Bisphenols—A Threat to the Natural Environment
Abstract
1. Introduction
2. Chemical Characterization of Bisphenols
| Acronym | MW g mol−1 | BCF a | logKAW b | logKOA b | logKOW b | logKOC b | SW b mg dm−3 | VP b (Pa) |
|---|---|---|---|---|---|---|---|---|
| BPA | 228.29 | 71.85 | −9.00 | 12.74 | 3.64 | 4.88 | 120 | 5.6 × 10−6 |
| BPF | 200.23 | 34.73 | −9.70 | 12.58 | 3.10 | 4.47 | 408 | 1.2 × 10−4 |
| BPS | 250.27 | 36.97 | −13.00 | 14.61 | 1.65 | 3.88 | 3518 | 6.4 × 10−8 |
3. Sources of Bisphenols in the Environment (Air, Water, Soil)
3.1. Air
3.2. Water
3.3. Soil
4. Microbiological and Biochemical Disturbances in Soils Exposed to Bisphenols
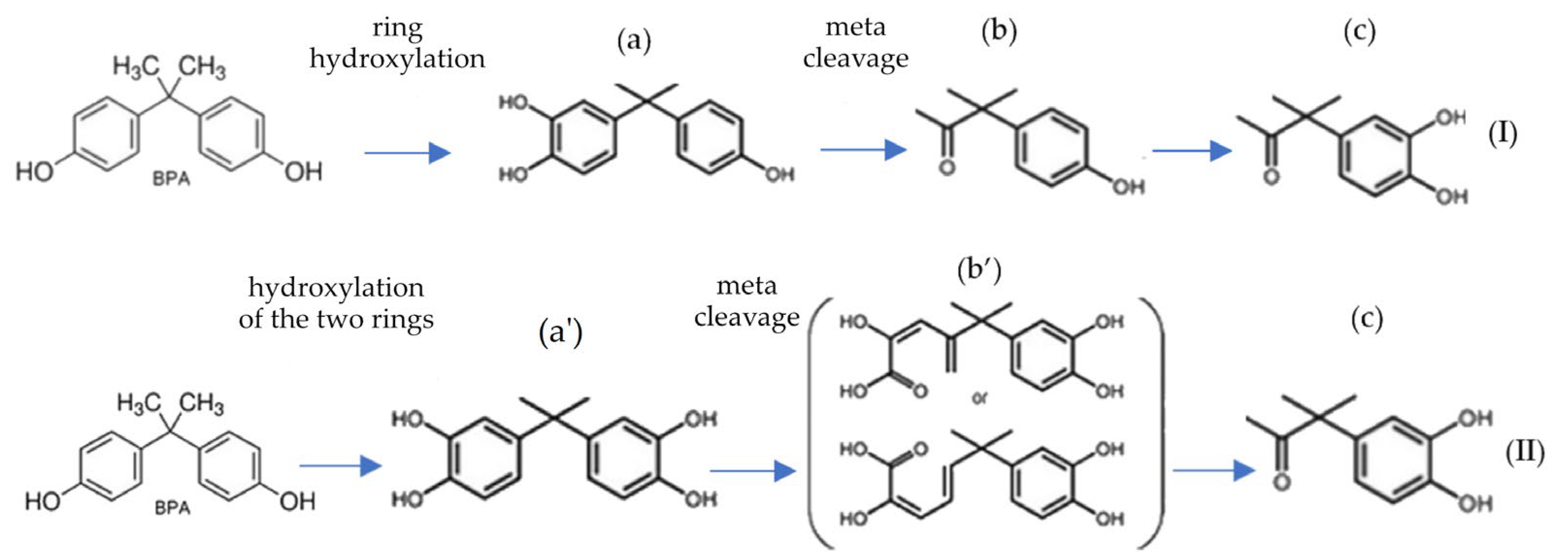
4.1. Microbiological Degradation of BPA, BPF, and BPS in the Soil Environment
4.2. The Response of the Soil Microbiome to Soil Contamination with BPA, BPF, and BPS
4.3. Sensitivity of Soil Enzymes to Soil Contamination with BPA, BPF, and BPS
5. Reaction of Crop Plants to Soil Contamination with Bisphenols
6. Toxicity of Bisphenols for Humans and Animals
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogs other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global assessment of bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal, and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Zhou, N.A.; Kjeldal, H.; Gough, H.L.; Nielsen, J.L. Identification of putative genes involved in bisphenol a degradation using differential protein abundance analysis of Sphingobium sp. BiD32. Environ. Sci. Technol. 2015, 49, 12232–12241. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin, R.G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Roosens, L.; Neels, H.; Covaci, A. Assessment of human exposure to Bisphenol-A, triclosan and tetrabromobisphenol A through indoor dust intake in Belgium. Chemosphere 2009, 76, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, W.; Kannan, K. Bisphenols, benzophenones, and bisphenol A diglycidyl ethers in textiles and infant clothing. Environ. Sci. Technol. 2017, 51, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- de Morais Farias, J.; Krepsky, N. Bacterial degradation of bisphenol analogs: An overview. Environ. Sci. Pollut. Res. 2022, 29, 76543–76564. [Google Scholar] [CrossRef]
- Intelligence, M. Bisphenol-A (BPA) Market-Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). 2022. Available online: https://www.researchandmarkets.com/reports/5318392/bisphenol-a-bpa-market-growth-trends-covid (accessed on 23 August 2023).
- Cydzik-Kwiatkowska, A.; Bernat, K.; Zielinska, M.; Bułkowska, K.; Wojnowska-Baryla, I. Aerobic granular sludge for bisphenol A (BPA) removal from wastewater. Int. Biodeterior. Biodegrad. 2017, 122, 1–11. [Google Scholar] [CrossRef]
- Statista. Production Forecast of Thermoplastics Worldwide from 2025 to 2050 (in Million Metric Tons). 2023. Available online: https://www.statista.com/statistics/664906/plastics-production-volume-forecast-worldwide/ (accessed on 23 August 2023).
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol. Lett. 2012, 211, 201–209. [Google Scholar] [CrossRef]
- Danzl, E.; Sei, K.; Soda, S.; Ike, M.; Fujita, M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int. J. Environ. Res. Public Health 2009, 6, 1472–1484. [Google Scholar] [CrossRef] [PubMed]
- ECHA. European Chemicals Agency. 2020. Available online: https://echa.europa.eu/substanceinformation/-/substanceinfo/100.001.133 (accessed on 23 August 2023).
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A targeted review on fate, occurrence, risk, and health implications of bisphenol analogs. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef]
- Durcik, M.; Gramec Skledar, D.; Tomašič, T.; Trontelj, J.; Mašič, P.L. Last piece in the puzzle of bisphenols BPA, BPS and BPF metabolism: Kinetics of the in vitro sulfation reaction. Chemosphere 2022, 303, 135133. [Google Scholar] [CrossRef] [PubMed]
- Vaccher, V.; Lopez, M.E.; Castaño, A.; Mol, H.; Haji-Abbas-Zarrabi, K.; Bury, D.; Koch, H.M.; Dvorakova, D.; Hajslova, J.; Nübler, S.; et al. European interlaboratory comparison investigations (ICI) and external quality assurance schemes (EQUAS) for the analysis of bisphenol A, S and F in human urine: Results from the HBM4EU project. Environ. Res. 2022, 210, 112933. [Google Scholar] [CrossRef] [PubMed]
- TEDX. List of Potential Endocrine Disruptors. 2018. Available online: https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list (accessed on 23 August 2023).
