Supported Gold Catalysts for Base-Free Furfural Oxidation: The State of the Art and Machine-Learning-Enabled Optimization
Abstract
:1. Introduction
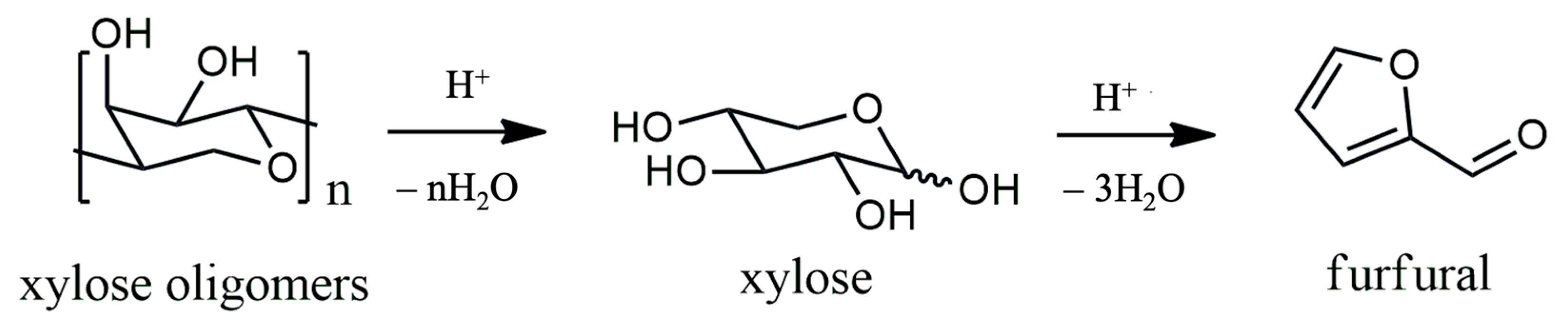
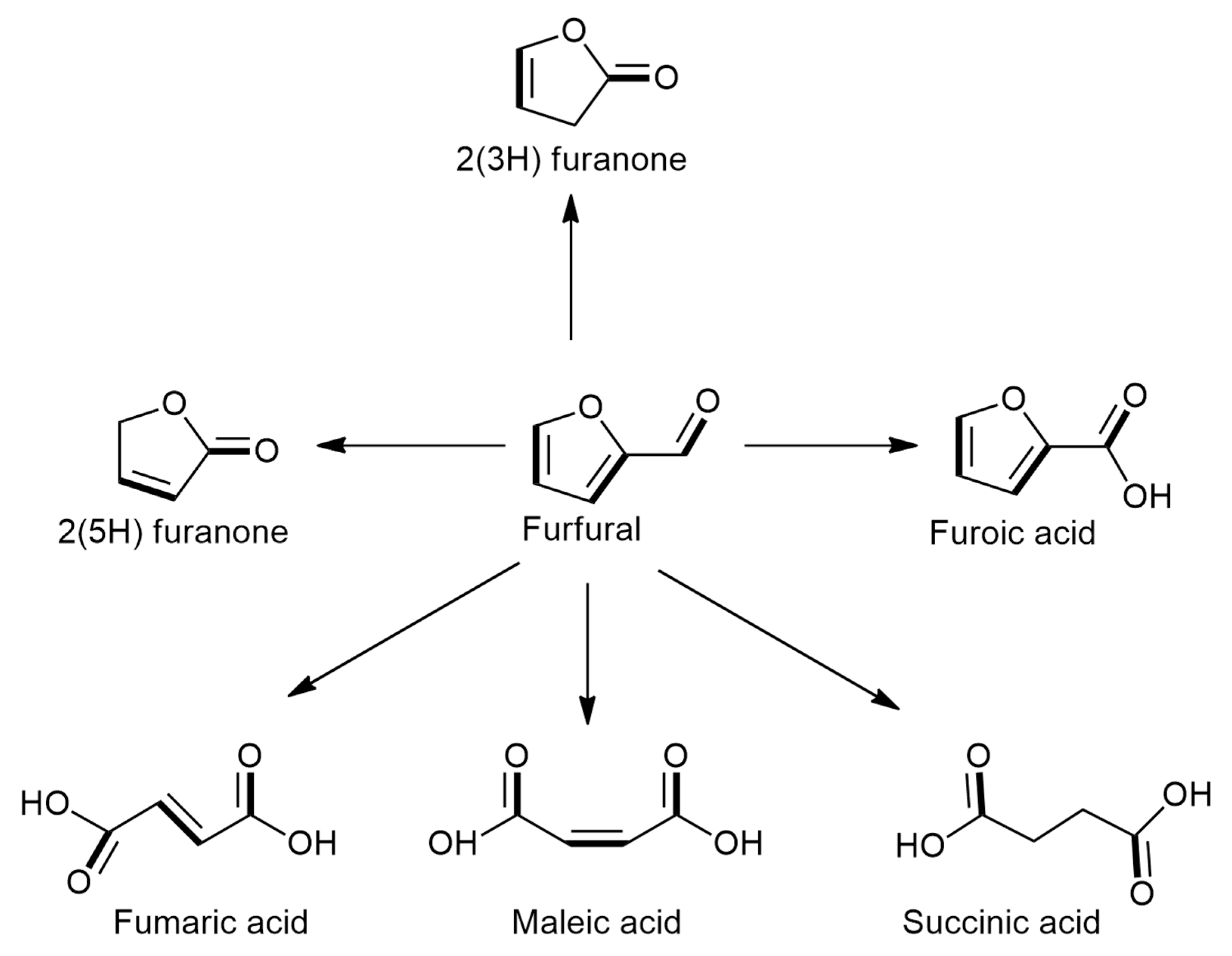
2. Catalytic Oxidation of Furfural
3. Furfural Oxidation on Basic Supports
4. Furfural Oxidation on Non-Basic Supports
5. Parameters Influencing Base-Free Furfural Oxidation: Machine Learning Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chheda, J.N.; Dumesic, J.A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 123, 59–70. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef]
- Renewables 2015 Global Status Report. Annual Reporting on Renewables: Ten Years of Excellence. Available online: http://docplayer.net/1033473-Renewables-2015-global-status-report-annual-reporting-on-renewables-ten-yearsof-excellence.html (accessed on 18 August 2023).
- Ravishankara, A.R.; Rudich, Y.; Pyle, J.A. Role of Chemistry in Earth’s Climate. Chem. Rev. 2015, 115, 3679–3681. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.C. Historical Developments in Hydroprocessing Bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Alves de Souza, L.; Magalhães de Souza, P.; Tozzi Wurzler, G.; Teixeira da Silva, V.; Azevedo, D.; Wojcieszak, R.; Bellot Noronha, F. Reductive Catalytic Fractionation of Lignocellulosic Biomass: Unveiling of the Reaction Mechanism. ACS Sustain. Chem. Eng. 2023, 11, 67–77. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Sadier, A.; Shi, D.; Mamede, A.S.; Paul, S.; Marceau, E.; Wojcieszak, R. Selective aqueous phase hydrogenation of xylose to xylitol over SiO2-supported Ni and Ni-Fe catalysts: Benefits of promotion by Fe. Appl. Catal. B Environ. 2021, 298, 120564. [Google Scholar] [CrossRef]
- De Souza Magalhães, P.; de Sousa Alves, L.; Noronha Bellot, F.; Wojcieszak, R. Dehydration of levoglucosan to levoglucosenone over solid acid catalysts. Tuning the product distribution by changing the acid properties of the catalysts. Mol. Catal. 2022, 529, 112564. [Google Scholar] [CrossRef]
- Araque-Marin, M.; Bellot Noronha, F.; Capron, M.; Dumeignil, F.; Friend, M.; Heuson, E.; Itabaiana, I.; Jalowiecki-Duhamel, L.; Katryniok, B.; Löfberg, A.; et al. Strengthening the Connection between Science, Society and Environment to Develop Future French and European Bioeconomies: Cutting-Edge Research of VAALBIO Team at UCCS. Molecules 2022, 27, 3889. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Itabaiana Junior, I.; Avelar do Nascimento, M.; de Souza Mendonça Alves, R.; Dufour, A.; Wojcieszak, R. Levoglucosan: A promising platform molecule? Green Chem. 2020, 22, 5859–5880. [Google Scholar] [CrossRef]
- Lange, J.-P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Biorefin. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Fossil Fuels Running out: Third Generation Micro Algal Biofuels Showing Light of Hope. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Dumeignil, F.; Capron, M.; Katryniok, B.; Wojcieszak, R.; Loefberg, A.; Girardon, J.S.; Desset, S.; Araque-Marin, M.; Jalowiecki-Duhamel, L.; Paul, S. Biomass-derived Platform Molecules Upgrading through Catalytic Processes: Yielding Chemicals and Fuels. J. Jpn. Petro. Inst. 2015, 58, 257–273. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Sadier, A.; Paul, S.; Wojcieszak, R. Selective Oxidation of Furfural at Room Temperature on a TiO2-Supported Ag Catalyst. Catalysts 2022, 12, 805. [Google Scholar] [CrossRef]
- Ferraz, C.; Kiméné, A.; Silva Vargas, K.; Heyte, S.; Durlin, C.; Simon, O.; Dumeignil, F.; Paul, S.; Wojcieszak, R. Efficient non-noble Ni–Cu based catalysts for the valorization of palmitic acid through a decarboxylation reaction. Catal. Sci. Tech. 2021, 11, 3025–3038. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis Goes Bio: From Carbohydrates and Sugar Alcohols to Platform Chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Drault, F.; Snoussi, Y.; Paul, S.; Itabaiana, I.; Wojcieszak, R. Recent Advances in Carboxylation of Furoic Acid into 2,5-Furandicarboxylic Acid: Pathways towards Bio-Based Polymers. ChemSusChem 2020, 13, 5164–5172. [Google Scholar] [CrossRef] [PubMed]
