Investigation of the Fabrication of Diamond/SiC Composites Using α-Si3N4/Si Infiltration
Abstract
1. Introduction
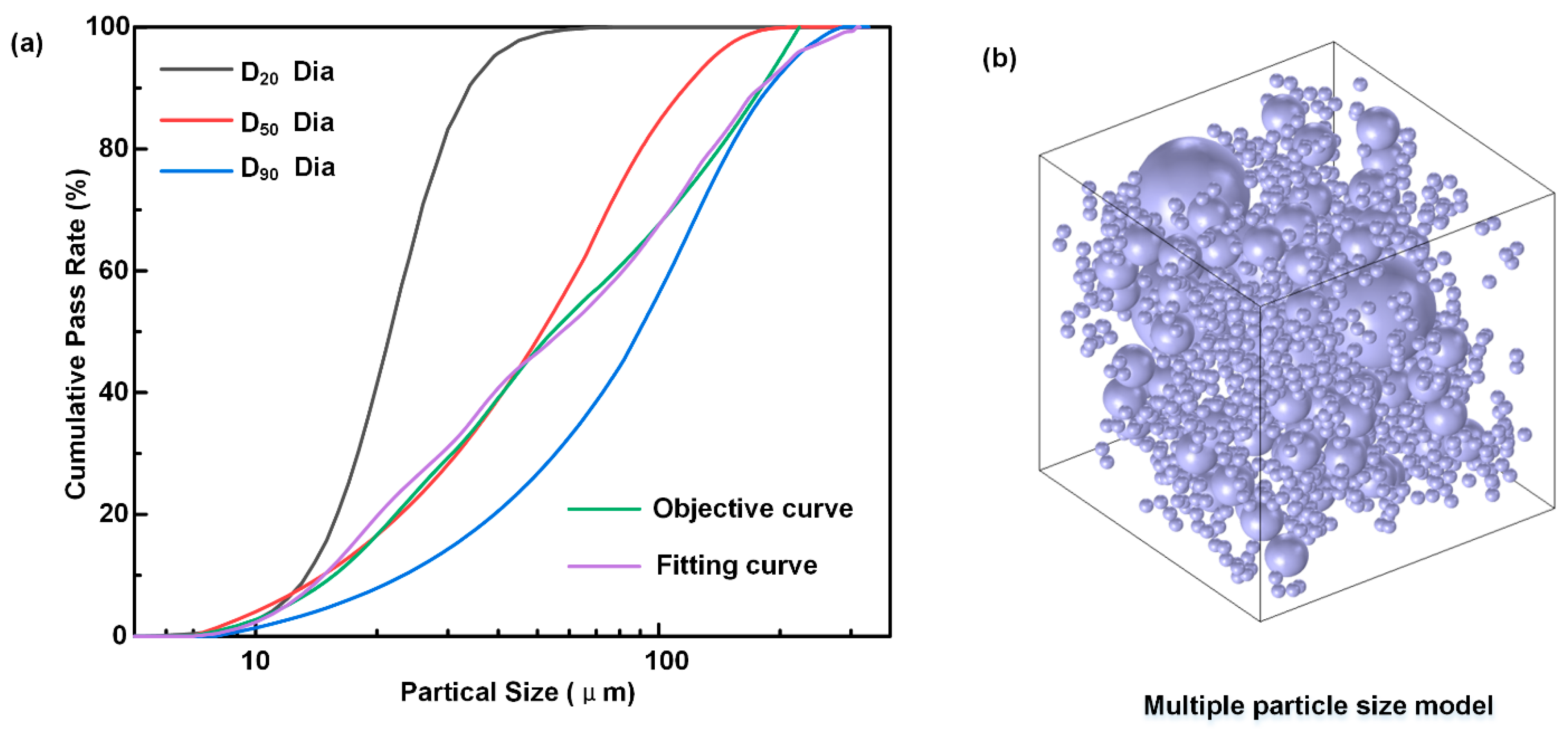
2. Optimization of Diamond Particle Gradation for Diamond/SiC Composites
3. Experimental Setup
3.1. Materials
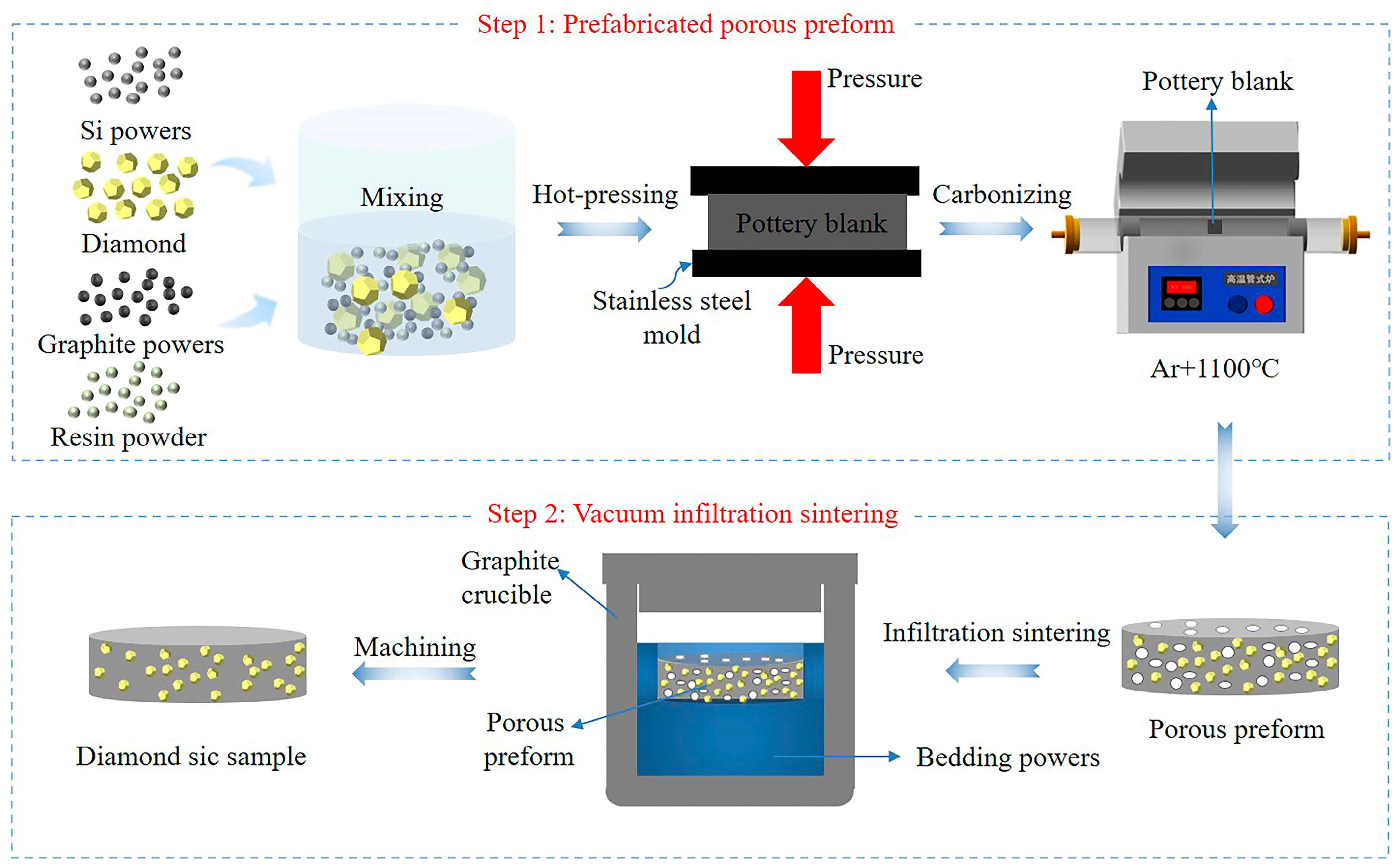
3.2. Preparation of Dia/SiC Composites
3.3. Characterization
4. Results and Discussion
4.1. Mechanism of Dia/SiC Sample Formation
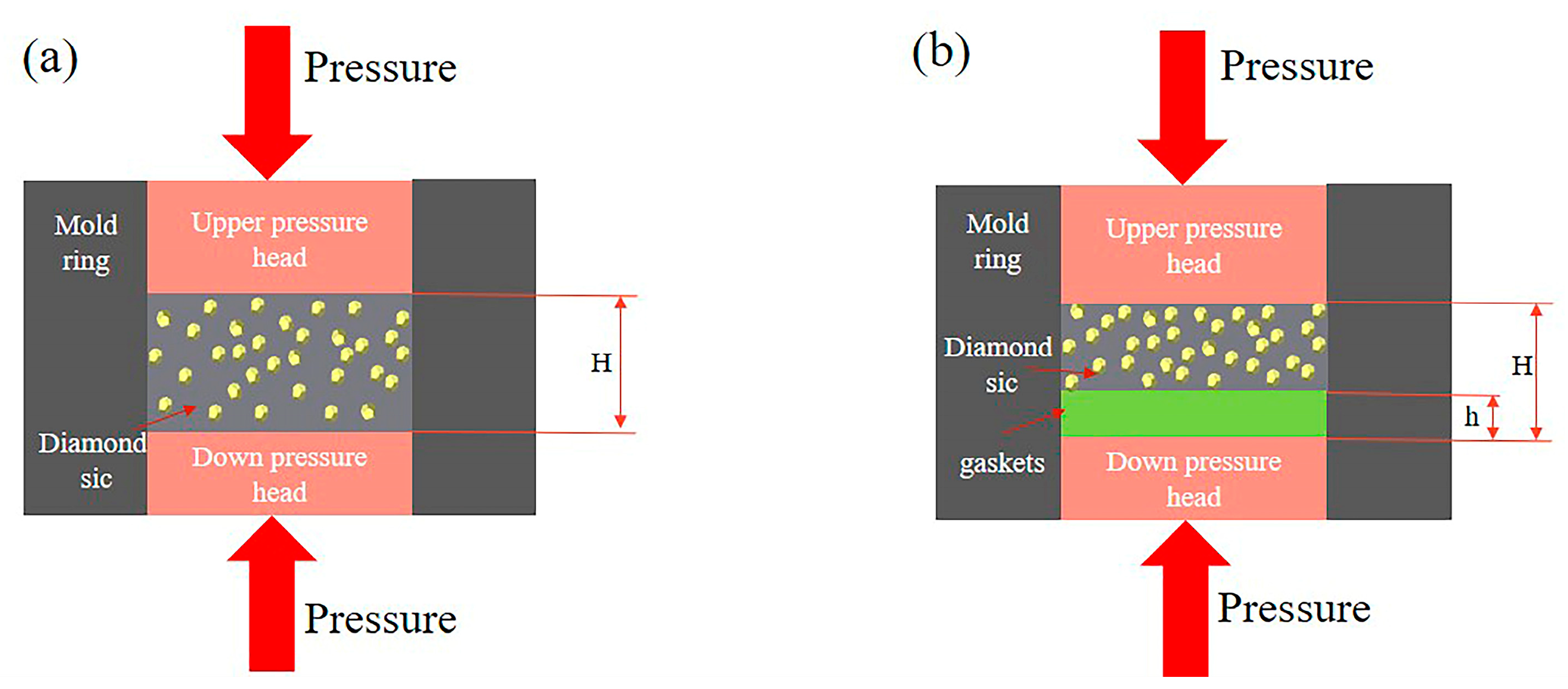
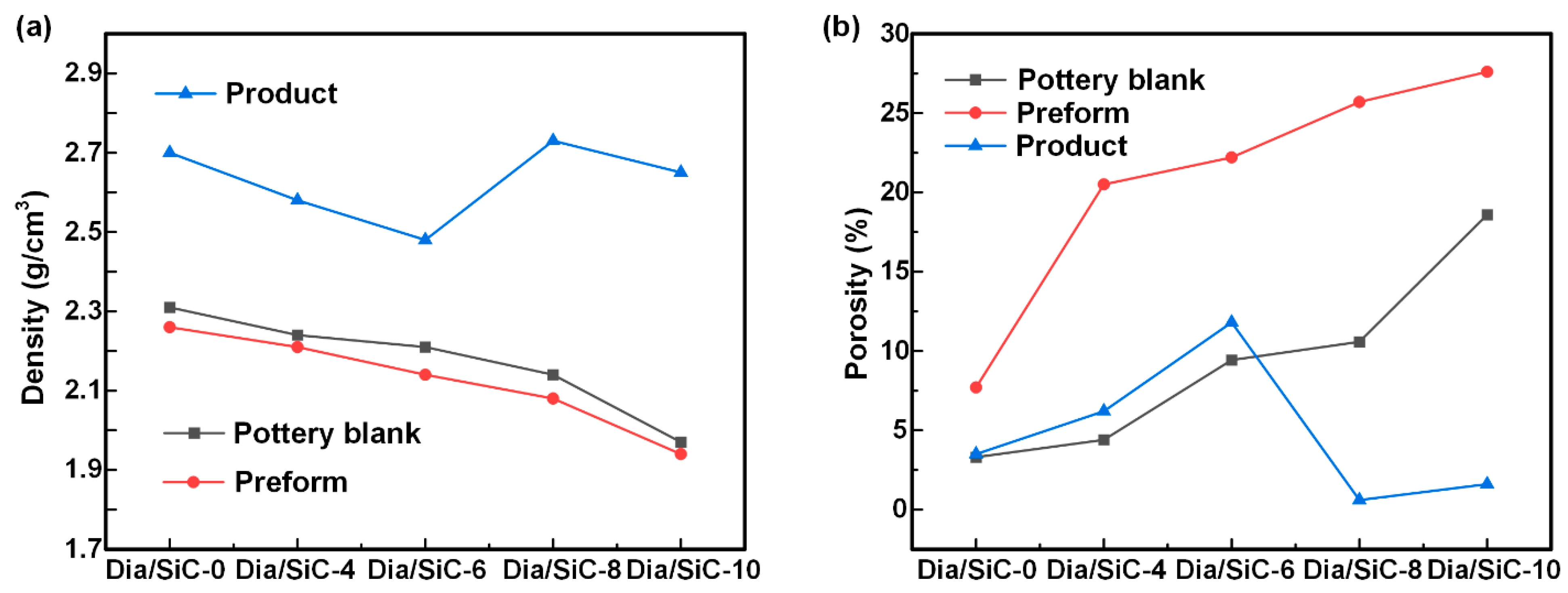
4.2. Influence of the Pressing Process on the Density and Porosity of the Dia/SiC Samples
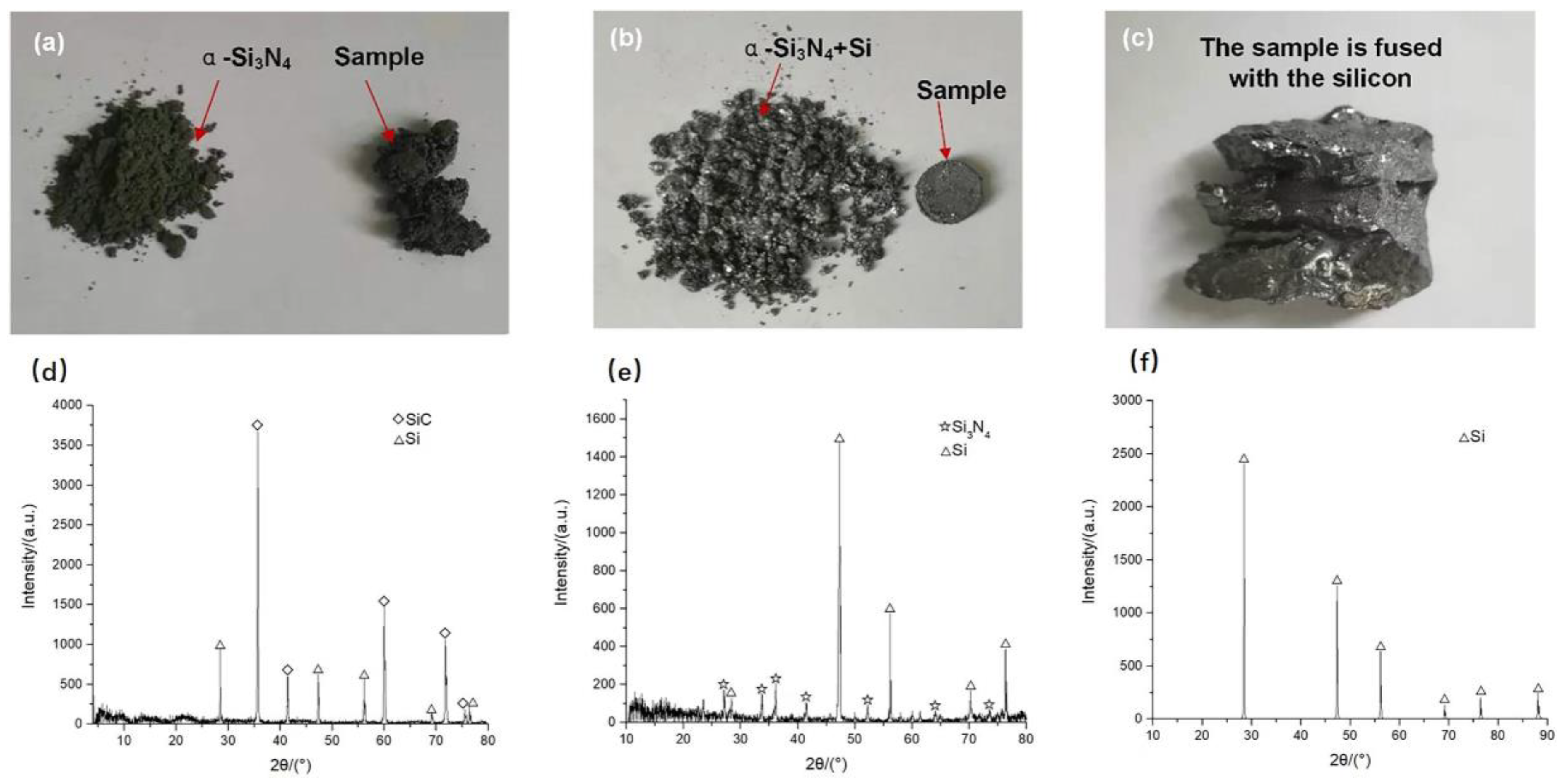
4.3. Influence of Bedding Powder Types on Dia/SiC Sample Preparation
4.4. Change Trend in Buried Powder Composition with the Increase of Sintering Temperature during Vacuum Infiltration
5. Conclusions
- (1)
- A multiscale diamond ratio optimization model based on the Dinger–Funk particle stacking theory was established, and the optimal volume ratio for diamonds with sizes of 20, 50, and 90 µm was 1:3:6.
- (2)
- Dia/SiC composites were prepared using the low-pressure siliconizing method at 1600 °C, and the effect of the preparation process on product density was investigated. The highest density and lowest porosity obtained were 2.73 g/cm3 and 0.6%, respectively.
