Luminescent Hybrid BPA.DA-NVP@Eu2L3 Materials: In Situ Synthesis, Spectroscopic, Thermal, and Mechanical Characterization
Abstract
1. Introduction
2. Results and Discussion
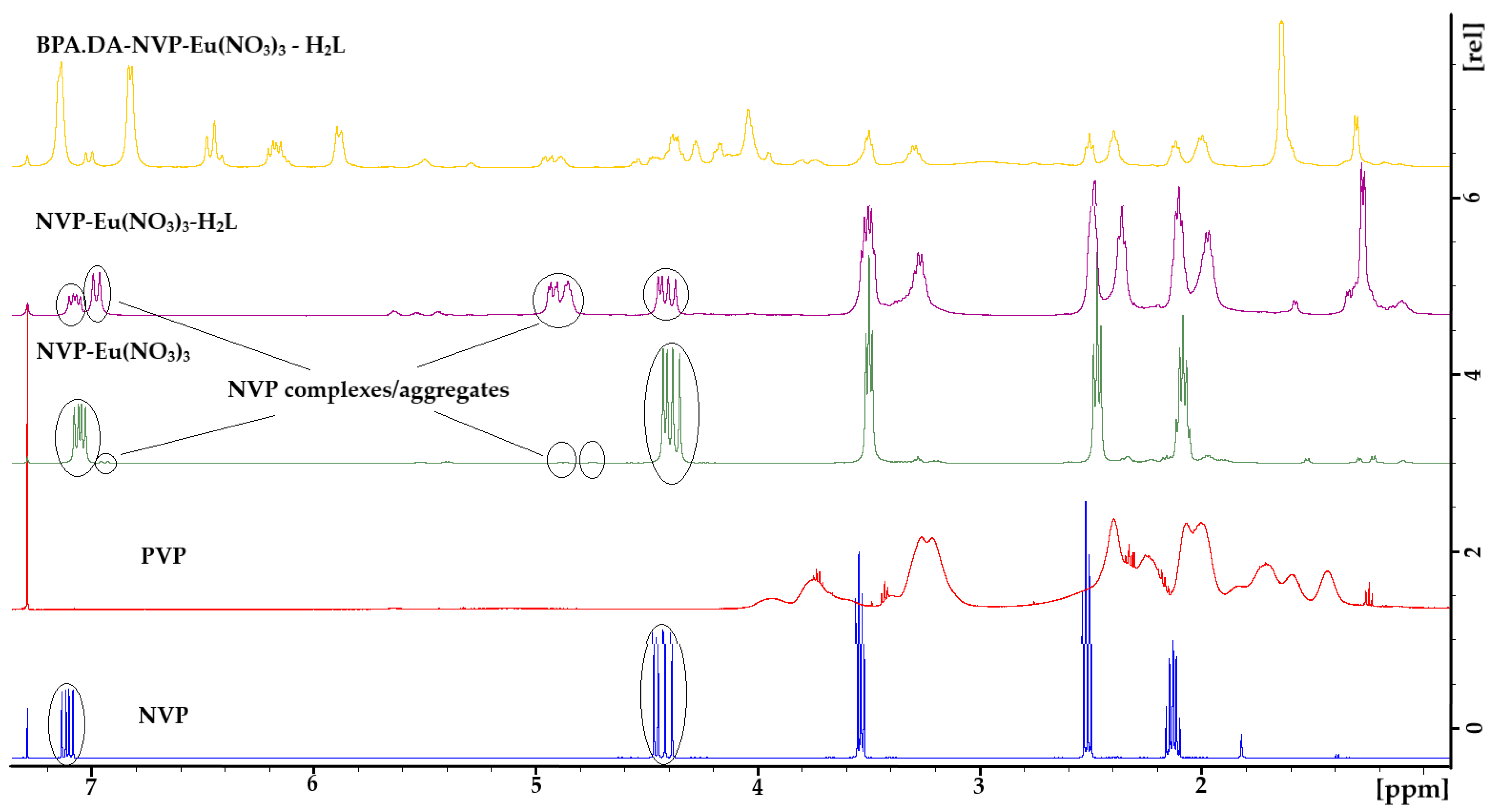
2.1. NMR Analysis
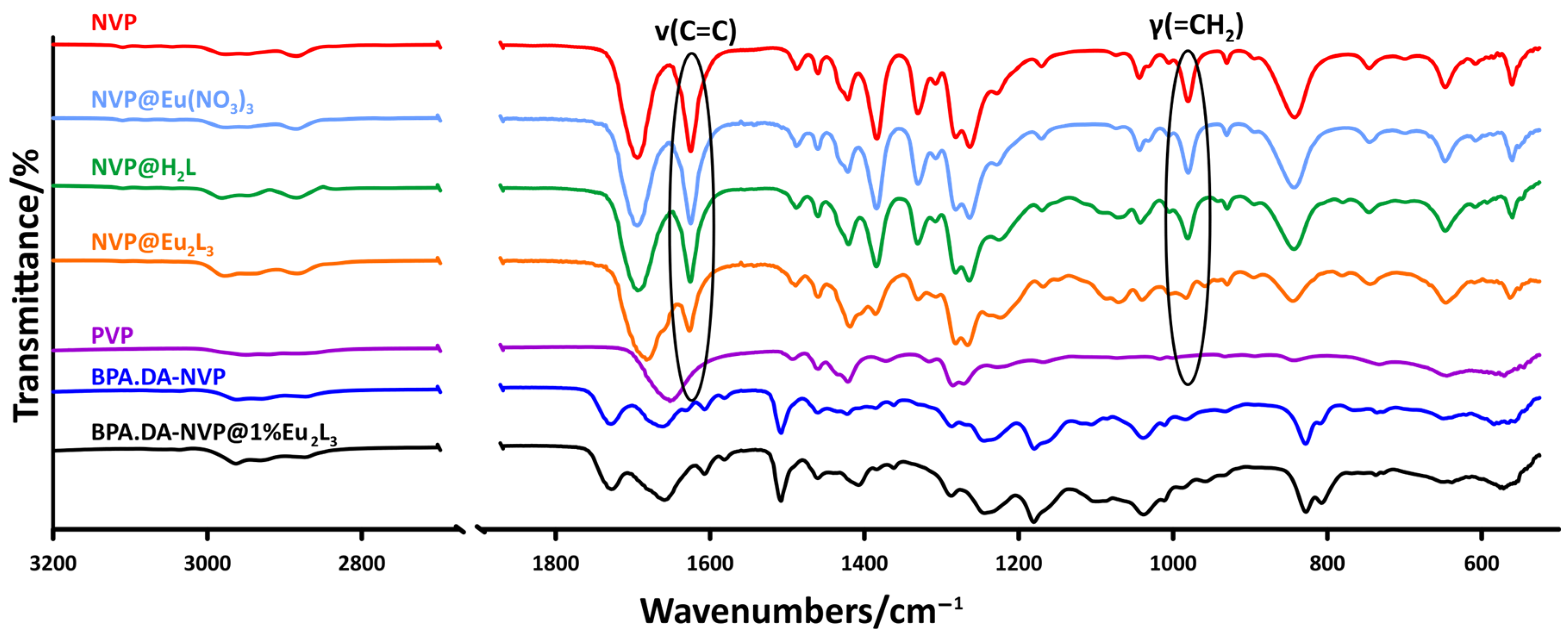
2.2. ATR/FTIR Analysis
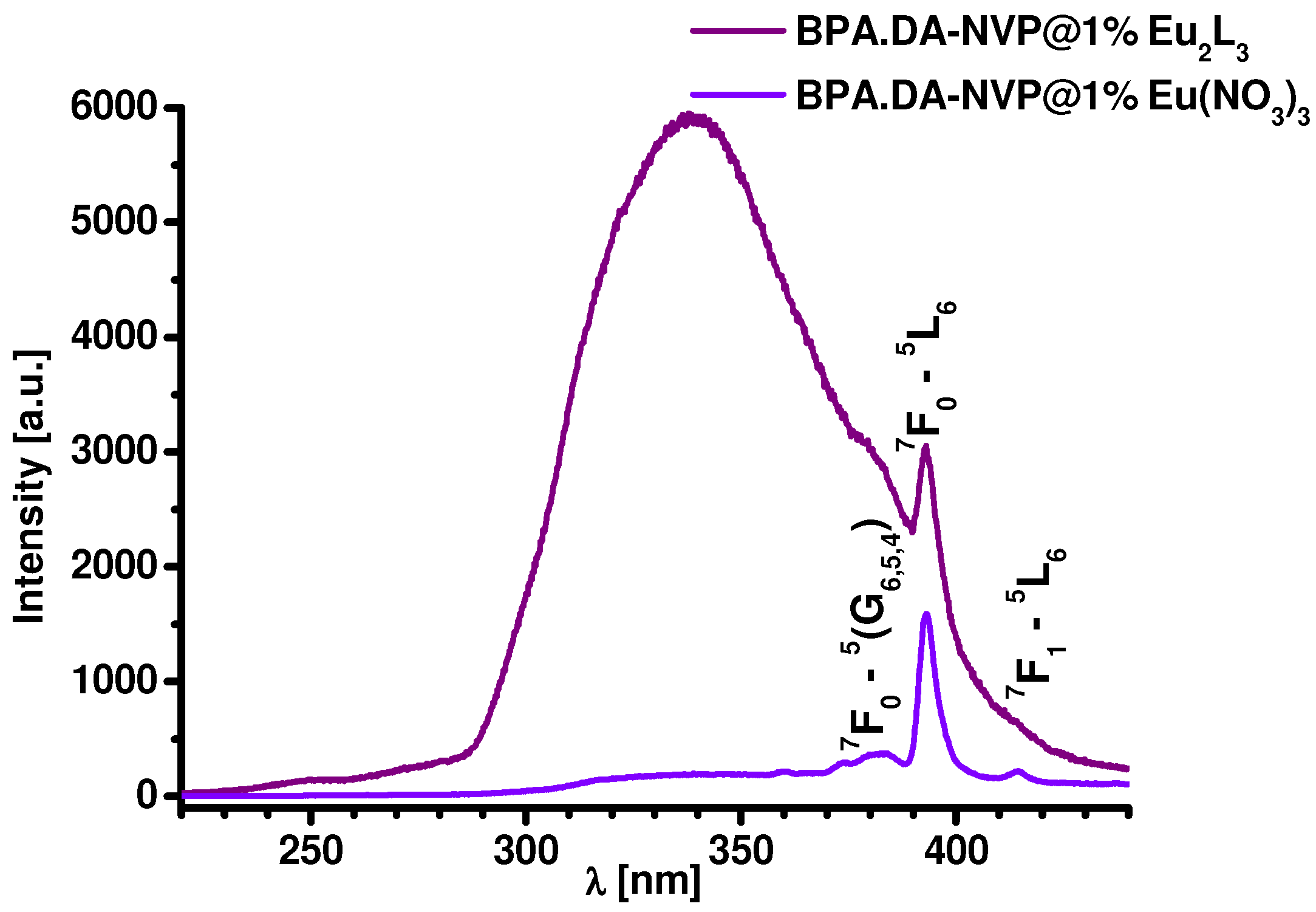
2.3. Luminescence Properties
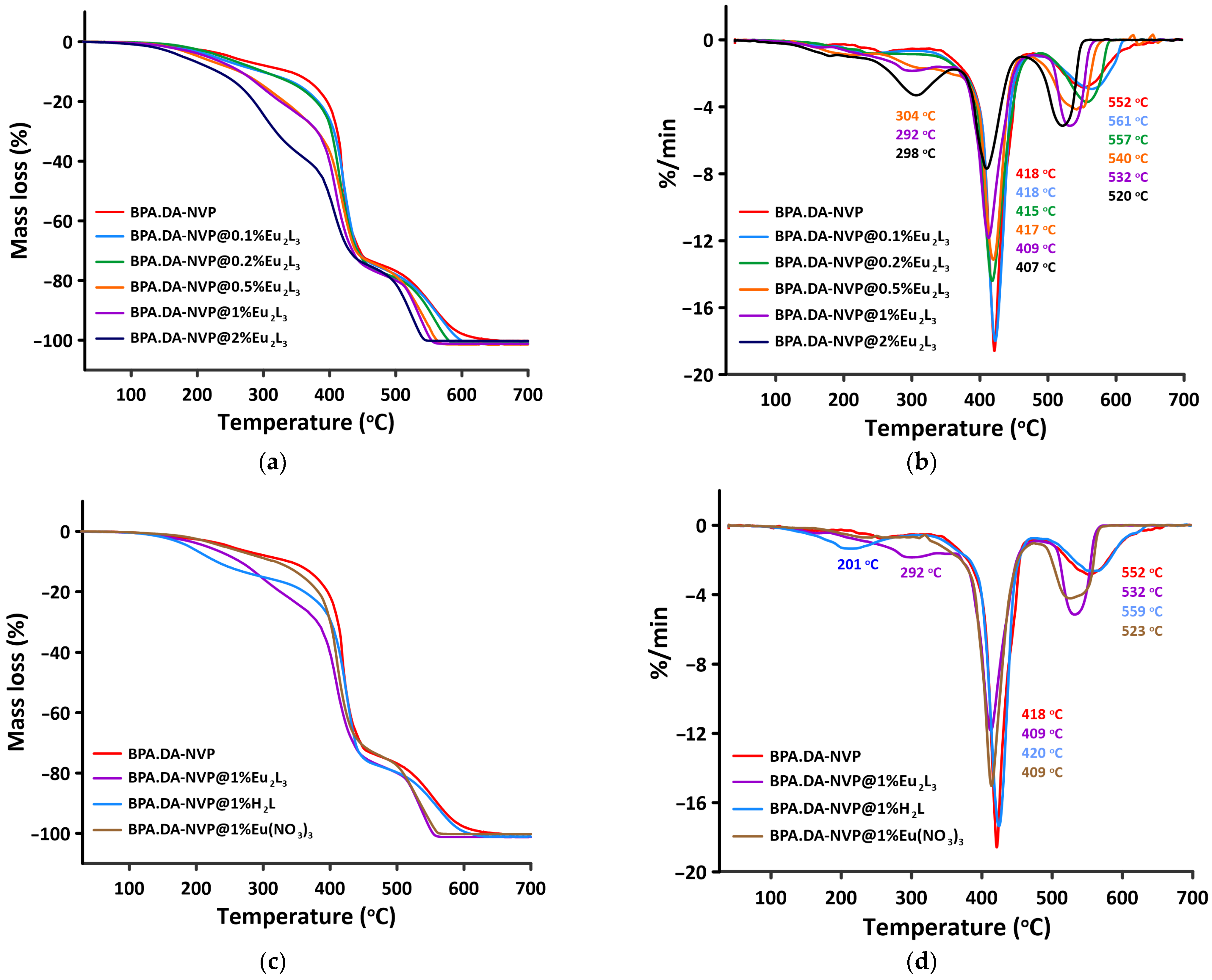
2.4. Thermal Analysis in Air Atmosphere
2.5. Thermal Analysis in Nitrogen
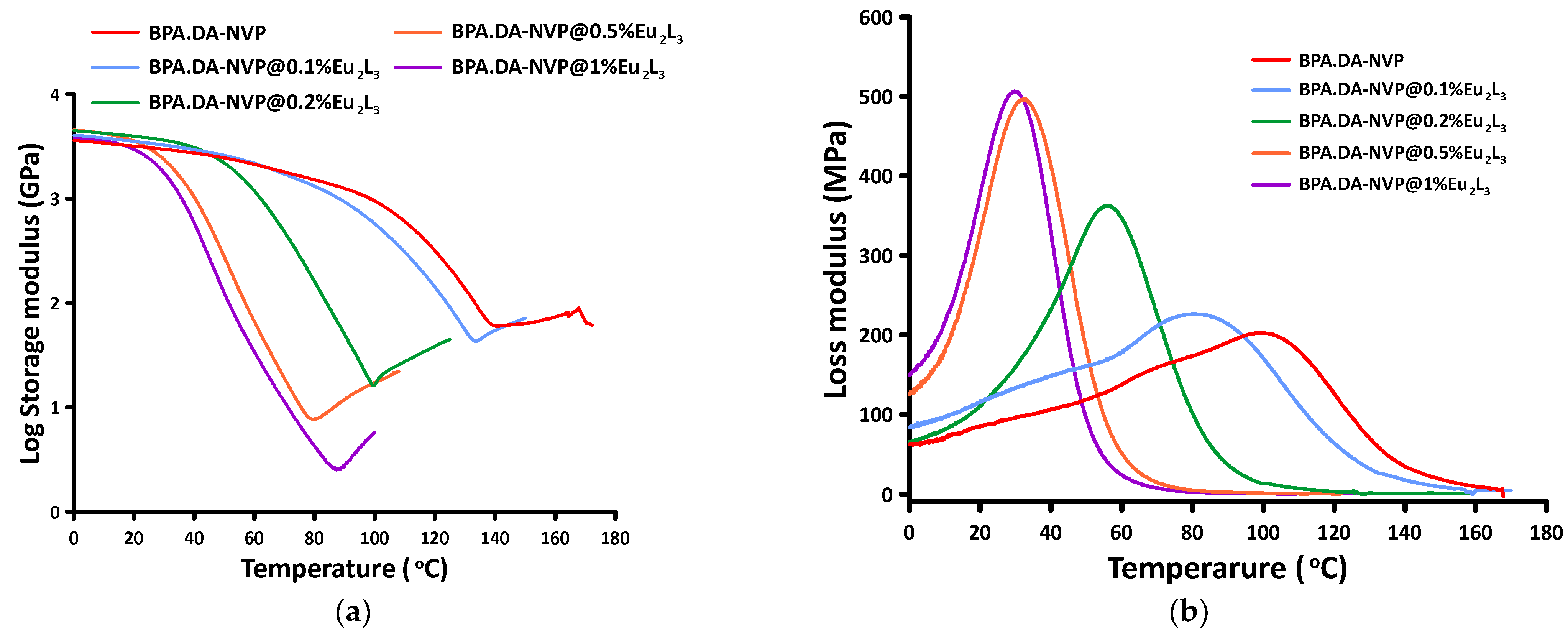
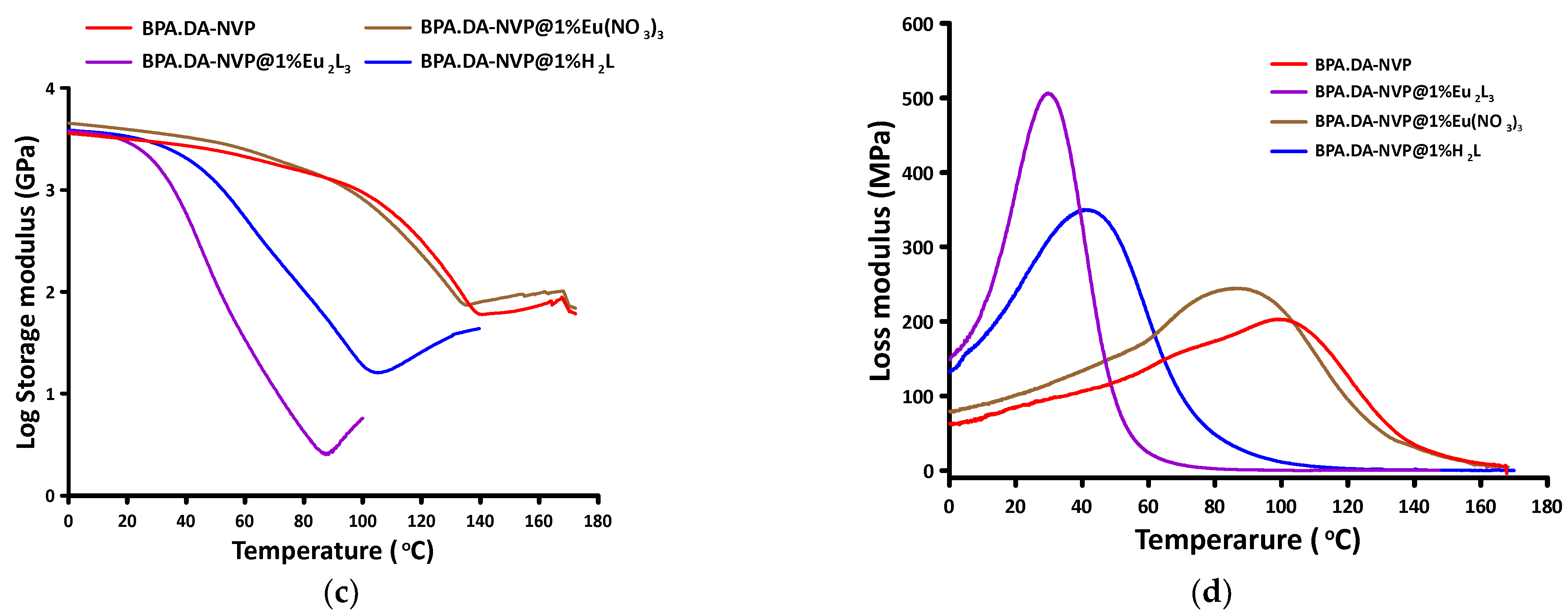
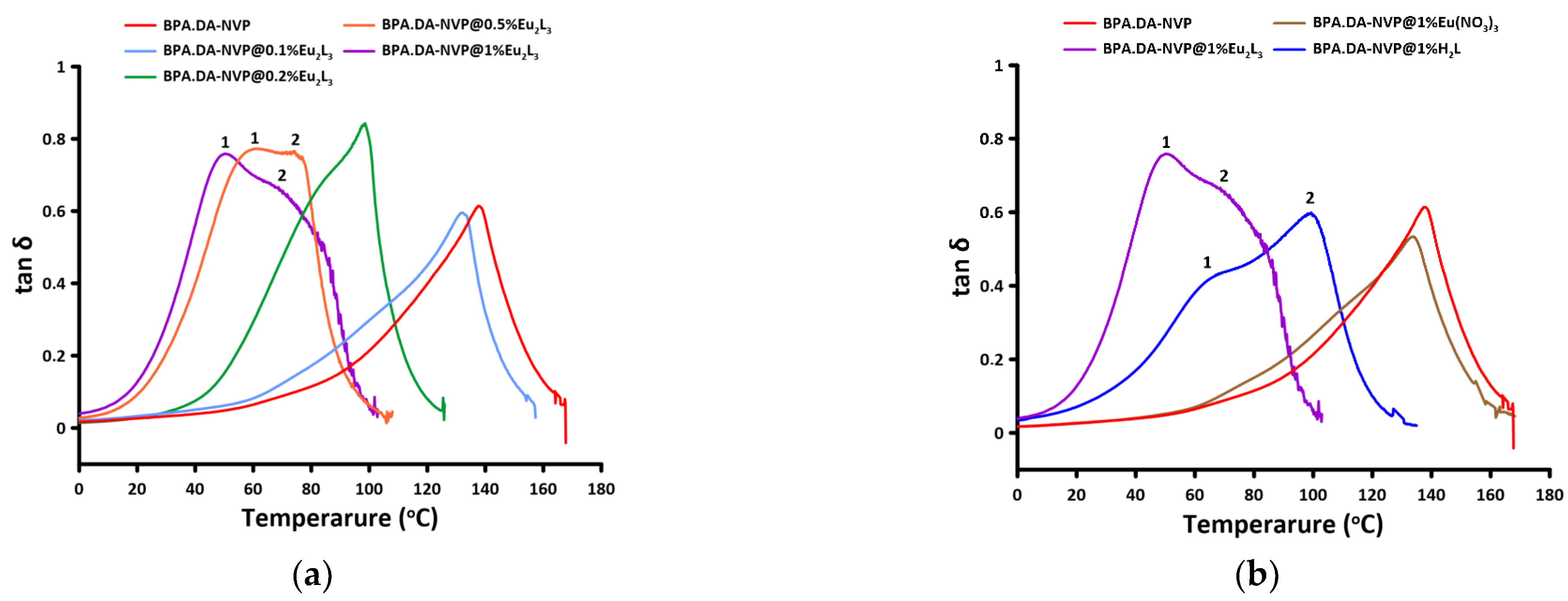
2.6. DMA Analysis
2.7. Flexural Test
2.8. Hardness Measurements
3. Materials and Methods
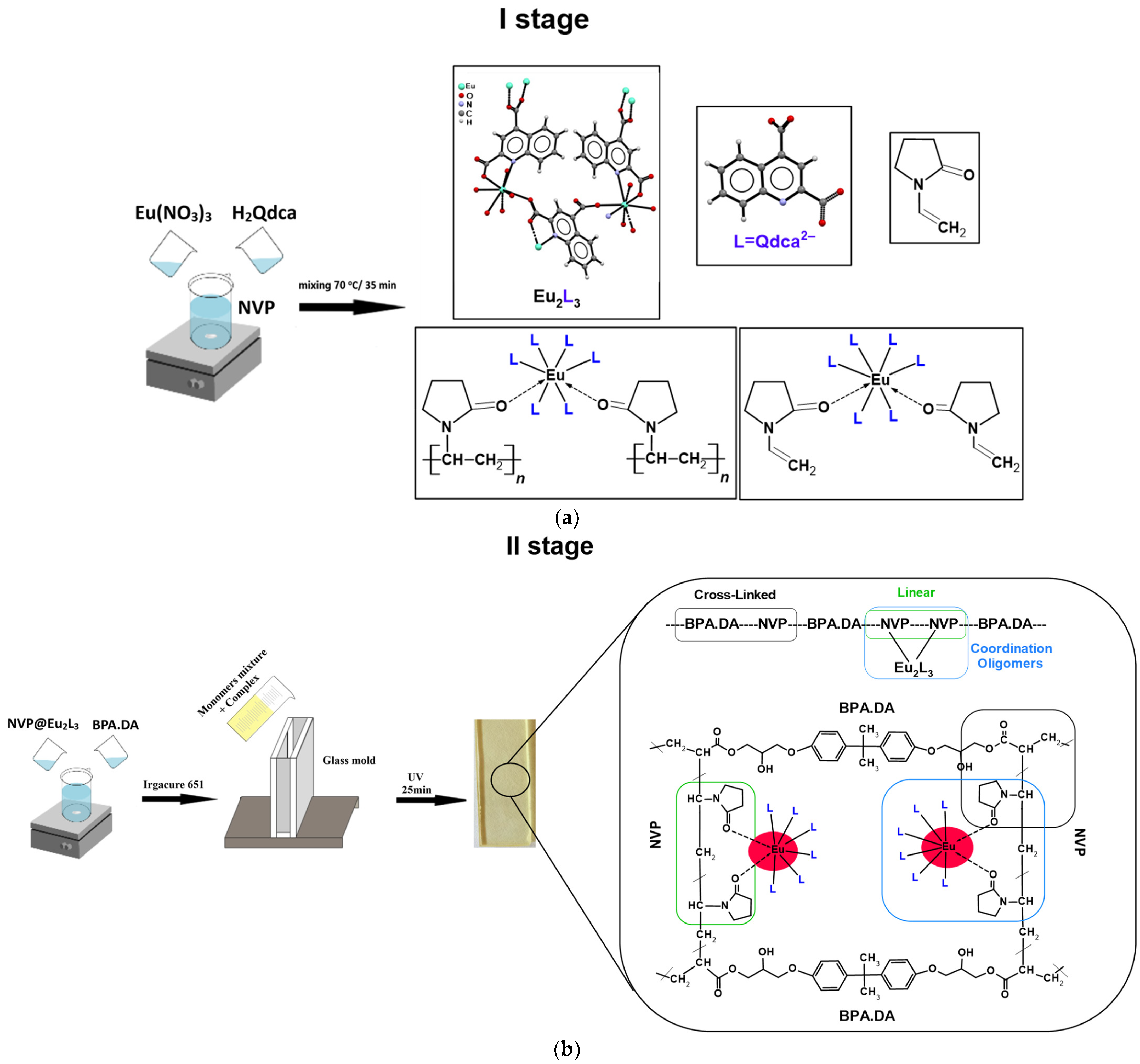
3.1. Synthesis of Hybrid Materials
3.2. Instrumentation and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Unterlass, M.M. Green Synthesis of Inorganic–Organic Hybrid Materials: State of the Art and Future Perspectives. Eur. J. Inorg. Chem. 2016, 2016, 1135–1156. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Tanaka, K.; Chujo, Y. Creative Synthesis of Organic–Inorganic Molecular Hybrid Materials. BCSJ 2017, 90, 463–474. [Google Scholar] [CrossRef]
