Wood-Poly(furfuryl Alcohol) Prepreg: A Novel, Ecofriendly Laminate Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preliminary Characterization
2.2. Prepreg Manufacturing
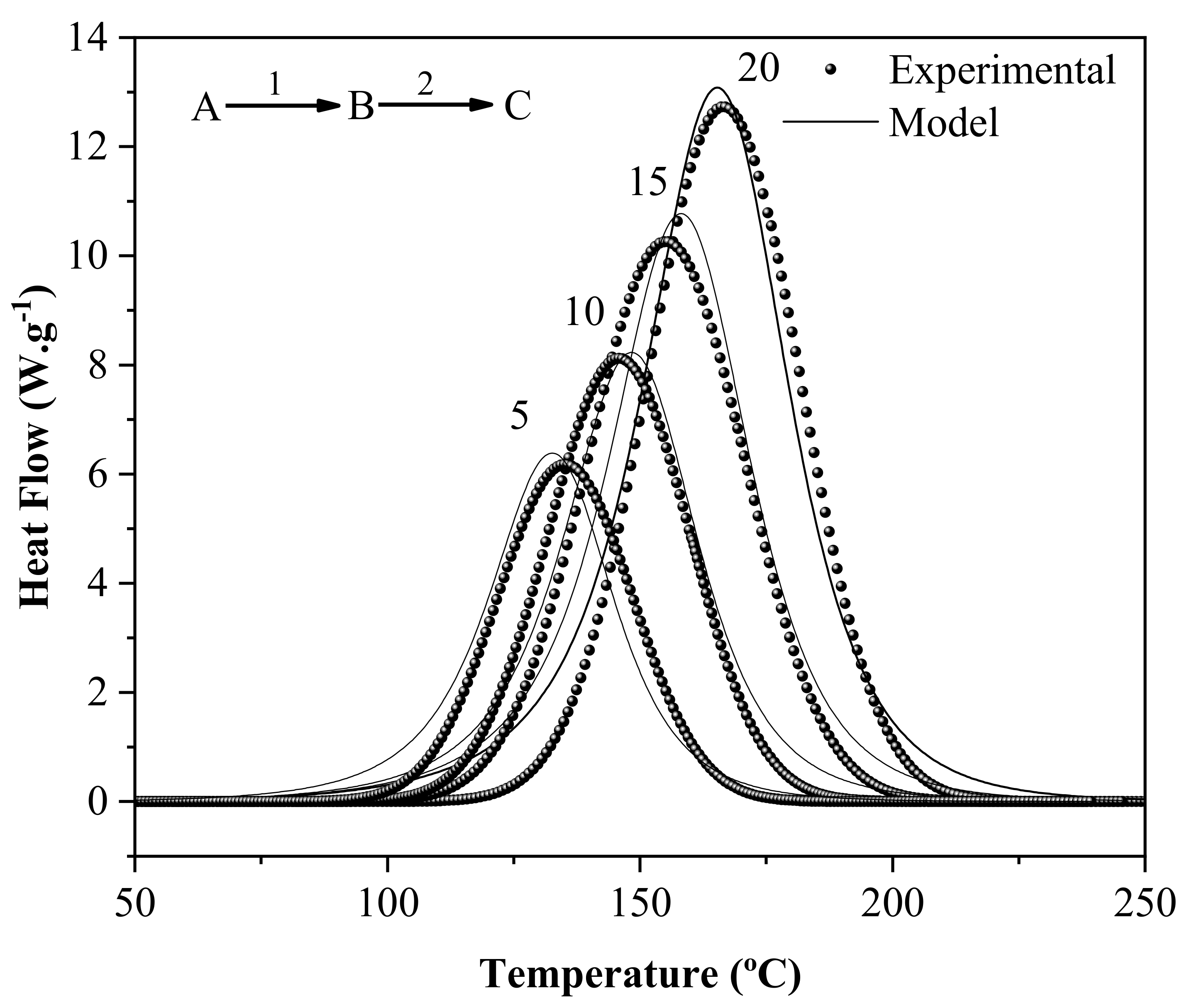
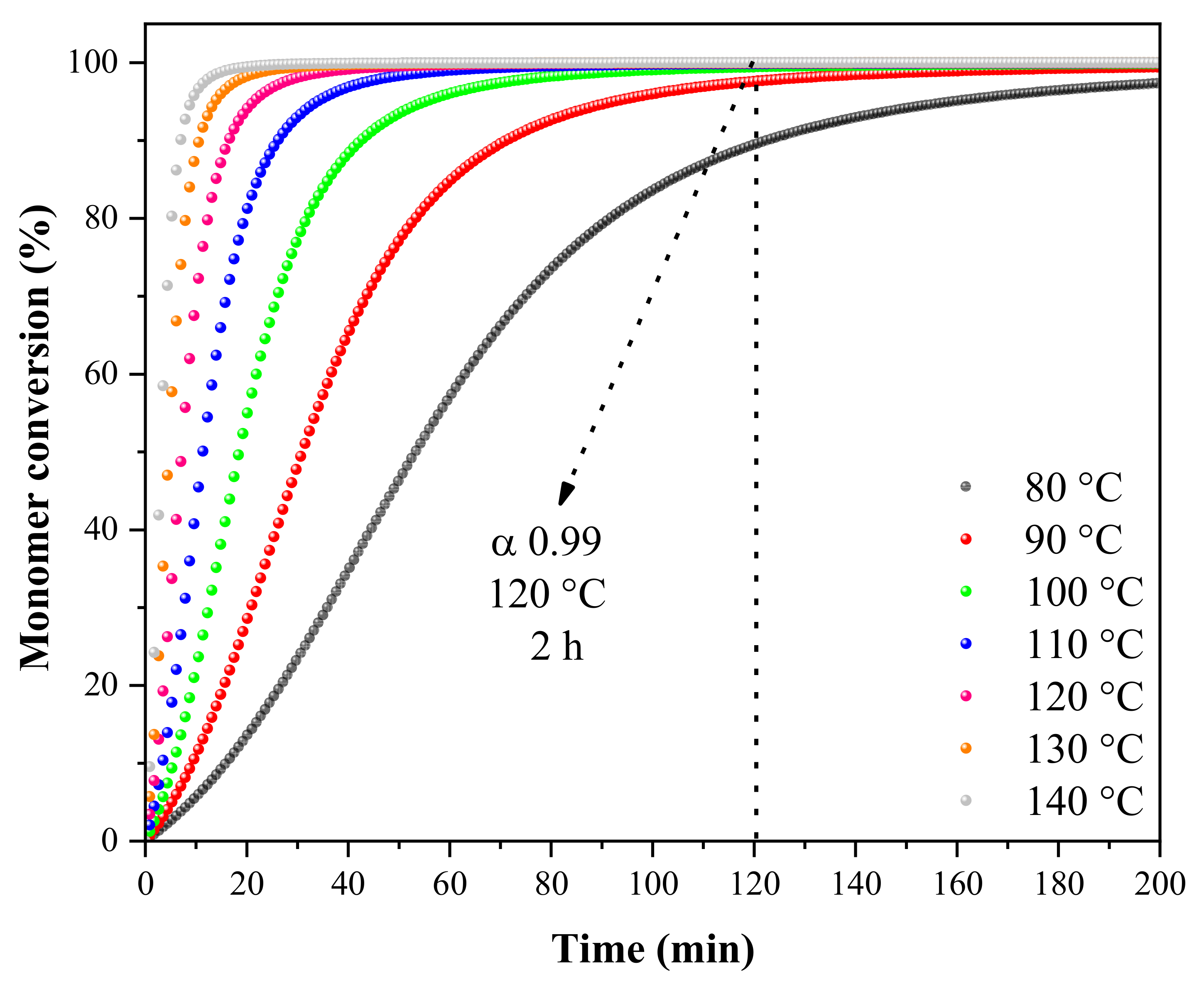
2.3. Polymerization Kinetics
2.4. Unidirectional Laminate Manufacturing
2.5. Laminate Characterization
2.6. Statistical Analysis
3. Results and Discussion
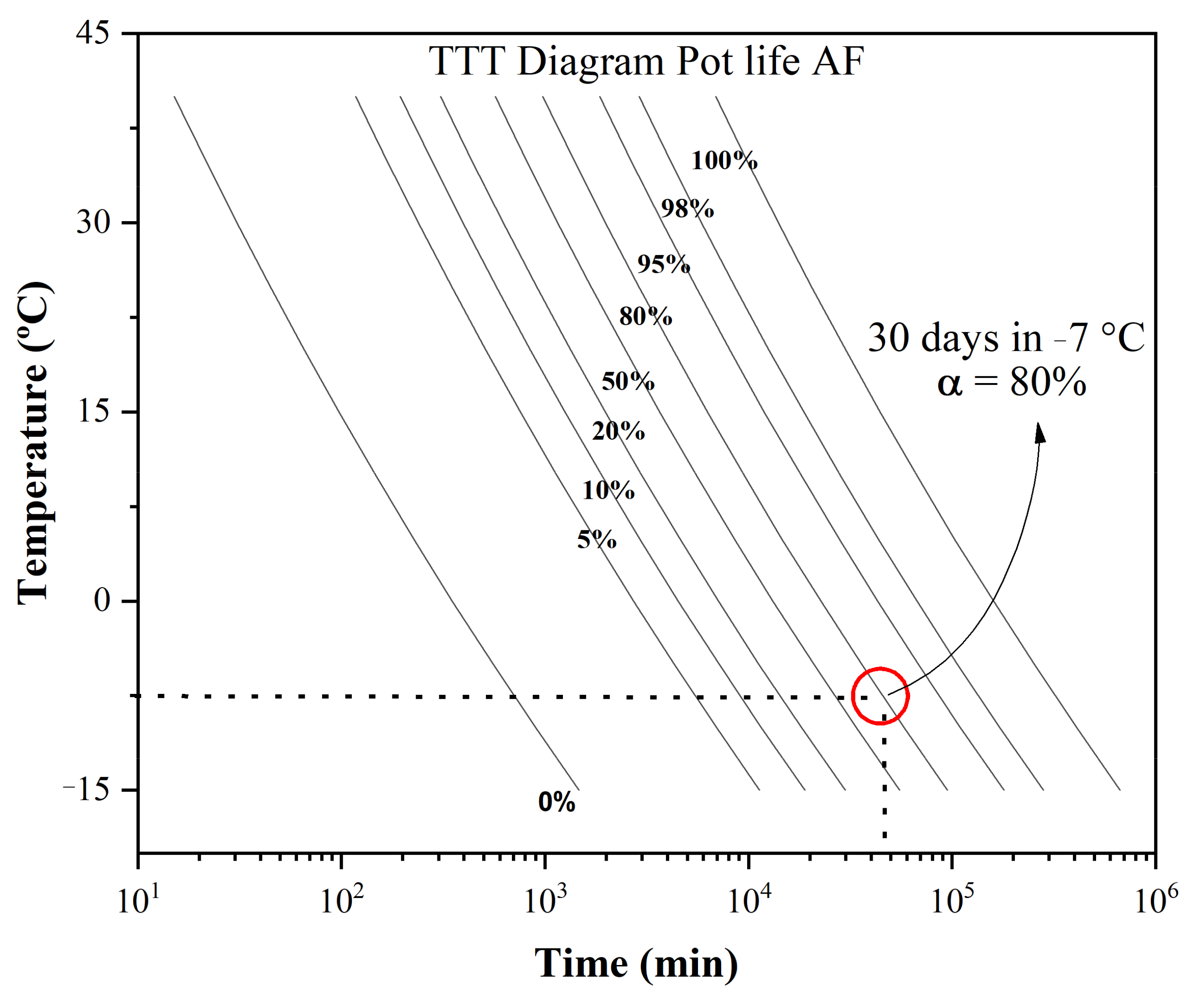
3.1. Polymerization Kinetics and Shelf Life
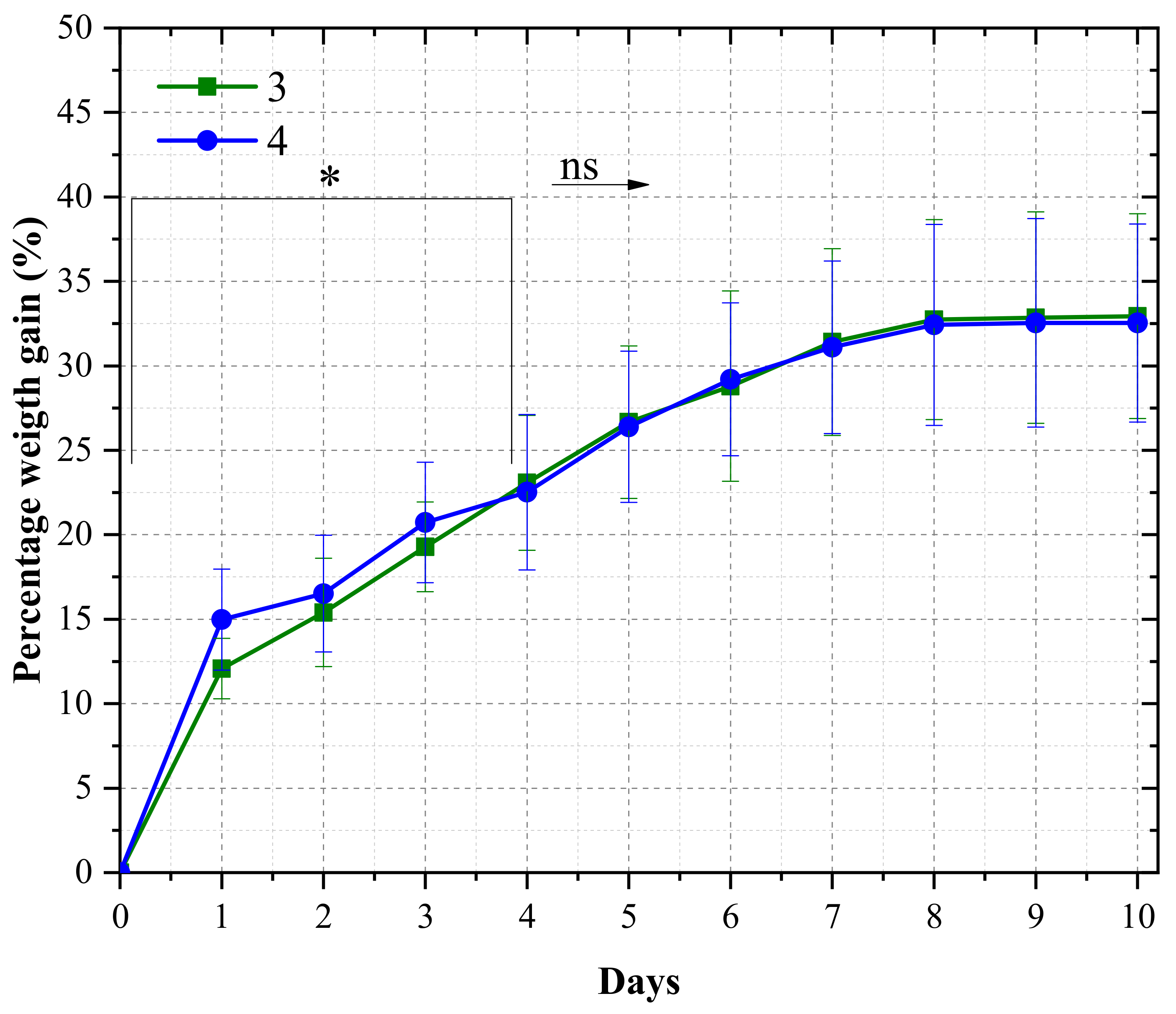
3.2. Laminate Morphology and Water Absorption
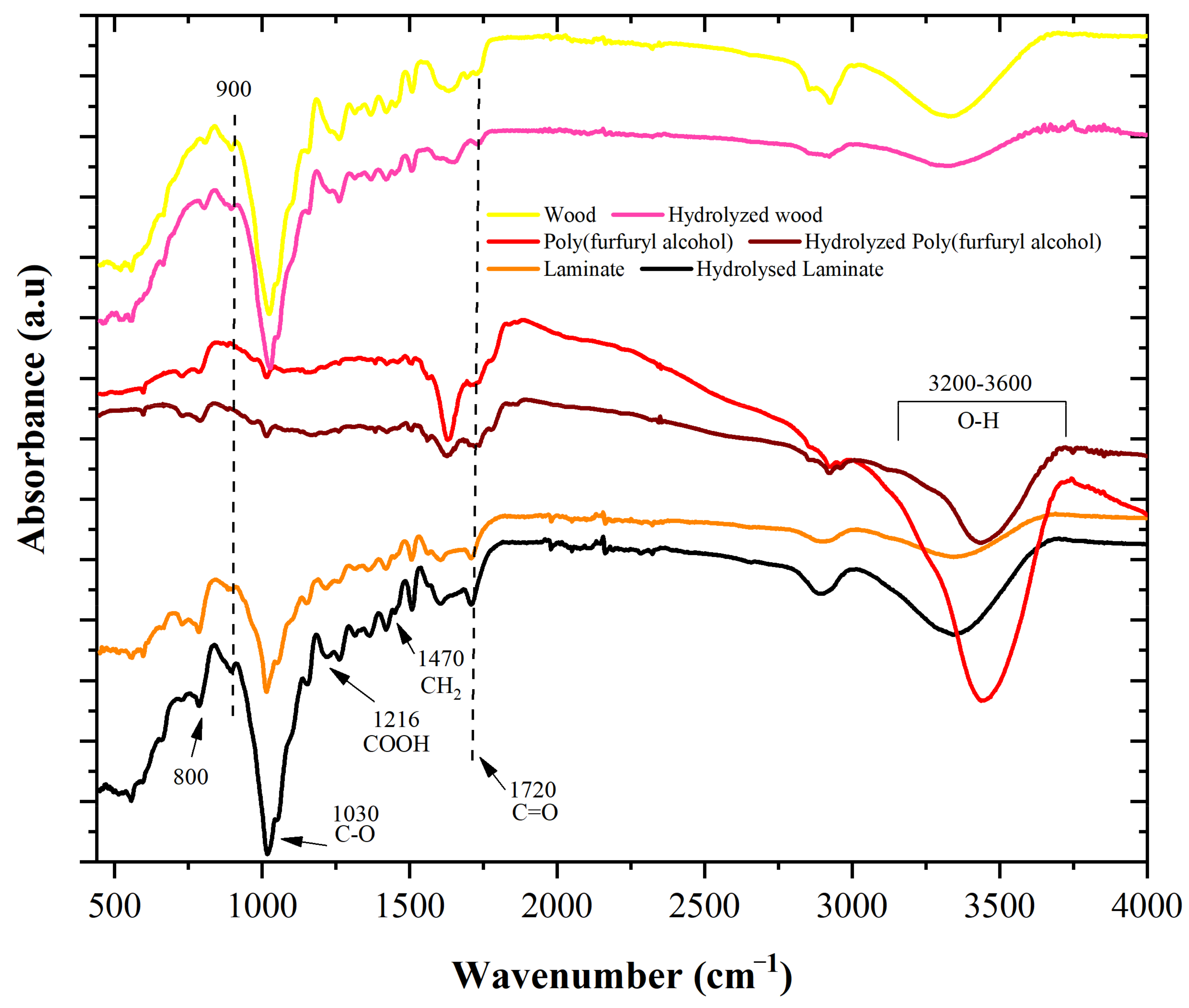
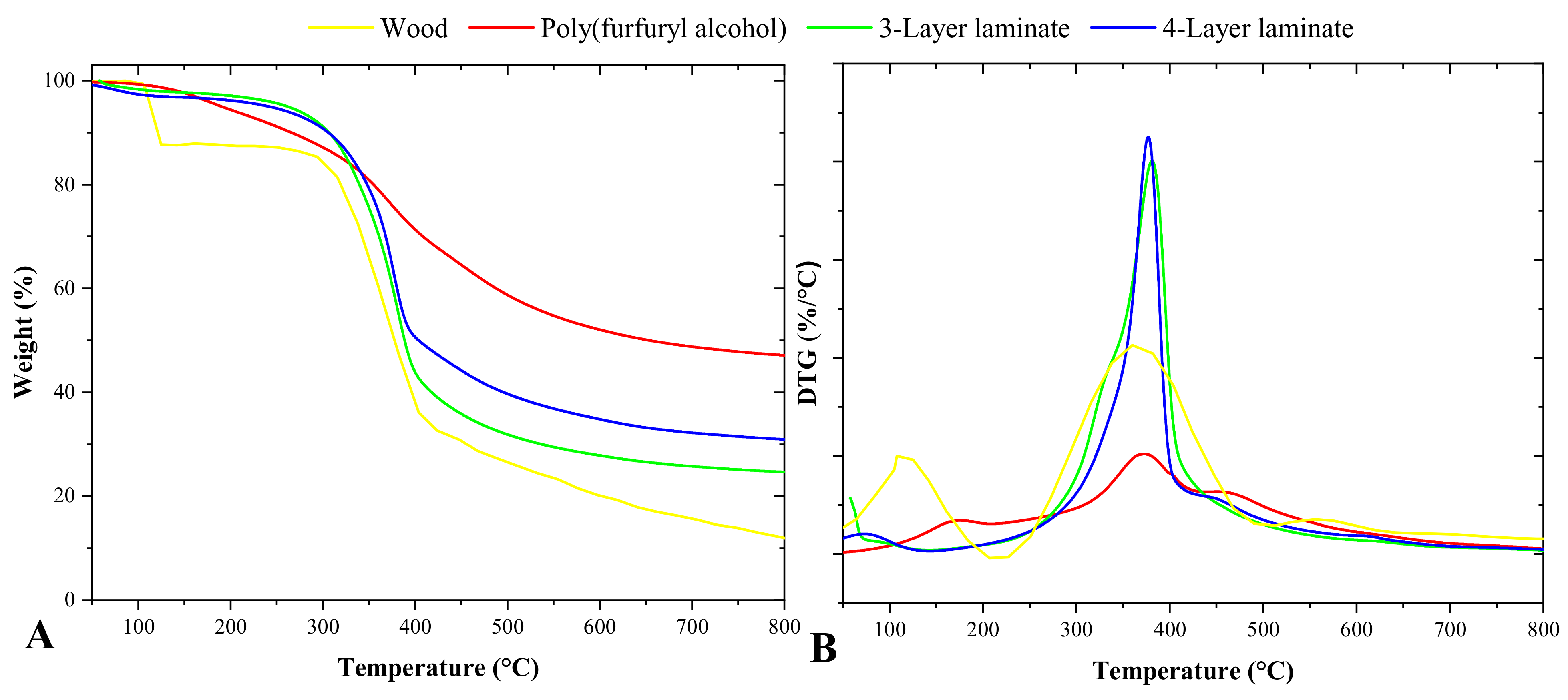
3.3. Chemical and Thermal Characterization
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budelmann, D.; Schmidt, C.; Meiners, D. Prepreg Tack: A Review of Mechanisms, Measurement, and Manufacturing Implication. Polym. Compos. 2020, 41, 3440–3458. [Google Scholar] [CrossRef]