- Verardi, A.; Ricca, E.; De Bari, I.; Calabro, V. Hydrolysis of Lignocellulosic Biomass: Current Status of Processes and Technologies and Future Perspectives; INTECH Open Access Publisher: London, UK, 2012; p. 99. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Yabushita, M.; Kobayashi, H.; Fukuoka, A. Quantitative evaluation of ball-milling effects on the hydrolysis of cellulose catalysed by activated carbon. Appl. Catal. B 2014, 145, 1–9. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A.; Eliot, D.; Lasure, L.; Jones, S. Top Value Added Chemicals From Biomass. Volume 1—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy Report; Pacific Northwest National Laboratory/U.S. Department of Energy: Oak Ridge, TN, USA, 2004. [Google Scholar]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000; 376p, ISBN 9780080528991. [Google Scholar]
- Wei, H.; He, Y.; Ye, J. Efficient Synthesis of Biobased Furoic Acid from Corncob via Chemoenzymatic Approach. Processes 2022, 10, 677. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.H.; Li, G. Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation. Biomass 2022, 2, 103–115. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Santarelli, F.; Paul, S.; Dumeignil, F.; Cavani, F.; Gonçalves, R. Recent developments in maleic acid synthesis from bio-based chemicals. Sustain. Chem. Proces. 2015, 3, 9. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Cuccovia, Y.; Silva, M.; Rossi, L. Selective oxidation of glucose to glucuronic acid by cesium-promoted gold nanoparticle catalyst. J. Mol. Catal. A Chem. 2016, 422, 35–42. [Google Scholar] [CrossRef]
- Solmi, S.; Morreale, C.; Ospitali, F.; Agnoli, S.; Cavani, F. Oxidation of d-Glucose to Glucaric Acid Using Au/C Catalysts. ChemCatChem 2017, 9, 2797–2806. [Google Scholar] [CrossRef]
- Novotný, O.; Cejpek, K.; VelíšeK, J. Formation of Carboxylic Acids during Degradation of Monosaccharides. Czech J. Food Sci. 2008, 26, 117–131. [Google Scholar] [CrossRef]
- Moulik, S.P.; Basu, D.; Bhattacharya, P.K. Effects of various conditions on the alkaline degradation of d-fructose and d-glucose. Carbohydr. Res. 1978, 63, 165–172. [Google Scholar] [CrossRef]
- Santarelli, F.; Paul, S.; Dumeignil, F.; Cavani, F.; Wojcieszak, F. Furoic Acid Preparation Method. World Patent WO2017158106A1, 21 September 2017. [Google Scholar]
- Roselli, A.; Carvalho, Y.; Dumeignil, F.; Cavani, F.; Paul, S.; Wojcieszak, R. Liquid Phase Furfural Oxidation under Uncontrolled pH in Batch and Flow Conditions: The Role of in Situ Formed Base. Catalysts 2020, 10, 73. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Ferraz, C.; Sha, J.; Houda, S.; Rossi, L.; Paul, S. Advances in Base-Free Oxidation of Bio-Based Compounds on Supported Gold Catalysts. Catalysts 2017, 7, 352. [Google Scholar] [CrossRef]
- Thuriot-Roukos, J.; Khadraoui, R.; Paul, S.; Wojcieszak, R. Raman Spectroscopy Applied to Monitor Furfural Liquid-Phase Oxidation Catalyzed by Supported Gold Nanoparticles. ACS Omega 2020, 5, 14283–14290. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.; Zieliński, M.; Pietrowski, M.; Heyte, S.; Dumeignil, F.; Rossi, L.; Wojcieszak, R. Influence of Support Basic Sites in Green Oxidation of Biobased Substrates Using Au Promoted Catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16332–16340. [Google Scholar] [CrossRef]
- Ferraz, C.; Braga, A.; Ghazzal, N.; Zieliński, M.; Pietrowski, M.; Itabaiana, I.; Dumeignil, F.; Rossi, L.; Wojcieszak, R. Efficient Oxidative Esterification of Furfural Using Au Nanoparticles Supported on Group 2 Alkaline Earth Metal Oxides. Catalysts 2020, 10, 430. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid under atmospHeric oxygen pressure. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Gallas-Hulin, A.; Kotni, R.K.; Nielsen, M.; Kegnæs, S. Catalytic Oxidation of Allylic Alcohols to Methyl Esters. Top. Catal. 2017, 60, 1380–1386. [Google Scholar] [CrossRef]