- ATSDR. Substance Priority List; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2017.
- Schimel, J.; Becerra, C.A.; Blankinship, J. Estimating decay dynamics for enzyme activities in soils from different ecosystems. Soil Biol. Biochem. 2017, 114, 5–11. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.M.; Li, Y.F.; Tan, Y.; Liu, N.; Liu, Y.J. The efficient hydroxy alkylation of phenol with formaldehyde to bisphenol F over thermoregulated phase-separable reaction system containing a water-soluble Brønsted acidic ionic liquid. RSC Adv. 2014, 4, 33466–33473. [Google Scholar] [CrossRef]
- Thoene, M.; Rytel, L.; Nowicka, N.; Wojtkiewicz, J. The state of bisphenol research in the lesser developed countries of the EU: A mini-review. Toxicol. Res. 2018, 7, 371–380. [Google Scholar] [CrossRef]
- Herrero, Ó.; Aquilino, M.; Sánchez-Argüello, P.; Planelló, R. The BPA-substitute bisphenol S alters the transcription of genes related to endocrine, stress response and biotransformation pathways in the aquatic midge Chironomus riparius (Diptera, Chironomidae). PLoS ONE 2018, 13, e0193387. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Harner, T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Zhang, J.; Huang, R.; Yin, H.; Dang, Z.; Wu, P.; Liu, Y. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci. Total Environ. 2019, 692, 107–116. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A. Soil microbiome response to contamination with Bisphenol A, Bisphenol F and Bisphenol S. Int. J. Mol. Sci. 2020, 21, 3529. [Google Scholar] [CrossRef] [PubMed]
- ChemSpider. Search and Share Chemistry, 2020. Available online: https://www.chemspider.com/ (accessed on 23 August 2023).
- Salgueiro-González, N.; Alda, M.J.L.; De Muniategui-Lorenzo, S.; Prada-Rodriguez, D.; Barcelo, D. Analysis and occurrence of endocrine-disrupting chemicals in airborne particles. Trends Anal. Chem. 2015, 66, 45–52. [Google Scholar] [CrossRef]
- Morin, N.; Arp, H.P.H.; Hale, S.E. Bisphenol a in solid waste materials, leachate water, and air particles from Norwegian waste-handling facilities: Presence and partitioning behavior. Environ. Sci. Technol. 2015, 49, 7675–7683. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.; Gullett, B.; Striebich, R.; Klosterman, J.; Contreras, J.; Devito, M. Endocrine disrupting chemical emissions from combustion sources: Diesel particulate emissions and domestic waste open burn emissions. Atmos. Environ. 2005, 39, 801–811. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K. Ubiquity of bisphenol A in the atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef]
- Salapasidou, M.; Samara, C.; Voutsa, D. Endocrine disrupting compounds in the atmosphere of the urban area of Thessaloniki, Greece. Atmos. Environ. 2011, 45, 3720–3729. [Google Scholar] [CrossRef]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P. Bisphenol A, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int. J. Environ. Res. Public Health 2019, 16, 2025. [Google Scholar] [CrossRef]
- Arp, H.P.H.; Morin, N.A.O.; Hale, S.E.; Okkenhaug, G.; Breivik, K.; Sparrevik, M. The mass flow and proposed management of bisphenol A in selected Norwegian waste streams. Waste Manag. 2017, 60, 775–785. [Google Scholar] [CrossRef]
- Bi, X.; Simoneit, B.R.T.; Wang, Z.; Wang, X.; Sheng, G.; Fu, J. The major components of particles emitted during recycling of waste printed circuit boards in a typical e-waste workshop of South China. Atmos. Environ. 2010, 44, 4440–4445. [Google Scholar] [CrossRef]
- Graziani, N.S.; Carreras, H.; Wannaz, E. Atmospheric levels of BPA associated with particulate matter in an urban environment. Heliyon 2019, 5, e01419. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Guo, Y.; Moon, H.B.; Nakata, H.; Wu, Q.; Kannan, K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 2012, 46, 9138–9145. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Binh, T.; Moon, H.-B.; et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol a via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar] [CrossRef]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef]
- Shah, M.B.; Liu, J.; Zhang, Q.; Stout, C.D.; Halpert, J.R. Halogen–π interactions in the cytochrome P450 active site: Structural insights into human CYP2B6 substrate selectivity. ACS Chem. Biol. 2017, 12, 1204–1210. [Google Scholar] [CrossRef]
- Li, D.; Bi, R.; Chen, H.; Mu, L.; Zhang, L.; Chen, Q.; Xie, H.; Luo, Y.; Xie, L. The acute toxicity of bisphenol A and lignin-derived bisphenol in algae, daphnids, and Japanese medaka. Environ. Sci. Pollut. Res. 2017, 24, 23872–23879. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Stepnowski, P. The quantification of bisphenols and their analogues in wastewaters and surface water by an improved solid-phase extraction gas chromatography/mass spectrometry method. Environ. Sci. Pollut. Res. 2020, 27, 28829–28839. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.; Liu, Z.H.; Zeng, F.; Zhang, J.; Dang, Z. Occurrence, spatial distribution, and main source identification of ten bisphenol analogues in the dry season of the Pearl River, South China. Environ. Sci. Pollut. Res. 2022, 29, 27352–27365. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, N.; Zhang, Y.; Hu, H.; Zhao, M.; Jin, H. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 2022, 287, 132218. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Moon, H.B.; Yamashita, N.; Yun, S.; Kannan, K. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: Spatial and temporal distributions. Environ. Sci. Technol. 2012, 46, 11558–11565. [Google Scholar] [CrossRef]
- Ozhan, K.; Kocaman, E. Temporal and Spatial Distributions of Bisphenol A in Marine and Freshwaters in Turkey. Arch. Environ. Contam. Toxicol. 2019, 76, 246–254. [Google Scholar] [CrossRef]
- Heemken, O.P.; Reincke, H.; Stachel, B.; Theobald, N. The occurrence of xenoestrogens in the Elbe river and the North Sea. Chemosphere 2001, 45, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Gorga, M.; Petrovic, M.; González-Alonso, S.; Barceló, D.; Valcárcel, Y. Analysis and occurrence of endocrine disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci. Total Environ. 2014, 466–467, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, F.L.; Paun, I.; Pirvu, F.; Pascu, L.F.; Galaon, T. Occurrence and Fate of Bisphenol A and its Congeners in Two Wastewater Treatment Plants and Receiving Surface Waters in Romania. Environ. Toxicol. Chem. 2021, 40, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, D.; Ruan, Y.; Taniyasu, S.; Yamazaki, E.; Kumar, N.J.I.; Lam, P.K.S.; Wang, X. Nobuyoshi Yamashita Nationwide distribution and potential risk of bisphenol analogues in Indian waters. Ecotoxicol. Environ. Saf. 2020, 200, 110718. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Feng, Q.; Hu, G.; Gao, Z.; Meng, Q.; Zhu, X. Occurrence, distribution, and risk assessment of bisphenol analogues in Luoma Lake and its inflow rivers in Jiangsu Province, China. Environ. Sci. Pollut. Res. 2022, 29, 1430–1445. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.S.; Moon, H.B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef]
- Peng, Z.E.; Wu, F.; Deng, N. Photodegradation of bisphenol A in simulated lake water containing algae, humic acid and ferric ions. Environ. Pollut. 2006, 144, 840–846. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Rashid, A.; Hu, A.; Xin, K.; Li, H.; Adyari, B.; Wang, Y.; Yu, C.-P.; Sun, Q. Bisphenol A attenuation in natural microcosm: Contribution of ecological components and identification of transformation pathways through stable isotope tracing. J. Hazard. Mater. 2019, 385, 121584. [Google Scholar] [CrossRef] [PubMed]
- NCBI. National Center for Biotechnology Information. PubChem Database. Bisphenol A, CID=6623, 2020. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol-A (accessed on 23 August 2023).
