Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures
Abstract
:1. Introduction
2. Experimental Programs
2.1. Materials
2.2. Test Specimens for Repair Coating
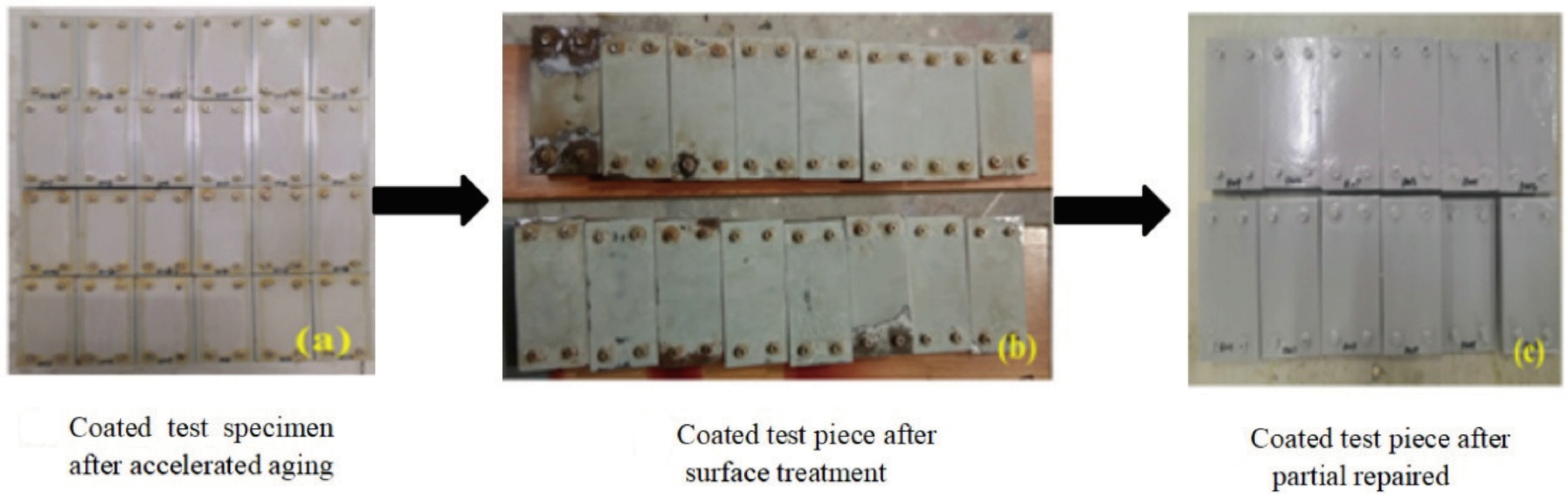
