Highly Efficient SnIn4S8@ZnO Z-Scheme Heterojunction Photocatalyst for Methylene Blue Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO
2.2. Synthesis of SnIn4S8@ZnO Heterojunction
2.3. Characterizations
2.4. Photocatalytic Degradation of MB
3. Results and Discussion
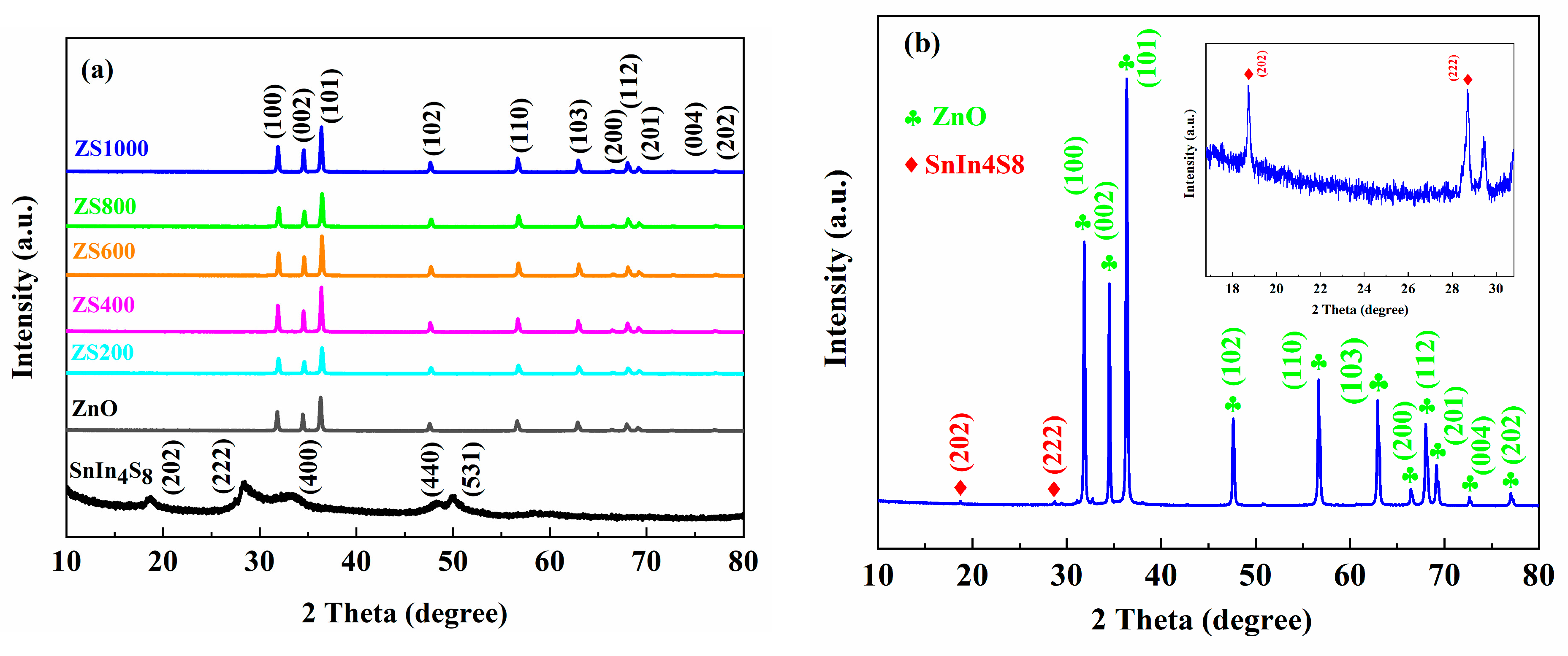
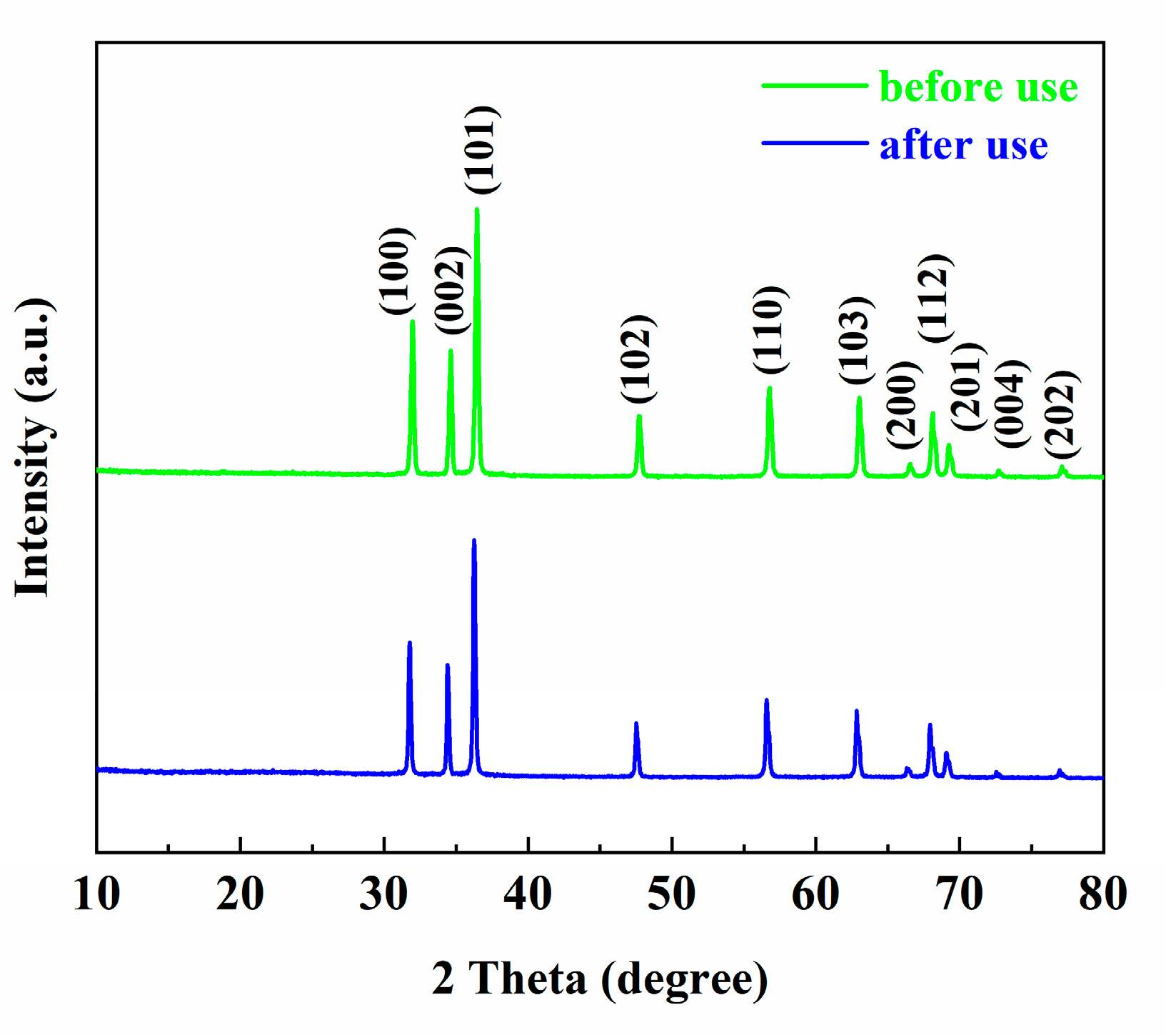
3.1. XRD Analysis
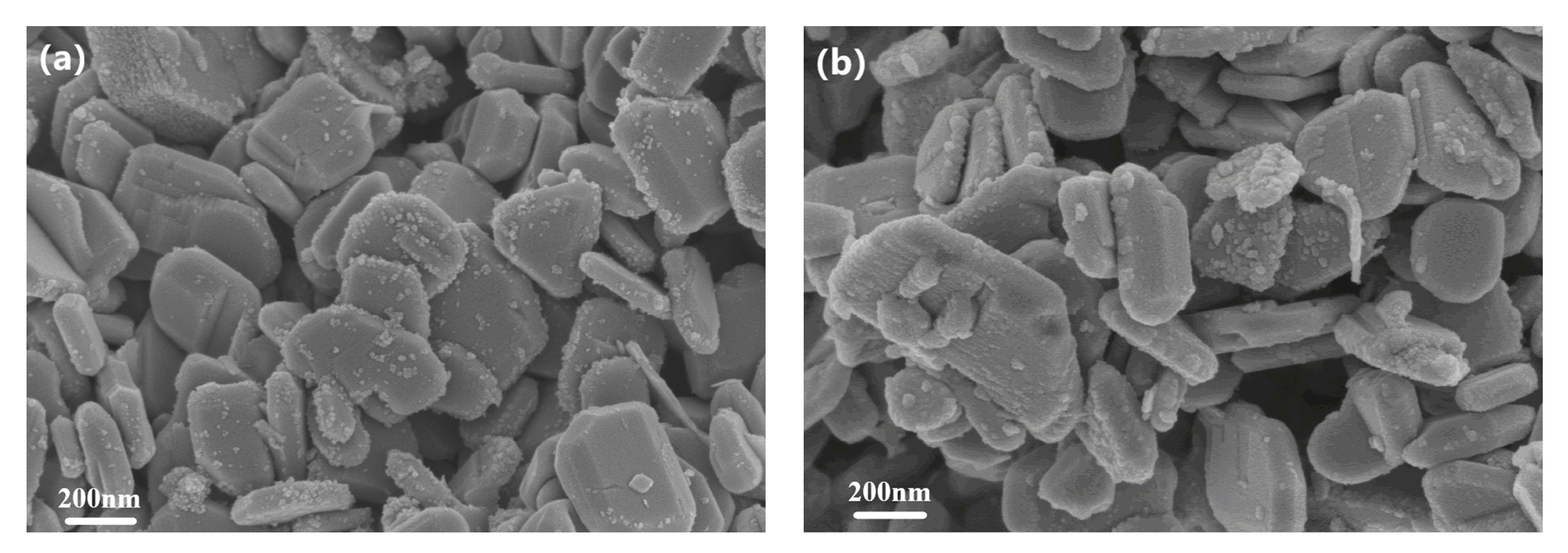
3.2. Morphological Analysis
3.3. BET Surface Area Analysis
3.4. UV-Vis Absorption Spectra Analysis
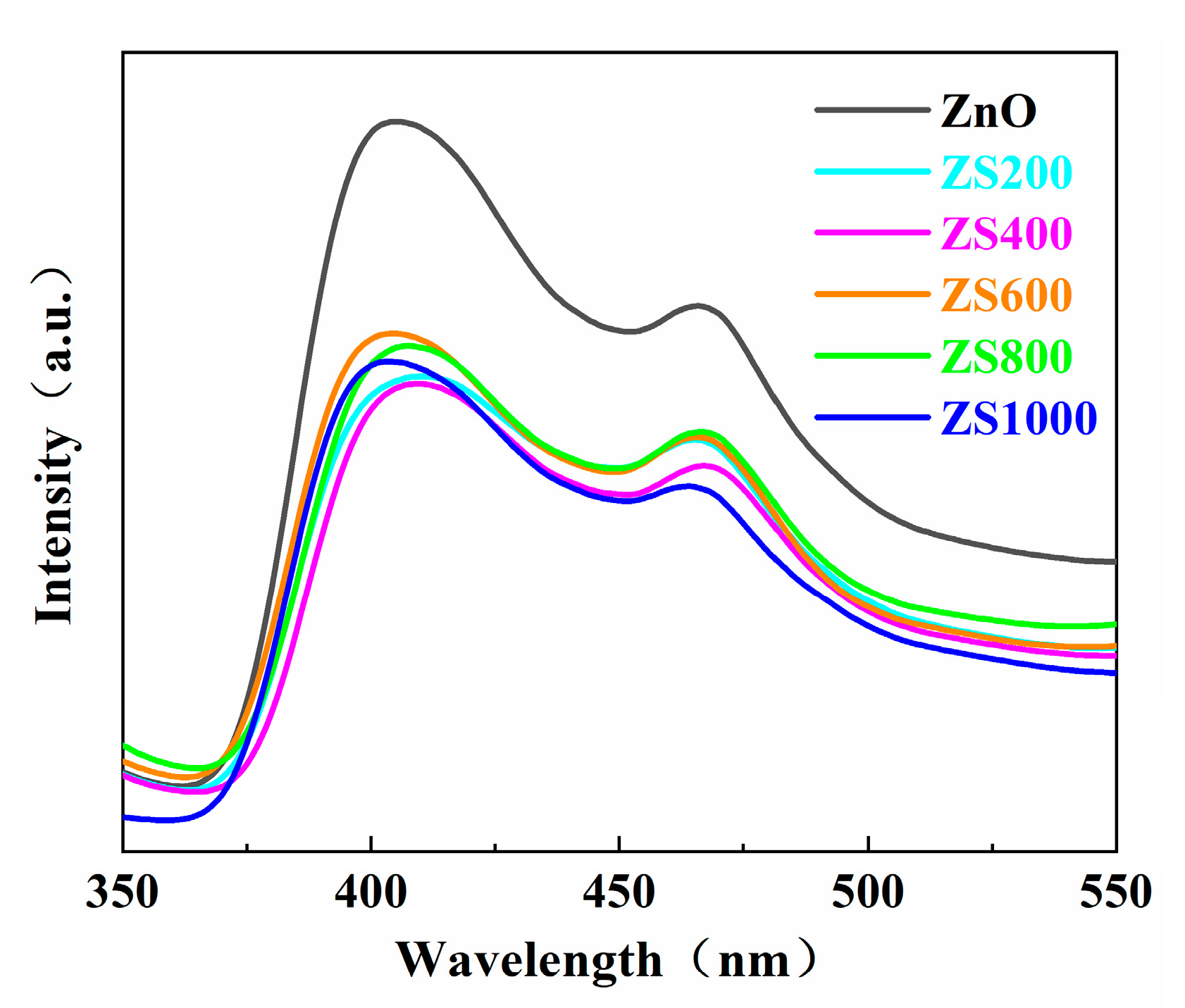
3.5. PL Spectra Analysis
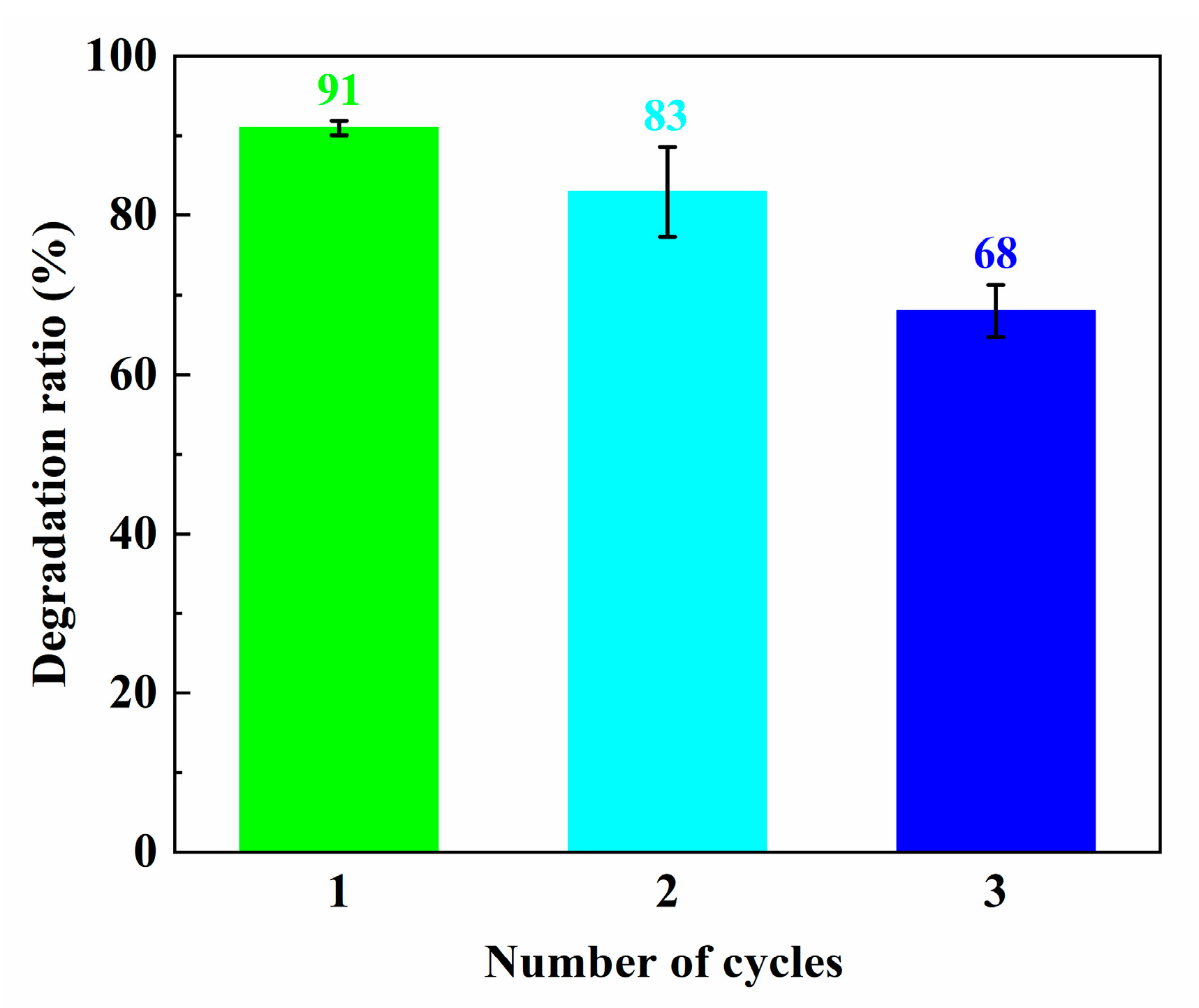
3.6. Photocatalytic Activities of the Specimens
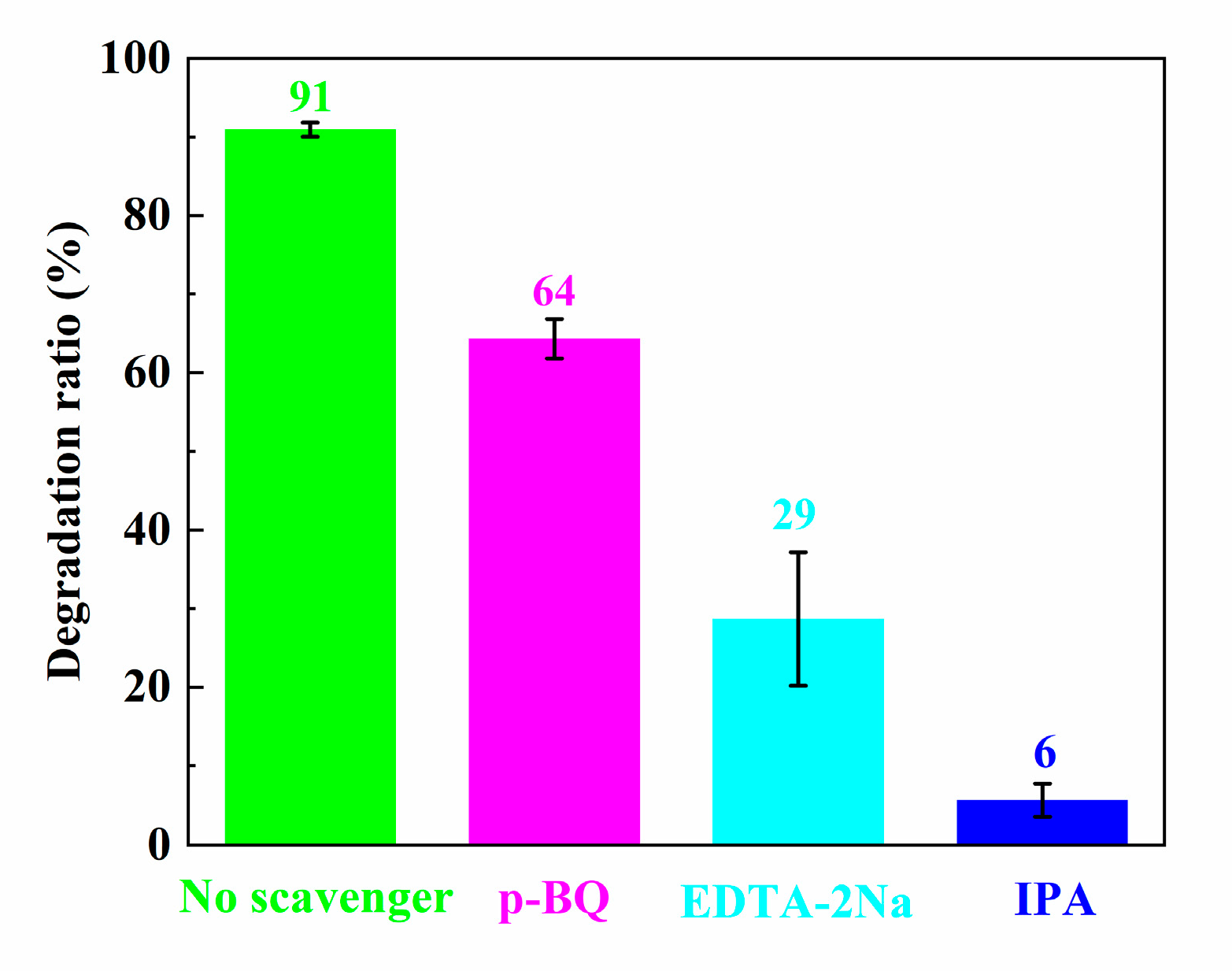
3.7. Photocatalytic Mechanism Analysis
- (1)
- ;
- (2)
- ;
- (3)
- ;
- (4)
- ;
- (5)
- .
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2023, 127, 234–250. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, B.; Sun, C. Can payment vehicle influence public willingness to pay for environmental pollution control? Evidence from the CVM survey and PSM method of China. J. Clean. Prod. 2022, 365, 132648. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, A.; Yang, Y.; Cao, Y.; Dong, F.; Zhou, Y. Surface modification to control the secondary pollution of photocatalytic nitric oxide removal over monolithic protonated g-C3N4/graphene cxide aerogel. J. Hazard. Mater. 2020, 397, 122822. [Google Scholar] [CrossRef]
