Morphology Controlled Deposition of Vanadium Oxide (VOx) Nanoparticles on the Surface of Highly Reduced Graphene Oxide for the Photocatalytic Degradation of Hazardous Organic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HRG–VOx Nanocomposites
2.3. Photocatalytic Activity Studies
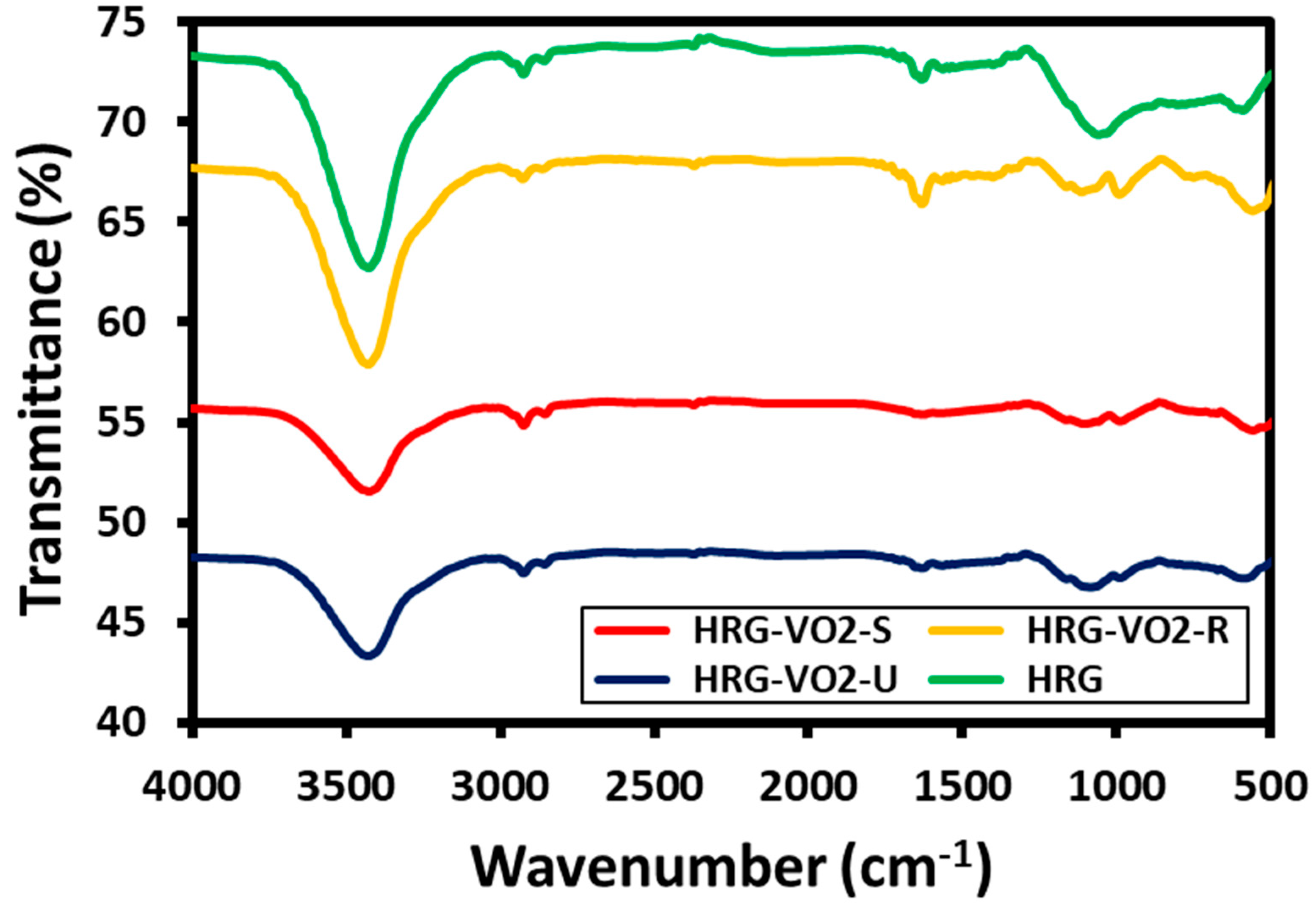
2.4. Characterization
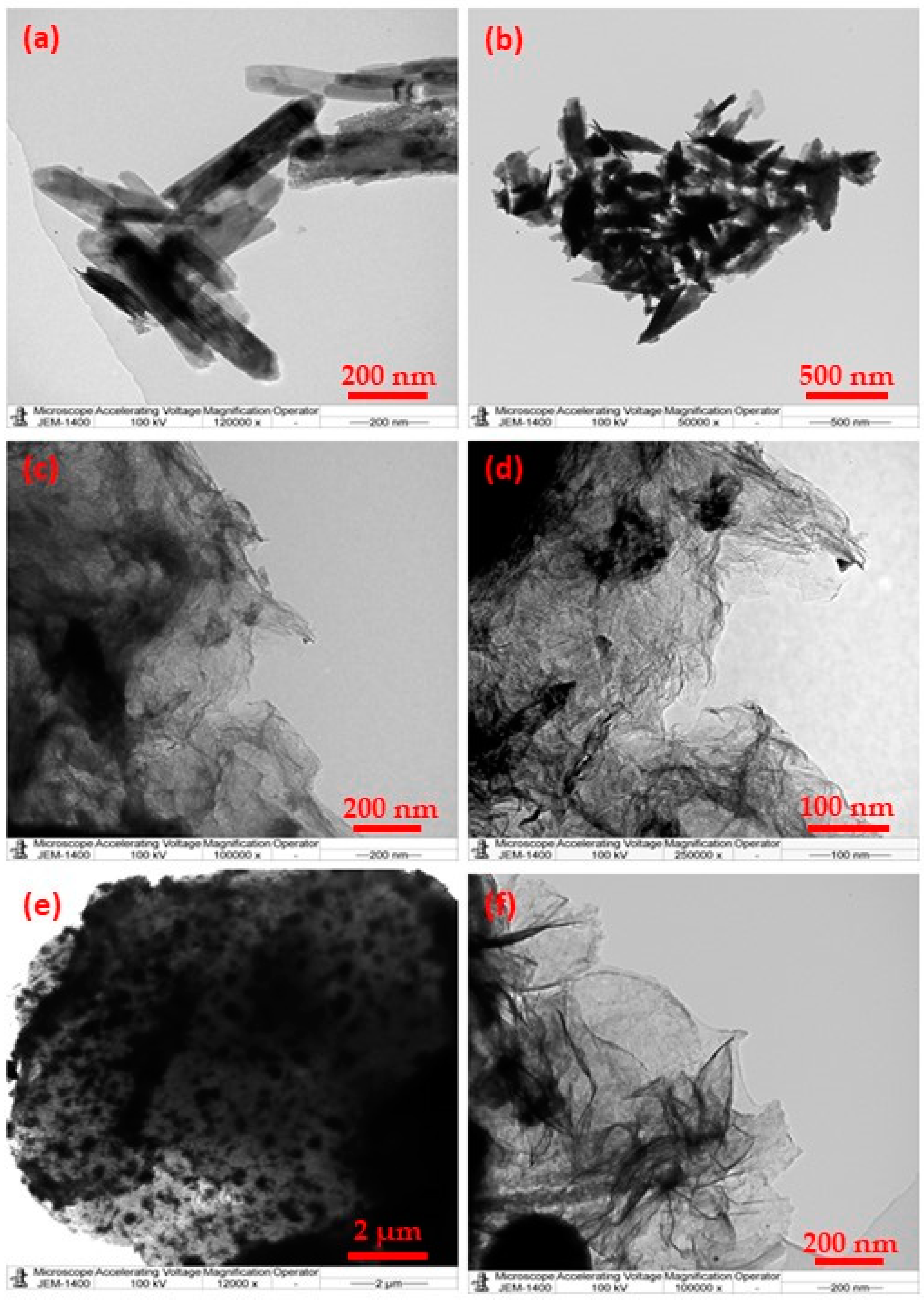
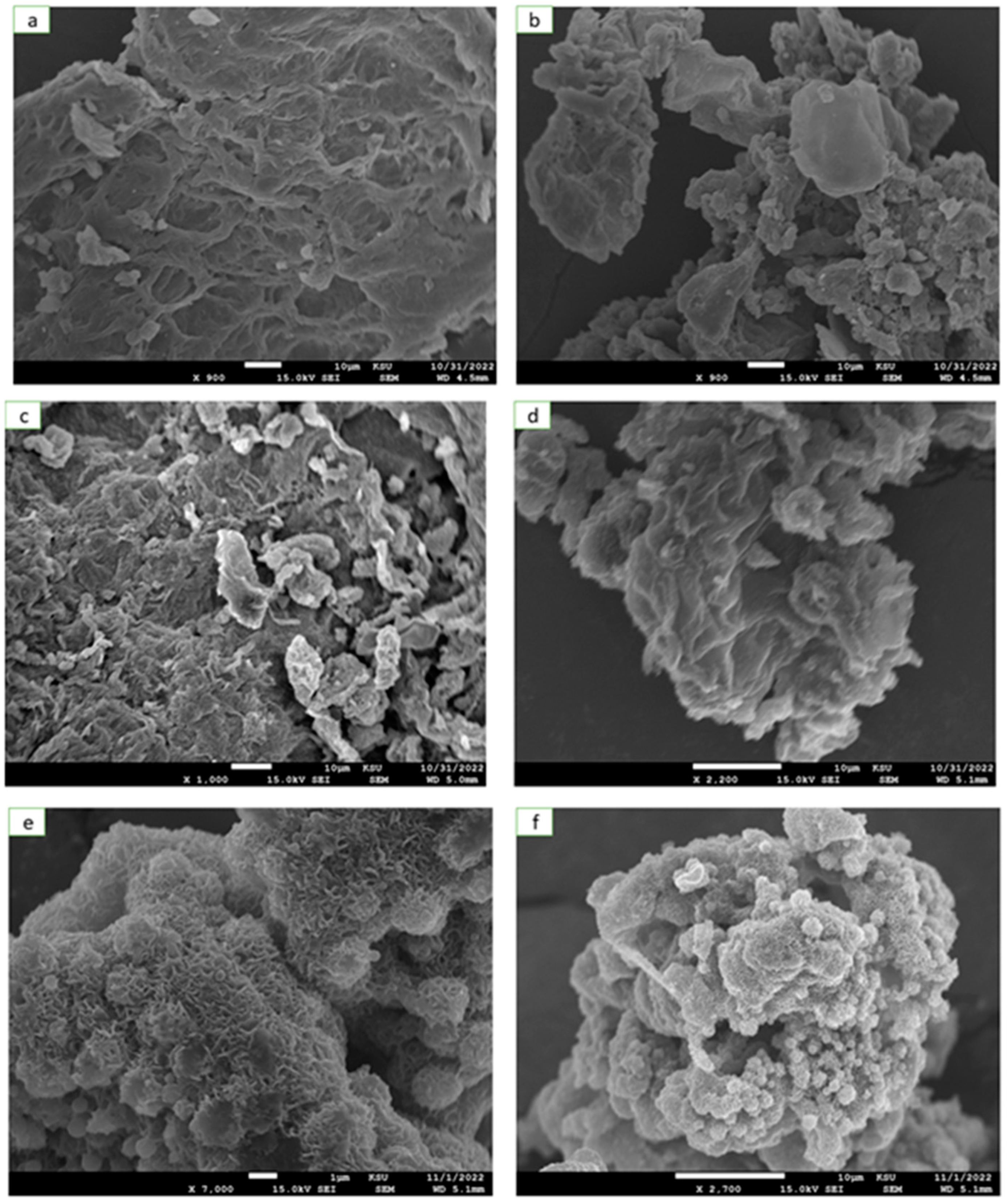
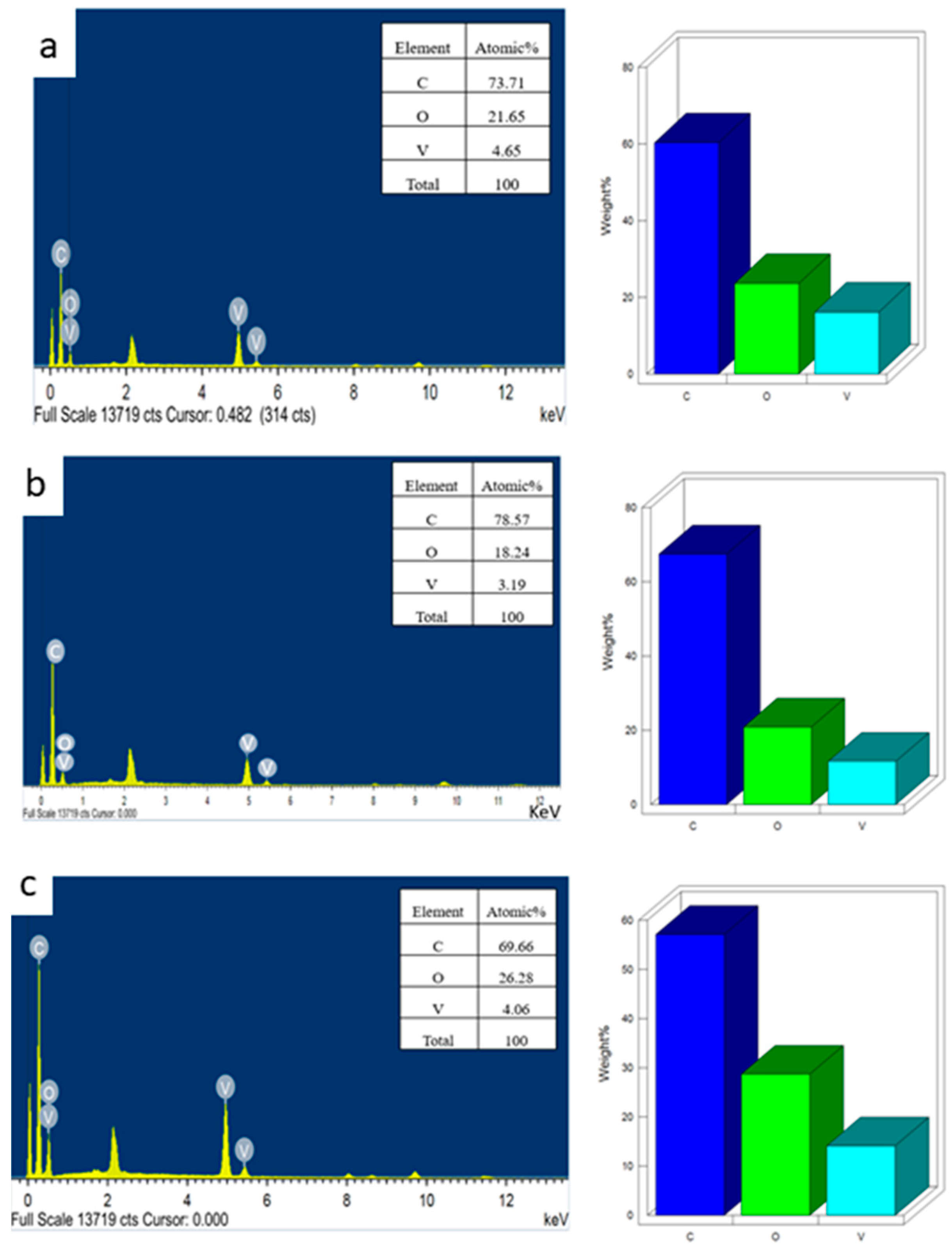
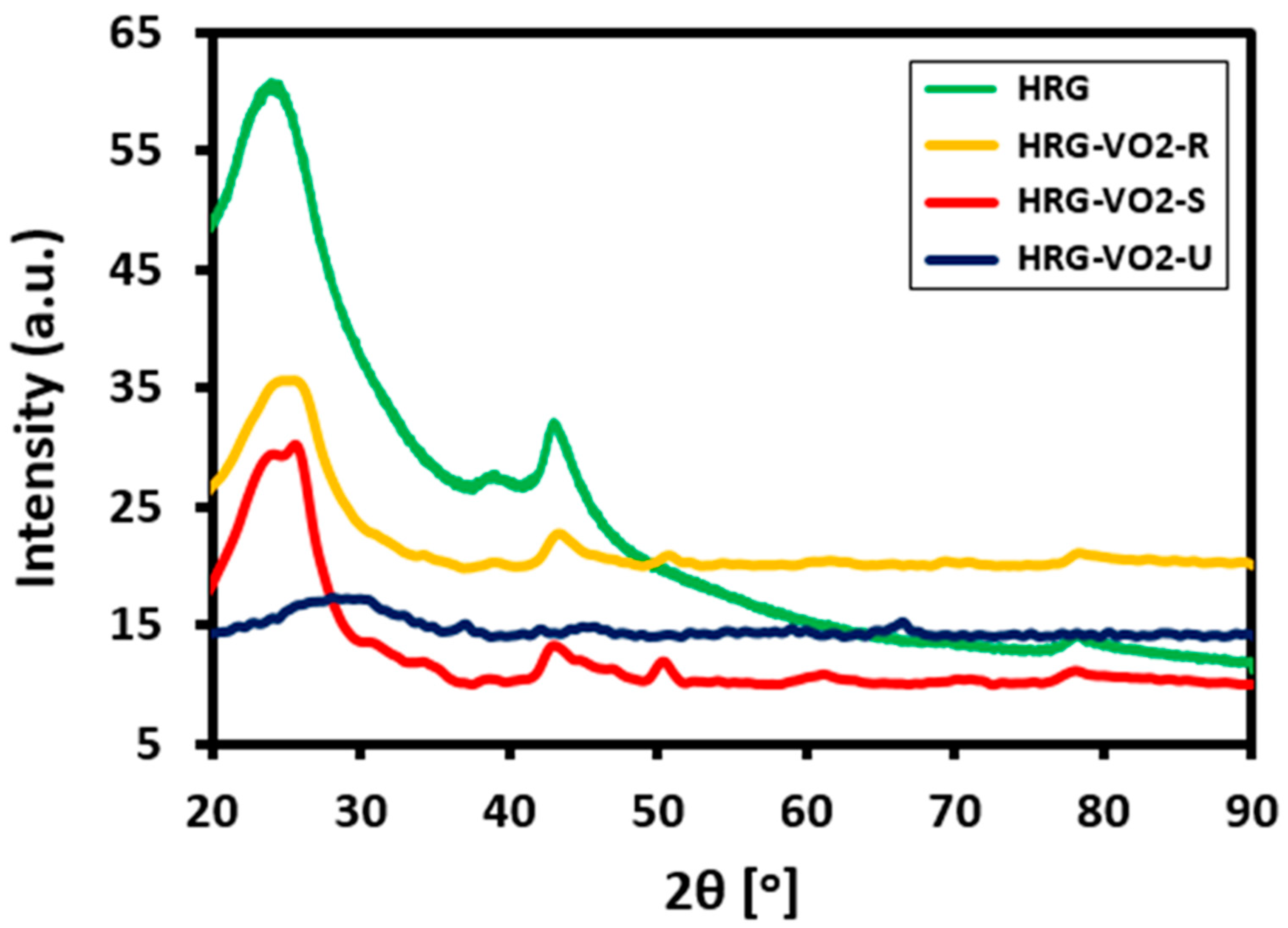
3. Results and Discussions
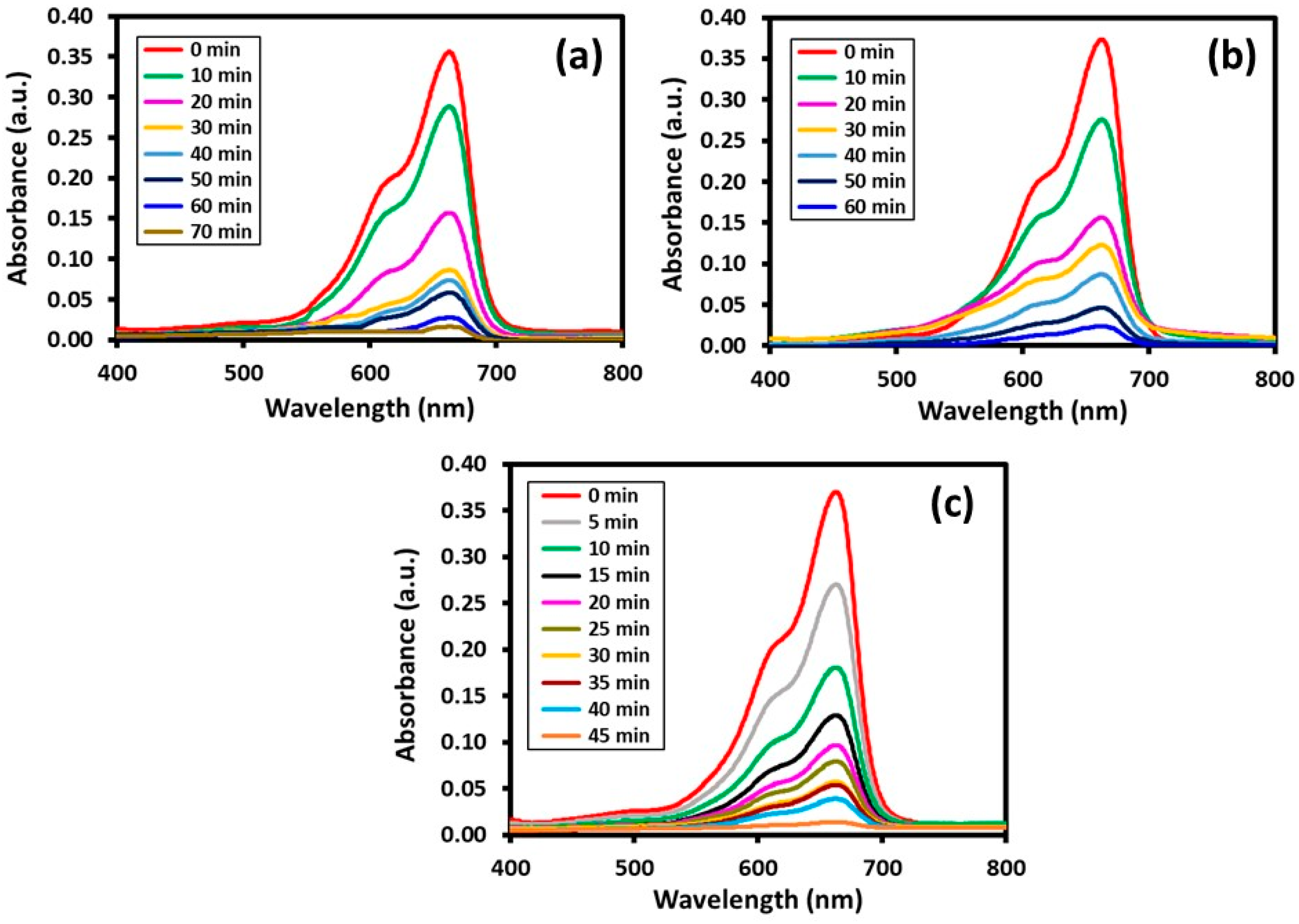
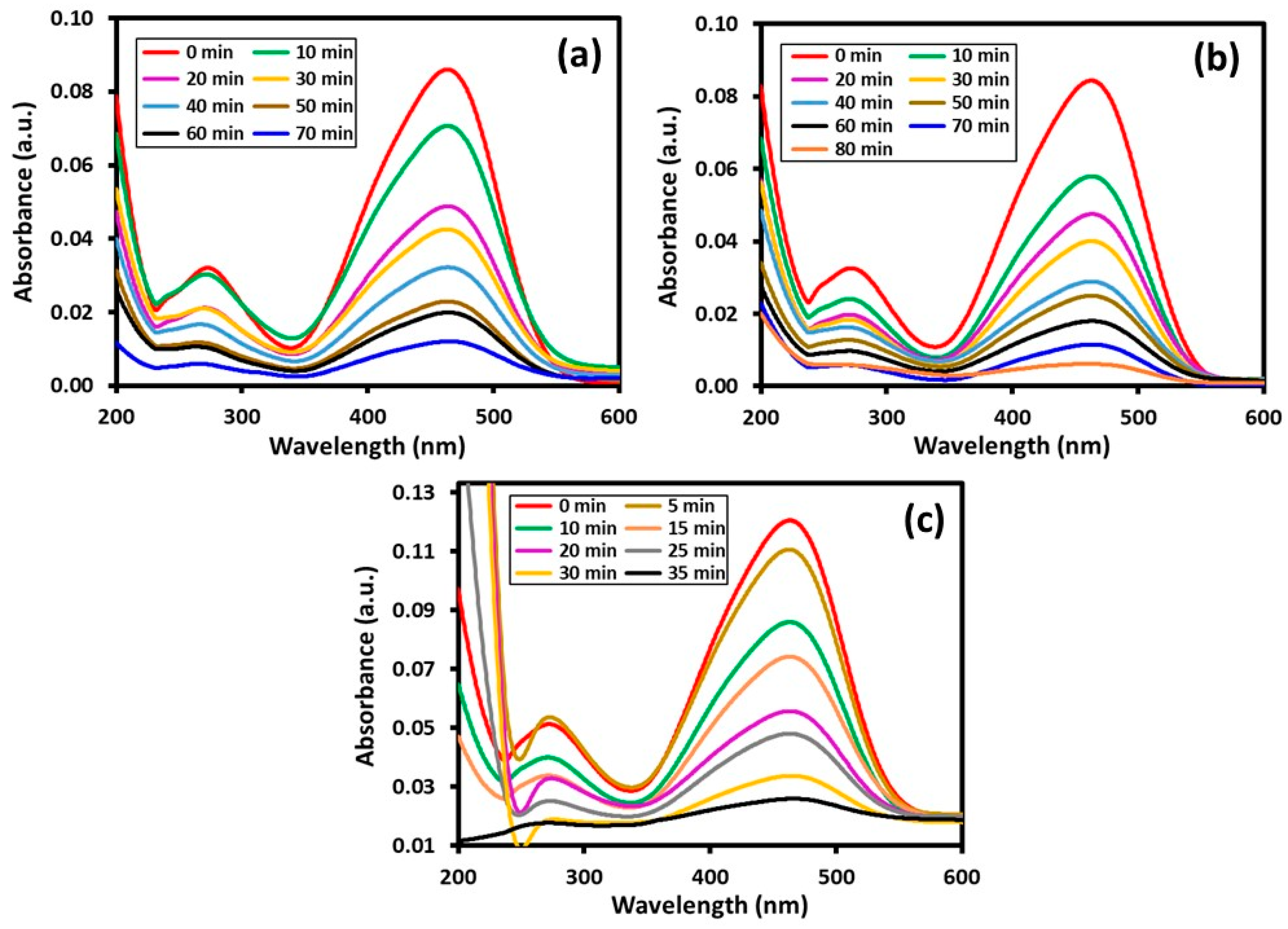
Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Lou, X.W. Design of heterostructured hollow photocatalysts for solar-to-chemical energy conversion. Adv. Mater. 2019, 31, 1900281. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ai, X.; Chen, Y.; Liu, P.; Lin, C.; Lu, K.; Jiang, W.; Wu, J.; Song, X. Improved Photocatalytic Activity via N-Type ZnO/p-Type NiO Heterojunctions. Nanomater. 2022, 12, 3665. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Iftekhar, S.; Park, Y.; Joseph, J.; Srivastava, V.; Khan, M.A.; Makvandi, P.; Sillanpaa, M.; Varma, R.S. An overview on non-spherical semiconductors for heterogeneous photocatalytic degradation of organic water contaminants. Chemosphere 2021, 280, 130907. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, B.; Bhatti, M.A.; Shah, A.A.; Tahira, A.; Shah, A.K.; Usto, A.; Aftab, U.; Bukhari, S.I.; Alshehri, S.; Shah Bukhari, S.N.U. Mn3O4@ ZnO hybrid material: An excellent photocatalyst for the degradation of synthetic dyes including methylene blue, methyl orange and malachite green. Nanomater. 