2.2.1. Details of the Methods for Partial Repair of Coatings
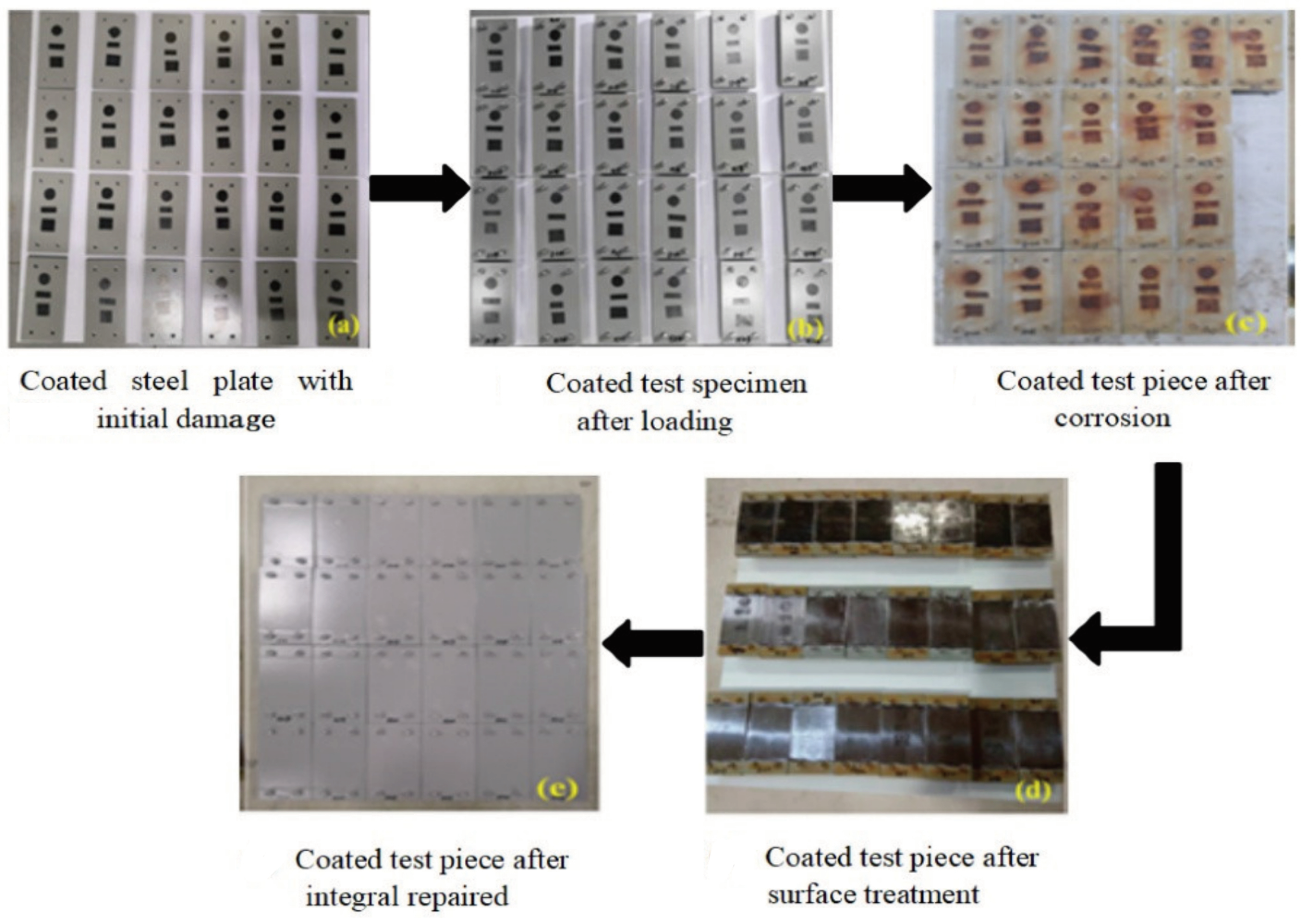
2.2.2. Details of the Methods for Integral Repair of Coatings
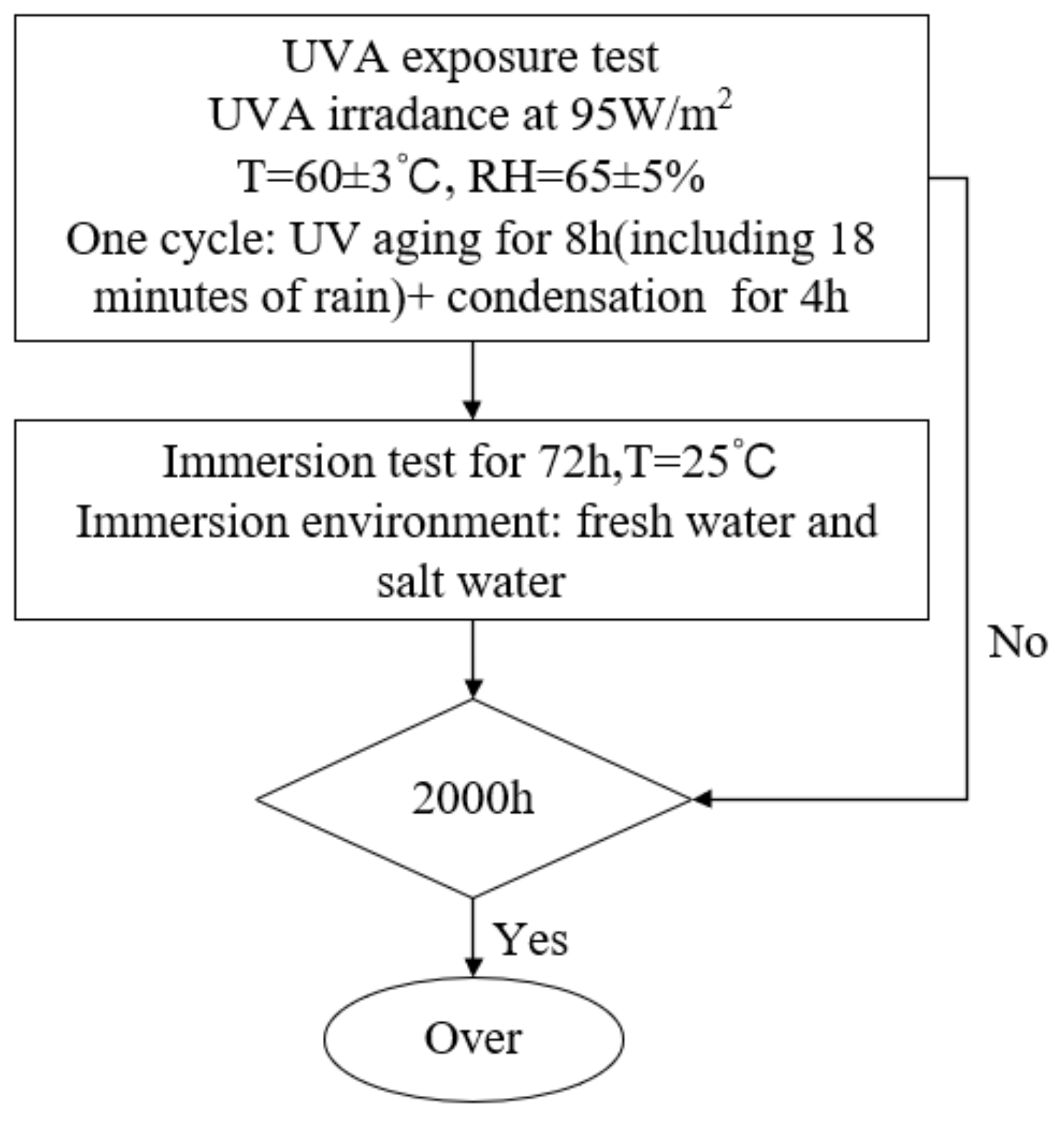