- Nuño, M.; Pesce, G.L.; Bowen, C.R.; Xenophontos, P.; Ball, R.J. Environmental performance of nano-structured Ca(OH)2/TiO2 photocatalytic coatings for buildings. Build. Environ. 2015, 92, 734–742. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Taher, M.A.; Tamaddon, A.M. Facile synthesis and characterization of magnetic nanocomposite ZnO/CoFe2O4 hetero-structure for rapid photocatalytic degradation of imidacloprid. Heliyon 2019, 5, e02870. [Google Scholar] [CrossRef]
- Xu, J.; Wen, Q.; Zhang, X.; Li, Y.; Cui, Z.; Li, P.; Pan, C. One-step construction of multi-walled CNTs loaded with Alpha-Fe2O3 nanoparticles for efficient photocatalytic properties. Materials 2021, 14, 2820. [Google Scholar] [CrossRef]
- Huang, R.; Wu, J.; Zhang, M.; Liu, B.; Zheng, Z.; Luo, D. Strategies to enhance photocatalytic activity of graphite carbon nitride-based photocatalysts. Mater. Des. 2021, 210, 110040. [Google Scholar] [CrossRef]
- Das, A.; Nair, R.G. Effect of aspect ratio on photocatalytic performance of hexagonal ZnO nanorods. J. Alloys Compd. 2020, 817, 153277. [Google Scholar] [CrossRef]
- Bhavsar, K.S.; Labhane, P.K.; Dhake, R.B.; Sonawane, G.H. Solvothermal synthesis of activated carbon loaded CdS nanoflowers: Boosted photodegradation of dye by adsorption and photocatalysis synergy. Chem. Phys. Lett. 2020, 744, 137202. [Google Scholar] [CrossRef]
- He, S.; Chai, J.; Lu, S.; Mu, X.; Liu, R.; Wang, Q.; Chen, F.; Li, Y.; Wang, J.; Wang, B. Solution-phase vertical growth of aligned NiCo2O4 nanosheet arrays on Au nanosheets with weakened oxygen-hydrogen bonds for photocatalytic oxygen evolution. Nanoscale 2020, 12, 6195–6203. [Google Scholar] [CrossRef] [PubMed]