- (3)
- A mixed powder of α-Si3N4 and Si was used as the bedding powder to effectively avoid the adhesion of the product to molten silicon. α-Si3N4 was used as a silicon source to generate silicon carbide at high temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, Y.; Guo, X.; Qian, Y.; Gao, H.; Weber, D.H.; Zhang, L.; Goodenough, J.B.; Yu, G. In Situ Formation of Liquid Metals via Galvanic Replacement Reaction to Build Dendrite-Free Alkali-Metal-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 12170–12177. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Song, Z.; Hao, H.; Yu, Z.; Cao, M.; Zhang, S.; Lanagan, M.T.; Liu, H. Homogeneous/Inhomogeneous-Structured Dielectrics and Their Energy-Storage Performances. Adv. Mater. 2017, 29, 1601727. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, M.Y.; Andreev, A.A.; Ezubchenko, I.S.; Chernykh, I.A.; Mayboroda, I.O.; Kolobkova, E.M.; Khrapovitskaya, Y.V.; Grishchenko, J.V.; Perminov, P.A.; Sedov, V.S.; et al. GaN-based Heterostructures with CVD Diamond Heat Sinks: A New Fabrication Approach Towards Efficient Electronic Devices. Appl. Mater. Today 2022, 26, 101338. [Google Scholar] [CrossRef]
- Raza, K.; Khalid, F.A. Optimization of Sintering Parameters for Diamond–Copper Composites in Conventional Sintering and their Thermal Conductivity. J. Alloys Compd. 2014, 615, 111–118. [Google Scholar] [CrossRef]