2.3. Performance Testing
3. Results and Discussion
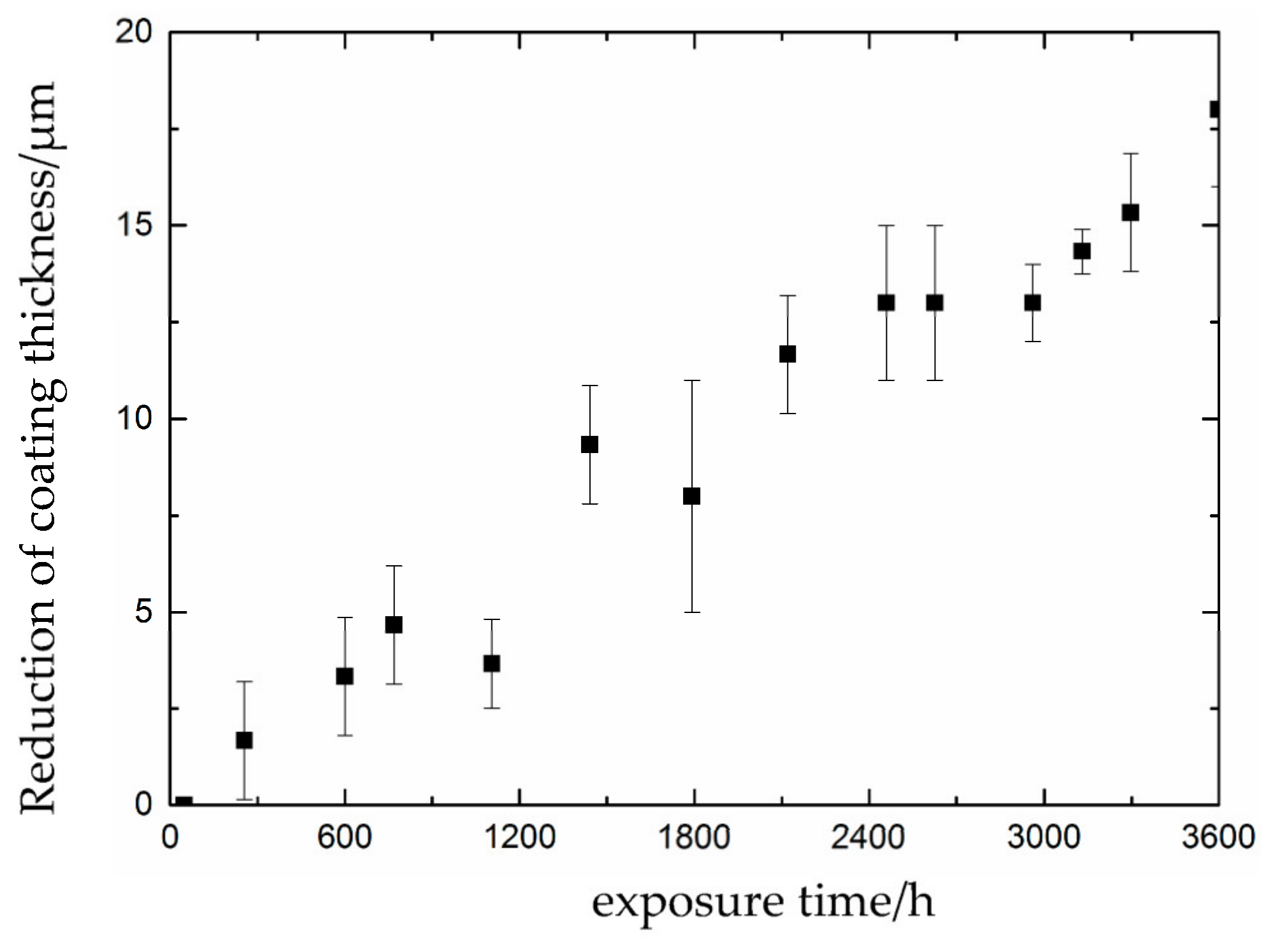
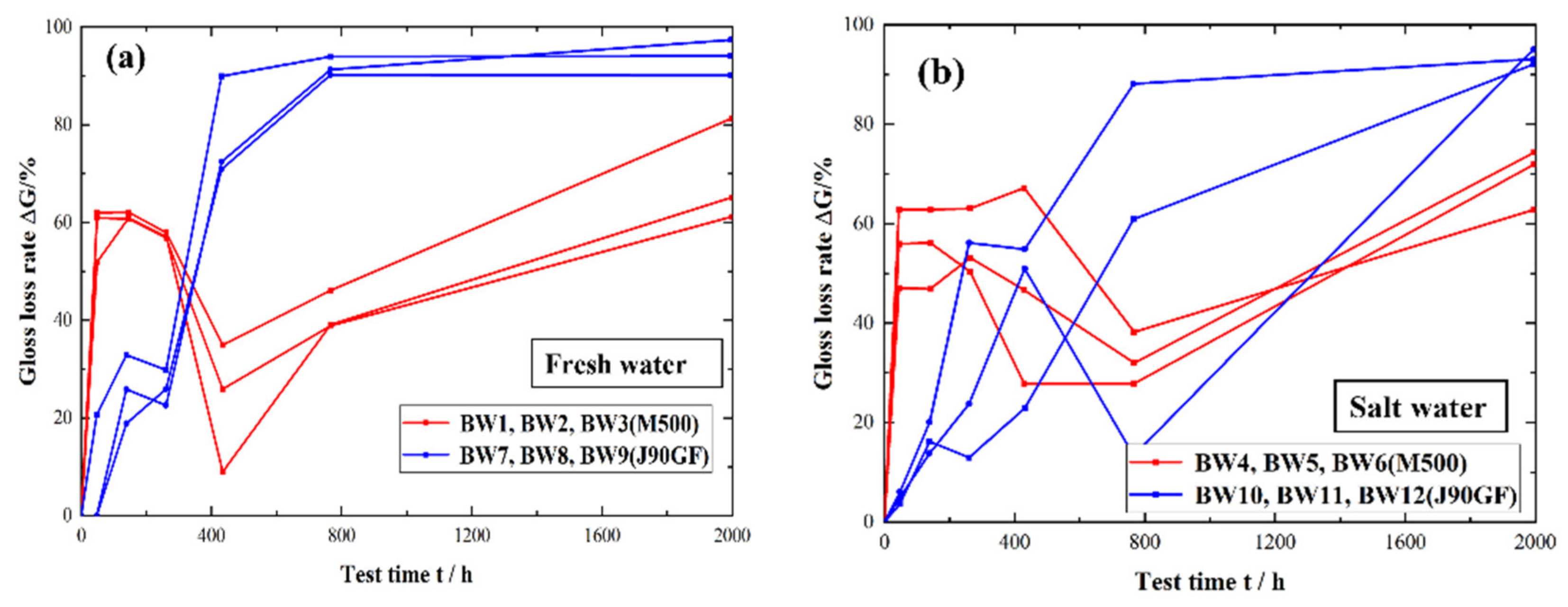
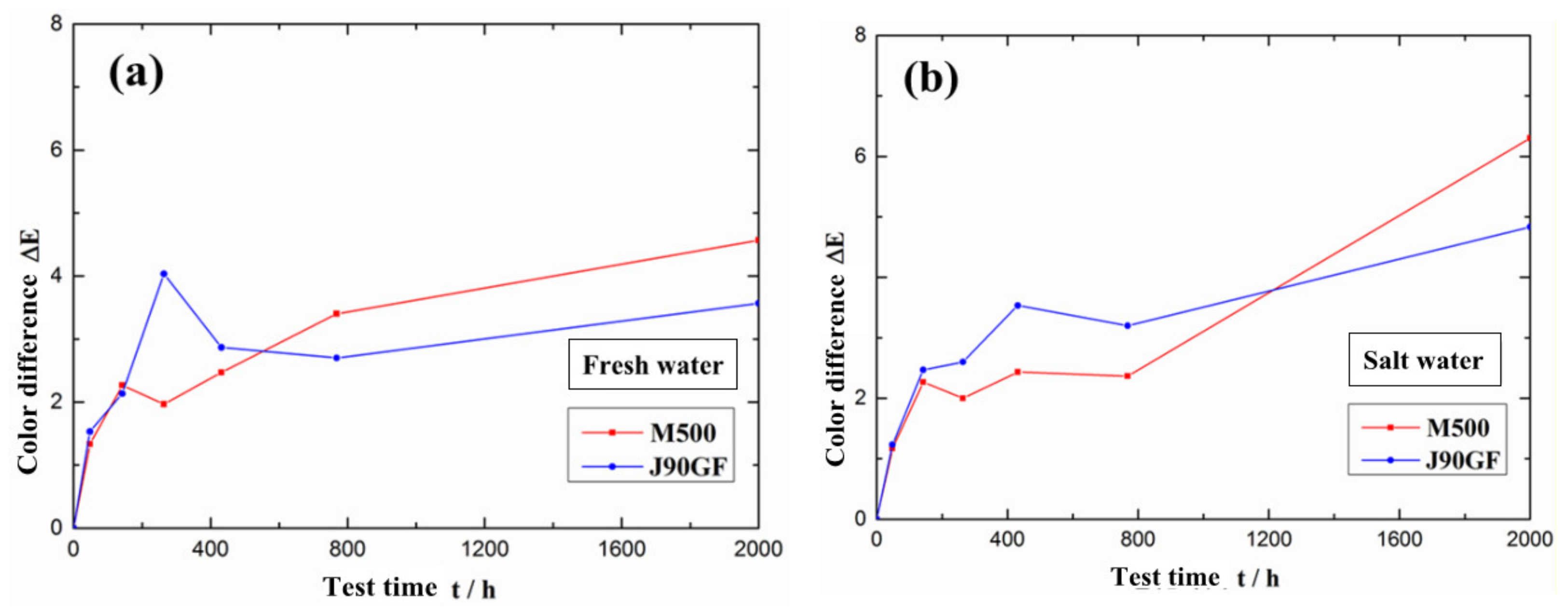
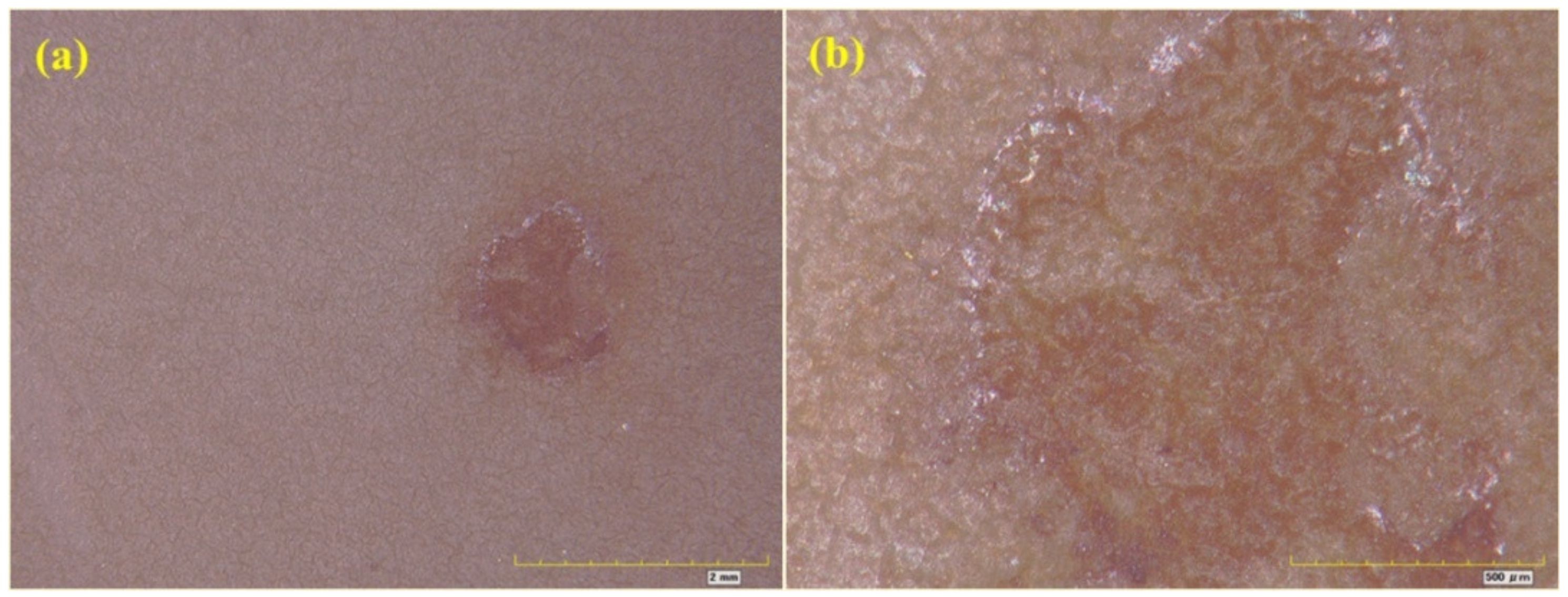