- Chumha, N.; Pudkon, W.; Chachvalvutikul, A.; Luangwanta, T.; Randorn, C.; Inceesungvorn, B.; Ngamjarurojana, A.; Kaowphong, S. Photocatalytic activity of CuInS2 nanoparticles synthesized via a simple and rapid microwave heating process. Mater. Res. Express 2020, 7, 015074. [Google Scholar] [CrossRef]
- Gao, G.; Xi, Q.; Zhou, H.; Zhao, Y.; Wu, C.; Wang, L.; Guo, P.; Xu, J. Novel inorganic perovskite quantum dots for photocatalysis. Nanoscale 2017, 9, 12032–12038. [Google Scholar] [CrossRef]
- Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Lennartz, B. Hydrothermal synthesis of heterostructured g-C3N4/Ag-TiO2 nanocomposites for enhanced photocatalytic degradation of organic pollutants. Materials 2023, 16, 5497. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wen, L.; Huang, S.; Li, Y.; Zhang, Y.; Lu, Y. Electronic properties of red and black phosphorous and their potential application as photocatalysts. RSC Adv. 2016, 6, 80872–80884. [Google Scholar] [CrossRef]
- Kumar, S.; Dhiman, A.; Sudhagar, P.; Krishnan, V. ZnO-graphene quantum dots heterojunctions for natural sunlight-driven photocatalytic environmental remediation. Appl. Surf. Sci. 2018, 447, 802–815. [Google Scholar] [CrossRef]