- Kondakci, E.; Solak, N. Enhanced Thermal Conductivity and Long-Term Stability of Diamond/Aluminum Composites Using SiC-coated Diamond Particles. J. Mater. Sci. 2022, 57, 3430–3440. [Google Scholar] [CrossRef]
- Voronin, G.A.; Zerda, T.W.; Qian, J.; Zhao, Y.; Dub, S.N. Diamond–SiC Nanocomposites Sintered from a Mixture of Diamond and Silicon Nanopowders. Diam. Relat. Mater. 2003, 12, 1477–1481. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Gierlotka, S.; Gromnitskaya, E.L.; Kozubowski, J.A.; Palosz, B.; Lojkowski, W.; Naletov, A.M. Mechanical Properties and Microstructure of Diamond–SiC Nanocomposites. Inorg. Mater. 2002, 38, 1117–1122. [Google Scholar] [CrossRef]
- Ohtaka, O.; Shimono, M.; Ohnishi, N.; Fukui, H.; Takebe, H.; Arima, H.; Yamanaka, T.; Kikegawa, T.; Kume, S. HIP Production of a diamond/SiC Composite and Application to High-Pressure Anvils. Phys. Earth Planet. Inter. 2004, 143–144, 587–591. [Google Scholar] [CrossRef]
- Wu, Q.; Peng, F.; Li, Q.; Zhang, W.; Guan, J.; Dou, Z. Study on the Thermal Conductivity of HTHP Diamond/SiC Ceramic Heat Sink Material. Diam. Abras. Eng. 2011, 31, 40–43. [Google Scholar]