3.1. Partial Repair Test Results for Epoxy Anti-Corrosion Coating
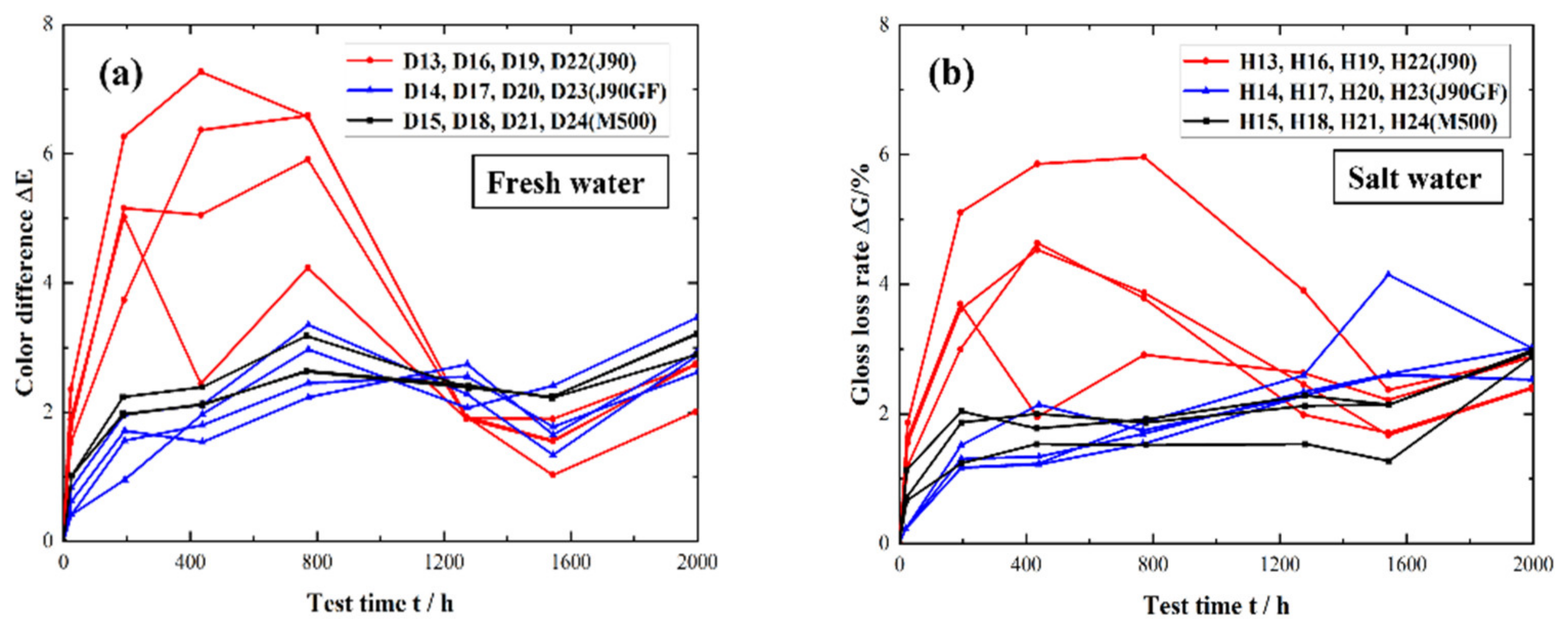
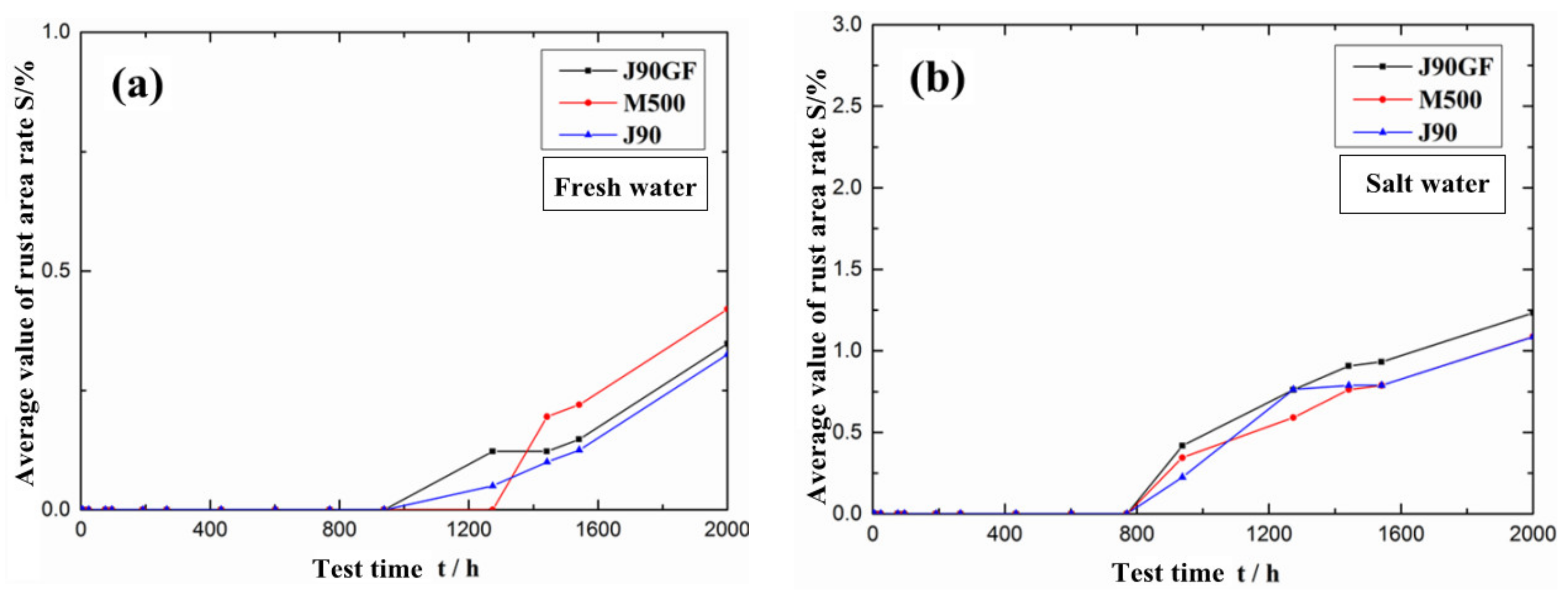
3.2. Integral Repair Test Results for Epoxy Anti-Corrosion Coating
3.3. Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures
4. Conclusions
- (1)
- From the standpoints of light loss rate, color difference and rust area rate, it was found that Marathon 500 super wear-resistant epoxy paint and Jotamastic 90 GF low surface treatment epoxy wear-resistant glass flake paint are suitable for the partial repair of epoxy anti-corrosion coatings on steel structures.