- Ren, T.; Jin, Z.; Yang, J.; Hu, R.; Zhao, F.; Gao, X.; Zhao, C. Highly efficient and stable p-LaFeO3/n-ZnO heterojunction photocatalyst for phenol degradation under visible light irradiation. J. Hazard. Mater. 2019, 377, 195–205. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Cao, L.; Li, J.; Ou Yang, H.; Yao, C. Microwave hydrothermal synthesis of Sr2+ doped ZnO crystallites with enhanced photocatalytic properties. Ceram. Int. 2014, 40, 2647–2653. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Meng, S.; Wang, Y.; Li, D. Regulating charge transfer over 3D Au/ZnO hybrid inverse opal toward efficiently photocatalytic degradation of bisphenol A and photoelectrochemical water splitting. Chem. Eng. J. 2020, 393, 124676. [Google Scholar] [CrossRef]
- Kuriakose, S.; Sahu, K.; Khan, S.A.; Tripathi, A.; Avasthi, D.K.; Mohapatra, S. Facile synthesis of Au-ZnO plasmonic nanohybrids for highly efficient photocatalytic degradation of methylene blue. Opt. Mater. 2017, 64, 47–52. [Google Scholar] [CrossRef]
- Ma, M.; Huang, Y.; Liu, J.; Liu, K.; Wang, Z.; Zhao, C.; Qu, S.; Wang, Z. Engineering the photoelectrochemical behaviors of ZnO for efficient solar water splitting. J. Semicond. 2020, 41, 091702. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.H.; Park, J.W.; Yip, A.C.K. Photocatalysts for degradation of dyes industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.; Sun, D.; Zou, Y.; Cheng, L.; He, C.; Wang, H.; Niu, C.; Wang, L. Au decorated hollow ZnO@ZnS heterostructure for enhanced photocatalytic hydrogen evolution: The insight into the roles of hollow channel and Au nanoparticles. Appl. Catal. B Environ. 2019, 244, 748–757. [Google Scholar] [CrossRef]