- Nauyoks, S.; Wieligor, M.; Zerda, T.W.; Balogh, L.; Ungar, T.; Stephens, P. Stress and Dislocations in Diamond–SiC Composites Sintered at High Pressure, High Temperature Conditions. Compos. Part Appl. Sci. Manuf. 2009, 40, 566–572. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Su, Z.A.; Fang, C.; Zeng, G.; Huang, Q. Fabrication and Performance of Micro-Diamond Modified C/SiC Composites Via Precursor Impregnation and Pyrolysis Process. Ceram. Int. 2018, 44, 9601–9608. [Google Scholar] [CrossRef]
- Zhu, C.; Lang, J.; Ma, N. Preparation of Si–Diamond–SiC Composites by in-Situ Reactive Sintering and Their Thermal Properties. Ceram. Int. 2012, 38, 6131–6136. [Google Scholar] [CrossRef]
- Shimono, M.; Kazehaya, K.; Kume, S. HIP-Sintering of Si-Mixed Diamond Powder (Special Issue on High Pressure). J. Soc. Mater. Sci. Jpn. 1998, 47, 990–993. [Google Scholar] [CrossRef]
- Mashhadikarimi, M.; Gomes, U.U.; Oliveira, M.P.; Guimarães, R.D.S.; Filgueira, M. Study HTHP Sintered WC/Co Hardmetal. Mater. Res. 2017, 20, 138–143. [Google Scholar] [CrossRef][Green Version]
- Gordeev, S.K.; Zhukov, S.G.; Danchukova, L.V. Low-Pressure Fabrication of Diamond–SiC–Si Composites. Inorg. Mater. 2001, 37, 579–583. [Google Scholar] [CrossRef]
- Mlungwane, K.; Herrmann, M.; Sigalas, I. The Low-Pressure Infiltration of Diamond by Silicon to Form Diamond–Silicon Carbide Composites. J. Eur. Ceram. Soc. 2008, 28, 321–326. [Google Scholar] [CrossRef]
