A Preliminary Study on the Use of Highly Aromatic Pyrolysis Oils Coming from Plastic Waste as Alternative Liquid Fuels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials to Be Pyrolyzed
2.2. Pyrolysis Experiments
2.3. Analytical Techniques
3. Results and Discussion
3.1. WEEE Sample Characterization
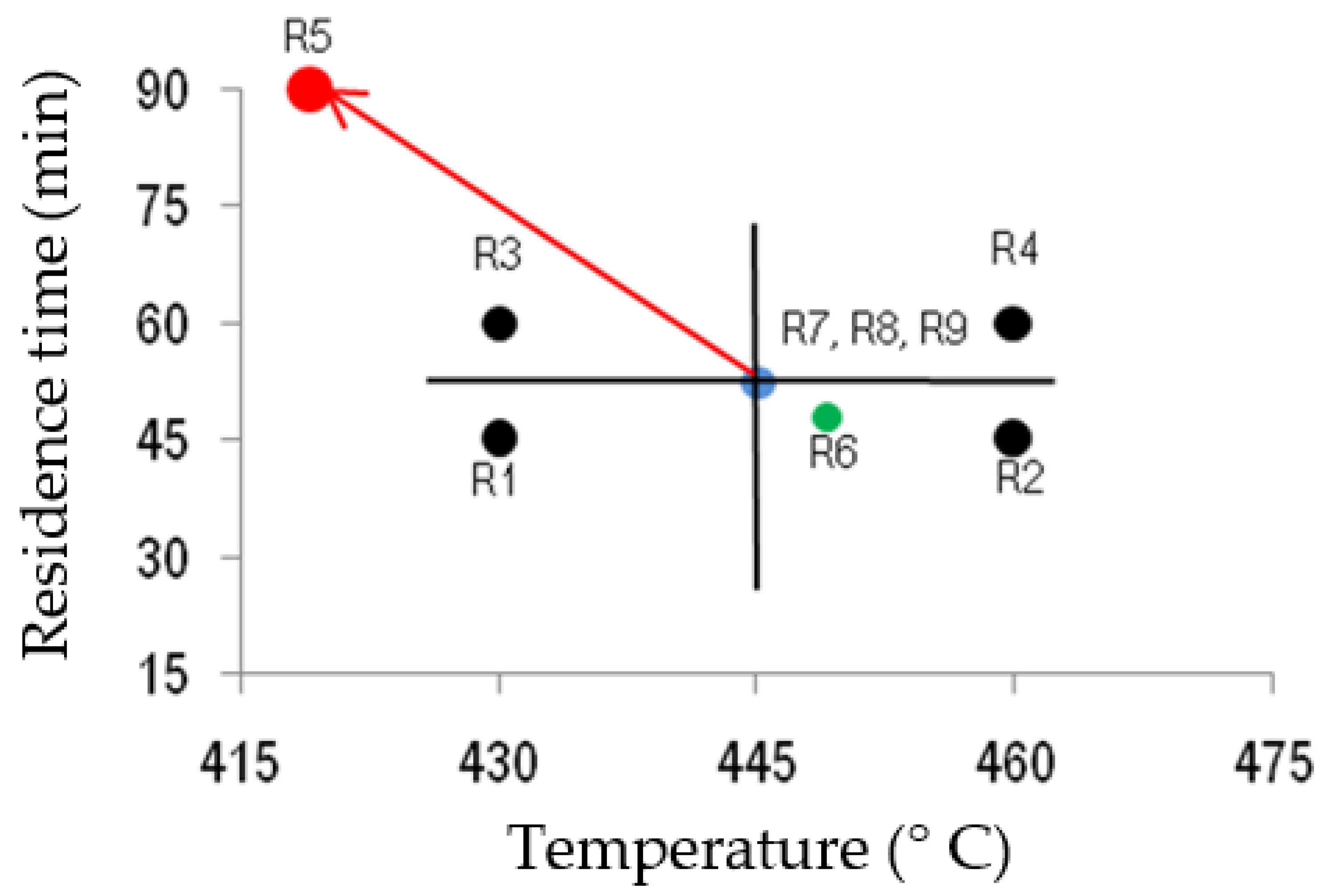