2022, 12, 3754. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Cardoso, R.M.; da Silva, J.C.E. Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomater. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Kong, E.D.H.; Chau, J.H.F.; Lai, C.W.; Khe, C.S.; Sharma, G.; Kumar, A.; Siengchin, S.; Sanjay, M.R. GO/TiO2-related nanocomposites as photocatalysts for pollutant removal in wastewater treatment. Nanomater. 2022, 12, 3536. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-dimensional hybrid nanostructures for heterogeneous photocatalysis and photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Leung, Y.H.; Ng, A.M.C. Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz. 2014, 1, 400–410. [Google Scholar] [CrossRef]
- Díaz, C.; Segovia, M.; Valenzuela, M.L. Solid state nanostructured metal oxides as photocatalysts and their application in pollutant degradation: A review. Photochem 2022, 2, 609–627. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R.; Attri, P. Transition metal oxides and their composites for photocatalytic dye degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Marschall, R.; Wang, L. Non-metal doping of transition metal oxides for visible-light photocatalysis. Catal. Today 2014, 225, 111–135. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem., Int. Ed. Engl. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Zhou, B.X.; Ding, S.S.; Yang, K.X.; Zhang, J.; Huang, G.F.; Pan, A.; Hu, W.; Li, K.; Huang, W.Q. Generalized synthetic strategy for amorphous transition metal oxides-based 2D heterojunctions with superb photocatalytic hydrogen and oxygen evolution. Adv. Funct. Mater. 