- Menegazzo, F.; Fantinel, T.; Signoretto, M.; Pinna, F.; Manzoli, M. On the process for furfural and HMF oxidative esterification over Au/ZrO2. J. Catal. 2014, 319, 61–70. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into chemicals: Aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, N.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Zope, B.N.; Davis, S.E.; Davis, R.J. Influence of Reaction Conditions on Diacid Formation During Au-Catalyzed Oxidation of Glycerol and Hydroxymethylfurfural. Top. Catal. 2012, 55, 24–32. [Google Scholar] [CrossRef]
- Bettahar, M.; Wojcieszak, R.; Monteverdi, S. NiAg catalysts prepared by reduction of Ni2+ ions in aqueous hydrazine: II. Support effect. J. Colloid. Interface Sci. 2009, 332, 416–424. [Google Scholar] [CrossRef]
- Ferraz, C.; da Silva Marques, A.; Rodrigues, T.; Camargo, P.; Paul, S.; Wojcieszak, R. Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances. Appl. Sci. 2018, 8, 1246. [Google Scholar] [CrossRef]
- Da Silva, A.G.; Rodrigues, T.; Candido, E.; de Freitas, I.; da Silva, A.; Fajardo, H.; Balzer, R.; Gomes, J.; Assaf, J.; de Oliveira, D.; et al. Combining active phase and support optimization in MnO2-Au nanoflowers: Enabling high activities towards green oxidation. J. Colloid Interface Sci. 2018, 530, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, H.; Jia, L.; Xiao, Y.; Hou, B.; Li, D. Effect of MnO2 Crystal Type on the Oxidation of Furfural to Furoic Acid. Catalysts 2023, 13, 663. [Google Scholar] [CrossRef]
- Gupta, N.; Fukuoka, A.; Nakajima, K. Metal-Free and Selective Oxidation of Furfural to Furoic Acid with an N-Heterocyclic Carbene Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 3434–3442. [Google Scholar] [CrossRef]
- Douthwait, M.; Huang, X.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Kondrat, S.A.; Edwards, J.K.; Sankar, M.; Knight, D.W.; Bethell, D.; et al. The controlled catalytic oxidation of furfural to furoic acid using AuPd/Mg(OH)2. Catal. Sci. Technol. 2017, 7, 5284–5293. [Google Scholar] [CrossRef]
- Vuyyuru, K.R.; Strasser, P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 2012, 195, 144–154. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P.; Cozzolino, A. Effects of mineral and monocarboxylic acids on the molecular association of dissolved humic substances. Eur. J. Soil. Sci. 1999, 50, 687–694. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Ghazzal, M. Getting Greener with the Synthesis of Nanoparticles and Nanomaterials. Nanomaterials 2022, 12, 2452. [Google Scholar] [CrossRef]
- Monti, E.; Ventimiglia, A.; Garcia Soto, A.; Martelli, F.; Rodríguez-Aguado, E.; Cecilia Juan, A.; Sadier, A.; Ospitali, F.; Tabanelli, T.; Albonetti, S.; et al. Effect of the Colloidal Preparation Method for Supported Preformed Colloidal Au Nanoparticles for the Liquid Phase Oxidation of 1,6-Hexanediol to Adipic Acid. Catalysts 2022, 12, 196. [Google Scholar] [CrossRef]
- Wang, L.-C.; Liu, Y.-M.; Chen, M.; Cao, Y.; He, H.Y.; Fan, K.-N. MnO2 Nanorod Supported Gold Nanoparticles with Enhanced Activity for Solvent-free Aerobic Alcohol Oxidation. J. Phys. Chem. C 2008, 112, 6981–6987. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Kisukuri, C.M.; Rodrigues, T.S.; Candido, E.G.; de Freitas, I.C.; da Silva, A.H.M.; Assaf, J.M.; Oliveira, D.C.; Andrade, L.H.; Camargo, P.H.C. MnO2 nanowires decorated with Au ultrasmall nanoparticles for the green oxidation of silanes and hydrogen production under ultralow loadings. Appl. Catal. B 2016, 184, 35–43. [Google Scholar] [CrossRef]
- Pastrin, F.A.C.; da Silva, A.G.M.; Dourado, A.H.B.; de Lima Batista, A.P.; de Oliveira-Filho, A.G.S.; Quiroz, J.; de Oliveira, D.C.; Camargo, P.H.C.; Cordoba de Torresi, S.I. Why Could the Nature of Surface Facets Lead to Differences in the Activity and Stability of Cu2O-Based Electrocatalytic Sensors? ACS Catal. 2018, 8, 6265–6272. [Google Scholar] [CrossRef]