- (2)
- From the standpoints of light loss rate, color difference and rust area rate, it was found that Jotamastic 90 low surface treatment epoxy wear-resistant coating and Marathon 500 super wear-resistant epoxy paint are more suitable for the integral repair of epoxy anticorrosion coatings on steel structures.
- (3)
- Based on the results of coating repair tests, the repair criteria for partial and integral repairs were defined, respectively, and the repair strategy for epoxy anticorrosion coatings on steel structures was put forward.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abass, A.O. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Kim, I.T.; Itoh, Y. Accelerated exposure tests as evaluation tool for estimating life of organic coatings on steel bridges. Corros. Eng. Sci. Technol. 2007, 42, 242–252. [Google Scholar] [CrossRef]
- Hirohata, M.; Takemi, J.; Itoh, Y. Corrosion accelerated exposure experiment simulating under seawater environment for organic coated steel materials. Corros. Eng. Sci. Technol. 2014, 50, 449–461. [Google Scholar] [CrossRef]
- Yang, L.X.; Li, X.G.; Cheng, X.; Deng, H. Diffusion and transport behavior of water and chloride ions in acrylic polyurethane coatings. Chin. J. Corros. Prot. 2006, 26, 6–10. (In Chinese) [Google Scholar]
- Mansfeld, F.; Kendig, M.W.; Tsai, S. Evaluation of Corrosion Behavior of Coated Metals with AC Impedance Measurements. Corrosion 1982, 38, 478–485. [Google Scholar] [CrossRef]
- Hu, J.; Gao, J.; Li, X.; Du, C. Effect of ultraviolet light on aging of acrylic polyurethane varnish. Chin. J. Corros. Prot. 2009, 29, 371–375. (In Chinese) [Google Scholar]
- Liu, T.; Ai, J.; Zhang, L.; Zhang, P.; Yang, C.; Xu, C. Experimental study on life prediction of anticorrosion coating on steel box girder based on image processing technology. Chin. J. Corros. Prot. 2013, 33, 407–412. (In Chinese) [Google Scholar]
- Kallias Alexandros, N.; Chryssanthopoulos, M.K.; Imam, B. Performance profiles of metallic bridges subject to coating degradation and atmospheric corrosion. Struct. Infrastruct. Eng. 2017, 13, 440–453. [Google Scholar] [CrossRef]
- Kere, K.J.; Huang, Q. Life-Cycle Cost Comparison of Corrosion Management Strategies for Steel Bridges. J. Bridge Eng. 2019, 24, 1–16. [Google Scholar] [CrossRef]
- Geng, G.Q.; Lin, J.; Liu, L.; Cui, J. Prediction method of anticorrosion coating life of steel bridge. J. Chang. Univ. (Nat. Sci. Ed.) 2006, 26, 43–47. (In Chinese) [Google Scholar]
- Andreas, W.M.; Sascha, B.; Peter, P.; Marquardt, T.; Winkels, I.; Viertel, J. Statistical effects of surface preparation and coating type on the corrosion protection performance of repair coatings for offshore wind power constructions. Mater. Corros. 2018, 69, 460–471. [Google Scholar]
- Nicolaia, R.P.; Frenkb, J.B.G.; Dekker, R. Modelling and optimizing imperfect repair of coatings on steel structures. Struct. Saf. 2009, 31, 234–244. [Google Scholar] [CrossRef]
- Bayliss, D.A.; Deacon, D.H. Corrosion Control of Steel Structure; Chemical Industry Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Viertel, J.; Neuer, L.; Mauch, B.; Czyborra, T. Project RepaKorr: Development of a novel single coat, direct to metal repair coating with outstanding protection and colour retention performance for offshore structures. Mater. Corros. 2017, 68, 1321–1325. [Google Scholar] [CrossRef]
- GB/T 8923-2011; Preparation of steel substrates before application of paints and related products—Visual assessment of surface cleanliness—Part 1: Rust grades and preparation grades of uncoated steel substrates and of steel substrates after overall removal of previous coatings. Standardization Administration of the People’s Republic of China: Beijing, China, 2011.