- Im, J.; Löffler, F.E. Fate of bisphenol A in terrestrial and aquatic environments. Environ. Sci. Technol. 2016, 50, 8403–8416. [Google Scholar] [CrossRef] [PubMed]
- Azizullah, A.; Khan, S.; Gao, G.; Gao, K. The interplay between bisphenol A and algae—A review. J. King Saud Univ. Sci. 2022, 34, 102050. [Google Scholar] [CrossRef]
- Hirano, T.; Honda, Y.; Watanabe, T.; Kuwahara, M. Degradation of bisphenol A by the lignin-degrading enzyme, manganese peroxidase, produced by the white-rot basidiomycete, Pleurotus ostreatus. Biosci. Biotechnol. Biochem. 2000, 64, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Y.; Pu, K.B.; Bai, J.R.; Ma, P.C.; Cai, W.F.; Guo, K.; Chen, Q.Y.; Wang., Y.H. Boosted biodegradation of recalcitrant bisphenol S by mix-cultured microbial fuel cells under micro-aerobic conditions. Biochem. Eng. J. 2023, 197, 108968. [Google Scholar] [CrossRef]
- Uzer, A.; Ercag, E.; Parlar, H.; Apak, R.; Filik, H. Spectrophotometric determination of 4,6-dinitro-o-cresol (DNOC) in soil and lemon juice. Anal. Chim. Acta 2006, 580, 83–90. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research Needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Baldè, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor—2017; United Nations University (UNU), International Telecommunication Union (ITU) and International Solid Waste Association: Bonn, Germany; Geneva, Switzerland; Vienna, Austria, 2017; pp. 1–116. [Google Scholar]
- Gupta, R.K.; Schuh, R.A.; Fiskum, G.; Flaws, J.A. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol. Appl. Pharmacol. 2006, 216, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Sad, M.E.; Padro, C.L.; Apestegut, C.R. Synthesis of cresols by alkylation of phenol with methanol on solid acids. Catal. Today 2008, 133–135, 720–728. [Google Scholar] [CrossRef]
- Landau, M.V.; Kaliya, M.L.; Herskowitz, M. Ammoxidation of p-cresol to p-hydroxybenzonitrile high-performance boria-phosphoria supported catalysts. Appl. Catal. A Gen. 2001, 208, 21–34. [Google Scholar] [CrossRef]
- Pèrez, R.A.; Albero, B.; Ferriz, M.; Tadeo, J.L. Rapid multi residue determination of bisphenol analogues in soil with on-line derivatization. Anal. Bioanal. Chem. 2017, 409, 4571–4580. [Google Scholar] [CrossRef]
- Staples, C.; van der Hoeven, N.; Clark, K.; Mihaich, E.; Woelz, J.; Hentges, S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 2018, 201, 448–458. [Google Scholar] [CrossRef]
- Yu, X.; Xue, J.; Yao, H.; Wu, Q.; Venkatesan, A.K.; Halden, R.U.; Kannan, K. Occurrence and estrogenic potency of eight bisphenol analogs in sewage sludge from the US EPA targeted national sewage sludge survey. J. Hazard. Mater. 2015, 299, 733–739. [Google Scholar] [CrossRef]
- Lee, S.; Liao, C.; Song, G.J.; Ra, K.; Kannan, K.; Moon, H.B. Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere 2015, 119, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef]
- USEPA. Bisphenol Action Plan. United States Environmental Protection Agency, Cincinnati, Ohio. 2010. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/bpa_action_plan.pdf (accessed on 26 August 2023).
- Bisphenol, S. National Toxicology Program. 2014. Available online: https://ntpsearch.niehs.nih.gov/?query=bisphenol+S&e=False&suffixes=false (accessed on 20 August 2023).
- Huang, D.Y.; Zhao, H.Q.; Liu, C.P.; Sun, C.X. Characteristics, sources, and transport of tetrabromobisphenol A and bisphenol A in soils from a typical e-waste recycling area in South China. Environ. Sci. Pollut. Res. 2014, 21, 5818–5826. [Google Scholar] [CrossRef] [PubMed]
- Setlhare, B.; Kumar, A.; Mokoena, M.P.; Pillay, B.; Olaniran, A.O. Phenol hydroxylase from Pseudomonas sp. KZNSA: Purification, characterization, and prediction of three-dimensional structure. Int. J. Biol. Macromol. 2020, 146, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A dangerous pollutant distorting the biological properties of soil. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Zhao, Y.; Wang, L.; Ma, Y.; Schäffer, A.; Ji, R. Fate of bisphenol S (BPS) and characterization of non-extractable residues in soil: Insights into persistence of BPS. Environ. Int. 2020, 143, 105908. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, L.S. Aerobic soil biodegradation of bisphenol (BPA) alternatives bisphenol S and bisphenol AF compared to BPA. Environ. Sci. Technol. 2017, 51, 13698–13704. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, L.; Liu, X.; Lv, J. Adsorption and aerobic biodegradation of four selected endocrine disrupting chemicals in soil–water system. Int. Biodeterior. Biodegrad. 2013, 76, 3–7. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Sun, F.; Zhou, D.; Guo, R.; Dong, T.; Chen, Y.; Ji, R.; Chen, J. Fate of 14C-bisphenol F isomers in an oxic soil and the effects of earthworm. Sci. Total Environ. 2019, 657, 254–261. [Google Scholar] [CrossRef]
- Lees, K.; Fitzsimons, M.; Snape, J.; Tappin, A.; Comber, S. Pharmaceuticals in soils of lower income countries: Physico-chemical fate and risks from wastewater irrigation. Environ. Int. 2016, 94, 712–723. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Q.; Yang, W.-X.; Dinh, Q.T.; Tran, T.A.T.; Zhao, X.-D.; Wu, J.-T.; Liu, Y.-X.; Liang, D.-L. DOM derivations determine the distribution and bioavailability of DOM-Se in selenate applied soil and mechanisms. Environ. Pollut. 2020, 259, 113899. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.H.; Yan, Z.R.; Ma, Y.F.; Zhu, Y.Y.; Li, X.Y.; Xu, J.; Zhang, W. pH dependence of the binding interactions between humic acids and bisphenol A-A thermodynamic perspective. Environ. Pollut. 2019, 255, 113292. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Park, B.J. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques. Rev. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Barceló, D. Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues–A review. Sci. Total Environ. 2019, 689, 160–177. [Google Scholar] [CrossRef]