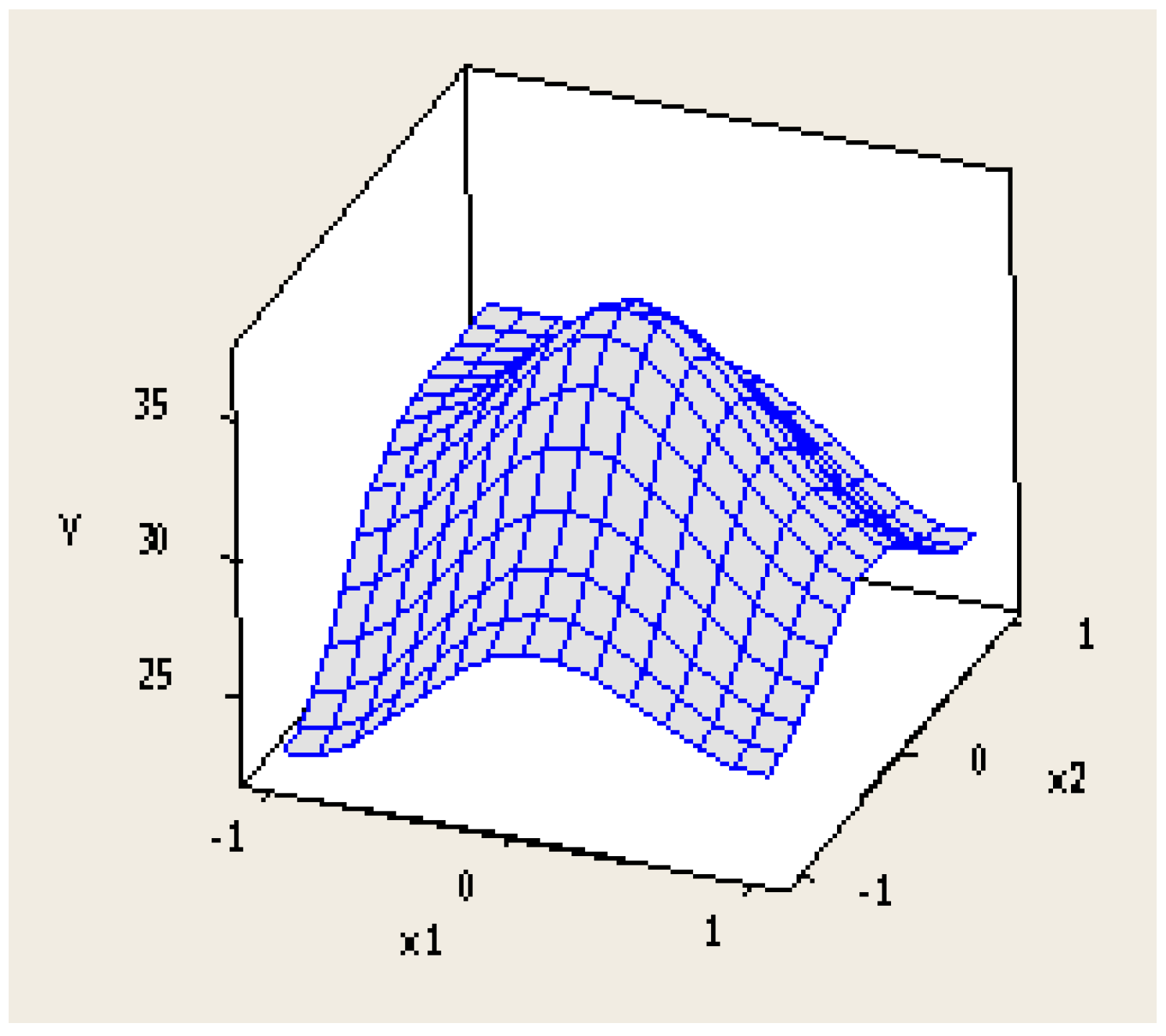
3.2. Pyrolysis Experiments
3.3. Pyrolysis Oils
4. Conclusions
- ▪
- The rejected streams that are produced in WEEE recycling facilities are plastic-rich mixtures, mainly constituted by styrenic polymers and polyolefins. Moreover, other materials such as metals or wood can be found.