- Matthey, B.; Höhn, S.; Wolfrum, A.; Mühle, U.; Motylenko, M.; Rafaja, D.; Michaelis, A.; Herrmann, M. Microstructural Investigation of diamond-SiC Composites Produced by Pressureless Silicon Infiltration. J. Eur. Ceram. Soc. 2017, 37, 1917–1928. [Google Scholar] [CrossRef]
- Zheng, W.; He, X.; Wu, M.; Ren, S.; Cao, S.; Guan, D.; Liu, R.; Qu, X. Thermal Expansion Coefficient of Diamond/SiC Composites Prepared by Silicon Vapor Infiltration in Vacuum. Vacuum 2019, 159, 507–515. [Google Scholar] [CrossRef]
- Qian, J.M.; Jin, Z.H.; Wang, X.W. Porous SiC Ceramics Fabricated by Reactive Infiltration of Gaseous Silicon into Charcoal. Ceram. Int. 2004, 30, 947–951. [Google Scholar] [CrossRef]
- Qian, J.; Wang, J.; Jin, Z. Preparation and Properties of Porous Microcellular SiC Ceramics by Reactive Infiltration of Si Vapor into Carbonized Basswood. Mater. Chem. Phys. 2003, 82, 648–653. [Google Scholar] [CrossRef]
- Herrmann, M.; Matthey, B.; Höhn, S.; Kinski, I.; Rafaja, D.; Michaelis, A. Diamond-Ceramics Composites—New Materials for a Wide Range of Challenging Applications. J. Eur. Ceram. Soc. 2012, 32, 1915–1923. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, C.Y.; Karandikar, P.; Ni, C. Interfacial Zone Surrounding the Diamond in Reaction Bonded Diamond/SiC Composites: Interphase Structure and Formation Mechanism. J. Eur. Ceram. Soc. 2019, 39, 5190–5196. [Google Scholar] [CrossRef]
