Study on Early Hydration Mechanism of Double-Liquid Grouting Material Modified by Composite Early Strength Agent
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
- (1)
- A material: the choice of sulfoaluminate cement clinker, from Zhengzhou City Jianwen Special Material Technology Co., Ltd. (Zhengzhou, China) production, with a setting time of 5–6 min, 1 d strength of 35 MPa, and 3 d strength of 45 MPa. The specific chemical composition is shown in Table 1.
- (2)
- B material: gypsum hemihydrate (CaSO4·0.5H2O) from Hongli Gypsum Factory in Quwo County, China. Chemical composition is shown in Table 1. Quicklime was obtained from the Yiyang Taojiang Lime Factory (Yiyang, China), and the fineness (10 mesh throughput) was 95.89%. The product was tested and met the requirements of national industry standards, such as GB/T8576-2010. Slaked lime was obtained from Ganzhou Fangsheng Building Materials Lime Factory Limited (Ganzhou, China), and the fineness (200 mesh throughput) was 96.14%.
- (3)
- Admixtures: VAE emulsion, polycarboxylic acid water reducing agent (PBS), HPMC, borax (BX), sodium citrate (SCE), calcium formate, sodium sulfate, and lithium carbonate were analytically pure reagents. Among them, calcium formate was derived from Tianjin Damao Chemical Reagent Factory (Tianjin, China) as a white crystalline powder with a molecular weight of 130.11 and a content ≥ 98%. Sodium sulfate was obtained from Tianjin Dengfeng Chemical Reagent Factory (Tianjin, China) as a white crystalline powder with a molecular weight of 142.04 and a content ≥ 99%. Lithium carbonate was obtained from Xilong Science Co., Ltd. (Shantou, China) as a lightweight white powder, with a molecular weight of 73.89 and a content ≥ 98%.
2.2. Pilot Program
2.3. Test Methods
- (1)
- Determination of setting time: The Vicat meter produced by Zhejiang Dadi Instrument Co., Ltd. (Wenling, China) was used to determine the setting time of the slurry, and the specific determination method refers to the provisions of GB/T 1346-2011.
- (2)