- ▪
- The optimum operational parameters for the maximisation of the liquid yield from WEEE plastics in the lab-scale pyrolysis plant used in this research are temperatures in the range of 430–450 °C and residence times in the range of 45–60 min. In such conditions, the oil yield is 30–35 wt.%.
- ▪
- Polyolefins and polystyrene are the plastics that maximise oil production in the pyrolysis process, without the generation of a solid product. On the other hand, styrenic co-polymers produce lower quantities of oil and a significant proportion of char.
- ▪
- The pyrolysis oils of WEEE plastics are lighter than commercial fuel oil 6 and present similar higher heating values (≈40 MJ kg−1). In this case, polyolefins maximise the heating value but also the presence of heavy substances. In contrast, styrenics reduce the heating value at the same time they generate light substances.
- ▪
- In combustion conditions, the pyrolysis oils of WEEE show the same toxicity profile in fumes as that of the commercial fuel oil 6.
- ▪
- Future research must be focused on determining specific properties of the pyrolysis oils that can limit their use as alternative fuels, and among others, the exact concentration of halogens or the solid formation after combustion. Another interesting area of investigation is the distillation of pyrolysis oils in order to obtain liquids with narrower carbon atom distribution, which are more easily comparable to liquid fossil fuels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Veksha, A.; Chan, W.P.; Giannis, A.; Lisak, G. Chemical recycling of plastic waste for sustainable material management: A prospective review on catalysts and processes. Renew. Sustain. Energy Rev. 2022, 154, 111866. [Google Scholar] [CrossRef]
- Lase, I.S.; Tonini, D.; Caro, D.; Albizzati, P.F.; Cristóbal, J.; Roosen, M.; Kusenberg, M.; Ragaert, K.; Van Geem, K.M.; Dewulf, J.; et al. How much can chemical recycling contribute to plastic waste recycling in Europe? An assessment using material flow analysis modeling. Resour. Conserv. Recycl. 2023, 192, 106916. [Google Scholar] [CrossRef]
- Vaishnavi, M.; Vasanth, P.M.; Rajkumar, S.; Gopinath, K.P.; Devarajan, Y. A critical review of the correlative effect of process parameters on pyrolysis of plastic wastes. J. Anal. Appl. Pyrolysis 2023, 170, 105907. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Kusenberg, M.; Roosen, M.; Zayoud, A.; Djokic, M.R.; Thi, H.D.; De Meester, S.; Ragaert, K.; Kresovic, U.; Van Geem, K.M. Assessing the feasibility of chemical recycling via steam cracking of untreated plastic waste pyrolysis oils: Feedstock impurities, product yields and coke formation. Waste Manag. 2022, 141, 104–114. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/1616 of 15 September 2022 on Recycled Plastic Materials and Articles Intended to Come into Contact with Foods, and Repealing Regulation (EC) No 282/2008. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R1616 (accessed on 13 September 2023).
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019L0904&qid=1689325508014 (accessed on 13 September 2023).
- Silva, A.P.; Bahú, J.O.; Soccol, R.; Rodríguez-Urrego, L.; Fajardo-Moreno, W.S.; Moya, H.; León-Pulido, J.; Concha, V.O.C. Naphtha Characterization (PIONA, Density, Distillation Curve and Sulfur Content): An Origin Comparison. Energies 2023, 16, 3568. [Google Scholar] [CrossRef]
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Catalytic pyrolysis of waste plastic from electrical and electronic equipment. J. Anal. Appl. Pyrolysis 2015, 113, 332–339. [Google Scholar] [CrossRef]
- Fulgencio-Medrano, L.; García-Fernández, S.; Asueta, A.; Lopez-Urionabarrenechea, A.; Perez-Martinez, B.B.; Arandes, J.M. Oil Production by Pyrolysis of Real Plastic Waste. Polymers 2022, 14, 553. [Google Scholar] [CrossRef]
- Shafaghat, H.; Gulshan, S.; Johansson, A.-C.; Evangelopoulos, P.; Yang, W. Selective recycling of BTX hydrocarbons from electronic plastic wastes using catalytic fast pyrolysis. Appl. Surf. Sci. 2022, 605, 154734. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Abdullah, T.; Inuwa, I. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Das, S.; Liang, C.; Dunn, J.B. Plastics to fuel or plastics: Life cycle assessment-based evaluation of different options for pyrolysis at end-of-life. Waste Manag. 2022, 153, 81–88. [Google Scholar] [CrossRef] [PubMed]
- ASTM D396-21; American Society for Testing and Materials (ASTM International). Standard Specification for Fuel Oils. ASTM: West Conshohocken, PA, USA, 2021.