- Gaur, V.K.; Gautam, K.; Sharma, P.; Gupta, S.; Pandey, A.; You, S.; Varjani, S. Carbon-based catalyst for environmental bioremediation and sustainability: Updates and perspectives on techno-economics and life cycle assessment. Environ. Res. 2022, 209, 112793. [Google Scholar] [CrossRef]
- Quintella, C.M.; Mata, A.M.T.; Lima, L.C.P. Overview of bioremediation with technology assessment and emphasis on fungal bioremediation of oil contaminated soils. J. Environ. Manag. 2019, 241, 156–166. [Google Scholar] [CrossRef]
- Eltoukhy, A.; Jia, Y.; Nahurira, R.; Abo-Kadoum, M.A.; Khokhar, I.; Wang, J.; Yan, Y. Biodegradation of endocrine disruptor Bisphenol A by Pseudomonas putida strain YC-AE1 isolated from polluted soil, Guangdong, China. BMC Microbiol. 2020, 20, 11. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Distribution and interactions of pentachlorophenol in soils: The roles of soil iron oxides and organic matter. J. Contam. Hydrol. 2016, 191, 99–106. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Zhou, Y.; Pang, S.; Gao, Y.; Jiang, C.; Ma, J.; Jin, Y.; Yang, Y.; Liu, G. Kinetics of oxidation of iodide (I-) and hypoiodous acid (HOI) by peroxymonosulfate (PMS) and formation of iodinated products in the PMS/I-/NOM system. Environ. Sci. Technol. Lett. 2017, 4, 76–82. [Google Scholar] [CrossRef]
- Jia, Y.; Eltoukhy, A.; Wang, J.; Li, X.; Hlaing, T.S.; Aung, M.M.; Nwe, M.T.; Lamraoui, I.; Yan, Y. Biodegradation of bisphenol A by Sphingobium sp. YC-JY1 and the essential role of cytochrome P450 monooxygenase. Int. J. Mol. Sci. 2020, 21, 3588. [Google Scholar] [CrossRef]
- Im, J.; Prevatte, C.; Campagna, S.; Löffler, F. Identification of 4-hydroxycumyl alcohol as the major MnO2-mediated bisphenol A transformation product and evaluation of its environmental fate. Environ. Sci. Technol. 2015, 49, 6214–6221. [Google Scholar] [CrossRef] [PubMed]
- Liebeg, E.W.; Cutright, T.J. The investigation of enhanced bioremediation through the addition of macro and micro nutrients in PAH contaminated soil. Int. Biodeter. Biodegr. 1999, 44, 55–64. [Google Scholar] [CrossRef]
- Kong, X.; Gao, H.; Song, X.; Deng, Y.; Zhang, Y. Adsorption of phenol on porous carbon from Toona sinensis leaves and its mechanism. Chem. Phys. Lett. 2020, 739, 137046. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, D.; Zhang, C.; Li, F.; Chu, G.; Wu, M.; Pan, B. Steinberg ChEW. The contrasting role of minerals in biochars in bisphenol A and sulfamethoxazole sorption. Chemosphere 2021, 264, 128490. [Google Scholar] [CrossRef] [PubMed]
- Louati, I.; Dammak, M.; Nasri, R.; Belbahri, L.; Nasri, M.; Abdelkafi, S.; Mechichi, T. Biodegradation and detoxifcation of bisphenol A by bacteria isolated from desert soils. 3 Biotech 2019, 9, 228. [Google Scholar] [CrossRef]
- Shobnam, N.; Sun, Y.; Mahmood, M.; Löffler, F.E.; Im, J. Biologically mediated abiotic degradation (BMAD) of bisphenol A by manganeseoxidizing bacteria. J. Hazard. Mater. 2021, 417, 125987. [Google Scholar] [CrossRef]
- Noszczyńska, M.; Chodór, M.; Jałowiecki, Ł.; Piotrowska-Seget, Z. A comprehensive study on bisphenol A degradation by newly isolated strains Acinetobacter sp. K1MN and Pseudomonas sp. BG12. Biodegradation 2021, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thathola, P.; Agnihotri, V.; Pandey, A.; Upadhyay, S.K. Biodegradation of bisphenol A using psychrotolerant bacterial strain Pseudomonas palleroniana GBPI_508. Arch. Microbiol. 2022, 204, 272. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.; Ma, L.; Weng, Z.; Zhou, J. Simultaneous removal of bisphenol F and nitrate by a novel isolated strain Pseudomonas sp. ZH-FAD. AIP Conf. Proc. 2022, 2474, 020003. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hosokawa, C.; Sasaki-Mori, M.; Akahira, A.; Fukunaga, K.; Ikeuchi, T.; Oshima, K.I.; Tsuchido, T. Isolation and characterization of novel bisphenol–A-degrading bacteria from soils. Biocontrol Sci. 2009, 14, 161–169. [Google Scholar] [CrossRef]
- Matsumura, Y.; Akahira-Moriya, A.; Sasaki-Mori, M. Bioremediation of bisphenol-a polluted soil by Sphingomonas bisphenolicum AO1 and the microbial community existing in the soil. Biocontrol Sci. 2015, 20, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Suyamud, B.; Inthorn, D.; Panyapinyopol, B.; Thiravetyan, P. Biodegradation of bisphenol A by a newly isolated Bacillus megaterium strain ISO-2 from a polycarbonate industrial wastewater. Water Air Soil Pollut. 2018, 229, 348. [Google Scholar] [CrossRef]
- Zühlke, M.K.; Schlüter, R.; Henning, A.K.; Lipka, M.; Mikolasch, A.; Schumann, P.; Giersberg, M.; Kunze, G.; Schauer, F. A novel mechanism of conjugate formation of bisphenol A and its analogues by Bacillus amyloliquefaciens: Detoxifcation and reduction of estrogenicity of bisphenols. Int. Biodeterior. Biodegrad. 2016, 109, 165–173. [Google Scholar] [CrossRef]
- Toyama, T.; Ojima, T.; Tanaka, Y.; Mori, K.; Morikawa, M. Sustainable biodegradation of phenolic endocrine-disrupting chemicals by Phragmites australis rhizosphere bacteria association. Water Sci. Technol. 2013, 68, 522–529. [Google Scholar] [CrossRef]
- Ogata, Y.; Goda, S.; Toyama, T.; Sei, K.; Ike, M. The 4-tert-butylphenolutilizing bacterium Sphingobium fuliginis OMI can degrade bisphenols via phenolic ring hydroxylation and meta-cleavage pathway. Environ. Sci. Technol. 2013, 47, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Sato, Y.; Inoue, D.; Sei, K.; Chang, Y.C.; Kikuchi, S.; Ike, M. Biodegradation of bisphenol A and bisphenol F in the rhizosphere sediment of Phragmites australis. J. Biosci. Bioeng. 2009, 108, 147–150. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Yuan, L.; Yu, J.; Li, J.; Huang, G.; Xi, B.; Liu, H. Aerobic degradation of bisphenol A by Achromobacter xylosoxidans strain B-16 isolated from compost leachate of municipal solid waste. Chemosphere 2007, 68, 181–190. [Google Scholar] [CrossRef]