2021, 31, 2009230. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Srivastava, V.K.; Kumar, P.; Wang, J.; Yu, A. Graphene-supported 2D transition metal oxide heterostructures. J. Mater. Chem. A 2018, 6, 13509–13537. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Li, Y.; Fu, J.; Liang, B. Progress in graphene/metal oxide composite photocatalysts for degradation of organic pollutants. Catalysts 2020, 10, 921. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Tahir, M.N. Advances in graphene/inorganic nanoparticle composites for catalytic applications. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef]
- Khan, M.; Assal, M.E.; Tahir, M.N.; Khan, M.; Ashraf, M.; Hatshan, M.R.; Khan, M.; Varala, R.; Badawi, N.M.; Adil, S.F. Graphene/inorganic nanocomposites: Evolving photocatalysts for solar energy conversion for environmental remediation. J. Saudi Chem. Soc. 2022, 26, 101544. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Babar, B.M.; Pisal, K.B.; Sutar, S.H.; Mujawar, S.H.; Kadam, L.D.; Pathan, H.M.; Pawar, U.T.; Kadam, P.M.; Patil, P.S. Hydrothermally prepared vanadium oxide nanostructures for photocatalytic application. Energy Environ. 2022, 15, 82–91. [Google Scholar] [CrossRef]
- Hu, P.; Hu, P.; Vu, T.D.; Li, M.; Wang, S.; Ke, Y.; Zeng, X.; Mai, L.; Long, Y. Vanadium Oxide: Phase Diagrams, Structures, Synthesis, and Applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xie, Y. Promising vanadium oxide and hydroxide nanostructures: From energy storage to energy saving. Energy Environ. Sci. 2010, 3, 1191–1206. [Google Scholar] [CrossRef]