- Ma, Q.; Han, X.; Lv, K.; Dong, R.; Hang, Z.; Xin, B.; Zheng, K. Ultrasound-enhanced preparation and photocatalytic properties of graphene-ZnO nanorod composite. Sep. Purif. Technol. 2021, 259, 118131. [Google Scholar]
- Fu, Y.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Xing, L.; Ma, J.; Wang, H.; Xue, X. Direct Z-scheme heterojunction of ZnO/MoS2 nanoarrays realized by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances. Appl. Catal. B Environ. 2021, 285, 119785. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Li, G.; Wang, Y.; Hu, W.; Guan, X. Porous SnIn4S8 microspheres in a new polymorph that promotes dyes degradation under visible light irradiation. J. Hazard. Mater. 2011, 186, 272–279. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Zhang, W.; Xiao, Y.; Xu, H.; Liu, Y.; Liu, Z.; Zhang, J.; Jiang, Y. Visible-light-driven double-shell SnIn4S8/TiO2 heterostructure with enhanced photocatalytic activity for MO removal and Cr(VI) cleanup. Appl. Surf. Sci. 2022, 587, 152867. [Google Scholar] [CrossRef]
- Sun, M.; Li, F.; Su, M.; Wei, D.; Yang, Q.; Yan, T.; Li, D. Fabrication of MOF-derived tubular In2O3@SnIn4S8 hybrid: Heterojunction formation and promoted photocatalytic reduction of Cr(VI) under visible light. J. Colloid Interf. Sci. 2021, 596, 278–287. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Y.; Fan, J.; Tang, Z. Construction of novel Z-scheme flower-like Bi2S3/SnIn4S8 heterojunctions with enhanced visible light photodegradation and bactericidal activity. Appl. Surf. Sci. 2019, 465, 212–222. [Google Scholar] [CrossRef]