- Fouda, A. Biodegradation of bisphenol A by some bacterial species and significance role of plasmids. Int. J. Adv. Res. Biol. Sci. 2015, 2, 93–108. [Google Scholar]
- Zühlke, M.K.; Schlüter, R.; Mikolasch, A.; Henning, A.K.; Giersberg, M.; Lalk, M.; Kunze, G.; Schweder, T.; Urich, T.; Schauer, F. Biotransformation of bisphenol A analogues by the biphenyl-degrading bacterium Cupriavidus basilensis-a structure-biotransformation relationship. Appl. Microbiol. Biotechnol. 2020, 104, 3569–3583. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Subramanya, N.; Zhao, F.; Yu, C.P.; Sandt, J.; Chu, K.H. Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 2009, 77, 1084–1089. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Soil enzyme response to bisphenol F contamination in the soil bioaugmented using bacterial and mould fungal consortium. Environ. Monit. Assess. 2020, 192, 20. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Biochemical activity of soil contaminated with BPS, bioaugmented with a mould fungi consortium and a bacteria consortium. Environ. Sci. Pollut. Res. 2019, 26, 37054–37069. [Google Scholar] [CrossRef]
- Carvalho, M.B.; Tavares, S.; Medeiros, J.; Núñez, O.; Gallart-Ayala, H.; Leitão, M.C.; Galceran, M.T.; Hursthouse, A. Pereira CS Degradation pathway of pentachlorophenol by Mucor plumbeus involves phase II conjugation and oxidation–reduction reactions. J. Hazard. Mater. 2011, 198, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Franchi, O.; Rosenkranz, F.; Chamy, R. Key microbial populations involved in anaerobic degradation of phenol and p-cresol using different inocula. Electron. J. Biotechnol. 2018, 35, 33–38. [Google Scholar] [CrossRef]
- Xue, F.; Ya, X.; Tong, Q.; Xiu, Y.; Huang, H. Heterologous overexpression of Pseudomonas umsongensis halohydrin dehalogenase in Escherichia coli and its application in epoxide asymmetric ring opening reactions. Process Biochem. 2018, 75, 139–145. [Google Scholar] [CrossRef]
- Wilson, B.; Zhu, J.; Cantwell, M.; Olsen, C.R. Short-term dynamics and retention of triclosan in the lower Hudson river estuary. Mar. Pollut. Bull. 2008, 56, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Gilevska, T.; Wetzig, F.; Dorer, C.; Richnow, H.H.; Vogt, C. Characterization of phenol and cresol biodegradation by compound specific stable isotope analysis. Environ. Pollut. 2016, 210, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Dub, D.; Zhou, W.; Zeng, X.; Cheng, G. Phenol degradation and genotypic analysis of dioxygenase genes in bacteria isolated from sediments. Braz. J. Microbiol. 2017, 48, 305–313. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Xu, L.; Zhu, K.; Feng, Y.; Pan, J.; Si, M.; Zhang, L.; Shen, X. Transcriptional control of the phenol hydroxylase gene phe of Corynebacterium glutamicum by the AraC-type regulator PheR. Microbiol. Res. 2018, 209, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Akahira, A.; Oshima, K.; Tsuchido, T.; Matsumura, Y. Purification of cytochrome P450 and ferredoxin, involved in bisphenol A degradation, from Sphingomonas sp. strain AO1. Appl. Environ. Microbiol. 2005, 71, 8024–8030. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, Q.H.; Wu, Y.C.; Lin, X.G. Oxidation of polycyclic aromatic hydrocarbons using Bacillus subtilis CotA with high laccase activity and copper independence. Chemosphere 2016, 148, 1–7. [Google Scholar] [CrossRef]
- Lubbers, R.J.M.; Dilokpimol, A.; Visser, J.; Makel, M.R.; Hilden, K.S.; de Vries, R.P. A comparison between the homocyclic aromatic metabolic pathways from plant-derived compounds by bacteria and fungi. Biotechnol. Adv. 2019, 37, 107396. [Google Scholar] [CrossRef]
- Harwood, C.S.; Burckhardt, G.; Herrmann, H.; Fuchs, G. Anaerobic metabolism of aromatic compounds via the benzoyl-CoA pathway. FEMS Microbiol. Rev. 1999, 22, 439–458. [Google Scholar] [CrossRef]
- Fischer, J.; Kappelmeyer, U.; Kastner, M.; Schauer, F.; Heipieper, H.J. The degradation of bisphenol A by the newly isolated bacterium Cupriavidus basilensis JF1 can be enhanced by biostimulation with phenol. Int. Biodeterior. Biodegrad. 2010, 64, 324–330. [Google Scholar] [CrossRef]
- McCormick, J.M.; Van Es, T.; Cooper, K.R.; White, L.A.; Haggblom, M.M. Microbially mediated O-methylation of bisphenol A results in metabolites with increased toxicity to the developing zebrafish (Danio rerio) embryo. Environ. Sci. Technol. 2011, 45, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Colbert, C.L.; Couture, M.M.J.; Eltis, L.D.T.; Bolin, J.A. A cluster exposed: Structure of the Rieske ferredoxin from biphenyl dioxygenase and the redox properties of Rieske Fe-S proteins. Structure 2000, 8, 1267–1278. [Google Scholar] [CrossRef]
- Chakraborty, J.; Das, S. Molecular perspectives and recent advances in microbial remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2016, 23, 16883–16903. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Li, G.; Mai, B.; An, T. Spore cells from BPA degrading bacteria Bacillus sp. GZB displaying high laccase activity and stability for BPA degradation. Sci. Total Environ. 2018, 640–641, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, V.; Izzo, V.; Scognamiglio, R.; Notomista, E.; Capasso, P.; Casbarra, A.; Pucci, P.; Donato, D. Phenol hydroxylase and toluene/o -xylene monooxygenase from Pseudomonas stutzeri OX1: Interplay between two enzymes. Appl. Environ. Microbiol. 2004, 70, 2211–2219. [Google Scholar] [CrossRef]
- Telke, A.A.; Kalyani, D.C.; Jadhav, U.U.; Parshetti, G.K.; Govindwar, S.P. Purification and characterization of an extracellular laccase from a Pseudomonas sp. LBC1 and its application for the removal of bisphenol A. J. Mol. Catal. B Enzym. 2009, 61, 252–260. [Google Scholar] [CrossRef]