- Basu, R.; Ghosh, S.; Bera, S.; Das, A.; Dhara, S. Phase-pure VO2 nanoporous structure for binder-free supercapacitor performances. Sci. Rep. 2019, 9, 4621. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhu, Y.; Li, Z.; Vajtai, R.; Ci, L.; Ajayan, P.M. Nanostructured VO2 photocatalysts for hydrogen production. ACS Nano 2008, 2, 1492–1496. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, P.; Ansari, S.A.; Manippady, S.R.; Jaiswal, A.; Saxena, M. VO2 nanostructures for batteries and supercapacitors: A review. Small 2021, 17, 2006651. [Google Scholar] [CrossRef]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal synthesis of VO2 polymorphs: Advantages, challenges and prospects for the application of energy efficient smart windows. Small 2017, 13, 1701147. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size-and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, J.; Zhu, B.; Yu, J. Shape-dependent photocatalytic hydrogen evolution activity over a Pt nanoparticle coupled gC3N4 photocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 19457–19463. [Google Scholar] [CrossRef]
- Nguyen, T.-D. From formation mechanisms to synthetic methods toward shape-controlled oxide nanoparticles. Nanoscale 2013, 5, 9455–9482. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Rhee, K.Y.; Jaleh, B.; Park, S.J. Altering the structure and properties of iron oxide nanoparticles and graphene oxide/iron oxide composites by urea. Appl. Surf. Sci. 2016, 364, 686–693. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Kuniyil, M.; Ashraf, M.; Frerichs, H.; Sarif, M.A.; Siddiqui, M.R.H.; Al–Warthan, A.; Labis, J.P. Facile synthesis of Pd@ graphene nanocomposites with enhanced catalytic activity towards Suzuki coupling reaction. Sci. Rep. 2020, 10, 11728. [Google Scholar] [CrossRef]
- Al-Hamoud, K.; Shaik, M.R.; Khan, M.; Alkhathlan, H.Z.; Adil, S.F.; Kuniyil, M.; Assal, M.E.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N. Pulicaria undulata extract-mediated eco-friendly preparation of TiO2 nanoparticles for photocatalytic degradation of methylene blue and methyl Orange. ACS Omega 2022, 7, 4812–4820. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chiang, Y.-W.; Huang, M.H. Synthesis of diverse Ag2O crystals and their facet-dependent photocatalytic activity examination. ACS Appl. Mater. Interfaces 2016, 8, 19672–19679. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, M.; Sangpour, P.; Hosseinzadeh, G. Morphology dependent photocatalytic activity of WO3 nanostructures. J. Energy Chem. 2015, 24, 171–177. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Al-Mufti, S.; Almontasser, A.; Rizvi, S. Single and double thermal reduction processes for synthesis reduced graphene oxide assisted by a muffle furnace: A facile robust synthesis and rapid approach to enhance electrical conductivity. AIP Adv. 2022, 12, 125306. [Google Scholar] [CrossRef]