- GB/T 9754-2007; Paints and varnishes—Determination of specular gloss of non-metallic paint films at 20°, 60° and 85°. Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- GB/T 1766-2008; Paints and varnishes—Rating schemes of degradation of coats. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Scrinzi, E.; Rossi, S.; Kamarchik, P.; Deflorian, F. Evaluation of durability of nano-silica containing clear coats for automotive applications. Prog. Org. Coat. 2011, 71, 384–390. [Google Scholar] [CrossRef]


| Parameters | Coating Systems | ||
|---|---|---|---|
| J90 | J90GF | M500 | |
| Volume solid content | 80 ± 2% | 80 ± 2% | 85 ± 2% |
| Gloss (GU 60°) | Semi-gloss (35–70) | Semi-gloss (35–70) | Gloss (70–85) |
| Flash point | 35 °C | 35 °C | 43 °C |
| Density | 1.4 kg/L | 1.4 kg/L | 1.6 kg/L |
| VOC | 246 g/L | 224 g/L | 162 g/L |
| Color | Grey | Grey | Grey |
| Mixing ratio (volume) | A:B = 3.5:1 | A:B = 3.5:1 | A:B = 5:1 |
| Diluent | Jotun 17# diluent | Jotun 17# diluent | Jotun 17# diluent |
| Test | Coating System | Coating Thickness (µm) | Surface Treatment Grade | Corrosive Environment | Number | Label |
|---|---|---|---|---|---|---|
| Partial repair test | M500 | 290 ± 20 (Bottom integration) | St3 | Fresh water | 3 | BW1, BW2, BW3 |
| Saline water | 3 | BW4, BW5, BW6 | ||||
| J90GF | Fresh water | 3 | BW7, BW8, BW9 | |||
| Saline water | 3 | BW10, BW11, BW12 |
| Test | Coating System | Coating Thickness (µm) | Surface Treatment Grade | Corrosive Environment | Number | Label |
|---|---|---|---|---|---|---|
| Integral repair test | J90GF | 290 ± 20 (Bottom integration) | St3 | Fresh water | 4 | D13, D16, D19, D22 |
| Saline water | 4 | H13, H16, H19, H22 | ||||
| M500 | Fresh water | 4 | D14, D17, D20, D23 | |||
| Saline water | 4 | H14, H17, H20, H23 | ||||
| J90 | Fresh water | 4 | D15, D18, D21, D24 | |||
| Saline water | 4 | H15, H18, H21, H24 |
| Item | Exposure/h | Pulverization | Cracking | Blistering | Rust | Spalling | Comprehensive Rating |
|---|---|---|---|---|---|---|---|
| D15 | 434 | 0 | 0 | 0 | Ri0 | 0 | 0 |
| 1106 | 1 | 0 | 0 | Ri1 | 0 | 1 | |
| 2000 | 2 | 0 | 0 | Ri1 | 0 | 2 | |
| H15 | 434 | 0 | 0 | 0 | Ri0 | 0 | 0 |
| 1106 | 2 | 0 | 1(S1) | Ri2 | 0 | 2 | |
| 2000 | 3 | 0 | 1(S1) | Ri3 | 0 | 4 |
| Repair Strategy | Repair Criteria | Repair Coating | Repair Method |
|---|---|---|---|
| Partial repair |
| M500, J90GF | Remove the finish coating and clean the coating in the severely rusted area to the undamaged area, groove the damaged and undamaged area joints, treat the surface to Sa2 or St3, and apply a certain thickness of low surface treatment repair coating. |
| Integral repair |
| J90, M500 | Conduct thorough surface treatment on the corroded coating, remove the old paint layer to the bolt anchorage area, mechanically polish the exposed metal matrix, and apply a certain thickness of low surface treatment repair coating on the surface of the treated steel structure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, K.; Wu, J.; Zhao, X.; Zhang, L.; Zhang, Z. Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures. Materials 2023, 16, 6257. https://doi.org/10.3390/ma16186257
Tu K, Wu J, Zhao X, Zhang L, Zhang Z. Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures. Materials. 2023; 16(18):6257. https://doi.org/10.3390/ma16186257
Chicago/Turabian StyleTu, Kun, Jin Wu, Xing Zhao, Lifang Zhang, and Zehui Zhang. 2023. "Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures" Materials 16, no. 18: 6257. https://doi.org/10.3390/ma16186257
APA StyleTu, K., Wu, J., Zhao, X., Zhang, L., & Zhang, Z. (2023). Repair Strategy for Epoxy Anticorrosive Coatings on Steel Structures. Materials, 16(18), 6257. https://doi.org/10.3390/ma16186257