- Ferraz, C.; Costa, N.; Teixeira-Neto, E.; Teixeira-Neto, A.; Liria, C.; Thuriot-Roukos, J.; Machini, T.; Froidevaux, R.; Dumeignil, F.; Rossi, L.; et al. 5-Hydroxymethylfurfural and Furfural Base-Free Oxidation over AuPd Embedded. Bimetallic Nanoparticles. Catalysts 2020, 10, 75. [Google Scholar] [CrossRef]
- Gesesse, G.; Wang, C.; Chang, B.; Tai, S.-H.; Beaunier, P.; Wojcieszak, R.; Remita, H.; Colbeau-Justin, C.; Ghazzal, N. A soft-chemistry assisted strong metal–support interaction on a designed plasmonic core–shell pHotocatalyst for enhanced pHotocatalytic hydrogen production. Nanoscale 2020, 12, 7011–7023. [Google Scholar] [CrossRef]
- Ferraz, C.; Navarro-Jaén, S.; Rossi, L.; Dumeignil, F.; Ghazzal, N.; Wojcieszak, R. Enhancing the activity of gold supported catalysts by oxide coating: Towards efficient oxidations. Green Chem. 2021, 23, 8453–8457. [Google Scholar] [CrossRef]
- Al Rawas, H.; Ferraz, C.; Thuriot-Roukos, J.; Heyte, S.; Paul, S.; Wojcieszak, R. Influence of Pd and Pt Promotion in Gold Based Bimetallic Catalysts on Selectivity Modulation in Furfural Base-Free Oxidation. Catalysts 2021, 11, 1226. [Google Scholar] [CrossRef]
- Al Rawas, H. Bimetallic Au Based Catalysts for Base-Free Oxidation of Furfural: Benefits of Promotion by Pd and Pt. Master’s Thesis, University of Lille, Lille, France, July 2021. [Google Scholar]



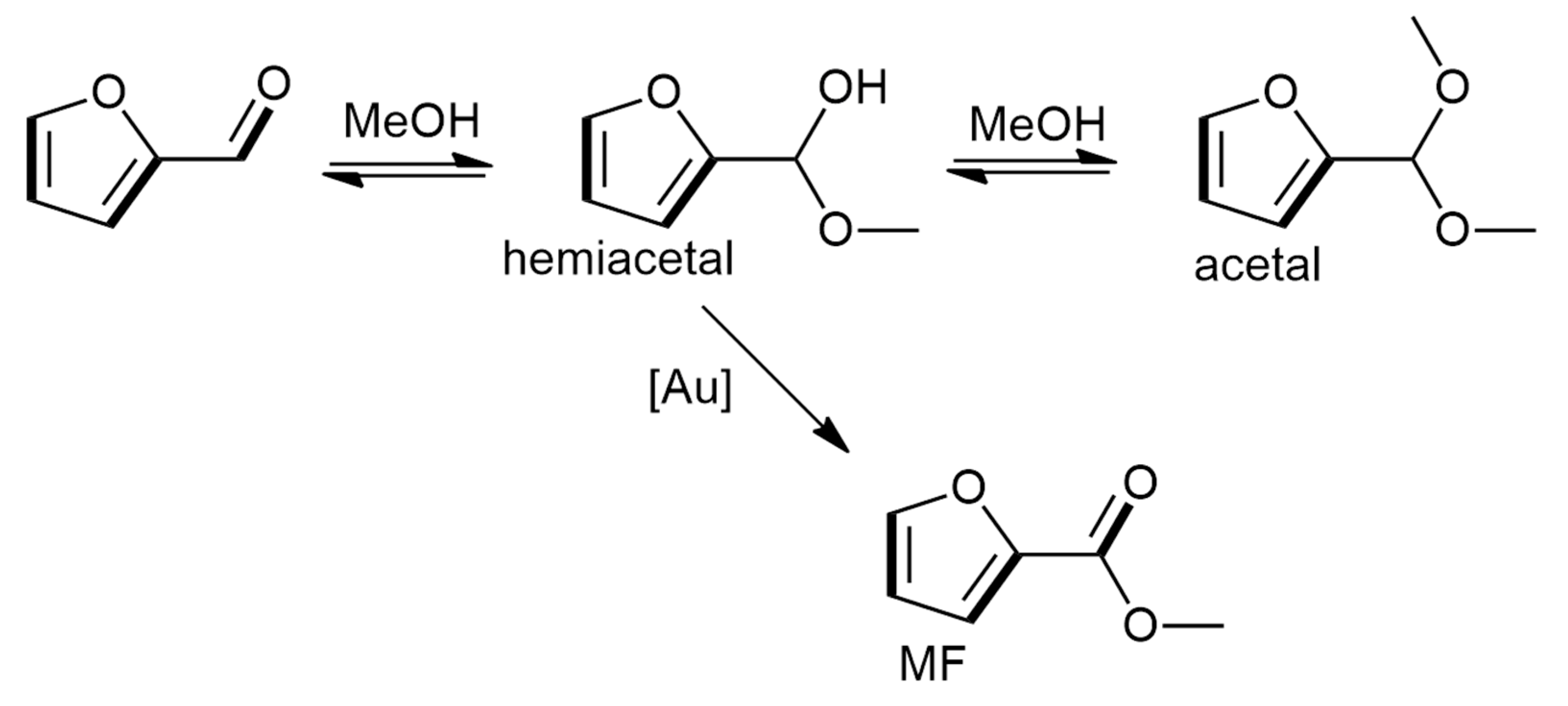

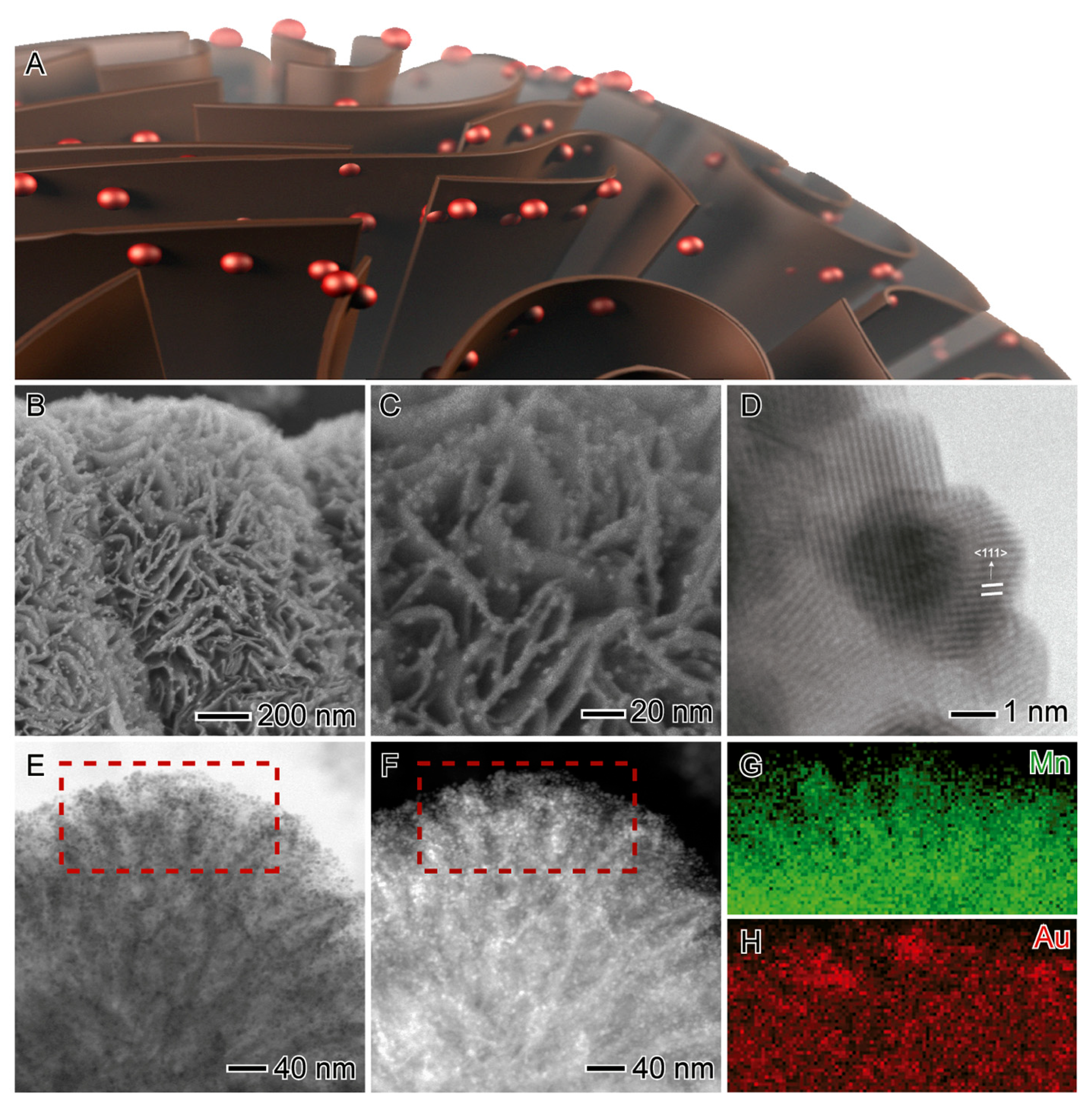
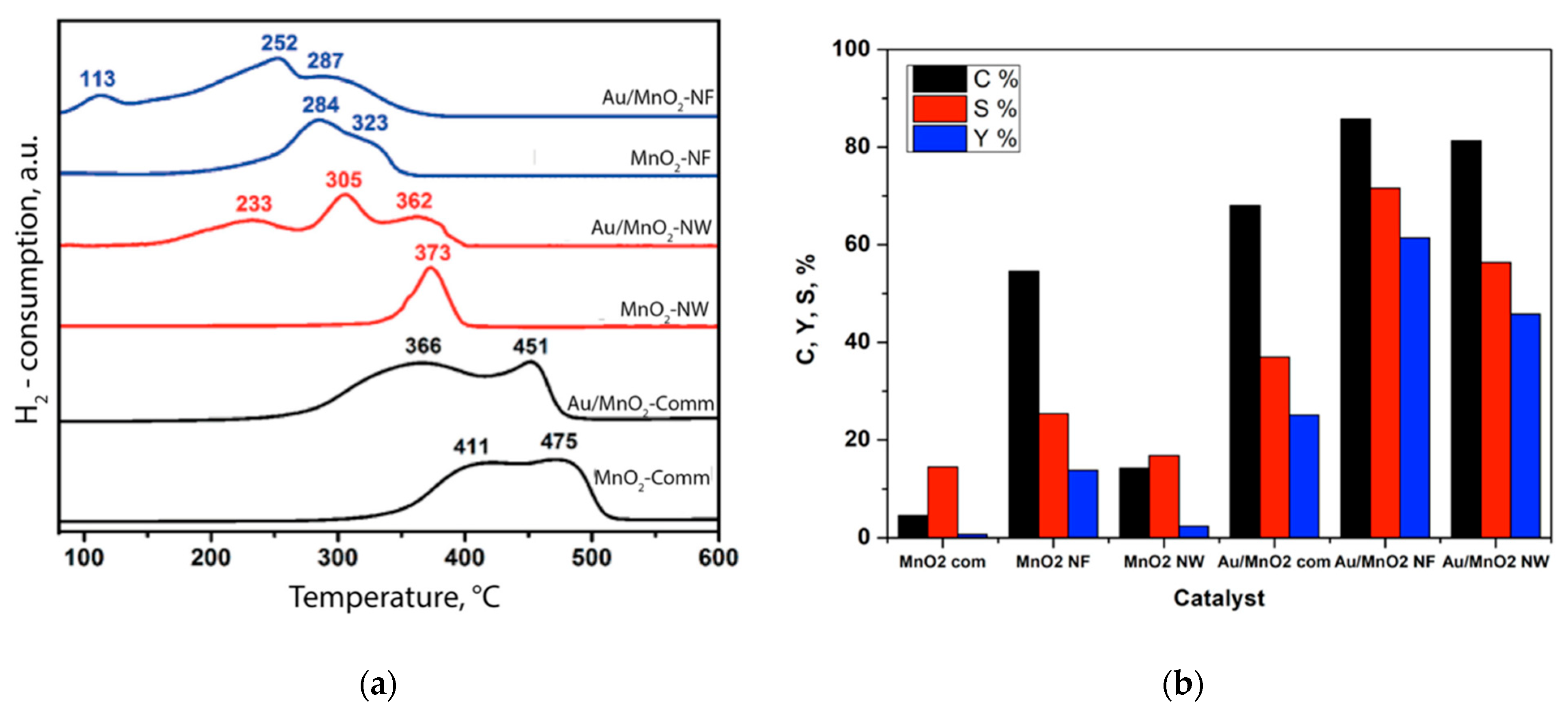
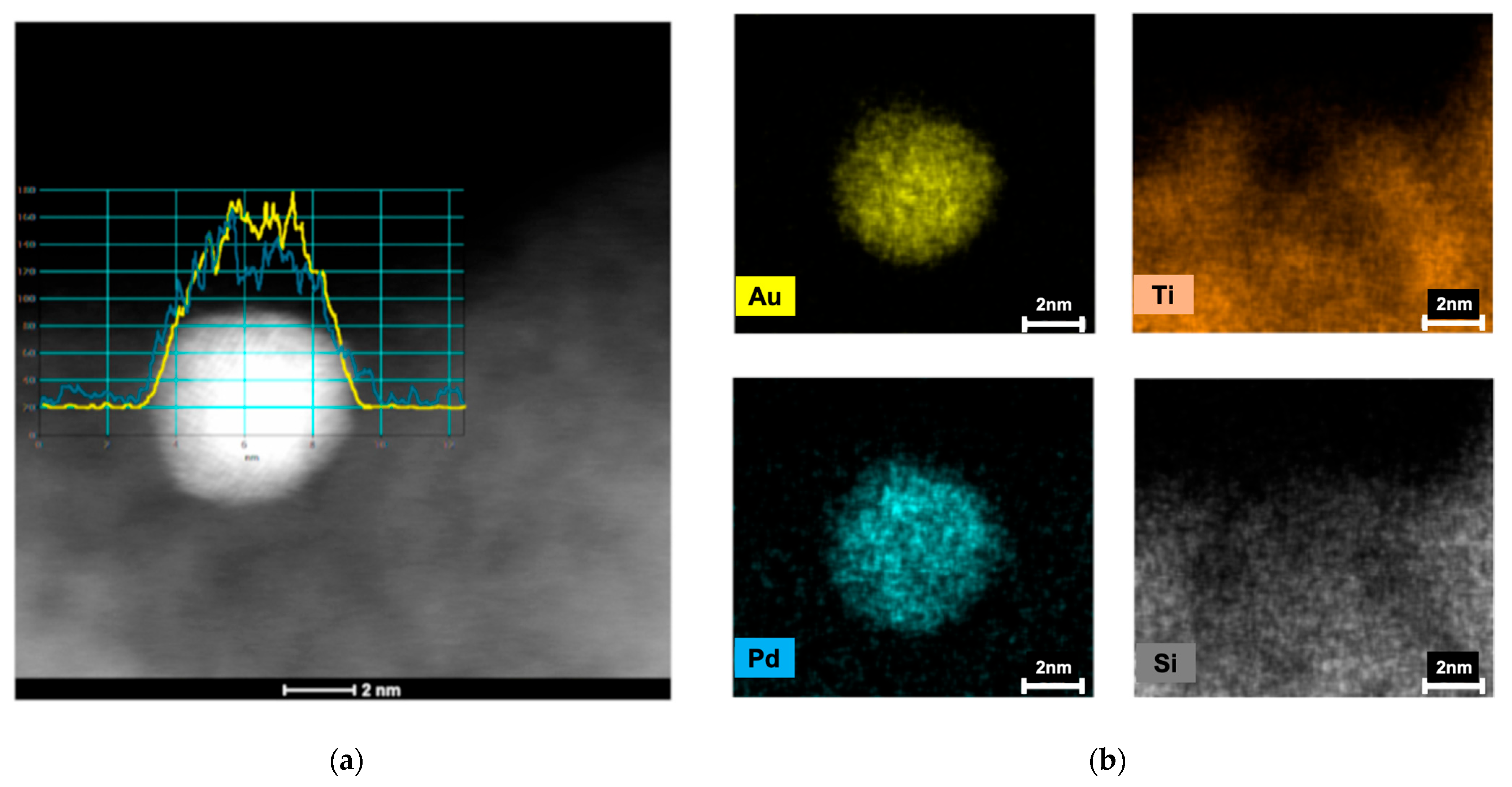
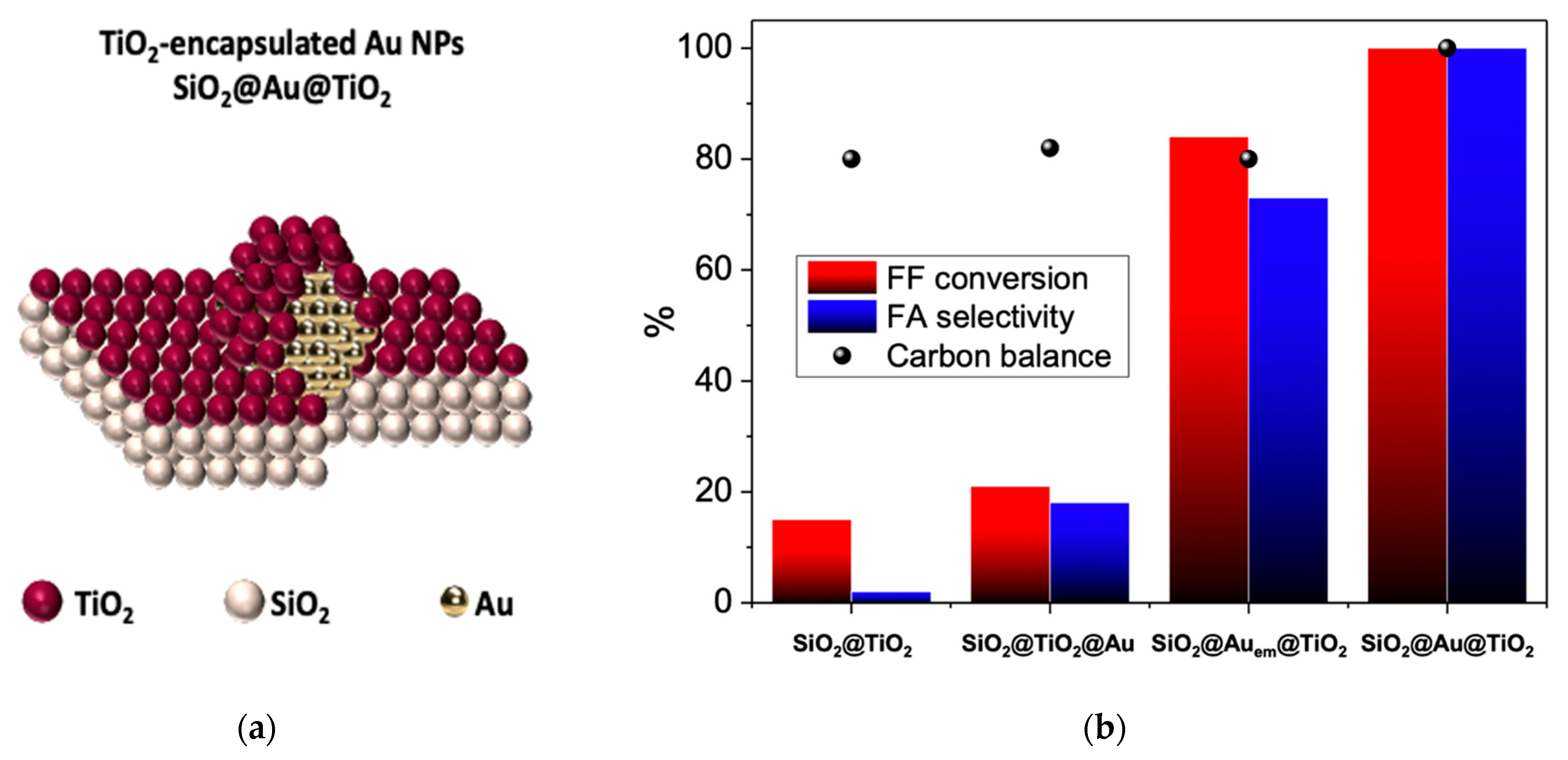
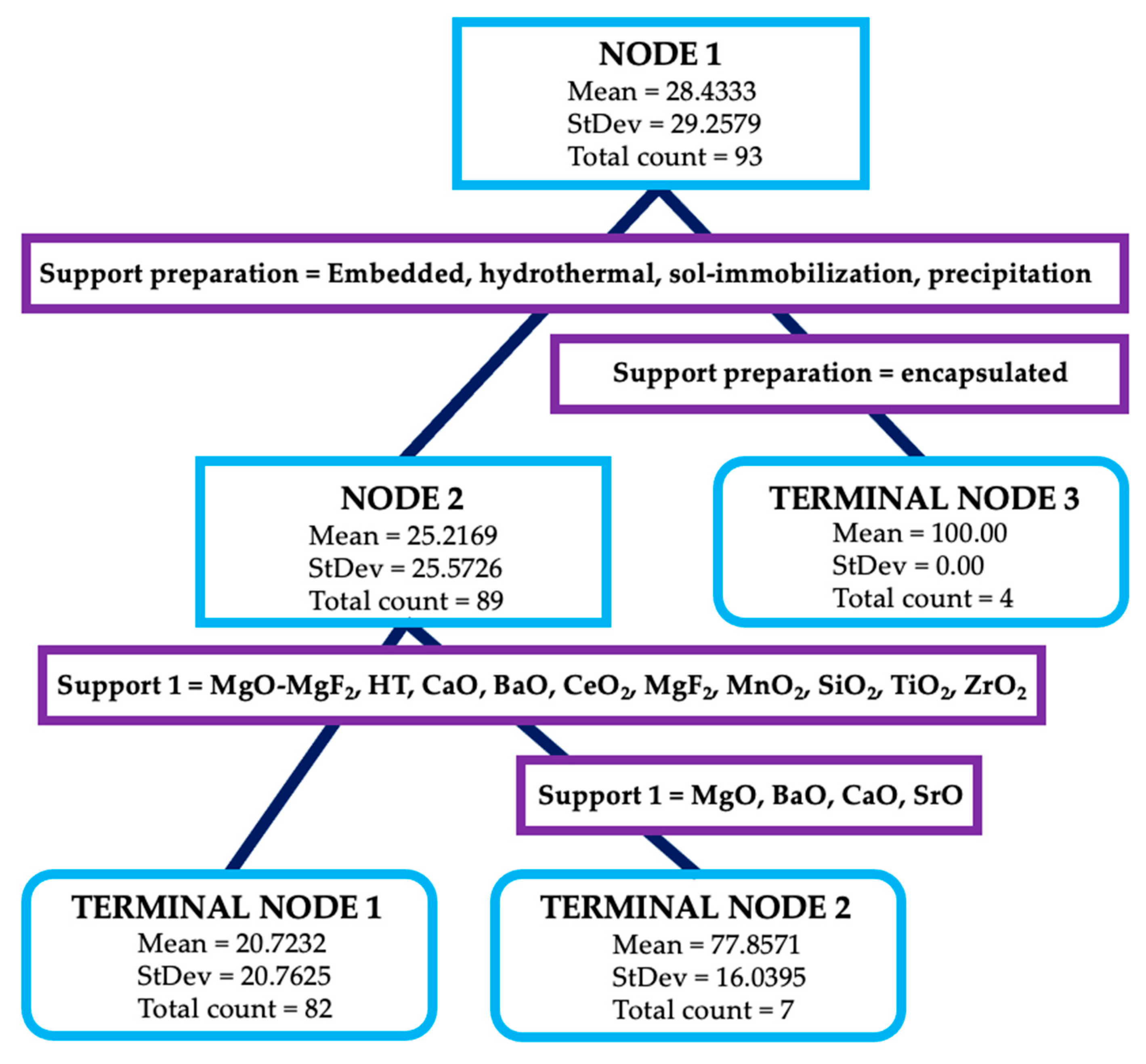
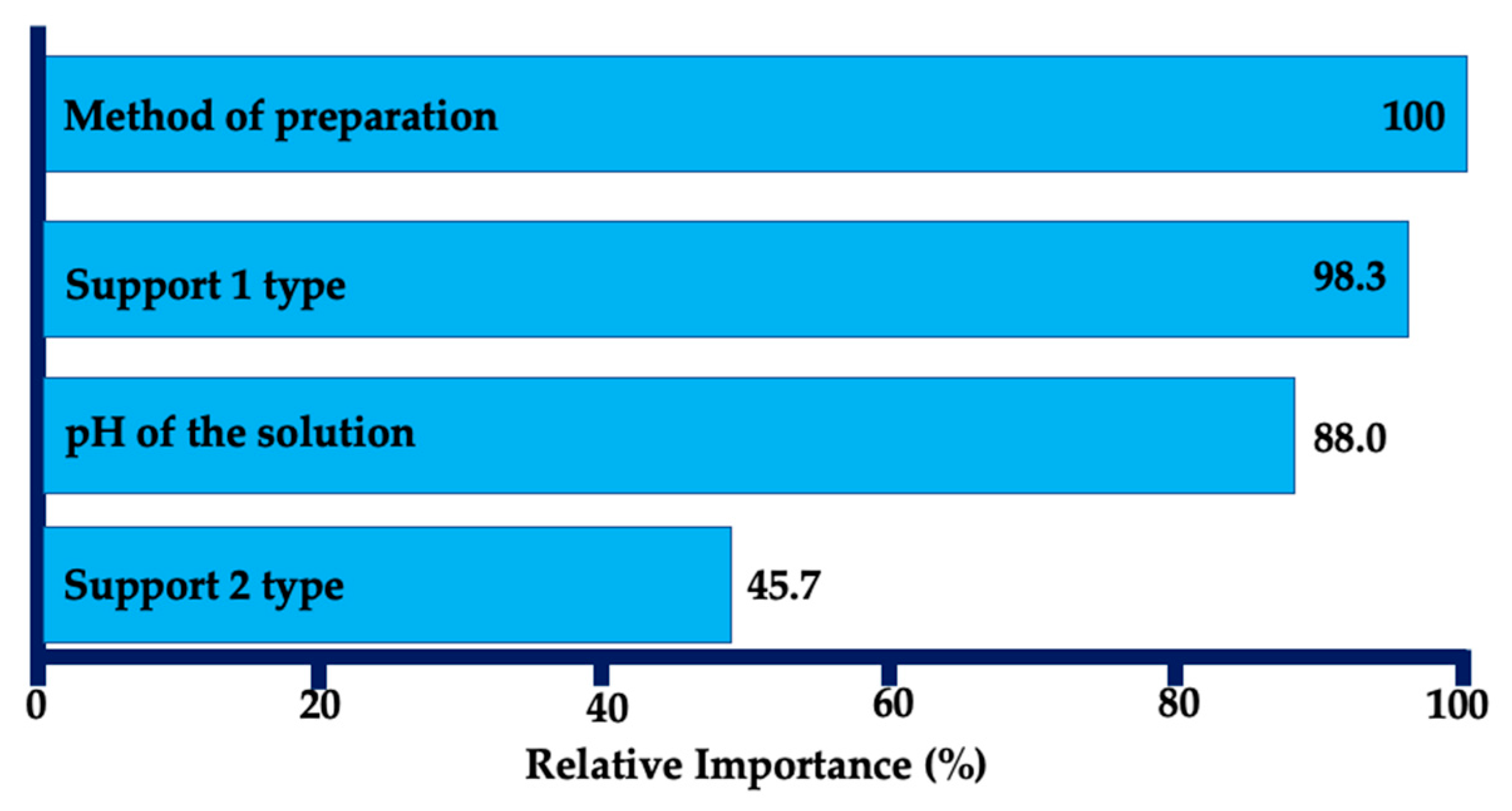

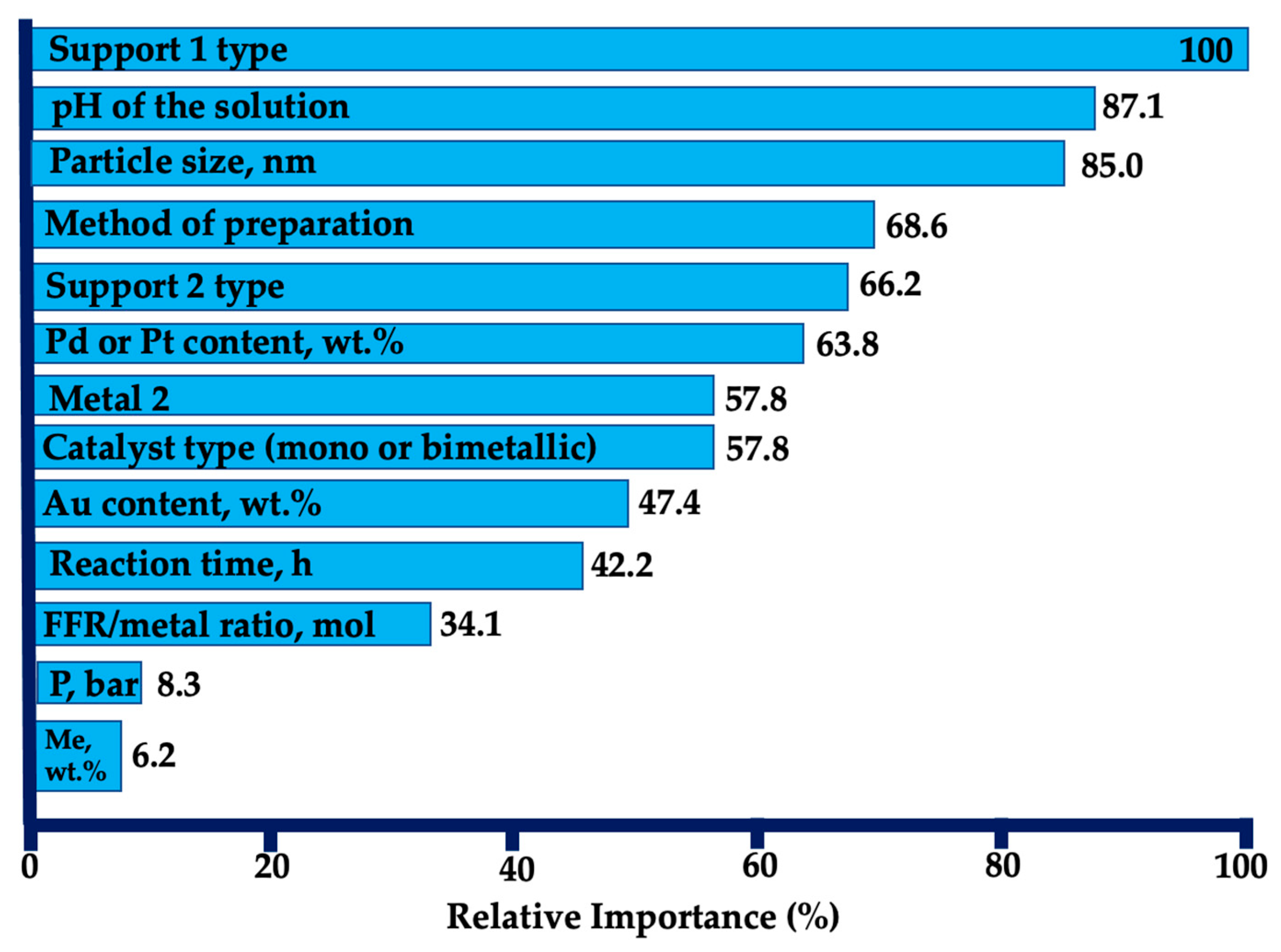
| Entry | Substrate/Solvent | FFR Conversion (%) | Yield (%) |
|---|---|---|---|
| 1 | ethanol | 45 | 45 |
| 2 | iso-propanol | 32 | 31 |
| 3 | n-butanol | 92 | 92 |
| 4 | iso-pentanol | 68 | 66 |
| Catalysts | Au [%] | Particle Size [nm] | Surface Area [m2/g] | Pore Volume [cm3/g] | Pore Diameter [A] |
|---|---|---|---|---|---|
| Au/MnO2-NF | 1.3 | 2.6 | 196 | 0.42 | 6.1 |
| MnO2-NF | - | - | 194 | 0.41 | 6.1 |
| Au/MnO2-NW | 1.3 | 3.2 | 125 | 0.16 | 6.9 |
| MnO2-NW | - | - | 122 | 0.16 | 6.8 |
| Au/MnO2-Comm | 1.4 | 2.6 | 15 | 0.05 | 17.8 |
| MnO2-Comm | - | - | 14 | 0.05 | 17.8 |
| Catalysts | Conversion [%] | Selectivity [%] | Yield [%] | TON | Carbon Balance [%] |
|---|---|---|---|---|---|
| Au4Pd1@SiTi | 83 | 45 | 37 | 91 | 54 |
| Au1Pd1@SiTi | 77 | 30 | 23 | 80 | 47 |
| Au1Pd4@SiTi | 54 | 23 | 12 | 57 | 58 |
| Au4Pd1/SiTi | 99 | 48 | 48 | 50 | 49 |
| Au1Pd1/SiTi | 54 | 18 | 10 | 28 | 56 |
| Au1Pd4/SiTi | 38 | 11 | 4 | 19 | 66 |
| Au1Pd1PVA@SiTi | 87 | 41 | 36 | 44 | 48 |
| Au/TiO2 | 100 | 8 | 8 | 43 | 8 |
| Au/ZrO2 | 92 | 32 | 29 | 45 | 37 |
| blank | 52 | 0 | 0 | - | 48 |
| Au@SiTi | 30 | 32 | 10 | 15 | 80 |
| Pd@SiTi | 34 | 20 | 7 | 17 | 73 |
| Catalyst | Metal 1 | Metal 2 | Supp. 1 | Supp. 2 | Synthesis Method | Au wt.% | Pd or Pt wt.% | Metal wt.% | Particle Size [nm] | FA Yield [%] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Au4Pd1@SiTi | Au | Pd | SiO2 | TiO2 | Embedded | 1.68 | 0.42 | 2.11 | 4 | 37 | [62] |
| Au1Pd1@SiTi | Au | Pd | SiO2 | TiO2 | Embedded | 1..37 | 0.97 | 2.34 | 3.8 | 23 | [62] |
| Au1Pd4@SiTi | Au | Pd | SiO2 | TiO2 | Embedded | 0.69 | 1.6 | 2.29 | 4 | 12 | [62] |
| Au4Pd1/SiTi | Au | Pd | SiO2 | TiO2 | Embedded | 5.41 | 0.8 | 6.21 | 4 | 48 | [62] |
| Au1Pd1/SiTi | Au | Pd | SiO2 | TiO2 | Embedded | 2.3 | 1.31 | 3.61 | 3.8 | 10 | [62] |
| Au1Pd4/SiTi | Au | Pd | SiO2 | TiO2 | Embedded | 1.21 | 2.21 | 3.34 | 4 | 4 | [62] |
| Au@SiTi | Au | No | SiO2 | TiO2 | Embedded | 6.4 | 0 | 6.4 | 4 | 10 | [62] |
| Pd@SiTi | Pd | No | SiO2 | TiO2 | Embedded | 0 | 3.1 | 3.1 | 4 | 7 | [62] |
| HT-4:1 | No | No | 0.8 MgO | 0.2 Al2O3 | Precipitation | 0 | 0 | 0 | 0 | 1 | [40] |