- Raji, R.; Gopchandran, K.G. Plasmonic photocatalytic activity of ZnO: Au nanostructures: Tailoring the plasmon absorption and interfacial charge transfer mechanism. J. Hazard. Mater. 2019, 368, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shaya, J.; Polychronopoulou, K.; Golovko, V.B.; Tesana, S.; Wang, S.; Lu, C. Photocatalytic degradation of ethiofencarb by a visible light-driven SnIn4S8 photocatalyst. Nanomaterials 2021, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, W.; Cui, X.; Li, Y.; Hou, B.; Cheng, L.; Zhang, P. Preparation of SnIn4S8/TiO2 nanotube photoanode and its photocathodic protection for Q235 carbon steel under visible light. Nanoscale Res. Lett. 2021, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, S.; Zhong, S.; Su, X.; Ma, C. A direct Z-scheme polypyrrole/Bi2WO6 nanoparticles with boosted photogenerated charge separation for photocatalytic reduction of Cr (VI): Characteristics, performance, and mechanisms. J. Clean. Prod. 2022, 337, 130577. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Q.; Li, X.; Chen, X. Characterization and photocatalytic activity of large-area single crystalline anatase TiO2 nanotube films hydrothermal synthesized on plasma electrolytic oxidation seed layers. J. Alloys Compd. 2014, 597, 101–109. [Google Scholar] [CrossRef]