- Joshi, S.J.; Patel, K.S.; Shah, D.B.; Patel, K.M.; Mawandiya, B.K. Development and Performance Analysis of out-of-Autoclave Curing Process for CFRP Composites. Adv. Mater. Process. Technol. 2022, 8, 1593–1603. [Google Scholar] [CrossRef]
- Stewart, A.L.; Poursartip, A. Characterization of Fibre Alignment in as-Received Aerospace Grade Unidirectional Prepreg. Compos. Part A Appl. Sci. Manuf. 2018, 112, 239–249. [Google Scholar] [CrossRef]
- Kupčák, R.; Zouhar, J. Application of Composite Materials in Sports Optics. Manuf. Technol. 2020, 20, 200–209. [Google Scholar] [CrossRef]
- Fang, W.; Yang, S.; Wang, X.-L.; Yuan, T.-Q.; Sun, R.-C. Manufacture and Application of Lignin-Based Carbon Fibers (LCFs) and Lignin-Based Carbon Nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Yadama, V.; Smith, L.V. Wood-Based Prepreg For Composite Laminates. Wood Fiber Sci. 2022, 54, 122–133. [Google Scholar] [CrossRef]
- Cheng, F.; Hu, Y.; Li, L. Interfacial Properties of Glass Fiber/unsaturated Polyester Resin/poplar Wood Composites Prepared with the Prepreg/press Process. Fibers Polym. 2015, 16, 911–917. [Google Scholar] [CrossRef]