| Au/HT-4:1 | Au | No | 0.8 MgO | 0.2 Al2O3 | Precipitation | 1.64 | 0 | 1.64 | 3.5 | 9 | [40] |
| Au/HT-H2O2-4:1 | Au | No | 0.8 MgO | 0.2 Al2O3 | Precipitation | 1.64 | 0 | 1.64 | 3.5 | 19 | [40] |
| Au/HT-4:1 | Au | No | 0.8 MgO | 0.2 Al2O3 | Precipitation | 1.64 | 0 | 1.64 | 3.5 | 27 | [40] |
| HT-2:1 | No | No | 0.6 MgO | 0.4 Al2O3 | Precipitation | 0 | 0 | 0 | 0 | 0 | [40] |
| Au/HT-2:1 | Au | No | 0.6 MgO | 0.4 Al2O3 | Precipitation | 1.52 | 0 | 1.52 | 3.5 | 6 | [40] |
| Au/HT-H2O2-2:1 | Au | No | 0.6 MgO | 0.4 Al2O3 | Precipitation | 1.52 | 0 | 1.52 | 3.5 | 17 | [40] |
| Au/HT-2:1 | Au | No | 0.6 MgO | 0.4 Al2O3 | Precipitation | 1.52 | 0 | 1.52 | 3.5 | 20 | [40] |
| HT-1:1 | No | No | 0.5 MgO | 0.5 Al2O3 | Precipitation | 0 | 0 | 0 | 0 | 0 | [40] |
| Au/HT-1:1 | Au | No | 0.5 MgO | 0.5 Al2O3 | Precipitation | 1.83 | 0 | 1.83 | 3.5 | 6 | [40] |
| Au/HT-H2O2-1:1 | Au | No | 0.5 MgO | 0.5 Al2O3 | Precipitation | 1.83 | 0 | 1.83 | 3.5 | 22 | [40] |
| Au/HT-1:1 | Au | No | 0.5 MgO | 0.5 Al2O3 | Precipitation | 1.83 | 0 | 1.83 | 3.5 | 25 | [40] |
| HT-1:5 | No | No | 0.2 MgO | 0.8 Al2O3 | Precipitation | 0 | 0 | 0 | 0 | 0 | [40] |
| Au/HT-1:5 | Au | No | 0.2 MgO | 0.8 Al2O3 | Precipitation | 1.9 | 0 | 1.9 | 3.5 | 0 | [40] |
| Au/HT-H2O2-1:5 | Au | No | 0.2 MgO | 0.8 Al2O3 | Precipitation | 1.9 | 0 | 1.9 | 3.5 | 5 | [40] |
| Au/HT-1:5 | Au | No | 0.2 MgO | 0.8 Al2O3 | Precipitation | 1.9 | 0 | 1.9 | 3.5 | 3 | [40] |
| Au/TiO2 | Au | No | TiO2 | No | Sol-PVA a | 2.09 | 0 | 2.09 | 3 | 40.48 | [65] |
| Pt/TiO2 | Pt | No | TiO2 | No | Sol-PVA a | 0 | 0.91 | 0.91 | 3 | 0.99 | [65] |
| Pd/TiO2 | Pd | No | TiO2 | No | Sol-PVA a | 0 | 1.98 | 1.98 | 3 | 1.45 | [65] |
| 0.5%Pt1-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.24 | 0.16 | 0.4 | 3 | 21.8 | [65] |
| 1.25%Pt1-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.62 | 0.42 | 1.04 | 3 | 30.1 | [65] |
| 2%Pt1-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 1.02 | 0.75 | 1.77 | 3 | 24.9 | [65] |
| 0.5%Pt1-Au3/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.37 | 0.12 | 0.49 | 3 | 23.6 | [65] |
| 1.25%Pt1-Au3/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.9 | 0.29 | 1.18 | 3 | 32.7 | [65] |
| 2%Pt1-Au3/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 1.36 | 0.43 | 1.79 | 3 | 37.5 | [65] |
| 0.5%Pt3-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.13 | 0.19 | 0.32 | 3 | 15.2 | [65] |
| 1.25%Pt3-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.26 | 0.5 | 0.77 | 3 | 22.8 | [65] |
| 2%Pt3-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.5 | 1.19 | 1.69 | 3 | 15.6 | [65] |
| 0.5%Pd1-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.31 | 0.18 | 0.49 | 3 | 22.8 | [65] |
| 1.25%Pd1-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.89 | 0.48 | 1.37 | 3 | 29.9 | [65] |
| 2%Pd1-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 1.29 | 0.68 | 1.97 | 3 | 34.5 | [65] |
| 0.5%Pd1-Au3/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.4 | 0.09 | 0.49 | 3 | 19.8 | [65] |
| 1.25%Pd1-Au3/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 1.15 | 0.22 | 1.37 | 3 | 31.5 | [65] |
| 2%Pd1-Au3/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 1.59 | 0.29 | 1.88 | 3 | 34.1 | [65] |
| 0.5%Pd3-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.17 | 0.3 | 0.47 | 3 | 11.4 | [65] |
| 1.25%Pd3-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.45 | 0.72 | 1.17 | 3 | 14.8 | [65] |
| 2%Pd3-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.78 | 1.21 | 1.99 | 3 | 17.6 | [65] |
| 0.5%Pt1-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.24 | 0.16 | 0.4 | 3 | 8.8 | [65] |
| 1.25%Pt1-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.62 | 0.42 | 1.04 | 3 | 0 | [65] |
| 2%Pt1-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 1.02 | 0.75 | 1.77 | 3 | 0 | [65] |
| 0.5%Pt1-Au3/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.37 | 0.12 | 0.49 | 3 | 28.8 | [65] |
| 1.25%Pt1-Au3/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.9 | 0.29 | 1.18 | 3 | 0.9 | [65] |