- Das, P.; Deoghare, A.B.; Maity, S.R. A novel approach to synthesize reduced graphene oxide (RGO) at low thermal conditions. Arab. J. Sci. Eng. 2021, 46, 5467–5475. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Song, H.J.; Choi, M.; Kim, J.-C.; Park, S.; Lee, C.W.; Hong, S.-H.; Kim, B.-K.; Kim, D.-W. Enhanced lithium storage in reduced graphene oxide-supported M-phase vanadium (IV) dioxide nanoparticles. Sci. Rep. 2016, 6, 30202. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. An XPS study on the surface reduction of V2O5 (001) induced by Ar+ ion bombardment. Surf. Sci. 2006, 600, 3512–3517. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms that control the adsorption–desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.; Zoltan, T.; Yadarola, C.; Mosquera, E.; Gracia, F.; García, A. The influence of the morphology of 1D TiO2 nanostructures on photogeneration of reactive oxygen species and enhanced photocatalytic activity. J. Mol. Liq. 2019, 281, 59–69. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Green synthesis of ZnO nanoparticles and its application in the degradation of some dyes. J. Am. Ceram. Soc. 2015, 98, 1739–1746. [Google Scholar] [CrossRef]
- Mirgane, N.A.; Shivankar, V.S.; Kotwal, S.B.; Wadhawa, G.C.; Sonawale, M.C. Degradation of dyes using biologically synthesized zinc oxide nanoparticles. Mater. Today Proc. 2021, 37, 849–853. [Google Scholar] [CrossRef]
- Ancy, K.; Bindhu, M.; Bai, J.S.; Gatasheh, M.K.; Hatamleh, A.A.; Ilavenil, S. Photocatalytic degradation of organic synthetic dyes and textile dyeing waste water by Al and F co-doped TiO2 nanoparticles. Environ. Res. 2022, 206, 112492. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Alamelu, K.; Raja, V.; Shiamala, L.; Ali, B.J. Biphasic TiO2 nanoparticles decorated graphene nanosheets for visible light driven photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2018, 430, 145–154. [Google Scholar] [CrossRef]