- Flowability test: A 60 × 36 × 60 mm net slurry mold produced by Cangzhou Zhulong Engineering Instrument Co., Ltd. (Cangzhou, China) was used to determine the flowability of the B liquid, and the specific determination method referred to the provisions of GB/T 8077-2012.
- (3)
- Compression test: The compressive strength of the mortar specimens at specific ages (3 h, 6 h, 1 d, 3 d, 7 d, and 28 d) was tested using a hydraulic press manufactured by Wuxi Building Material Testing Instrument Co., Ltd. (Wuxi, China) The specific determination method was operated in accordance with the provisions of GB/T 17671-2021.
- (4)
- Viscosity test: The U.S. Bohlefeld Brookfield DV2TLV viscometer (BROOKFIELD, Massachusetts, USA.)was used, and the No. 6 rotor and RTD temperature probe were selected for real-time monitoring of the viscosity of the ethyl-liquid and temperature. The rotor speed was set to 6.0 RPM.
- (5)
- Scanning electron microscope (SEM) test: The British Cressington automatic ion sputtering instrument 108AUTO was used on the surface of the sample to form a conductive layer of gold spraying, improving the conductivity and stability of the sample. A German Merlin Compact (Carl Zeiss AG, Oberkochen, Germany) scanning electron microscope was selected under low vacuum conditions, and the microstructure of the sample hydrated for 3 h was observed using secondary electron (SE) mode at an accelerating voltage of 15.0 kV.
- (6)
- X-ray (XRD) diffraction test: Quantitative analysis of the hydration of the 3 h sample was performed using the SmartLab rotating-target X-ray diffractometer (Japan Neo- Co., Ltd. Tokyo, Japan). The scanning range was set from 5° to 65°, with a scanning rate of 0.04°/s.
3. Results and Discussion
3.1. Effect of Early Strength Agent on Performance of SACDL
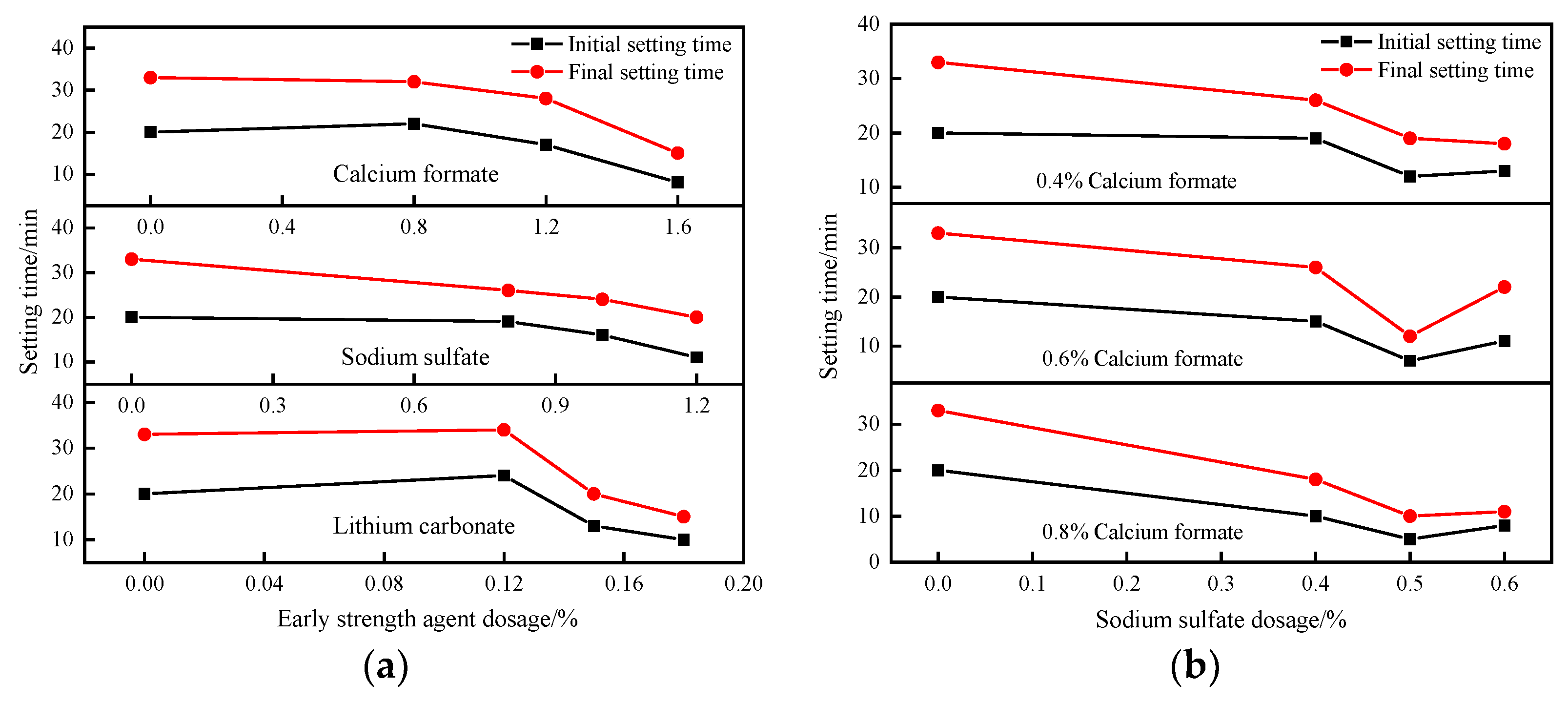
3.1.1. Effect of Early Strengthening Agents on SACDL Setting Time
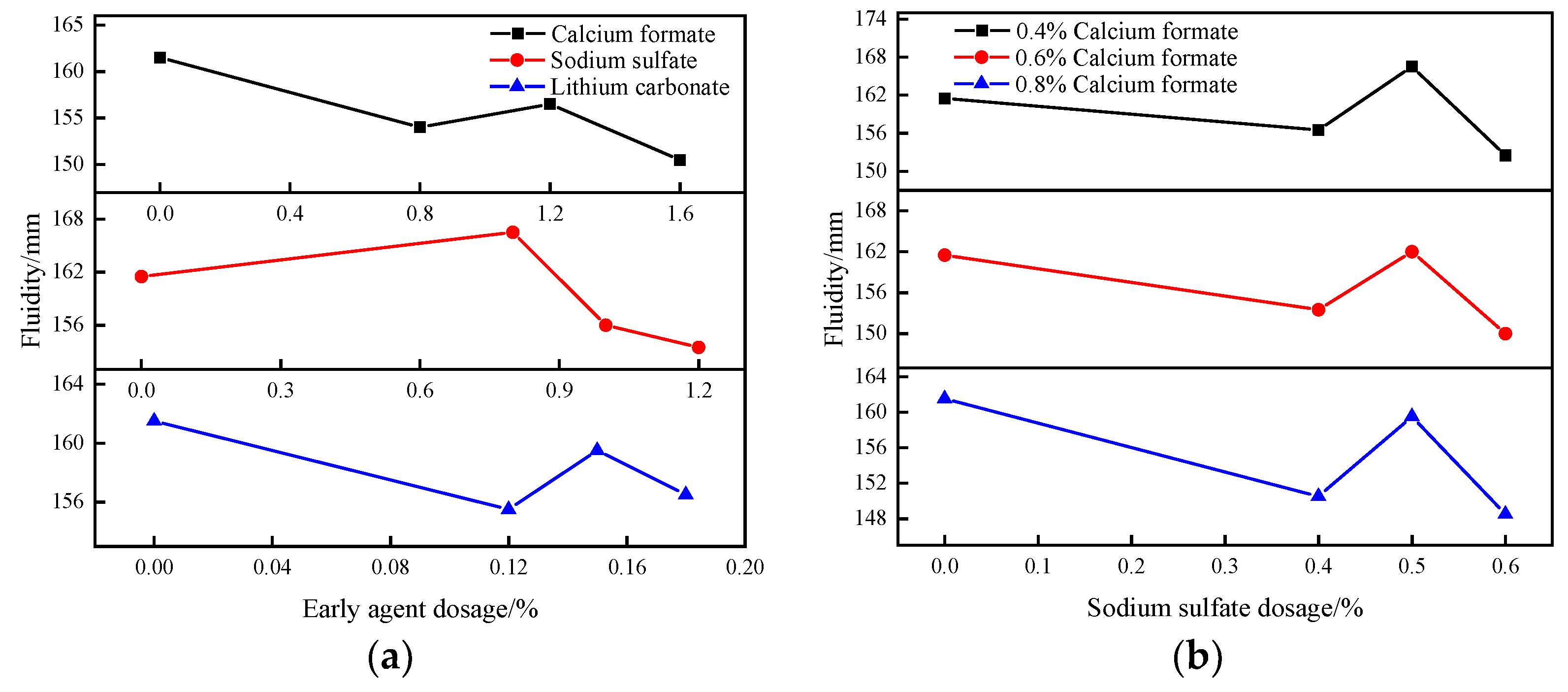
3.1.2. Effect of Early Strength Agent on the Flowability of B-Liquid
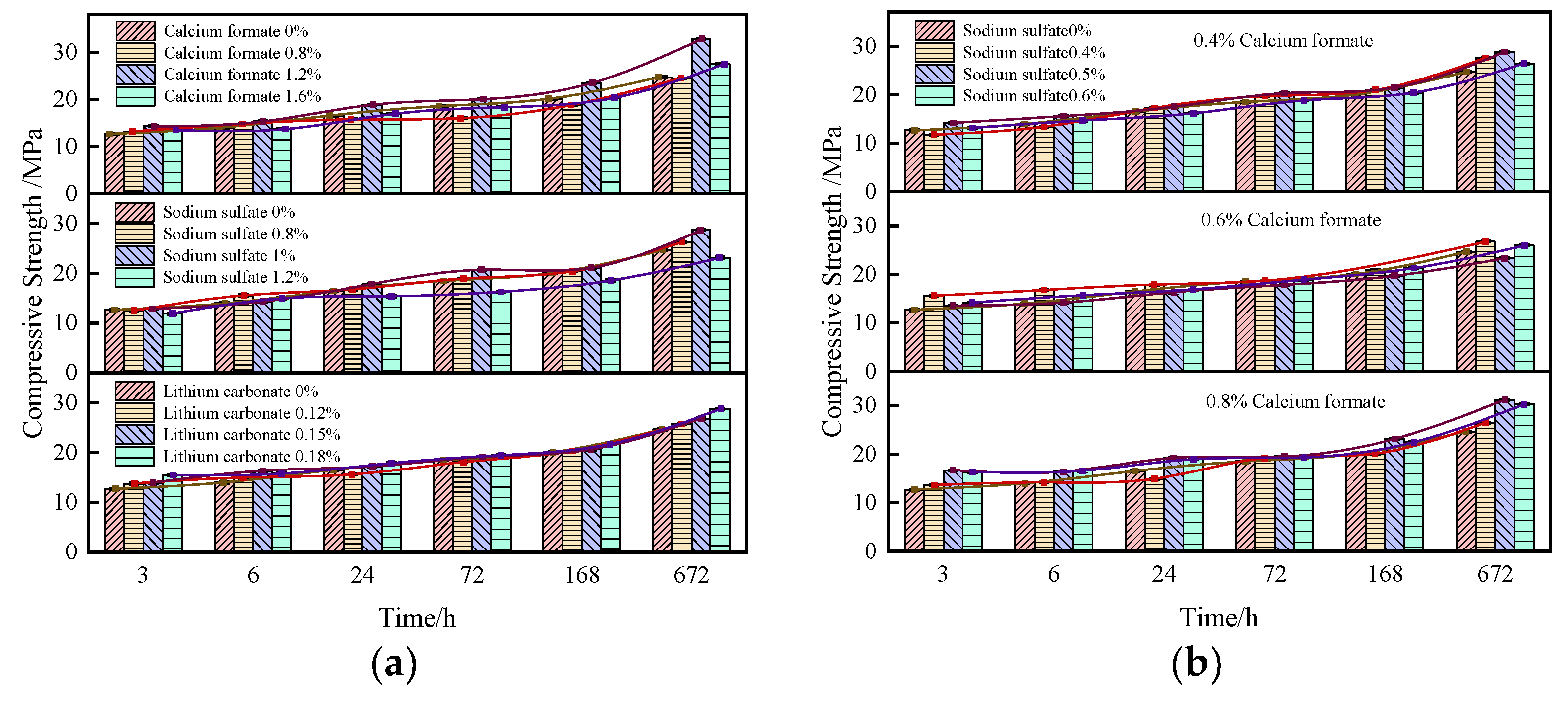
3.1.3. Effect of Early Strength Agent on SACDL Strength
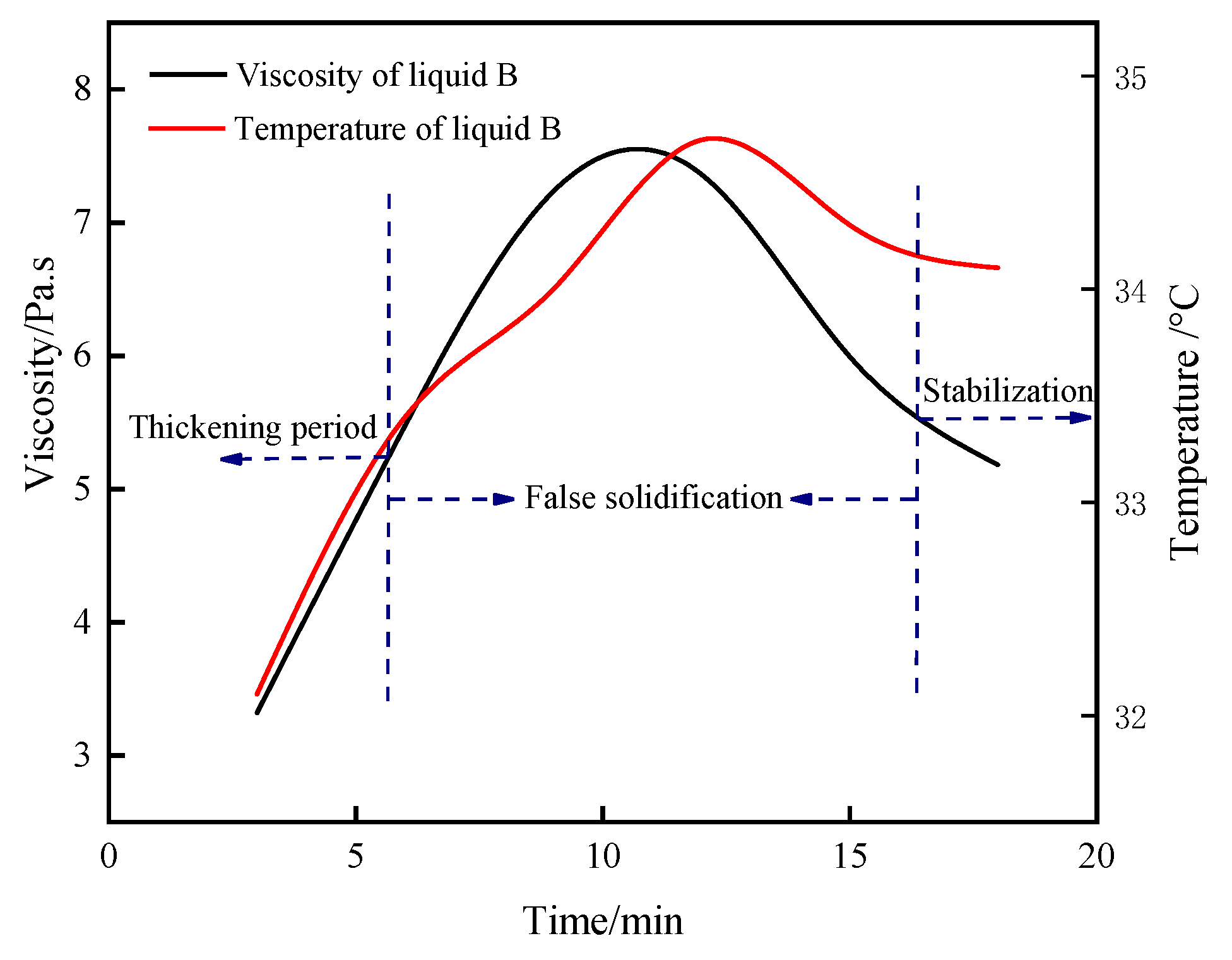
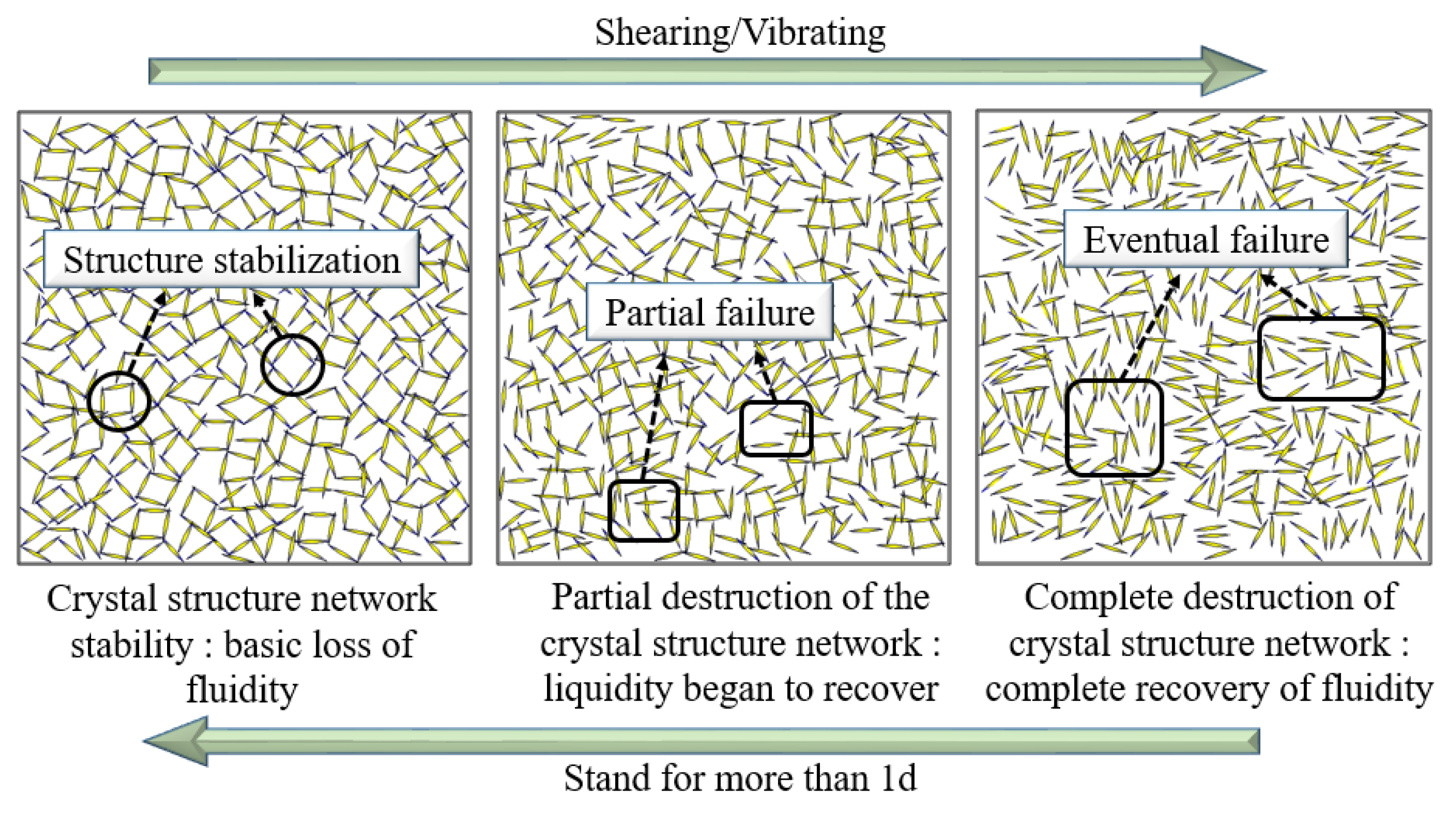
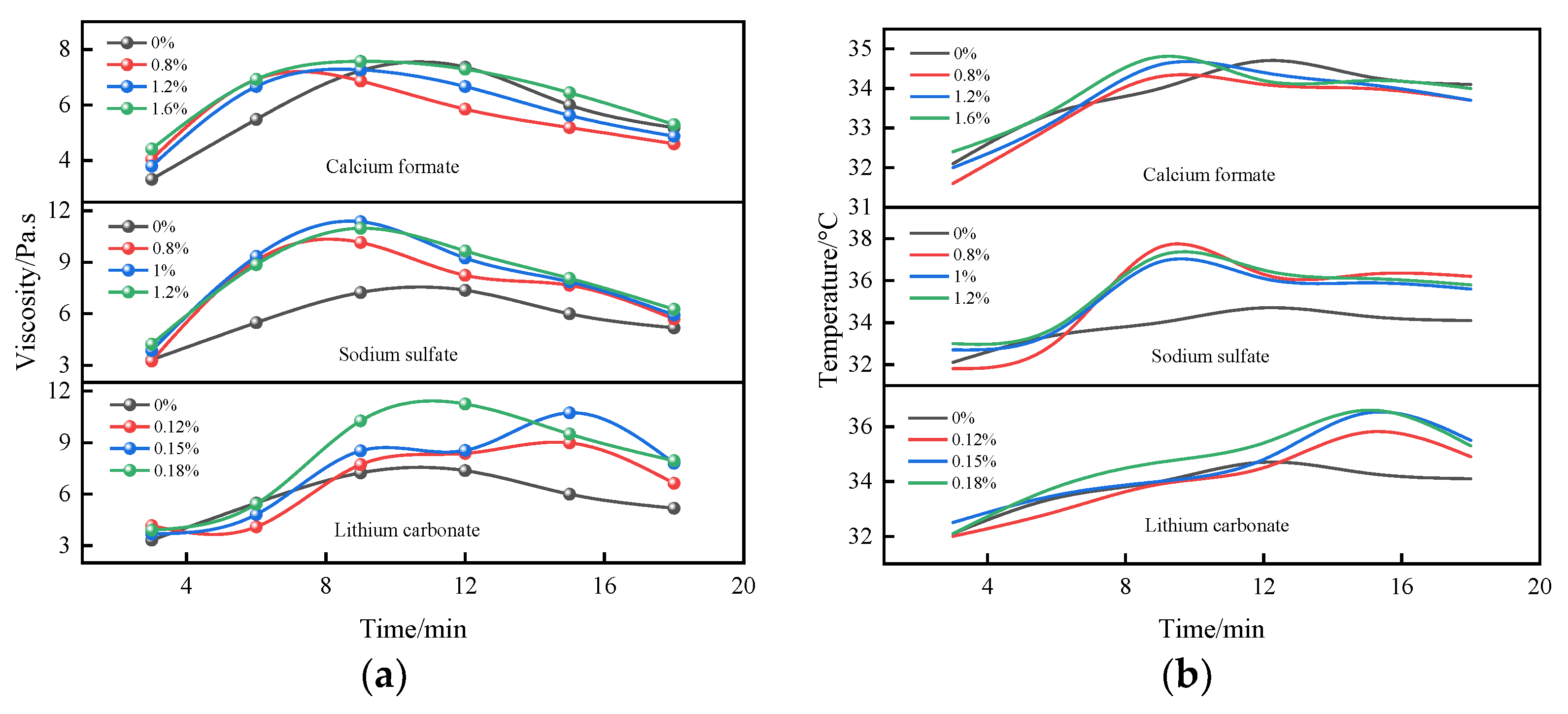