- Kalyani, D.C.; Telke, A.A.; Surwase, S.N.; Jadhav, S.B.; Lee, J.K.; Jadhav, J.P. Effectual decolorization and detoxification of triphenylmethane dye Malachite Green (MG) by Pseudomonas aeruginosa NCIM 2074 and its enzyme system. Clean Technol. Environ. Policy 2012, 14, 989–1001. [Google Scholar] [CrossRef]
- Torres-García, J.L.; Ahuactzin-Pérez, M.; Fernández, F.J.; Cortés-Espinosa, D.V. Bisphenol A in the environment and recent advances in biodegradation by fungi. Chemosphere 2022, 303, 134940. [Google Scholar] [CrossRef]
- Mitbaá, R.; Hernández, D.R.O.; Pozo, C.; Nasri, M.; Mechichi, T.; González, J.; Aranda, E. Degradation of bisphenol A and acute toxicity reduction by different thermo-tolerant ascomycete strains isolated from arid soils. Ecotoxicol. Environ. Saf. 2018, 156, 87–96. [Google Scholar] [CrossRef]
- Leitão, A.L.; Duarte, M.P.; Santos Oliveira, J. Degradation of phenol by a halotolerant strain of Penicillium chrysogenum. Int. Biodeter. Biodegr. 2007, 59, 220–225. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Dhanve, R.; Jadhav, J. Biodegradation of triphenylmethane dye cotton blue by Penicillium ochrochloron MTCC 517. J. Hazard. Mater. 2008, 157, 472–479. [Google Scholar] [CrossRef]
- Hugentobler, K.G.; Müller, M. Towards semisynthetic natural compounds with a biaryl axis: Oxidative phenol coupling in Aspergillus niger. Bioorg. Med. Chem. 2018, 26, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Ruprich-Robert, G.; Silar, P.; Herbert, E.; Ferrari, R.; Chapeland-Leclerc, F. Characterization of three multicopper oxidases in the filamentous fungus Podospora anserina: A new role of an ABR1-like protein in fungal development? Fungal Genet. Biol. 2018, 116, 1–13. [Google Scholar] [CrossRef]
- Xie, N.; Chapeland-Leclerc, F.; Silar, P.; Ruprich-Robert, G. Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina. Environ. Microbiol. 2014, 16, 141–161. [Google Scholar] [CrossRef]
- Gulve, R.M.; Deshmukh, A.M. Antimicrobial activity of the marine Actinomycetes. Inter. Multidiscip. Res. J. 2012, 2, 16–22. [Google Scholar]
- Xu, J.; Sheng, G.P.; Ma, Y.; Wang, L.F.; Yu, H.Q. Roles of extracellular polymeric substances (EPS) in the migration and removal of sulfamethazine in activated sludge system. Water Res. 2013, 47, 5298–5306. [Google Scholar] [CrossRef]
- Li, C.; Lu, Q.; Ye, J.; Qin, H.; Long, Y.; Wang, L.; Ou, H. Metabolic and proteomic mechanism of bisphenol A degradation by Bacillus thuringiensis. Sci. Total Environ. 2018, 640–641, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Dercová, K.; Certík, M.; Malová, A.; Sejáková, Z. Effect of chlorophenols on the membrane lipids of bacterial cells. Int. Biodeter. Biodegr. 2004, 54, 251–254. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.; Hu, H.; Zhang, X. Reaction mechanism and degradation pathway of rhodamine 6G by photocatalytic treatment. Water Air Soil Pollut. 2017, 228, 291–301. [Google Scholar] [CrossRef]
- Heider, J.; Fuchs, G. Microbial anaerobic aromatic metabolism. Anaerobe 1997, 3, 1–22. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effect of separate and combined toxicity of bisphenol A and zinc on the soil microbiome. Int. J. Mol. Sci. 2022, 23, 5937. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Gryta, A.; Kalembasa, S.; Kalembasa, D. Variation in soil microbial population and enzyme activities under faba bean as affected by pentachlorophenol. Appl. Soil Ecol. 2020, 150, 103466. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Pingree, M.R.A.; Gao, S. Chapter 16-Assessing soil biological health in forest soils. In Developments in Soil Science; Busse, M., Christian, P., Giardina, D.M., Debbie, M., Page-Dumroese, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 397–426. [Google Scholar] [CrossRef]
- Kucharski, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J. Enzyme activity and microorganisms diversity in soil contaminated with the Boreal 58 WG. J. Environ. Sci. Health Part B 2016, 51, 446–454. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Shuler, M.; Kargi, F. Bioprocess Engineering Basic Concepts; Prentice-Hall Incorporation: Englewood Cliffs, NJ, USA, 2010; 576p, ISBN 9780130819085. [Google Scholar]
- Mo, L.; Wang, Q.; Bi, E. Effects of endogenous and exogenous dissolved organic matter on sorption behaviours of bisphenol A onto soils. J. Environ. Manag. 2021, 287, 112312. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Applicability of biochemical indices to quality assessment of soil polluted with heavy metal. J. Elem. 2013, 18, 733–756. [Google Scholar] [CrossRef]
- Campos, J.A.; Peco, J.D.; García-Noguero, E. Antigerminative comparison between naturally occurring naphthoquinones and commercial pesticides. Soil dehydrogenase activity used as bioindicator to test soil toxicity. Sci. Total Environ. 2019, 694, 133672. [Google Scholar] [CrossRef] [PubMed]
- Perotti, E.B.R. Impact of hydroquinone used as a redox effector model on potential denitrification, microbial activity and redox condition of a cultivable soil. Rev. Argent. Microbiol. 2015, 47, 212–218. [Google Scholar] [CrossRef]
- Borisov, V.B.; Verkhovsky, M.I. Oxygen as acceptor. EcoSal Plus 2015, 6. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Role of Chlorella sp. and rhamnolipid 90 in maintaining homeostasis in soil contaminated with bisphenol A. J. Soils Sediments 2021, 21, 27–41. [Google Scholar] [CrossRef]
- Daudzai, Z.; Treesubsuntorn, C.; Thiravetyan, P. Inoculated Clitoria ternatea with Bacillus cereus ERBP for enhancing gaseous ethylbenzene phytoremediation: Plant metabolites and expression of ethylbenzene degradation genes. Ecotox. Environ. Saf. 2018, 164, 50–60. [Google Scholar] [CrossRef]