- Wahab, N.H.A.; Tahir, P.M.; Yunus, N.Y.M.; Ashaari, Z.; Yong, A.C.C.; Ibrahim, N.A. Influence of Resin Molecular Weight on Curing and Thermal Degradation of Plywood Made from Phenolic Prepreg Palm Veneers. J. Adhes. 2014, 90, 210–229. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Biziks, V.; Mai, C.; Militz, H. Modification of Beech Veneers with Lignin Phenol Formaldehyde Resins in the Production of Laminated Veneer Lumber (LVL). Eur. J. Wood Wood Prod. 2018, 76, 843–851. [Google Scholar] [CrossRef]
- Silva, S.O.; Teixeira, L.A.; Gontijo, A.B.; Luz, S.M. Processing Characterization of Sisal/Epoxy Prepregs. J. Res. Updat. Polym. Sci. 2021, 10, 42–50. [Google Scholar] [CrossRef]
- Lee, B.J.; McDonald, A.G.; James, B. Influence of Fiber Length on the Mechanical Properties of Wood-Fiber/polypropylene Prepreg Sheets. Mater. Res. Innov. 2001, 4, 97–103. [Google Scholar] [CrossRef]
- Libera, V.D.; Teixeira, L.A.; Leão, R.M.; Luz, S.M. Evaluation of Thermal Behavior and Cure Kinetics of a Curauá Fiber Prepreg by the Non-Isothermal Method. Mater. Today Proc. 2019, 8, 839–846. [Google Scholar] [CrossRef]