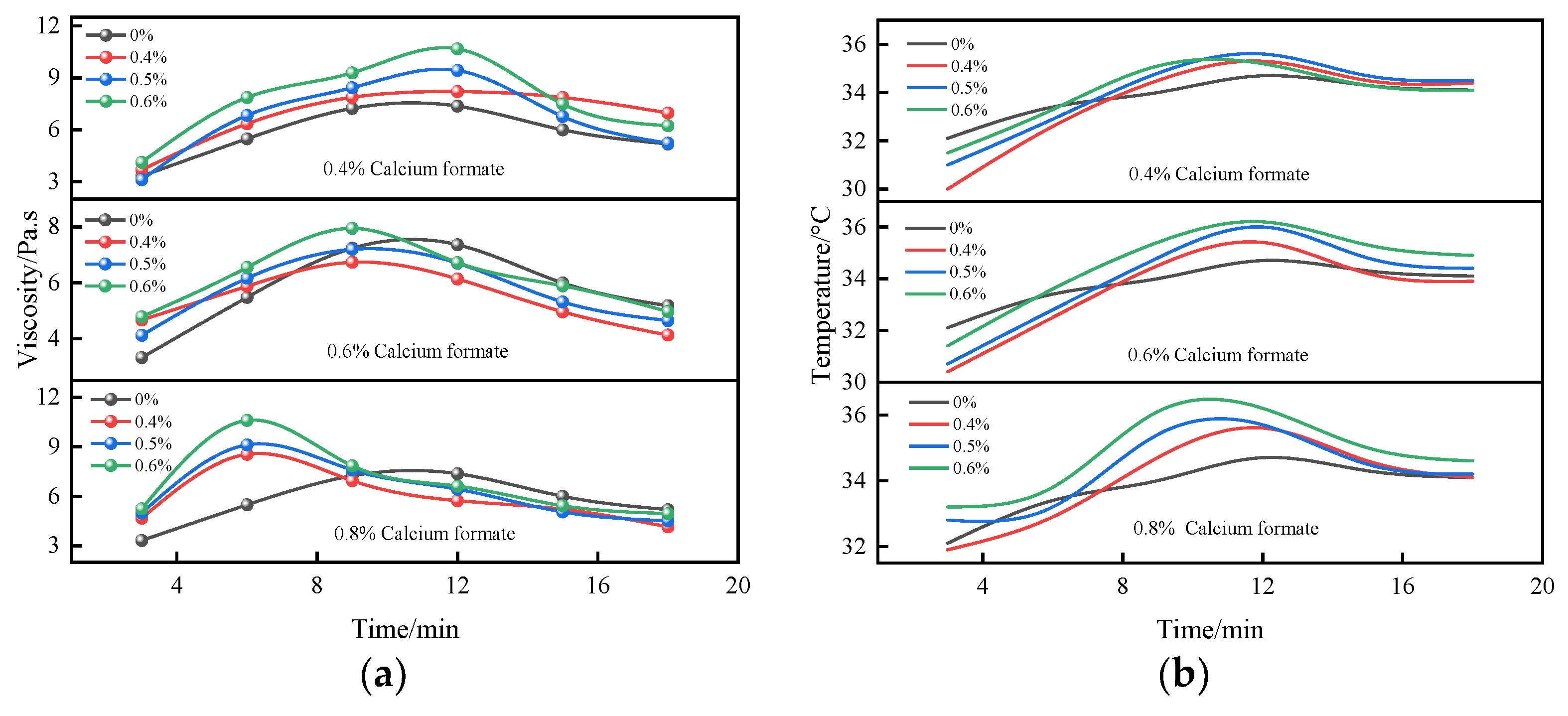
3.1.4. Time-Varying Characteristic Curve of Viscosity and Temperature of B Liquid under the Action of Early Strength Agents
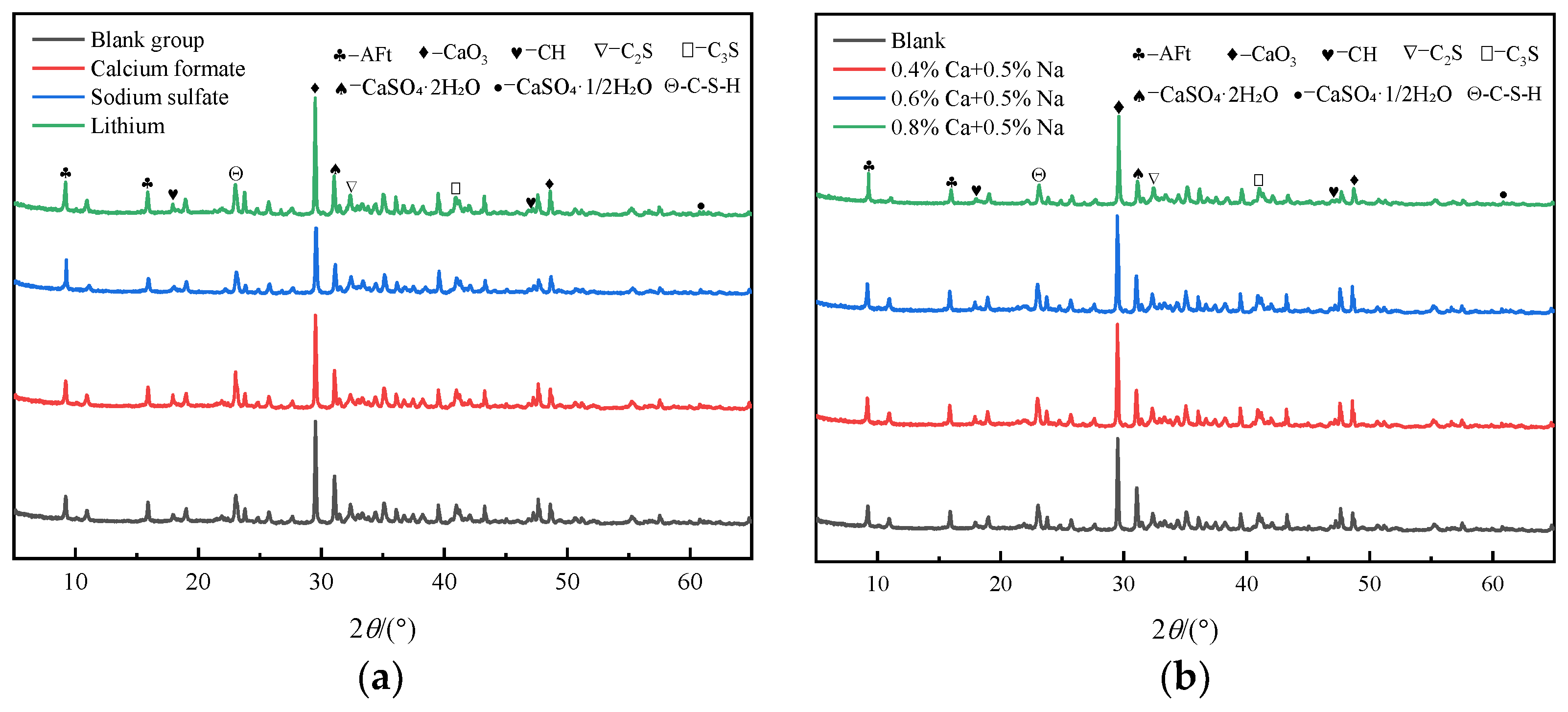
3.2. XRD Analysis of Hydration Products
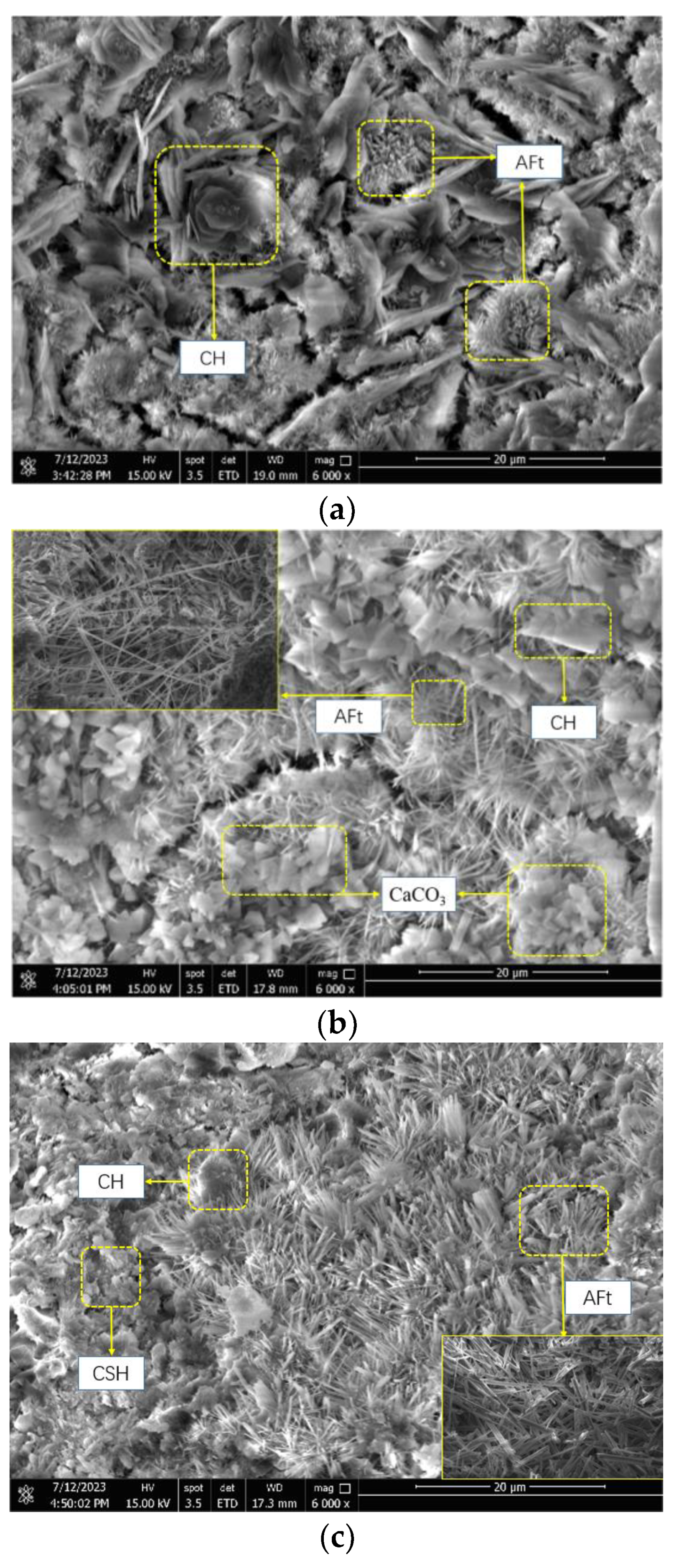
3.3. Observation of Microscopic Morphology of Hydration Products
4. Conclusions
- (1)
- The adsorption–permeation effect of calcium formate, the complex decomposition reaction of sodium sulfate, the precipitation polarization effect of lithium carbonate, and the synergistic effects of composite early strength agents can accelerate the early hydration rate of SACDL, shorten the setting time of SACDL slurry while ensuring the fluidity of the B liquid, and improve its early strength. Our comprehensive analysis shows that the composite early-strength agent had the best effect on the modification of SACDL; 0.8% calcium formate was compounded with 0.5% sodium sulfate to promote coagulation. The early-strength effect was significant; the initial and final coagulation durations of SACDL were shortened to 5 min and 10 min, respectively; and the compressive strength of 3 h reached up to 16.7 MPa, 31% higher than that of the blank group.
- (2)
- The composite early strength agent was able to simultaneously increase the concentrations of SO42− and Ca2+ in the liquid phase and accelerate the formation of the CaSO4·2H2O colloid and the crystal growth rate, thereby shortening the thickening period and pseudo-condensation period of the B liquid. Under the combined action of 0.8% calcium formate and 0.4% sodium sulfate, the viscosity of liquid B tended to be stable within 9 min, effectively shortening the mixing times of liquid A and liquid B and greatly improving the efficiency of double liquid grouting engineering.
- (3)
- The solubility of C3A and CaSO4·2H2O increased under the condition of a low-doped composite early strength agent, which increased the rate of AFt generation. HCOO− was able to penetrate into the hydration layer of C3S and C2S and to accelerate the dissolution of C3S and C2S, which, thus, jointly promoted the early hydration of SACDL.