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Luda, M.P. Pyrolysis of WEEE plastics. In Waste Electrical and Electronic Equipment (WEEE) Handbook, 1st ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 239–263. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Arato, S.; Persson, H.; Kantarelis, E.; Yang, W. Reduction of brominated flame retardants (BFRs) in plastics from waste electrical and electronic equipment (WEEE) by solvent extraction and the influence on their thermal decomposition. Waste Manag. 2019, 94, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, P.; Persson, H.; Kantarelis, E.; Yang, W. Performance analysis and fate of bromine in a single screw reactor for pyrolysis of waste electrical and electronic equipment (WEEE). Process. Saf. Environ. Prot. 2020, 143, 313–321. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Lappas, A.A.; Achilias, D.S. Thermo-chemical recycling of plastics retrieved from waste electric and electronic equipment (WEEE) by pyrolysis: Identification of the polymer type, removal of bromine compounds from plastics based on an environmentally-friendly process and characterization of the pyrolysates. Sustain. Chem. Pharm. 2023, 35. [Google Scholar] [CrossRef]
- Santella, C.; Cafiero, L.; De Angelis, D.; La Marca, F.; Tuffi, R.; Ciprioti, S.V. Thermal and catalytic pyrolysis of a mixture of plastics from small waste electrical and electronic equipment (WEEE). Waste Manag. 2016, 54, 143–152. [Google Scholar] [CrossRef]
- Marino, A.; Aloise, A.; Hernando, H.; Fermoso, J.; Cozza, D.; Giglio, E.; Migliori, M.; Pizarro, P.; Giordano, G.; Serrano, D.P. ZSM-5 zeolites performance assessment in catalytic pyrolysis of PVC-containing real WEEE plastic wastes. Catal. Today 2022, 390–391, 210–220. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; Wiley: New York City, NY, USA, 2019; ISBN 978-1-119-49244-3. [Google Scholar]
- Perez-Martinez, B.B.; Lopez-Urionabarrenechea, A.; Serras-Malillos, A.; Acha, E.; Martínez-Santos, M.I.; Iriondo, A.; Caballero, B.M. Dechlorination of plastic-rich fractions rejected from waste electric and electronic equipment recycling plants by means of stepwise pyrolysis for valorization. WIT Trans. Ecol. Environ. 2021, 254, 81–90. Available online: https://www.witpress.com/elibrary/wit-transactions-on-ecology-and-the-environment/254/38128 (accessed on 13 September 2023).