- César dos Santos, J.; Ávila de Oliveira, L.; Panzera, T.H.; Remillat, C.D.L.; Farrow, I.; Placet, V.; Scarpa, F. Ageing of Autoclaved Epoxy/flax Composites: Effects on Water Absorption, Porosity and Flexural Behaviour. Compos. Part B Eng. 2020, 202, 108380. [Google Scholar] [CrossRef]
- Tomo, H.S.S.; Ujianto, O.; Rizal, R.; Pratama, Y. Effects of Number of Ply, Compression Temperature, Pressure and Time on Mechanical Properties of Prepreg Kenaf-Polypropilene Composites. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012026. [Google Scholar] [CrossRef]
- Pantaloni, D.; Bourmaud, A.; Baley, C.; Clifford, M.J.; Ramage, M.H.; Shah, D.U. A Review of Permeability and Flow Simulation for Liquid Composite Moulding of Plant Fibre Composites. Materials 2020, 13, 4811. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Tjong, J.; Nayak, S.K.; Sain, M. Studies on Permeability of Sisal Fibre Mat during Thermoset Resin Filling in Vacuum Infusion Process. Can. J. Chem. Eng. 2015, 93, 1364–1370. [Google Scholar] [CrossRef]
- Acosta, A.P.; Barbosa, K.T.; da Silva, A.A.X.; Gatto, D.A.; de Avila Delucis, R.; Amico, S.C. Vacuum Infusion as a Novel Method to Determine Wood Permeability. Eur. J. Wood Wood Prod. 2022, 81, 33–34. [Google Scholar] [CrossRef]
- Xie, L.; Xu, H.; Wang, Z.-P.; Li, X.-J.; Chen, J.-B.; Zhang, Z.-J.; Yin, H.-M.; Zhong, G.-J.; Lei, J.; Li, Z.-M. Toward Faster Degradation for Natural Fiber Reinforced Poly(lactic Acid) Biocomposites by Enhancing the Hydrolysis-Induced Surface Erosion. J. Polym. Res. 2014, 21, 357. [Google Scholar] [CrossRef]
- Acosta, A.P.; Schulz, H.R.; Barbosa, K.T.; Zanol, G.S.; Gallio, E.; Delucis, R.D.A.; Gatto, D.A. Dimensional Stability and Colour Responses of Pinus Elliottii Wood Subjected to Furfurylation Treatments. Maderas. Cienc. y Tecnol. 2020, 22, 303–310. [Google Scholar] [CrossRef]