- Lange, F.; Mörtel, H.; Rudert, V. Dense Packing of Cement Pastes and Resulting Consequences on Mortar Properties. Cem. Concr. Res. 1997, 27, 1481–1488. [Google Scholar] [CrossRef]
- Zhu, P.; Mao, X.Q.; Qu, W.; Li, Z.; Ma, Z.J. Investigation of Using Recycled Powder from Waste of Clay Bricks and Cement Solids in Reactive Powder Concrete. Constr. Build. Mater. 2016, 113, 246–254. [Google Scholar] [CrossRef]
- Mao, L.; Han, J.; Zhao, D.; Song, N.; Shi, L.; Wang, J. Particle Packing Theory Guided Thermal Conductive Polymer Preparation and Related Properties. ACS Appl. Mater. Interfaces 2018, 10, 33556–33563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, C.; Feng, W.; Men, J.; Cheng, L.; Zhang, L. Microstructure and Properties of Diamond/SiC Composites Prepared by Tape-Casting and Chemical Vapor Infiltration Process. J. Eur. Ceram. Soc. 2014, 34, 3489–3498. [Google Scholar] [CrossRef]
- Ramesh, P.D.; Rao, K.J. Carbothermal Reduction and Nitridation Reaction of SiOx and Preoxidized SiOx: Formation of α-Si3N4 Fibers. J. Mater. Res. 1994, 9, 2330–2340. [Google Scholar] [CrossRef]