- Nassar, M.Y.; NourEldien, M.S.; Ibrahim, I.M.; Aly, H.M. A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Processes 2023, 11, 1322. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Three isomeric Zn (II)–Sn (IV)–Zn (II) porphyrin-triad-based supramolecular nanoarchitectures for the morphology-dependent photocatalytic degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef]
- Shee, N.K.; Jo, H.J.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn (IV) porphyrins with Ag (I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhang, Z.; Amin, N.; Zhai, H. Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surf. Interfaces 2020, 19, 100502. [Google Scholar] [CrossRef]


| Percentage of Degradation | ||||
|---|---|---|---|---|
| Catalyst | MB | MO | MB | MO |
| HRG–VOx-R | 70 min | 70 min | 97.9% | 86.0% |
| HRG–VOx-S | 60 min | 80 min | 93.5% | 92.8% |
| HRG–VOx-U | 45 min | 35 min | 99.1% | 97.2% |
| Percentage of Degradation | References | ||||
|---|---|---|---|---|---|
| Type of Catalyst | MB | MO | MB | MO | |
| ZnO NPs | 35 min | 35 min | ~60.0% | ~98.0% | [48] |
| ZnO NPs | 60 min | 60 min | ~4.3% | ~4.7% | [49] |
| Al–F∕TiO2 NPs | 120 min | 120 min | ~61.0% | ~83.0% | [50] |
| Graphene–TiO2 (P25) | 60 min | nil | 65.0% | nil | [51] |
| Graphene–TiO2 (mixed phase) | 60 min | nil | 97.5% | nil | [52] |