- (4)
- Under the condition of a high dosage of the composite early strength agent, AFt was transformed from an elongated needle-like to a rod-like shape. A further increase in the Ca2+ concentration promoted the crystallization nodules and precipitation of CH. The C-S-H gel was rapidly formed and filled the gaps between a large number of rod-like AFt crystals, resulting in a denser structure and effectively improving the early strength of SACDL.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, S.; He, Y.; Liu, L.; Cui, X. Preparation of potassium methylsilicate modified one-part alkali-activated slag grouting material and its effect on adhesion properties of semi-flexible pavement. Constr. Build. Mater. 2023, 366, 130139. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, X.; Xu, S.; Wang, Z.; Li, W. Experimental study on properties of a new type of grouting material for the reinforcement of fractured seam floor. J. Mater. Res. Technol. 2019, 8, 5271–5282. [Google Scholar] [CrossRef]
- He, Y.S.; Lai, X.J.; Wang, X.L.; Wang, K. A literature review on properties and applications of grouts for shield tunnel. Constr. Build. Mater. 2020, 239, 117782. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zhang, Z.L.; Liu, T.R.; Li, C.S.; Zhang, Q.Q. Grouting mechanism of quick setting slurry in rock fissure with consideration of viscosity variation with space. Tunn. Undergr. Space Technol. 2017, 70, 262–273. [Google Scholar] [CrossRef]
- Li, J.X.; Hao, Y.J. Effects of mortar ratio on the properties of super early strength grouting materials by ternary complex system. Constr. Build. Mater. 2017, 152, 769–776. [Google Scholar] [CrossRef]
- Kaur, R.; Kothiyal, N.C. Comparative effects of sterically stabilized functionalized carbon nanotubes and graphene oxide as reinforcing agent on physico-mechanical properties and electrical resistivity of cement nanocomposites. Constr. Build. Mater. 2019, 202, 121–138. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, X.; Hou, P.; Ye, Z. Influences of nano-TiO2 on the properties of cement-based materials: Hydration and drying shrinkage. Constr. Build. Mater. 2015, 81, 35–41. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Qian, X. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.; Corr, D.J.; Shah, S.P. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Liu, A.; Wang, X. Effect of Nano-CaCO3 on Properties of Cement Paste. Energy Procedia 2012, 16, 991–996. [Google Scholar] [CrossRef]
- Wang, S.L.; Shen, Q.A.; Lyu, H.Z.; Guo, C.Y.; He, M.Z.; Mou, G.; Wei, Y.Z. Rapid regeneration cement-stabilized macadam: Preparation, mechanical properties, and dry shrinkage performance. Constr. Build. Mater. 2022, 341, 127901. [Google Scholar] [CrossRef]
- Wang, J.; Qian, C.; Qu, J.; Guo, J. Effect of lithium salt and nano nucleating agent on early hydration of cement based materials. Constr. Build. Mater. 2018, 174, 24–29. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, R.Z.; Zhao, X.; Xu, R.W.; Niu, T.D. Effect of nano calcium carbonate on hydration characteristics and microstructure of cement-based materials: A review. J. Build. Eng. 2022, 50, 104220. [Google Scholar] [CrossRef]
- Cao, M.; Ming, X.; He, K.; Li, L.; Shen, S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites—A Review. Materials 2019, 12, 781. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.K.; Xu, G.; Yang, J.; Zhao, N.Y.; Sun, J.Y. Study on mechanical and shrinkage properties of ES-UHPC. Constr. Build. Mater. 2023, 377, 131137. [Google Scholar] [CrossRef]
- Zhou, J.H.; Qi, X.; Ma, C.; Fang, Z.; Lou, J. Effect and mechanism of composite early-strength agents on sulfoaluminate cement-based UHPC. Case Stud Constr Mat. 2023, 18, e01768. [Google Scholar] [CrossRef]
- Yu, X.; Yu, C.; Jiang, Q.; Liu, P.J.; Yang, B. Study on the effect of early strength agent on the early hydration of cement using in situ XRD. Materials Herald. 2020, 34, 2058–2062. [Google Scholar]
- Lin, M.; Wang, J.W.; Li, Y. Preparation of inorganic-based composite early strength agent and its effect on the performance of water-stabilized materials. Materials Herald. 2018, 32, 511–516. [Google Scholar]
- Wang, Y.; Sun, L.; Liu, S.; Li, S.; Guan, X.; Luo, S. Development of a Novel Double-Sulfate Composite Early Strength Agent to Improve the Hydration Hardening Properties of Portland Cement Paste. Coatings 2022, 12, 1485. [Google Scholar] [CrossRef]
- Zou, F.; Hu, C.; Wang, F.; Ruan, Y.; Hu, S. Enhancement of early-age strength of the high content fly ash blended cement paste by sodium sulfate and C–S–H seeds towards a greener binder. J. Clean Prod. 2020, 244, 118566. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, S.; Chen, H. Early Strength-Promoting Mechanism of Inorganic Salts on Limestone-Calcined Clay Cement. Sustainability 2023, 15, 5286. [Google Scholar] [CrossRef]
- Etcheverry, J.M.; Villagran-Zaccardi, Y.A.; Heede, P.V.; Hallet, V.; Belie, N.D. Effect of sodium sulfate activation on the early age behaviour and microstructure development of hybrid cementitious systems containing Portland cement, and blast furnace slag. Cem. Concr. Compos. 2023, 141, 105101. [Google Scholar] [CrossRef]
- Ren, G.; Tian, Z.; Wu, J.; Gao, X. Effects of combined accelerating admixtures on mechanical strength and microstructure of cement mortar. Constr. Build. Mater. 2021, 304, 124642. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Dell’Aversano, R.; Gazzaniga, G.; Pastore, T. Influence of Lithium Carbonate and Sodium Carbonate on Physical and Elastic Properties and on Carbonation Resistance of Calcium Sulphoaluminate-Based Mortars. Appl. Sci. 2020, 10, 176. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, T.; Xiong, Z.; Sun, Y. Effects of lithium carbonate on performances of sulphoaluminate cement-based dual liquid high water material and its mechanisms. Constr. Build. Mater. 2018, 161, 374–380. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Moeen, M.; Wang, X.; Luo, S. Insights into the effect mechanism of nano LiAl-LDHs on the early hydration of sulphoaluminate cement-based materials. J. Nanopart Res. 2023, 25, 65. [Google Scholar] [CrossRef]