- Zhu, G.; Li, X.; Wang, H.; Zhang, L. Microwave assisted synthesis of reduced graphene oxide incorporated MOF-derived ZnO composites for photocatalytic application. Catal. Commun. 2017, 88, 5–8. [Google Scholar] [CrossRef]
- Umar, A.; Karunagaran, B.; Suh, E.K.; Hahn, Y.B. Structural and optical properties of single-crystalline ZnO nanorods grown on silicon by thermal evaporation. Nanotechnology 2006, 17, 4072–4077. [Google Scholar] [CrossRef]
- Dai, L.; Chen, X.L.; Wang, W.J.; Zhou, T.; Hu, B.Q. Growth and luminescence characterization of large-scale zinc oxide nanowires. J. Phys. Condens. Matter 2003, 15, 2221–2226. [Google Scholar] [CrossRef]
- Yu, C.; Tong, Z.; Li, S.; Yin, Y. Enhancing the photocatalytic activity of ZnO by using tourmaline. Mater. Lett. 2019, 240, 161–164. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Liu, Z.; Xia, P.; Xie, Y.; Xiong, D. Synthesis of the 0D/3D CuO/ZnO heterojunction with enhanced photocatalytic activity. J. Phys. Chem. C 2018, 122, 9531–9539. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Zuo, Y.; He, G.; Chen, Q.; Meng, Q.; Chen, H. Reduced graphene oxide supported ZnO/CdS heterojunction enhances photocatalytic removal efficiency of hexavalent chromium from aqueous solution. Chemosphere 2022, 286, 131738. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, Y.; Xu, X.; Li, X.; Wen, X.; Liu, Z.; Xing, R.; Guo, H.; Fei, Z. Efficient photocatalytic H2 evolution and Cr (VI) reduction under visible light using a novel Z-scheme SnIn4S8/CeO2 heterojunction photocatalysts. J. Hazard. Mater. 