- Wu, X.C.; Song, W.H.; Huang, W.D.; Pu, M.H.; Zhao, B.; Sun, Y.P.; Du, J.J. Simultaneous Growth of α-Si3N4and β-SiC Nanorods. Mater. Res. Bull. 2001, 36, 847–852. [Google Scholar] [CrossRef]
- Umebayashi, K.K. Room Temperature Strength and Fracture Toughness of β-Sialon(z = 0.5)·SiC Composite Fabricated from α-Si3N4, Aluminum-Isopropoxide and β-SiC. J. Mater. Sci. Lett. 1996, 15, 1990–1993. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, B.; Zhang, Y.; Zhao, J.; Wang, J.; Huang, G. Investigation of the Fabrication of Diamond/SiC Composites Using α-Si3N4/Si Infiltration. Materials 2023, 16, 6252. https://doi.org/10.3390/ma16186252
Xing B, Zhang Y, Zhao J, Wang J, Huang G. Investigation of the Fabrication of Diamond/SiC Composites Using α-Si3N4/Si Infiltration. Materials. 2023; 16(18):6252. https://doi.org/10.3390/ma16186252
Chicago/Turabian StyleXing, Bo, Yingfan Zhang, Jinzhui Zhao, Jianyu Wang, and Guoqin Huang. 2023. "Investigation of the Fabrication of Diamond/SiC Composites Using α-Si3N4/Si Infiltration" Materials 16, no. 18: 6252. https://doi.org/10.3390/ma16186252
APA StyleXing, B., Zhang, Y., Zhao, J., Wang, J., & Huang, G. (2023). Investigation of the Fabrication of Diamond/SiC Composites Using α-Si3N4/Si Infiltration. Materials, 16(18), 6252. https://doi.org/10.3390/ma16186252