- Kim, T.; Assary, R.S.; Pauls, R.E.; Marshall, C.L.; Curtiss, L.A.; Stair, P.C. Thermodynamics and Reaction Pathways of Furfuryl Alcohol Oligomer Formation. Catal. Commun. 2014, 46, 66–70. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Madsen, B. Chemorheological Study of a Polyfurfuryl Alcohol Resin System-Pre-Gel Curing Stage. Ind. Crops Prod. 2014, 52, 321–328. [Google Scholar] [CrossRef]
- Acosta, A.P.; Xavier da Silva, A.A.; de Avila Delucis, R.; Amico, S.C. Wood and Wood-Jute Laminates Manufactured by Vacuum Infusion. J. Build. Eng. 2023, 64, 105619. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Cruz, J.A.; Amico, S.C.; Bianchi, O. Effect of the Aramid Pulp on the Physicochemical, Viscoelastic Properties and Rheokinetics of Polyurethanes. J. Polym. Res. 2023, 30, 12. [Google Scholar] [CrossRef]
- ASTM D6641; Standard Test Method for Compressive Properties of Polymer Matrix Composite Materials Using a Combined Loading Compression (CLC) Test Fixture. ASTM: West Conshohocken, PA, USA, 2014.
- ASTM D3039; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. ASTM: West Conshohocken, PA, USA, 2014.