- Kot, M.; Zaborska, W. Irreversible inhibition of jack bean urease by pyrocatechol. J. Enzym. Inhib. Med. Chem. 2003, 18, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Perveen, S.; Khan, A. Synthesis, enzyme inhibition and anticancer investigations of unsymmetrical 1,3-disubstituted ureas. J. Serb. Chem. Soc. 2014, 79, 1–10. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Response of soil alkaline phosphatase to biochar amendments: Changes in kinetic and thermodynamic Characteristics. Geoderma 2019, 337, 44–54. [Google Scholar] [CrossRef]
- Krych, J.; Gebicki, J.L.; Gebicka, L. Flavonoid-induced conversion of catalase to its inactive form–Compound II. Free Radic. Res. 2014, 48, 1334–1341. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Hong, J.; Wang, W.; Huang, K.; Yang, W.Y.; Zhao, Y.X.; Xiao, B.L.; Gao, Y.F.; Moosavi-Movahedi, Z.; Ghourchian, H.; Moosavi-Movahedi, A.A. A highly efficient nano-cluster artificial peroxidase and its direct electrochemistry on a nanocomplex modified glassy carbon electrode. Anal. Sci. 2012, 28, 711–716. [Google Scholar] [CrossRef][Green Version]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2001, 51, 51–106. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef]
- Ge, L.; Xie, Q.; Wei, X.; Li, Y.; Shen, W.; Hu, Y.; Yao, J.; Wang, S.; Du, X.; Zeng, X. Five undescribed plant-derived bisphenols from Artemisia capillaris aerial parts: Structure elucidation, anti-hepatoma activities and plausible biogenetic pathway. Arab. J. Chem. 2023, 16, 104580. [Google Scholar] [CrossRef]
- Schmidt, B.; Schuphan, I. Metabolism of the environmental estrogen bisphenol A by plant cell suspension cultures. Chemosphere 2002, 49, 51–59. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.W.; Chang, H.Q.; Li, Z.J.; Xue, J.M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Kondo, Y.; Shimoda, K.; Miyahara, K.; Hamada, H.; Hamada, H. Regioselective hydroxylation, reduction, and glycosylation of diphenyl compounds by cultured plant cells of Eucalyptus perriniana. Plant Biotechnol. 2006, 23, 291–296. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Wang, L.; Shen, F.; Zhou, Q.; Huang, X. Impacts of exogenous pollutant bisphenol A on characteristics of soybeans. Ecotoxicol. Environ. Saf. 2018, 157, 463–471. [Google Scholar] [CrossRef]
- Nie, L.; Wang, L.; Wang, Q.; Wang, S.; Zhou, Q.; Huang, X. Effects of bisphenol A on mineral nutrition in soybean seedling roots. Environ. Toxicol. Chem. 2015, 34, 133–140. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Hu, D.; Zhou, Q.; Huang, X. Effects of exogenous bisphenol A on the function of mitochondria in root cells of soybean (Glycine max L.) seedlings. Chemosphere 2019, 222, 619–627. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Cherianidou, A.; Eleftheriou, E.P. Effects of bisphenol A on the microtubule arrays in root meristematic cells of Pisum sativum L. Mut. Res. 2013, 750, 111–120. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Jadhav, A.S.; Chandagade, C.A.; Raut, P.D. Genotoxicity of bisphenol A on root meristem cells of Allium cepa: A cytogenetic approach. Asian J. Water Environ. Pollut. 2012, 9, 39–43. [Google Scholar]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Gu, Z.; Tao, Y.; Shen, C.; Zhou, Y.; Han, Y.; Yang, R. Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chem. X 2019, 2, 100023. [Google Scholar] [CrossRef]
- Samuilov, V.D.; Kiselevsky, D.B.; Oleskin, A.V. Mitochondria-targeted quinones suppress the generation of reactive oxygen species, programmed cell death and senescence in plants. Mitochondrion 2019, 46, 164–171. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Goeppert, N.; Dror, I.; Berkowitz, B. Fate and transport of free and conjugated estrogens during soil passage. Environ. Pollut. 2015, 206, 80–87. [Google Scholar] [CrossRef]
- Li, S.W.; Leng, Y.; Feng, L.; Zeng, X.Y. Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2014, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Hua, W.; Zhou, M.; Wang, Q.; Zhou, Q.; Huang, X. Effects of bisphenol A, an environmental endocrine disruptor, on the endogenous hormones of plants. Environ. Sci. Pollut. Res. 2015, 22, 17653–17662. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, L.H.; Zhou, Q.; Huang, X.H. Effects of bisphenol A on ammonium assimilation in soybean roots. Environ. Sci. Pollut. Res. 2013, 20, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kwak, J.I.; An, Y.J. Effects of bisphenol A in soil on growth, photosynthesis activity, and genistein levels in crop plants (Vigna radiata). Chemosphere 2018, 209, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Zhou, Q.; Huang, X. Reactive oxygen species initiate a protective response in plant roots to stress induced by environmental bisphenol A. Ecotoxicol. Environ. Saf. 2018, 154, 197–205. [Google Scholar] [CrossRef]
- Preisner, M.; Wojtasik, W.; Kostyn, K.; Boba, A.; Czuj, T.; Szopa, J.; Kulma, A. The cinnamyl alcohol dehydrogenase family in flax: Differentiation during plant growthand under stress conditions. J. Plant Physiol. 2018, 221, 132–143. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Jiang, N.; Yu, H.; Morohashi, K.; Ouma, W.Z.; Morales-Mantilla, D.E.; Gomez-Cano, F.A.; Mukundi, E.; Prada-Salcedo, L.D.; et al. A Maize Gene Regulatory Network for Phenolic Metabolism. Mol. Plant 2017, 10, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Hassan, F.; Raghib, J.; Bilal, A.; Aamir, R.; Khan, F.A.; Naushin, F. Chapter 9-Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Iqbal, M., Khan, R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, M.; Geng, X.; Liu, J.; Chen, J. Horizontal transfer of genetic determinants for degradation of phenol between the bacteria living in plant and its rhizosphere. Appl. Microbiol. Biotechnol. 2007, 77, 733–739. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientifc opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstufs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- EU. European Commission Regulation 2018/213. Of J. Eur. Union. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0213&from=EL (accessed on 20 August 2023).
