 8.82 | 5.14 | 3.80 | 15.81 | 0.1970 | 0.1970 |
| 35% WGP | 28 | 9.54 | 8.55 | 6.15 | 4.55 | 12.65 | 0.1479 | 0.2275 |
| Plain paste | 90 | 16.07 | 4.75 | 1.07 | 0.79 | 14.64 | 0.1883 | 0.1883 |
| 35% WGP | 90 | 10.81 | 4.32 | 1.93 | 1.42 | 10.79 | 0.1367 | 0.2103 |
| Groups | Content | Cost | Function | Value | ||
|---|---|---|---|---|---|---|
| Cement (kg) | WGP (kg) | Water (kg) | CO2 Emissions (kg) | Compressive Strength (MPa) | F/C | |
| Plain paste | 1367.00 | 0.00 | 546.80 | 1452.44 | 63.89 | 0.044 |
| 15% WGP | 1161.95 | 205.05 | 546.80 | 1235.14 | 57.26 | 0.046 |
| 20% WGP | 1093.60 | 273.40 | 546.80 | 1162.71 | 56.35 | 0.048 |
| 25% WGP | 1025.25 | 341.75 | 546.80 | 1090.28 | 57.70 | 0.053 |
| 30% WGP | 956.90 | 410.10 | 546.80 | 1017.85 | 58.61 | 0.058 |
| 35% WGP | 888.55 | 478.45 | 546.80 | 945.42 | 56.36 | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.; Zhang, X.; Lin, M.; Wang, Q.; Sun, J. Recycling Local Waste Glass Bottles into Cement Paste: Effect on Hydration, Microstructure, and CO2 Emission. Materials 2023, 16, 6195. https://doi.org/10.3390/ma16186195
Ren F, Zhang X, Lin M, Wang Q, Sun J. Recycling Local Waste Glass Bottles into Cement Paste: Effect on Hydration, Microstructure, and CO2 Emission. Materials. 2023; 16(18):6195. https://doi.org/10.3390/ma16186195
Chicago/Turabian StyleRen, Fengming, Xiwen Zhang, Mingxin Lin, Qing Wang, and Jing Sun. 2023. "Recycling Local Waste Glass Bottles into Cement Paste: Effect on Hydration, Microstructure, and CO2 Emission" Materials 16, no. 18: 6195. https://doi.org/10.3390/ma16186195
APA StyleRen, F., Zhang, X., Lin, M., Wang, Q., & Sun, J. (2023). Recycling Local Waste Glass Bottles into Cement Paste: Effect on Hydration, Microstructure, and CO2 Emission. Materials, 16(18), 6195. https://doi.org/10.3390/ma16186195