- Tondi, G.; Cefarin, N.; Sepperer, T.; D’Amico, F.; Berger, R.J.F.; Musso, M.; Birarda, G.; Reyer, A.; Schnabel, T.; Vaccari, L. Understanding the Polymerization of Polyfurfuryl Alcohol: Ring Opening and Diels-Alder Reactions. Polymers 2019, 11, 2126. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, D.; Jiang, P.; Li, G.; Yang, S.; Chu, F. Reaction Mechanisms of Furfuryl Alcohol Polymer with Wood Cell Wall Components. Holzforschung 2021, 75, 1150–1158. [Google Scholar] [CrossRef]
- Falco, G.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. FA Polymerization Disruption by Protic Polar Solvents. Polymers 2018, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New Insights on the Thermal Degradation Pathways of Neat Poly(furfuryl Alcohol) and Poly(furfuryl alcohol)/SiO2 Hybrid Materials. Polym. Degrad. Stab. 2009, 94, 908–913. [Google Scholar] [CrossRef]
- He, G.; Riedl, B.; Aït-Kadi, A. Model-Free Kinetics: Curing Behavior of Phenol Formaldehyde Resins by Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2003, 87, 433–440. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Chemorheological Analysis and Model-Free Kinetics of Acid Catalysed Furfuryl Alcohol Polymerization. Phys. Chem. Chem. Phys. 2007, 9, 5359–5366. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Grivel, J.C.; Madsen, B. Study on the Non-Isothermal Curing Kinetics of a Polyfurfuryl Alcohol Bioresin by DSC Using Different Amounts of Catalyst. Thermochim. Acta 2012, 529, 29–35. [Google Scholar] [CrossRef]
- Dias, T.D.C.; da Silva, A.A.X.; Tonatto, M.L.P.; Amico, S.C. Experimental Investigation on the Mechanical and Physical Properties of Glass/Jute Hybrid Laminates. Polymers 2022, 14, 4742. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xian, Y.; Deng, J.; Cheng, H.; Chen, F.; Wang, G. Evaluation of Water Absorption and Its Influence on the Physical-Mechanical Properties of Bamboo-Bundle Laminated Veneer Lumber. BioResources 2016, 11, 1359–1368. [Google Scholar] [CrossRef]
- De Melo, R.R.; Del Menezzi, C.H.S. Influence of Veneer Thickness on the Properties of LVL from Paricá (Schizolobium Amazonicum) Plantation Trees. Eur. J. Wood Wood Prod. 2014, 72, 191–198. [Google Scholar] [CrossRef]
- Wahab, R.; Samsi, H.W.; Mohamed, A. Utilization Potential of 30Year-Old Oil Palm Trunks Laminated Veneer Lumbers for Non-Structural Purposes. J. Sustain. Dev. 2009, 1, 109–113. [Google Scholar] [CrossRef]
- Salari, A.; Tabarsa, T.; Khazaeian, A.; Saraeian, A. Effect of Nanoclay on Some Applied Properties of Oriented Strand Board (OSB) Made from Underutilized Low Quality Paulownia (Paulownia Fortunei) Wood. J. Wood Sci. 2012, 58, 513–524. [Google Scholar] [CrossRef]
- Lunguleasa, A.; Ayrilmis, N.; Spirchez, C.; Özdemir, F. Investigation of the Effects of Heat Treatment Applied to Beech Plywood. Drv. Ind. 2018, 69, 349–355. [Google Scholar] [CrossRef]
- Chang, L.; Tang, Q.; Gao, L.; Fang, L.; Wang, Z.; Guo, W. Fabrication and Characterization of HDPE Resins as Adhesives in Plywood. Eur. J. Wood Wood Prod. 2018, 76, 325–335. [Google Scholar] [CrossRef]
- Valentino, H.A.S.; de Tarso Laia dos Reis e Silva Pupio, P.; Gandini, A.; Lacerda, T.M. Furfuryl Alcohol/tung Oil Matrix-Based Composites Reinforced with Bacterial Cellulose Fibres. Cellulose 2021, 28, 7109–7121. [Google Scholar] [CrossRef]
- González-Díaz, E.; Alonso-López, J.M. Characterization by Thermogravimetric Analysis of the Wood Used in Canary Architectural Heritage. J. Cult. Herit. 2017, 23, 111–118. [Google Scholar] [CrossRef]