- Zhao, C.; Xie, P.; Wang, H.; Cai, Z. Liquid chromatography-mass spectrometry-based metabolomics and lipidomics reveal toxicological mechanisms of bisphenol F in breast cancer xenografts. J. Hazard. Mater. 2018, 358, 503–507. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, L.; Qiu, Z.; Lee, D.Y.; Settle, S.J.; Que, H.S.; Telesca, D.; Yang, X.; Allard, P. Exposure to the BPA-substitute bisphenol S causes unique alterations of germline function. PLoS Genet. 2016, 12, e1006223. [Google Scholar] [CrossRef]
- Molina-Molina, J.M.; Amaya, E.; Grimaldi, M.; Saenz, J.M.; Real, M.; Fernandez, M.F. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Zelieann, C.; Wang, W.; Flaws, J. Bisphenol Ainhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod. Toxicol. 2013, 42, 58–67. [Google Scholar] [CrossRef]
- Cabaton, N.; Dumont, C.; Severinm, I.; Perdu, E.; Zalko, D.; Cherkaoui-Malki, M.; Chagnon, M.C. Genotoxic and endocrine activities of bis(hydroxyphenyl) methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 2009, 255, 15–24. [Google Scholar] [CrossRef]
- Rubin, B.S.; Soto, A.M. Bisphenol A: Perinatal exposure and body weight. Mol. Cell Endocrinol. 2009, 304, 55–62. [Google Scholar] [CrossRef]
- Stossi, F.; Bolt, M.J.; Ashcroft, F.J.; Lamerdin, J.E.; Melnick, J.S.; Powell, R.T.; Dandekar, R.D.; Mancini, M.D.; Walker, C.L.; Westwick, J.K.; et al. Defining estrogenic mechanisms of bisphenol A analogs through high throughput microscopy-based contextual assays. Chem. Biol. 2014, 21, 743–753. [Google Scholar] [CrossRef]
- Viñas, R.; Watson, C.S. Bisphenol S disrupts estradiol-induced nongenomic ~signaling in a rat pituitary cell line: Effects on cell functions. Environ. Health Perspect. 2013, 121, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Guo, L.; Ming, X. Bisphenol A exposure in children with autism spectrum disorders. Autism Res. 2015, 8, 272–283. [Google Scholar] [CrossRef]
- Castro, B.; Sánchez, P.; Torres, J.M.; Ortega, E. Bisphenol A, bisphenol F, and bisphenol S affect differently 5α-reductase expression and dopamine–serotonin system in the prefrontal cortex of juvenile female rats. Environ. Res. 2015, 142, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Nolte, E.L.R.; Wang, Y.; Margolis, A.E.; Calafat, A.M.; Wang, S.; Garcia, W.; Hoepner, L.A.; Peterson, B.S.; Rauh, V.; et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016, 151, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Karramass, T.; Sol, C.; Kannan, K.; Trasande, L.; Jaddoe, V.; Duijts, L. Bisphenol and phthalate exposure during pregnancy and the development of childhood lung function and asthma. The Generation R Study. Environ. Pollut. 2023, 32, 121853. [Google Scholar] [CrossRef]
- Rotimi, O.A.; Olawole, T.D.; DE Campos, O.C.; Adelani, I.B.; Rotimi, S.O. Bisphenol A in Africa: A review of environmental and biological levels. Sci. Total Environ. 2021, 764, 142854. [Google Scholar] [CrossRef]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martínez, D.; et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 2015, 135, 370–378. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.; Zhai, L.; Bai, Y.; Jia, L. Urinary bisphenol A and S are associated with diminished ovarian reserve in women from an infertility clinic in Northern China. Ecotoxicol. Environ. Saf. 2023, 256, 114867. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Petre, C.E.; Monk, K.R.; Puga, A.; Knudsen, K.E. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol. Cancer Ther. 2002, 1, 515–524. [Google Scholar] [PubMed]
- Song, H.; Zhang, T.; Yang, P.; Li, M.; Yang, Y.; Wang, Y.; Du, J.; Pan, K.; Zhang, K. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRγ signals. Toxicol. Vitr. 2015, 30, 521–528. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Alizadeh, S.; Raesi Vanani, A.; Dehghani, M.A.; Shirani, M.; Alipour, M.; Shahmohammadi, H.A.; Rafiei Asl, S. Effects of quercetin on bisphenol A-induced mitochondrial toxicity in rat liver. Iran. J. Basic Med. Sci. 2019, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jiao, Z.; Shi, J.; Li, M.; Guo, Q.; Shao, B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere 2016, 147, 9–19. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, R.; Zong, W. Bisphenol S interacts with catalase and induces oxidative stress in mouse liver and renal cells. J. Agric. Food Chem. 2016, 64, 6630–6640. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Li, Y.; Lin, C.; Chen, Y.; Zhang, X.; Yu, H. Melatonin inhibited the progression of gastric cancer induced by Bisphenol S via regulating the estrogen receptor 1. Ecotoxicol. Environ. Saf. 2023, 259, 115054. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental effects and estrogenicity of Bisphenol A alternatives in a zebrafish embryo model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K.; Xie, B.; Thompson, M.L.; Sung, S.; Ong, S.K.; van Leeuwen, J. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ. Sci. Technol. 2006, 40, 6537–6546. [Google Scholar] [CrossRef] [PubMed]


| Location | BPA | BPF | BPS | Sample Collection (Year) |
|---|---|---|---|---|
| Europe | ||||
| Iasi, Romania | 680 | 41 | 380 | 2012 |
| Athens, Komotini, Erateini, Greece | 1700 | 5500 | 1500 | 2014 |
| Asia | ||||
| Patna, India | 360 | 29 | <12 | 2014 |
| Jeddah, Saudi Arabia | 1100 | 160 | 110 | 2013 |
| Ansan, Anyang, South Korea | 1100 | 1300 | 10 | 2014 |
| North America | ||||
| Albany, United States | 3800 | 4400 | 2.1 | 2014 |
| South America | ||||
| Cartagena, Columbia | 420 | 69 | 3.7 | 2014 |
| Microorganisms | Kind of BP | References |
|---|---|---|
| Genus: Pseudomonas | ||
| Pseudomonas putida YC-AE1 | BPA, BPS, BPF | [91] |
| Pseudomonas aeruginosa Gb30 | BPA | [99] |
| Pseudomonas putida G320 | BPA | [100] |
| Pseudomonas sp. BG-12 | BPA | [101] |
| Pseudomonas palleroniana GBPI_508 | BPA | [102] |
| Pseudomonas sp. ZH-FAD | BPF | [103] |
| Genus: Sphingomonas Sphingomonas sp. SO11 | BPA | [104] |
| Sphingomonas sp. SO1a | BPA | [104] |
| Sphingomonas sp. SO4a | BPA | [104] |
| Sphingomonas bisphenolicum AO1 | BPA | [105] |
| Genus: Bacillus | ||
| Bacillus sp. YA27 | BPA | [104] |
| Bacillus megaterium ISO-2 | BPA | [106] |
| Bacillus amyloliquefaciens | BPA, BPF | [107] |
| Genus: Sphingobium | ||
| Sphingobium fuliginis TIK1 | BPA, BPS, BPF | [108] |
| Sphingobium sp. IT4 | BPA, BPS, BPF | [108] |
| Sphingobium fuliginis OMI | BPA, BPS, BPF | [109] |
| Sphingobium yanoikuyae TYF-1 | BPA, BPF | [110] |
| Sphingobium yanoikuyae FM-2 | BPF | [110] |
| other bacterial species: | ||
| Achromobacter xylosoxidans B-16 | BPA | [111] |
| Arthrobacter sp. YC-RL1 | BPA | [111] |
| Klebsiella pneumoniae J2 | BPA | [112] |
| Enterobacter asburiae L4 | BPA | [112] |
| Cupriavidus basilensis SBUG | BPA, BPF | [113] |
| BPA | BPF | BPS |
|---|---|---|
| Lysobacter | Caldilinea | Dactylosporangium |
| Steroidobacter | Arthrobacter | Geodermatophilus |
| Variovorax | Cellulosimicrobium | Sphingopyxis |
| Mycoplana | Prominomonospora |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenols—A Threat to the Natural Environment. Materials 2023, 16, 6500. https://doi.org/10.3390/ma16196500
Zaborowska M, Wyszkowska J, Borowik A, Kucharski J. Bisphenols—A Threat to the Natural Environment. Materials. 2023; 16(19):6500. https://doi.org/10.3390/ma16196500
Chicago/Turabian StyleZaborowska, Magdalena, Jadwiga Wyszkowska, Agata Borowik, and Jan Kucharski. 2023. "Bisphenols—A Threat to the Natural Environment" Materials 16, no. 19: 6500. https://doi.org/10.3390/ma16196500
APA StyleZaborowska, M., Wyszkowska, J., Borowik, A., & Kucharski, J. (2023). Bisphenols—A Threat to the Natural Environment. Materials, 16(19), 6500. https://doi.org/10.3390/ma16196500