- Bolbukh, Y.; Podkoscielna, B.; Lipke, A.; Bartnicki, A.; Gawdzik, B.; Tertykh, V. Immobilization of Polymeric Luminophor on Nanoparticles Surface. Nanoscale Res. Lett. 2016, 11, 206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Muntaser, A.A.; Pashameah, R.A.; Sharma, K.; Alzahrani, E.; Farea, M.O.; Morsi, M.A. α-MoO3 Nanobelts/CMC-PVA Nanocomposites: Hybrid Materials for Optoelectronic and Dielectric Applications. J. Polym. Res. 2022, 29, 274. [Google Scholar] [CrossRef]
- Ilyas, R.; Aisyah, H.; Nordin, A.; Ngadi, N.; Zuhri, M.; Asyraf, M.; Sapuan, S.; Zainudin, E.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef]
- Khdary, N.H.; Almuarqab, B.T.; El Enany, G. Nanoparticle-Embedded Polymers and Their Applications: A Review. Membranes 2023, 13, 537. [Google Scholar] [CrossRef]
- Al Rugaie, O.; Jabir, M.S.; Mohammed, M.K.A.; Abbas, R.H.; Ahmed, D.S.; Sulaiman, G.M.; Mohammed, S.A.A.; Khan, R.A.; Al-Regaiey, K.A.; Alsharidah, M.; et al. Modification of SWCNTs with Hybrid Materials ZnO–Ag and ZnO–Au for Enhancing Bactericidal Activity of Phagocytic Cells against Escherichia Coli through NOX2 Pathway. Sci. Rep. 2022, 12, 17203. [Google Scholar] [CrossRef]
- Khan, M.F.; Elahi, E.; Hassan, N.U.; Rehman, M.A.; Khalil, H.M.W.; Khan, M.A.; Rehman, S.; Hao, A.; Noh, H.; Khan, K.; et al. Bipolar Photoresponse of a Graphene Field-Effect Transistor Induced by Photochemical Reactions. ACS Appl. Electron. Mater. 2023, 5, 5111–5119. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in It, Their Composition and Applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef]
- Mastria, R.; Rizzo, A.; Giansante, C.; Ballarini, D.; Dominici, L.; Inganäs, O.; Gigli, G. Role of Polymer in Hybrid Polymer/PbS Quantum Dot Solar Cells. J. Phys. Chem. C 2015, 119, 14972–14979. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Wang, L.; Zhang, H.; Wang, Y.; Wang, Y. Organic/Inorganic Hybrid Sensors: A Review. Sens. Actuators B Chem. 2013, 182, 467–481. [Google Scholar] [CrossRef]