| 2%Pt1-Au3/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 1.36 | 0.43 | 1.79 | 3 | 0 | [65] |
| 0.5%Pt3-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.13 | 0.19 | 0.32 | 3 | 5.2 | [65] |
| 1.25%Pt3-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.26 | 0.5 | 0.77 | 3 | 0 | [65] |
| 2%Pt3-Au1/TiO2 | Au | Pt | TiO2 | No | Sol-PVA a | 0.5 | 1.19 | 1.69 | 3 | 0 | [65] |
| 0.5%Pd1-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.31 | 0.18 | 0.49 | 3 | 33.5 | [65] |
| 1.25%Pd1-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.89 | 0.48 | 1.37 | 3 | 24.5 | [65] |
| 2%Pd1-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 1.29 | 0.68 | 1.97 | 3 | 13.2 | [65] |
| 0.5%Pd1-Au3/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.4 | 0.09 | 0.49 | 3 | 51.6 | [65] |
| 1.25%Pd1-Au3/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 1.15 | 0.22 | 1.37 | 3 | 58.2 | [65] |
| 2%Pd1-Au3/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 1.59 | 0.29 | 1.88 | 3 | 58.7 | [65] |
| 0.5%Pd3-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.17 | 0.3 | 0.47 | 3 | 12.2 | [65] |
| 1.25%Pd3-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.45 | 0.72 | 1.17 | 3 | 0.9 | [65] |
| 2%Pd3-Au1/TiO2 | Au | Pd | TiO2 | No | Sol-PVA a | 0.78 | 1.21 | 1.99 | 3 | 0 | [65] |
| Au/MgO | Au | No | MgO | No | Sol-PVA a | 1.8 | 0 | 1.8 | 3.6 | 100 | [41] |
| Au/TiO2 | Au | No | TiO2 | No | Sol-PVA a | 1.8 | 0 | 1.8 | 3.6 | 30 | [41] |
| Au/ZrO2 | Au | No | ZrO2 | No | Sol-PVA a | 1.8 | 0 | 1.8 | 3.6 | 32 | [41] |
| Au/CeO2 | Au | No | CeO2 | No | Sol-PVA a | 1.8 | 0 | 1.8 | 3.6 | 18 | [41] |
| Au/MgF2 | Au | No | MgF2 | No | Precipitation | 1.8 | 0 | 1.8 | 3.6 | 2 | [41] |
| Au/0.6MgF2-0.4MgO | Au | No | 0.4 MgO | 0.6 MgF2 | Precipitation | 1.8 | 0 | 1.8 | 3.6 | 76 | [41] |
| Au/0.4MgF2-0.6MgO | Au | No | 0.6 MgO | 0.4 MgF2 | Precipitation | 1.8 | 0 | 1.8 | 3.6 | 99 | [41] |
| SiO2@TiO2 | No | No | SiO2 | TiO2 | Sol-PVA a | 0 | 0 | 0 | 5 | 0.3 | [64] |
| 0.25% SiO2@Au@TiO2 | Au | No | SiO2 | TiO2 | Encapsulated | 0.13 | 0 | 0.13 | 5 | 100 | [64] |
| 0.5% SiO2@Au@TiO2 | Au | No | SiO2 | TiO2 | Encapsulated | 0.53 | 0 | 0.53 | 5 | 100 | [64] |
| 1% SiO2@Au@TiO2 | Au | No | SiO2 | TiO2 | Encapsulated | 1.13 | 0 | 1.13 | 5 | 100 | [64] |
| 2% SiO2@Au@TiO2 | Au | No | SiO2 | TiO2 | Encapsulated | 2.02 | 0 | 2.02 | 5 | 100 | [64] |
| 0.25% SiO2@TiO2@Au | Au | No | SiO2 | TiO2 | Embedded | 0.28 | 0 | 0.28 | 5 | 8.4 | [64] |
| 0.5% SiO2@TiO2@Au | Au | No | SiO2 | TiO2 | Embedded | 0.56 | 0 | 0.56 | 5 | 41.8 | [64] |
| 1% SiO2@TiO2@Au | Au | No | SiO2 | TiO2 | Embedded | 1.26 | 0 | 1.26 | 5 | 19 | [64] |
| 2% SiO2@TiO2@Au | Au | No | SiO2 | TiO2 | Embedded | 1.37 | 0 | 1.37 | 5 | 3.8 | [64] |
| 2%Au/CaO | Au | No | CaO | No | Sol-PVA a | 2 | 0 | 2 | 3.5 | 81 | [66] |
| 2%Au/SrO | Au | No | SrO | No | Sol-PVA a | 2 | 0 | 2 | 3.6 | 66 | [66] |
| 2%Au/BaO | Au | No | BaO | No | Sol-PVA a | 2 | 0 | 2 | 3.7 | 54 | [66] |
| 2%Au/BeO | Au | No | BeO | No | Sol-PVA a | 2 | 0 | 2 | 3.8 | 32 | [66] |
| Au-HT2:1 | Au | No | 0.6MgO | 0.4Al2O3 | Precipitation | 1.3 | 0 | 1.3 | 3.7 | 72 | [38] |
| Au-HT3:1 | Au | No | 0.7MgO | 0.3Al2O3 | Precipitation | 1.7 | 0 | 1.7 | 3.7 | 68 | [38] |
| Au-HT4:1 | Au | No | 0.75MgO | 0.25Al2O3 | Precipitation | 1.2 | 0 | 1.2 | 3.7 | 100 | [38] |
| Au-HT5:1 | Au | No | 0.8MgO | 0.2Al2O3 | Precipitation | 1.4 | 0 | 1.4 | 3.7 | 100 | [38] |
| MnO2 com | No | No | MnO2 | No | Hydrothermal | 0 | 0 | 0 | 0 | 2 | [50] |
| MnO2 NF | No | No | MnO2 NF | No | Hydrothermal | 0 | 0 | 0 | 0 | 14 | [50] |
| MnO2 NW | No | No | MnO2 NW | No | Hydrothermal | 0 | 0 | 0 | 0 | 4 | [50] |
| Au/MnO2 com | Au | No | MnO2 | No | Hydrothermal | 1.4 | 0 | 1.4 | 2.6 | 25 | [50] |
| Au/MnO2 NF | Au | No | MnO2 NF | No | Hydrothermal | 1.3 | 0 | 1.3 | 2.6 | 60 | [50] |
| Au/MnO2 NW | Au | No | MnO2 NW | No | Hydrothermal | 1.3 | 0 | 1.3 | 3.2 | 45 | [50] |