2021, 416, 126217. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhen, Y.; Sun, Y.; Li, L.; Ding, D. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium (VI) removal. Chem. Eng. J. 2021, 415, 129002. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Cao, J.; Wang, Y.; Wang, F.; Wu, H. DFT-proved Z-type ZnO/SnIn4S8 heterojunction for detecting hexavalent chromium. J. Alloys Compd. 2022, 922, 166266. [Google Scholar] [CrossRef]




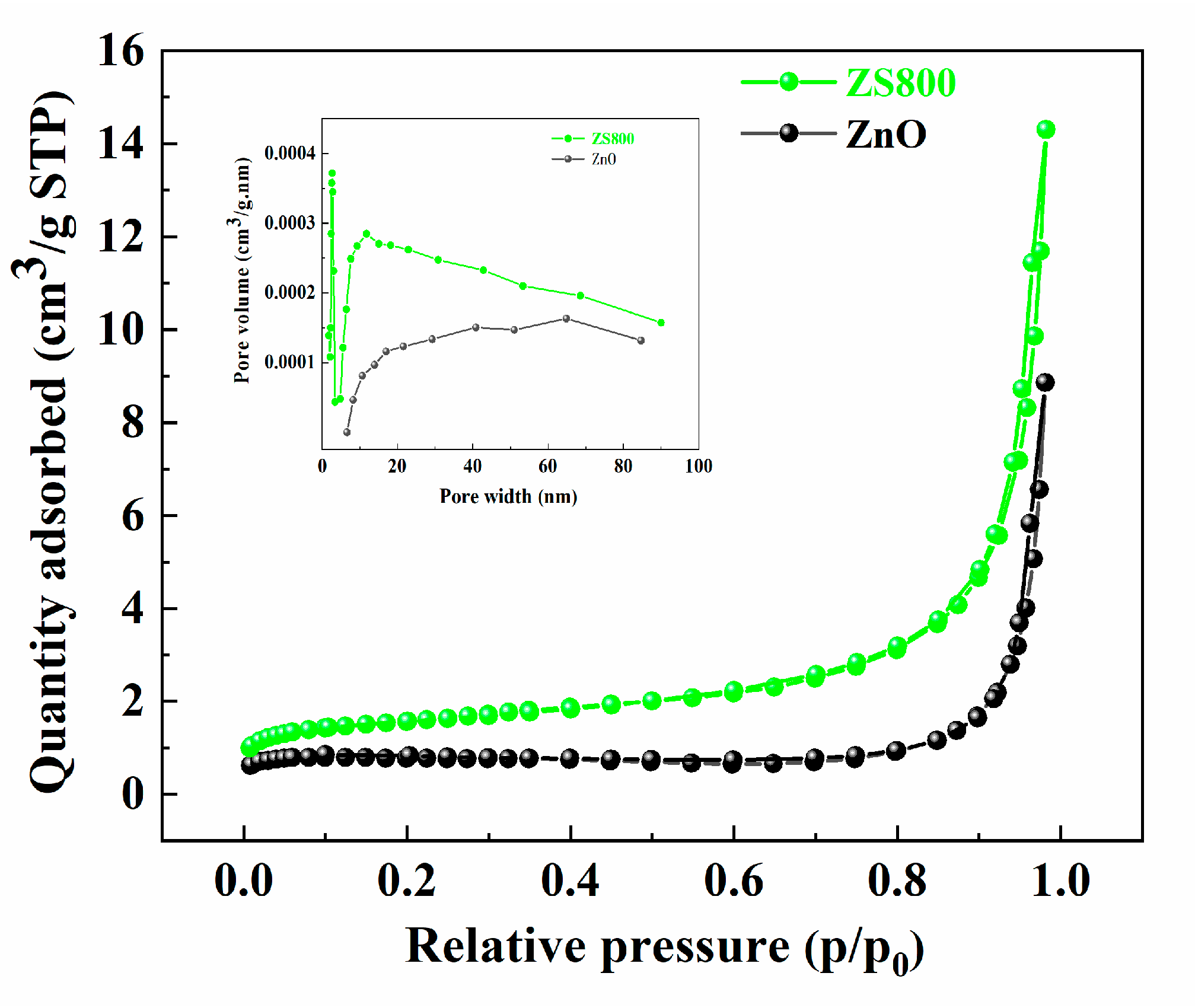




| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| ZnO | 3.2329 | 0.0137 | 17.0021 |
| ZS800 | 5.6337 | 0.0221 | 15.7473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Sun, C.; Zhao, J.; Cai, Q.; Yao, S. Highly Efficient SnIn4S8@ZnO Z-Scheme Heterojunction Photocatalyst for Methylene Blue Photodegradation. Materials 2023, 16, 6380. https://doi.org/10.3390/ma16196380
Luo Q, Sun C, Zhao J, Cai Q, Yao S. Highly Efficient SnIn4S8@ZnO Z-Scheme Heterojunction Photocatalyst for Methylene Blue Photodegradation. Materials. 2023; 16(19):6380. https://doi.org/10.3390/ma16196380
Chicago/Turabian StyleLuo, Qiang, Changlin Sun, Juan Zhao, Qizhou Cai, and Shanshan Yao. 2023. "Highly Efficient SnIn4S8@ZnO Z-Scheme Heterojunction Photocatalyst for Methylene Blue Photodegradation" Materials 16, no. 19: 6380. https://doi.org/10.3390/ma16196380