- Chujo, Y. Organic—Inorganic Hybrid Materials. Curr. Opin. Solid State Mater. Sci. 1996, 1, 806–811. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, G.; Ding, Y.; Zheng, J. Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide. Polymers 2018, 10, 716. [Google Scholar] [CrossRef]
- Aritake, Y.; Akitsu, T. The Role of Chiral Dopants in Organic/Inorganic Hybrid Materials Containing Chiral Schiff Base Ni(II), Cu(II) and Zn(II) Complexes. Polyhedron 2012, 31, 278–284. [Google Scholar] [CrossRef]
- Rehman, M.A.; Park, S.; Khan, M.F.; Bhopal, M.F.; Nazir, G.; Kim, M.; Farooq, A.; Ha, J.; Rehman, S.; Jun, S.C.; et al. Development of Directly Grown-graphene–Silicon Schottky Barrier Solar Cell Using Co-doping Technique. Int. J. Energy Res. 2022, 46, 11510–11522. [Google Scholar] [CrossRef]
- Hood, M.; Mari, M.; Muñoz-Espí, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid Organic-Inorganic Materials—In Search of Synergic Activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Tollan, C.; Yang, F.; Beltrán, M.; Qin, Y.; Knez, M. Conductive Polymer–Inorganic Hybrid Materials through Synergistic Mutual Doping of the Constituents. ACS Appl. Mater. Interfaces 2017, 9, 27964–27971. [Google Scholar] [CrossRef]