- Derakhshani, A.; Ghadi, A.; Vahdat, S.E. Study of the effect of calcium nitrate, calcium formate, triethanolamine, and triisopropanolamine on compressive strength of Portland-pozzolana cement. Case Stud. Constr. Mater. 2023, 18, e01799. [Google Scholar] [CrossRef]
- Zhang, P.S.; Xu, X.X.; Memon, S.A.; Dong, J.Z.; Li, X.D.; Cui, Z.H. Effect of calcium sulfate type and dosage on properties of calcium aluminate cement-based self-leveling mortar. Constr. Build. Mater. 2018, 167, 253–262. [Google Scholar] [CrossRef]
- Dong, M.; Li, S.J.; Lang, L.; Chen, X.; Jin, X.J.; Ma, W. Recycling thermal modified phosphogypsum in calcium sulfoaluminate cement: Evolution of engineering properties and micro-mechanism. Constr. Build. Mater. 2023, 373, 130823. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Z.; Zhang, D.; Wei, T. Study on Rheological Behavior and Surface Properties of Epoxy Resin Chemical Grouting Material Considering Time Variation. Materials 2019, 12, 3277. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, W.; Zhang, J.; Zhou, J. Epoxy-based grouting materials with super-low viscosities and improved toughness. Constr. Build Mater. 2020, 267, 121104. [Google Scholar] [CrossRef]
- Ding, X.; Wei, B.; Dai, R.; Chen, H.; Shan, Z. Effect of collagen hydrolysate obtained from leather waste on the setting, hydration and crystallization process of gypsum. J. Ind. Eng. Chem. 2022, 110, 158–167. [Google Scholar] [CrossRef]
- BüliChen, D.; Plank, J. Water retention capacity and working mechanism of methyl hydroxypropyl cellulose (MHPC) in gypsum plaster—Which impact has sulfate? Cement. Conceete. Res. 2013, 46, 66–72. [Google Scholar] [CrossRef]
- Hu, T.; Hao, J.Y.; Cheng, G.J.; Guo, B.; Li, X.J. Preparation and Hardening Performance of Lightweight Gypsum Mortar Based on Desulfurization Gypsum. Iran J. Sci. Technol. Trans. Civ. Eng. 2023, 47, 2717–2730. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Q.; Luo, T. Reuse of phosphogypsum as hemihydrate gypsum: The negative effect and content control of H3PO4. Resour. Conserv. Recy. 2021, 174, 105830. [Google Scholar] [CrossRef]
- Kamarou, M.; Korob, N.; Kwapinski, W.; Romanovski, V. High-quality gypsum binders based on synthetic calcium sulfate dihydrate produced from industrial waste. J. Ind. Eng. Chem. 2021, 100, 324–332. [Google Scholar] [CrossRef]
- Yang, M.; Qian, J. Activation of anhydrate phosphogypsum by K2SO4 and hemihydrate gypsum. J. Wuhan. Univ. Technol. 2011, 26, 1103–1107. [Google Scholar] [CrossRef]
- Yu, Q.L.; Brouwers, H.J.H. Microstructure and mechanical properties of β-hemihydrate produced gypsum: An insight from its hydration process. Constr. Build. Mater. 2011, 25, 3149–3157. [Google Scholar] [CrossRef]
- Frank, W.; Lukas, M.; Christian, M.; Barbara, L. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar]
- Ma, C.; Feng, S.; Zhang, Z. Effects of various gypsum on early-age hydration behavior of magnesium oxychloride cement (MOC). J. Therm. Anal. Calorim. 2023, 148, 3283–3294. [Google Scholar] [CrossRef]
- Li, X.; Hao, J. Orthogonal test design for optimization of synthesis of super early strength anchoring material. Constr. Build. Mater. 2018, 181, 42–48. [Google Scholar] [CrossRef]
- Luo, B.; Luo, Z.D.; Ren, Q.H. Effect of quicklime on early hydration and microstructure of low water-to-cement ratio slurry. Mater. Herald. 2023, 37, 91–97. [Google Scholar]
- Qu, P.Y. Research on the Effect of Calcium Formate on the Properties of Supersulfate Cement. Master’s Thesis, Jinan University, Jinan, China, 2022. [Google Scholar]
- Pang, M.C.; Tan, Y.Z.; Yang, Y.Z. A review of early strength agents in cement-based materials and their mechanism of action. Mater. Herald. 2023, 37, 80–90. [Google Scholar]
- Peng, R.; Chen, W.; Li, Q. Properties and microstructure of sodium sulfate-excited tailings filling materials. J. Build. Mater. 2020, 23, 685–691. [Google Scholar]
- Shen, S.Y.; Tang, L.M.; Shen, D.X. Growth habit of calcium hydroxide crystals in cement pastes. J. Silic. 2016, 44, 232–238. [Google Scholar]
| Composition | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | TiO2 | SO3 | H2O | Other |
|---|---|---|---|---|---|---|---|---|---|
| SAC/wt% | 29.72 | 9.90 | 3.99 | 42.36 | 1.32 | 1.33 | 9.77 | — | 1.61 |
| Gypsum/wt% | 0.41 | 2.14 | 0.18 | 34.85 | 0.21 | — | 48.83 | 10.75 | 2.63 |
| Liquid A | SAC | H2O | VAE | HPMC | PBS | BX | SCE |
| 1 | 0.45 | 0.2% | 0.1% | 0.15% | 0.3% | 0.4% | |
| Liquid B | CaSO4·0.5H2O | Quicklime | Slaked lime | H2O | VAE | HPMC | PBS |
| 0.8 | 0.1 | 0.1 | 0.45 | 0.2% | 0.1% | 0.2% |
| Level/Factor | Sodium Calcium Formate Dosage/% | Sodium Sulfate Dosage/% |
|---|---|---|
| A1 | 0.4 | 0.4 |
| A2 | 0.6 | 0.5 |
| A3 | 0.8 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, J.; Jiao, H.; Yang, Z.; Zheng, D.; Sun, J. Study on Early Hydration Mechanism of Double-Liquid Grouting Material Modified by Composite Early Strength Agent. Materials 2023, 16, 6475. https://doi.org/10.3390/ma16196475
Chen X, Wang J, Jiao H, Yang Z, Zheng D, Sun J. Study on Early Hydration Mechanism of Double-Liquid Grouting Material Modified by Composite Early Strength Agent. Materials. 2023; 16(19):6475. https://doi.org/10.3390/ma16196475
Chicago/Turabian StyleChen, Xinming, Jie Wang, Huazhe Jiao, Zhi Yang, Diantao Zheng, and Jinyu Sun. 2023. "Study on Early Hydration Mechanism of Double-Liquid Grouting Material Modified by Composite Early Strength Agent" Materials 16, no. 19: 6475. https://doi.org/10.3390/ma16196475
APA StyleChen, X., Wang, J., Jiao, H., Yang, Z., Zheng, D., & Sun, J. (2023). Study on Early Hydration Mechanism of Double-Liquid Grouting Material Modified by Composite Early Strength Agent. Materials, 16(19), 6475. https://doi.org/10.3390/ma16196475