- Caballero, B.M.; de Marco, I.; Adrados, A.; López-Urionabarrenechea, A.; Solar, J.; Gastelu, N. Possibilities and limits of pyrolysis for recycling plastic rich waste streams rejected from phones recycling plants. Waste Manag. 2016, 57, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Ciprioti, S.V. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seraji, S.M.; Song, P.; Varley, R.J.; Bourbigot, S.; Voice, D.; Wang, H. Fire-retardant unsaturated polyester thermosets: The state-of-the-art, challenges and opportunities. Chem. Eng. J. 2022, 430, 132785. [Google Scholar] [CrossRef]
- Dong, N.; Hui, H.; Li, S.; Du, L. Study on preparation of aromatic-rich oil by thermal dechlorination and fast pyrolysis of PVC. J. Anal. Appl. Pyrolysis 2023, 169, 105817. [Google Scholar] [CrossRef]
- López, F.A.; Martín, M.; Alguacil, F.; Rincón, J.M.; Centeno, T.; Romero, M. Thermolysis of fibreglass polyester composite and reutilisation of the glass fibre residue to obtain a glass–ceramic material. J. Anal. Appl. Pyrolysis 2012, 93, 104–112. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Van Krevelen, D.; Nijenhuis, K.T. Thermal Decomposition. In Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 3, pp. 763–777. [Google Scholar] [CrossRef]
- Oyekan, S.O. Catalytic Naphtha Reforming Process, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 378. [Google Scholar] [CrossRef]


| Organic/Inorganic | Element | Value (wt.% for CHNS/ClBrF and mg/kg for Metals) |
|---|---|---|
| Organic content (83.8 wt.%) | C | 75.0 |
| H | 7.9 | |
| N | 1.5 | |
| S | <0.02 | |
| Cl | 1.4 | |
| Br | 0.7 | |
| F | <0.02 | |
| Ash content 1 (16.2 wt.%) | Al | 2727 |
| Cu | 37,068 | |
| Zn | 1135 | |
| Ca | 7891 | |
| Sb | 3537 | |
| P | 1304 | |
| Sn | 1689 |
| Run | T (°C) | tr (min) | Liquid | Solid | Gas * |
|---|---|---|---|---|---|
| #1 | 430 | 45 | 22.6 | 28.4 | 49.0 |
| #2 | 460 | 45 | 25.2 | 32.8 | 42.0 |
| #3 | 460 | 60 | 24.8 | 31.2 | 44.0 |
| #4 | 430 | 60 | 29.7 | 30.6 | 39.7 |
| #5 | 420 | 90 | 25.4 | 46.4 | 28.2 |
| #6 | 450 | 48 | 30.6 | 30.2 | 39.2 |
| #7 | 445 | 53 | 35.1 | 32.9 | 32.0 |
| #8 | 445 | 53 | 36.0 | 31.8 | 32.2 |
| #9 | 445 | 53 | 36.8 | 31.2 | 32.0 |
| Sample | Nature | Liquid | Solid | Gas * |
|---|---|---|---|---|
| PP | Polyolefinic | 81.0 | 0.0 | 19.0 |
| HDPE | 78.9 | 0.0 | 21.1 | |
| 75 wt.% PP + 25 wt.% HDPE | 78.0 | 0.0 | 22.0 | |
| 50 wt.% PP + 50 wt.% HDPE | 80.0 | 0.0 | 20.0 | |
| 25 wt.% PP + 75 wt.% HDPE | 83.0 | 0.0 | 17.0 | |
| PS | Styrenic | 85.2 | 0.1 | 14.7 |
| ABS | 45.1 | 31.6 | 23.3 | |
| ASA | 56.6 | 18.8 | 24.6 | |
| SAN | 67.2 | 23.9 | 8.9 | |
| SB | 68.5 | 19.1 | 12.4 | |
| 60 wt.% ABS + 40 wt.% SB | 50.4 | 20.7 | 28.9 | |
| 40 wt.% ABS + 60 wt.% SB | 59.8 | 17.1 | 23.1 |
| Compound | Run #7 |
|---|---|
| Ethylbenzene | 30.0 |
| Benzene, 1,3-dimethyl- | 4.3 |
| Benzene, (1-methylethyl)- | 16.4 |
| Benzene, propyl | 4.3 |
| Benzene, 1,3,5-trimethyl- | 2.7 |
| Phenol | 3.2 |
| Benzene, 1-methyl-4-propyl- | 1.7 |
| Phenol, 2-methyl- | 1.9 |
| Phenol, 4-(1-methylethyl)- | 3.5 |