| Catalyst | FFR/Metal Ratio [mol/mol] | Temp [°C] | Pressure [bar] | Reaction Time [h] | Solvent | pH | FA Yield [%] | Leaching ** | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Au4Pd1@SiTi | 50 | 110 | 26 | 10 | Water | 3 | 37 | 2 | [62] |
| Au1Pd1@SiTi | 50 | 110 | 26 | 10 | Water | 3 | 23 | 2 | [62] |
| Au1Pd4@SiTi | 50 | 110 | 26 | 10 | Water | 3 | 12 | 2 | [62] |
| Au4Pd1/SiTi | 50 | 110 | 26 | 10 | Water | 3 | 48 | 2 | [62] |
| Au1Pd1/SiTi | 50 | 110 | 26 | 10 | Water | 3 | 10 | 2 | [62] |
| Au1Pd4/SiTi | 50 | 110 | 26 | 10 | Water | 3 | 4 | 2 | [62] |
| Au@SiTi | 50 | 110 | 26 | 10 | Water | 3 | 10 | 2 | [62] |
| Pd@SiTi | 50 | 110 | 26 | 10 | Water | 3 | 7 | 2 | [62] |
| HT-4:1 | 100 | 90 | 1 | 2 | Water | 7.6 | 1 | 1 | [40] |
| Au/HT-4:1 | 100 | 90 | 1 | 2 | Water | 7.6 | 9 | 1 | [40] |
| Au/HT-H2O2-4:1 | 100 | 90 | 1 | 2 | Water + H2O2 | 7.6 | 19 | 1 | [40] |
| Au/HT-4:1 | 100 | 90 | 1 | 6 | Water | 7.6 | 27 | 1 | [40] |
| HT-2:1 | 100 | 90 | 1 | 2 | Water | 7 | 0 | 1 | [40] |
| Au/HT-2:1 | 100 | 90 | 1 | 2 | Water | 7 | 6 | 1 | [40] |
| Au/HT-H2O2-2:1 | 100 | 90 | 1 | 2 | Water + H2O2 | 7 | 17 | 1 | [40] |
| Au/HT-2:1 | 100 | 90 | 1 | 6 | Water | 7 | 20 | 1 | [40] |
| HT-1:1 | 100 | 90 | 1 | 2 | Water | 6.5 | 0 | 1 | [40] |
| Au/HT-1:1 | 100 | 90 | 1 | 2 | Water | 6.5 | 6 | 1 | [40] |
| Au/HT-H2O2-1:1 | 100 | 90 | 1 | 2 | Water + H2O2 | 6.5 | 22 | 1 | [40] |
| Au/HT-1:1 | 100 | 90 | 1 | 6 | Water | 6.5 | 25 | 1 | [40] |
| HT-1:5 | 100 | 90 | 1 | 2 | Water | 6 | 0 | 1 | [40] |
| Au/HT-1:5 | 100 | 90 | 1 | 2 | Water | 6 | 0 | 1 | [40] |
| Au/HT-H2O2-1:5 | 100 | 90 | 1 | 2 | Water + H2O2 | 6 | 5 | 1 | [40] |
| Au/HT-1:5 | 100 | 90 | 1 | 6 | Water | 6 | 3 | 1 | [40] |
| Au/TiO2 | 50 | 110 | 15 | 2 | Water | 3 | 40.48 | 3 | [65] |
| Pt/TiO2 | 50 | 110 | 15 | 2 | Water | 3 | 0.99 | 3 | [65] |
| Pd/TiO2 | 50 | 110 | 15 | 2 | Water | 3 | 1.45 | 3 | [65] |
| 0.5%Pt1-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 21.8 | 3 | [65] |
| 1.25%Pt1-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 30.1 | 3 | [65] |
| 2%Pt1-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 24.9 | 3 | [65] |
| 0.5%Pt1-Au3/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 23.6 | 3 | [65] |
| 1.25%Pt1-Au3/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 32.7 | 3 | [65] |
| 2%Pt1-Au3/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 37.5 | 3 | [65] |
| 0.5%Pt3-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 15.2 | 3 | [65] |
| 1.25%Pt3-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 22.8 | 3 | [65] |
| 2%Pt3-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 15.6 | 3 | [65] |
| 0.5%Pd1-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 22.8 | 3 | [65] |
| 1.25%Pd1-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 29.9 | 3 | [65] |
| 2%Pd1-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 34.5 | 3 | [65] |
| 0.5%Pd1-Au3/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 19.8 | 3 | [65] |
| 1.25%Pd1-Au3/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 31.5 | 3 | [65] |
| 2%Pd1-Au3/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 34.1 | 3 | [65] |
| 0.5%Pd3-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 11.4 | 3 | [65] |
| 1.25%Pd3-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 14.8 | 3 | [65] |
| 2%Pd3-Au1/TiO2 | 50 | 80 | 15 | 4 | Water | 3 | 17.6 | 3 | [65] |
| 0.5%Pt1-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 8.8 | 3 | [65] |
| 1.25%Pt1-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0 | 3 | [65] |
| 2%Pt1-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0 | 3 | [65] |
| 0.5%Pt1-Au3/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 28.8 | 3 | [65] |
| 1.25%Pt1-Au3/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0.9 | 3 | [65] |
| 2%Pt1-Au3/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0 | 3 | [65] |
| 0.5%Pt3-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 5.2 | 3 | [65] |