| V2O5 | 360 min | nil | 60.3% | nil | [53] |
| HRG–VOx-U | 45 min | 35 min | 99.1% | 97.2% | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, M.R.; Aldhuwayhi, F.N.; Al-Mohaimeed, A.M.; Hatshan, M.R.; Kuniyil, M.; Adil, S.F.; Khan, M. Morphology Controlled Deposition of Vanadium Oxide (VOx) Nanoparticles on the Surface of Highly Reduced Graphene Oxide for the Photocatalytic Degradation of Hazardous Organic Dyes. Materials 2023, 16, 6340. https://doi.org/10.3390/ma16186340
Shaik MR, Aldhuwayhi FN, Al-Mohaimeed AM, Hatshan MR, Kuniyil M, Adil SF, Khan M. Morphology Controlled Deposition of Vanadium Oxide (VOx) Nanoparticles on the Surface of Highly Reduced Graphene Oxide for the Photocatalytic Degradation of Hazardous Organic Dyes. Materials. 2023; 16(18):6340. https://doi.org/10.3390/ma16186340
Chicago/Turabian StyleShaik, Mohammed Rafi, Fatimah N. Aldhuwayhi, Amal Mohammed Al-Mohaimeed, Mohammad Rafe Hatshan, Mufsir Kuniyil, Syed Farooq Adil, and Mujeeb Khan. 2023. "Morphology Controlled Deposition of Vanadium Oxide (VOx) Nanoparticles on the Surface of Highly Reduced Graphene Oxide for the Photocatalytic Degradation of Hazardous Organic Dyes" Materials 16, no. 18: 6340. https://doi.org/10.3390/ma16186340
APA StyleShaik, M. R., Aldhuwayhi, F. N., Al-Mohaimeed, A. M., Hatshan, M. R., Kuniyil, M., Adil, S. F., & Khan, M. (2023). Morphology Controlled Deposition of Vanadium Oxide (VOx) Nanoparticles on the Surface of Highly Reduced Graphene Oxide for the Photocatalytic Degradation of Hazardous Organic Dyes. Materials, 16(18), 6340. https://doi.org/10.3390/ma16186340