| Naphthalene 2-methyl- | 1.9 |
| TOTAL IDENTIFIED | 70.0 |
| NON-IDENTIFIED | 30.0 |
| Experiment | HHV | C7–C12 | C13–C16 | C17–C30 |
|---|---|---|---|---|
| #1 | 41.4 | 71.6 | 9.8 | 18.6 |
| #2 | 40.3 | 79.4 | 7.4 | 13.2 |
| #3 | 39.2 | 85.7 | 6.1 | 8.1 |
| #4 | 40.7 | 79.6 | 7.1 | 13.2 |
| #5 | 40.5 | 93.0 | 3.4 | 3.5 |
| #6 | 40.9 | 82.8 | 7.0 | 10.2 |
| #7 | 39.8 | 91.4 | 4.5 | 4.1 |
| #8 | 40.8 | 90.4 | 4.7 | 4.9 |
| #9 | 40.5 | 84.4 | 6.3 | 9.3 |
| Fuel oil 6 | 45.0 | 35.9 | 26.7 | 37.3 |
| Sample | HHV | C7–C12 | C13–C16 | C17–C30 |
|---|---|---|---|---|
| PP | 44.3 | 56.9 | 22.9 | 20.2 |
| HDPE | 46.1 | 32.4 | 27.4 | 40.2 |
| 75 wt.% PP + 25 wt.% HDPE | 41.7 | 50.4 | 23.0 | 26.6 |
| 50 wt.% PP + 50 wt.% HDPE | 43.3 | 50.3 | 23.2 | 25.6 |
| 25 wt.% PP + 75 wt.% HDPE | 43.9 | 40.3 | 25.2 | 34.5 |
| PS | 40.2 | 76.1 | 7.8 | 16.1 |
| ABS | 38.7 | 79.4 | 10.1 | 10.5 |
| ASA | 37.4 | 74.0 | 12.2 | 13.7 |
| SAN | 38.2 | 63.2 | 15.1 | 21.7 |
| 60 wt.% ABS + 40 wt.% SB | 39.8 | 63.0 | 14.5 | 22.5 |
| 40 wt.% ABS + 60 wt.% SB | 40.5 | 9.9 | 19.7 | 40.5 |
| Property | WEEE Oil #9 | Commercial No. 6 Fuel Oil | |
|---|---|---|---|
| Density (kg m−3) | 879 | 900 | |
| Viscosity (mm2 s−1) | 1.31 | 2.0–4.5 | |
| Halogens (mg g−1) | Cl | <0.35 | <0.35 |
| Br | <0.07 | <0.07 | |
| F | <0.07 | <0.07 | |
| Solid content (wt.%) | 5 | - | |
| Toxicity (mg g−1) | CO | 48.2 | 48.6 |
| CO2 | 406.5 | 466.5 | |
| HCl | <2.2 | <2.2 | |
| HBr | <1.1 | <1.1 | |
| SO2 | <2.2 | <2.2 | |
| HF | <0.1 | <0.1 | |
| HCN | <1.1 | 2.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asueta, A.; Fulgencio-Medrano, L.; Miguel-Fernández, R.; Leivar, J.; Amundarain, I.; Iruskieta, A.; Arnaiz, S.; Gutiérrez-Ortiz, J.I.; Lopez-Urionabarrenechea, A. A Preliminary Study on the Use of Highly Aromatic Pyrolysis Oils Coming from Plastic Waste as Alternative Liquid Fuels. Materials 2023, 16, 6306. https://doi.org/10.3390/ma16186306
Asueta A, Fulgencio-Medrano L, Miguel-Fernández R, Leivar J, Amundarain I, Iruskieta A, Arnaiz S, Gutiérrez-Ortiz JI, Lopez-Urionabarrenechea A. A Preliminary Study on the Use of Highly Aromatic Pyrolysis Oils Coming from Plastic Waste as Alternative Liquid Fuels. Materials. 2023; 16(18):6306. https://doi.org/10.3390/ma16186306
Chicago/Turabian StyleAsueta, Asier, Laura Fulgencio-Medrano, Rafael Miguel-Fernández, Jon Leivar, Izotz Amundarain, Ana Iruskieta, Sixto Arnaiz, Jose Ignacio Gutiérrez-Ortiz, and Alexander Lopez-Urionabarrenechea. 2023. "A Preliminary Study on the Use of Highly Aromatic Pyrolysis Oils Coming from Plastic Waste as Alternative Liquid Fuels" Materials 16, no. 18: 6306. https://doi.org/10.3390/ma16186306
APA StyleAsueta, A., Fulgencio-Medrano, L., Miguel-Fernández, R., Leivar, J., Amundarain, I., Iruskieta, A., Arnaiz, S., Gutiérrez-Ortiz, J. I., & Lopez-Urionabarrenechea, A. (2023). A Preliminary Study on the Use of Highly Aromatic Pyrolysis Oils Coming from Plastic Waste as Alternative Liquid Fuels. Materials, 16(18), 6306. https://doi.org/10.3390/ma16186306