| 1.25%Pt3-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0 | 3 | [65] |
| 2%Pt3-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0 | 3 | [65] |
| 0.5%Pd1-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 33.5 | 3 | [65] |
| 1.25%Pd1-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 24.5 | 3 | [65] |
| 2%Pd1-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 13.2 | 3 | [65] |
| 0.5%Pd1-Au3/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 51.6 | 3 | [65] |
| 1.25%Pd1-Au3/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 58.2 | 3 | [65] |
| 2%Pd1-Au3/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 58.7 | 3 | [65] |
| 0.5%Pd3-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 12.2 | 3 | [65] |
| 1.25%Pd3-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0.9 | 3 | [65] |
| 2%Pd3-Au1/TiO2 | 50 | 110 | 15 | 4 | Water | 3 | 0 | 3 | [65] |
| Au/MgO | 50 | 110 | 26 | 2 | Water | 10.5 | 100 | 1 | [41] |
| Au/TiO2 | 50 | 110 | 26 | 2 | Water | 3 | 30 | 1 | [41] |
| Au/ZrO2 | 50 | 110 | 26 | 2 | Water | 4.9 | 32 | 1 | [41] |
| Au/CeO2 | 50 | 110 | 26 | 2 | Water | 5 | 18 | 1 | [41] |
| Au/MgF2 | 50 | 110 | 26 | 2 | Water | 3.8 | 2 | 1 | [41] |
| Au/0.6MgF2-0.4MgO | 50 | 110 | 26 | 2 | Water | 7.8 | 76 | 1 | [41] |
| Au/0.4MgF2-0.6MgO | 50 | 110 | 26 | 2 | Water | 8.8 | 99 | 1 | [41] |
| SiO2@TiO2 | 100 | 110 | 24 | 2 | Water | 3 | 0.3 | 2 | [64] |
| 0.25% SiO2@Au@TiO2 | 100 | 110 | 24 | 2 | Water | 3 | 100 | 2 | [64] |
| 0.5% SiO2@Au@TiO2 | 100 | 110 | 24 | 2 | Water | 3 | 100 | 2 | [64] |
| 1% SiO2@Au@TiO2 | 100 | 110 | 24 | 2 | Water | 3 | 100 | 2 | [64] |
| 2% SiO2@Au@TiO2 | 100 | 110 | 24 | 2 | Water | 3 | 100 | 2 | [64] |
| 0.25% SiO2@TiO2@Au | 100 | 110 | 24 | 2 | Water | 3 | 8.4 | 2 | [64] |
| 0.5% SiO2@TiO2@Au | 100 | 110 | 24 | 2 | Water | 3 | 41.8 | 2 | [64] |
| 1% SiO2@TiO2@Au | 100 | 110 | 24 | 2 | Water | 3 | 19 | 2 | [64] |
| 2% SiO2@TiO2@Au | 100 | 110 | 24 | 2 | Water | 3 | 3.8 | 2 | [64] |
| 2%Au/CaO | 50 | 110 | 15 | 2 | Water | 10 | 81 | 1 | [66] |
| 2%Au/SrO | 50 | 110 | 15 | 2 | Water | 8.5 | 66 | 1 | [66] |
| 2%Au/BaO | 50 | 110 | 15 | 2 | Water | 8 | 54 | 1 | [66] |
| 2%Au/BeO | 50 | 110 | 15 | 2 | Water | 3.5 | 32 | 2 | [66] |
| Au-HT2:1 | 200 | 110 | 6 | 2 | Water | 7 | 72 | 1 | [38] |
| Au-HT3:1 | 200 | 110 | 6 | 2 | Water | 7 | 68 | 1 | [38] |
| Au-HT4:1 | 200 | 110 | 6 | 2 | Water | 7 | 100 | 1 | [38] |
| Au-HT5:1 | 200 | 110 | 6 | 2 | Water | 7 | 100 | 1 | [38] |
| MnO2 com * | 100 | 110 | 12 | 2 | Water | 5.5 | 2 | 3 | [50] |
| MnO2 NF * | 100 | 110 | 12 | 2 | Water | 5.5 | 14 | 3 | [50] |
| MnO2 NW * | 100 | 110 | 12 | 2 | Water | 5.5 | 4 | 3 | [50] |
| Au/MnO2 com | 100 | 110 | 12 | 2 | Water | 5.5 | 25 | 3 | [50] |
| Au/MnO2 NF | 100 | 110 | 12 | 2 | Water | 5.5 | 60 | 3 | [50] |
| Au/MnO2 NW | 100 | 110 | 12 | 2 | Water | 5.5 | 45 | 3 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuriot-Roukos, J.; Ferraz, C.P.; K. Al Rawas, H.; Heyte, S.; Paul, S.; Itabaiana Jr, I.; Pietrowski, M.; Zieliński, M.; Ghazzal, M.N.; Dumeignil, F.; et al. Supported Gold Catalysts for Base-Free Furfural Oxidation: The State of the Art and Machine-Learning-Enabled Optimization. Materials 2023, 16, 6357. https://doi.org/10.3390/ma16196357
Thuriot-Roukos J, Ferraz CP, K. Al Rawas H, Heyte S, Paul S, Itabaiana Jr I, Pietrowski M, Zieliński M, Ghazzal MN, Dumeignil F, et al. Supported Gold Catalysts for Base-Free Furfural Oxidation: The State of the Art and Machine-Learning-Enabled Optimization. Materials. 2023; 16(19):6357. https://doi.org/10.3390/ma16196357
Chicago/Turabian StyleThuriot-Roukos, Joëlle, Camila Palombo Ferraz, Hisham K. Al Rawas, Svetlana Heyte, Sébastien Paul, Ivaldo Itabaiana Jr, Mariusz Pietrowski, Michal Zieliński, Mohammed N. Ghazzal, Franck Dumeignil, and et al. 2023. "Supported Gold Catalysts for Base-Free Furfural Oxidation: The State of the Art and Machine-Learning-Enabled Optimization" Materials 16, no. 19: 6357. https://doi.org/10.3390/ma16196357