- Duriot, R.; Rescalvo, F.J.; Pot, G.; Denaud, L.; Girardon, S.; Frayssinhes, R. An Insight into Mechanical Properties of Heartwood and Sapwood of Large French Douglas-Fir LVL. Constr. Build. Mater. 2021, 299, 123859. [Google Scholar] [CrossRef]
- Aydm, I.; Çolak, S.; Çolakoǧlu, G.; Salih, E. A Comparative Study on Some Physical and Mechanical Properties of Laminated Veneer Lumber (LVL) Produced from Beech (Fagus Orientalis Lipsky) and Eucalyptus (Eucalyptus Camaldulensis Dehn.) Veneers. Holzforschung 2004, 62, 218–220. [Google Scholar] [CrossRef]


| Step 1/Step 2 | E1 (T) (kJ mol−1) | log A1 (s−1) | E2 (T) (kJ mol−1) | log A2 (s−1) | Correlation Coefficient (r) | F Test |
|---|---|---|---|---|---|---|
| Bna/D1 (n = 1.50, log Kcat = 1.25) | 55.91 | 4.04 | 99.98 | 1.29 | 0.97 | 1.00 |
| Bna (n = 1.22, a = 0.58)/D3 | 55.42 | 5.08 | 124.28 | −0.75 | 0.98 | 1.05 |
| C1B/D2 (n = 1.50, log Kcat = 1.26) | 55.29 | 3.95 | 99.92 | 0.98 | 0.98 | 1.14 |
| C1B/D3 (n = 1.50, log Kcat = 1.27) | 55.21 | 3.94 | 96.85 | 0.98 | 0.98 | 1.34 |
| C1B/D4 (n = 1.50, log Kcat = 1.27) | 55.18 | 3.93 | 90.35 | −1.44 | 0.95 | 2.34 |
| CnB/R2 (n = 1.50, log Kcat = 1.27) | 55.18 | 3.93 | 90.25 | 4.94 | 0.94 | 4.68 |
| CnB/R3 (n = 1.49, log Kcat = 1.26) | 55.22 | 3.94 | 102.08 | −14.68 | 0.91 | 7.23 |
| CnB/D1 (n = 1.06, log Kcat = -4.00) | 78.85 | 7.59 | 98.25 | 1.31 | 0.93 | 8.35 |
| CnB (n = 1.42, log Kcat = 1.29)/D2 | 55.24 | 3.92 | 25.21 | 5.45 | 0.97 | 16.63 |
| CnB (n = 1.42, log Kcat = 1.29)/D3 | 55.59 | 4.67 | 87.89 | 1.29 | 0.169 | 22.83 |
| C1B (log Kcat = 0.86)/D3 | 50.51 | 4.03 | 99.23 | 3.26 | 0.97 | 29.15 |
| C1B (log Kcat = 0.86)/D2 | 54.05 | 4.01 | 87.89 | 2.48 | 0.96 | 29.32 |
| Bna (n = 1.22, a = 0.58)/D1 | 47.76 | 3.76 | 54.22 | 1.06 | 0.75 | 36.32 |
| C1B/D1 (log Kcat = 1.06) | 47.76 | 3.76 | 41.33 | 1.06 | 0.26 | 45.40 |
| Bna/D1 (n = 1.06, a = 4.00) | 47.76 | 3.76 | 85.23 | 1.31 | 0.23 | 80.48 |
| B1 (n = 1.22, a = 0.58)/D2 | 55.42 | 5.08 | 29.78 | 98.19 | 0.98 | 111.75 |
| Group | T2% | T5% | T50% | Residue (%) |
|---|---|---|---|---|
| Wood | 106.60 | 113.24 | 366.40 | 12.60 |
| Poly(furfuryl alcohol) | 142.59 | 190.20 | 653.56 | 47.12 |
| 3-Layer laminate | 118.90 | 261.88 | 388.56 | 24.67 |
| 4-Layer laminate | 90.57 | 241.94 | 403.67 | 30.97 |
| Group | Density (g/cm3) | Compressive Strength (MPa) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Modulus (MPa) | Flexural Strength (MPa) |
|---|---|---|---|---|---|---|
| 3 | 0.937 (0.087 a) | 92.5 (7.25 a) | 43.8 (11.2 a) | 7151.0 (656 a) | 11953.00 (1021 a) | 108.15 (33.4 a) |
| 4 | 0.849 (0.084 a) | 100.0 (5.38 a) | 59.6 (11.4 a) | 7297.0 (976 a) | 13627.00 (843 a) | 105.54 (17.60 a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, A.P.; Esteves, B.; Cruz, J.A.d.; Aramburu, A.B.; Kairytė, A.; Członka, S.; Ramos, D.O.; Goularte, M.d.P.; Delucis, R.d.A.; Gatto, D.A.; et al. Wood-Poly(furfuryl Alcohol) Prepreg: A Novel, Ecofriendly Laminate Composite. Materials 2023, 16, 6237. https://doi.org/10.3390/ma16186237
Acosta AP, Esteves B, Cruz JAd, Aramburu AB, Kairytė A, Członka S, Ramos DO, Goularte MdP, Delucis RdA, Gatto DA, et al. Wood-Poly(furfuryl Alcohol) Prepreg: A Novel, Ecofriendly Laminate Composite. Materials. 2023; 16(18):6237. https://doi.org/10.3390/ma16186237
Chicago/Turabian StyleAcosta, Andrey Pereira, Bruno Esteves, Joziel Aparecido da Cruz, Arthur Behenck Aramburu, Agnė Kairytė, Sylwia Członka, Dionatan Orestes Ramos, Matheus de Paula Goularte, Rafael de Avila Delucis, Darci Alberto Gatto, and et al. 2023. "Wood-Poly(furfuryl Alcohol) Prepreg: A Novel, Ecofriendly Laminate Composite" Materials 16, no. 18: 6237. https://doi.org/10.3390/ma16186237