- Li, P.; Li, H. Recent Progress in the Lanthanide-Complexes Based Luminescent Hybrid Materials. Coord. Chem. Rev. 2021, 441, 213988. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Derkaoui, I.; Basyooni, M.A.; Achehboune, M.; Khenfouch, M.; Belaid, W.; Enculescu, M.; Matei, E. Reduced Graphene Oxide-Functionalized Zinc Oxide Nanorods as Promising Nanocomposites for White Light Emitting Diodes and Reliable UV Photodetection Devices. Mater. Chem. Phys. 2023, 306, 128063. [Google Scholar] [CrossRef]
- Majumdar, R.; Tantayanon, S. In-Situ Synthesis of Metal Nanoparticle Embedded Soft Hybrid Materials via Eco-Benign Approach. Pure Appl. Chem. 2022, 94, 999–1018. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid Materials for the Removal of Emerging Pollutants in Water: Classification, Synthesis, and Properties. Chem. Eng. J. Adv. 2022, 10, 100252. [Google Scholar] [CrossRef]
- Guo, Q.; Ghadiri, R.; Weigel, T.; Aumann, A.; Gurevich, E.; Esen, C.; Medenbach, O.; Cheng, W.; Chichkov, B.; Ostendorf, A. Comparison of in Situ and Ex Situ Methods for Synthesis of Two-Photon Polymerization Polymer Nanocomposites. Polymers 2014, 6, 2037–2050. [Google Scholar] [CrossRef]
- Morselli, D.; Bondioli, F.; Sangermano, M.; Messori, M. Epoxy Networks Reinforced with TiO2 Generated by Nonhydrolytic Sol-Gel Process: A Comparison between in Situ and Ex Situ Syntheses to Obtain Filled Polymers. Polym. Eng. Sci. 2015, 55, 1689–1697. [Google Scholar] [CrossRef]
- Adnan, M.; Dalod, A.; Balci, M.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef]
- Nimrodh Ananth, A.; Umapathy, S.; Sophia, J.; Mathavan, T.; Mangalaraj, D. On the Optical and Thermal Properties of in Situ/Ex Situ Reduced Ag NP’s/PVA Composites and Its Role as a Simple SPR-Based Protein Sensor. Appl. Nanosci. 2011, 1, 87–96. [Google Scholar] [CrossRef]
- Carraro, M.; Gross, S. Hybrid Materials Based on the Embedding of Organically Modified Transition Metal Oxoclusters or Polyoxometalates into Polymers for Functional Applications: A Review. Materials 2014, 7, 3956–3989. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current Issues in Research on Structure–Property Relationships in Polymer Nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Zhang, G.; Mei, L.; Ding, J.; Su, K.; Guo, Q.; Lv, G.; Liao, L. Recent Progress on Lanthanide Complexes/Clay Minerals Hybrid Luminescent Materials. J. Rare Earths 2022, 40, 1360–1370. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid Materials Based on Lanthanide Organic Complexes: A Review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Du, J.; Kou, J.; Zhang, Z.; Liu, F. Hierarchical Porous Cellulose/Lanthanide Hybrid Materials as Luminescent Sensor. J. Rare Earths 2018, 36, 1036–1043. [Google Scholar] [CrossRef]
- Tran, T.H.; Bentlage, M.; Lezhnina, M.M.; Kynast, U. Efficient Red Emission from Europium Chelate-Silicone Host-Guest Hybrids. Z. Für Naturforschung B 2014, 69, 210–216. [Google Scholar] [CrossRef]
- Liu, M.; Cai, Z.; Xu, Y.; Hu, J.; Sun, B. Europium(III)-Based Fluorescent Microspheres with Styrene Copolymerization toward an Enhanced Photoluminescence Performance. ACS Appl. Polym. Mater. 2022, 4, 8109–8117. [Google Scholar] [CrossRef]
- Singh, P.; Kachhap, S.; Singh, P.; Singh, S.K. Lanthanide-Based Hybrid Nanostructures: Classification, Synthesis, Optical Properties, and Multifunctional Applications. Coord. Chem. Rev. 2022, 472, 214795. [Google Scholar] [CrossRef]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Kumar, S.; Singh, D. Lanthanides β-Diketonate Complexes as Energy-Efficient Emissive Materials: A Review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Luminescence Behavior of PMMA Films Doped with Tb(III) and Eu(III) Complexes. J. Lumin. 2022, 242, 118609. [Google Scholar] [CrossRef]
- Dědek, I.; Kupka, V.; Jakubec, P.; Šedajová, V.; Jayaramulu, K.; Otyepka, M. Metal-Organic Framework/Conductive Polymer Hybrid Materials for Supercapacitors. Appl. Mater. Today 2022, 26, 101387. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Chen, C.; Ma, C.; Zhang, D.; Bo, S.; Zhen, Z. Optical Gain at 1535nm in LaF3: Er,Yb Nanoparticle-Doped Organic-Inorganic Hybrid Material Waveguide. Appl. Phys. Lett. 2007, 91, 161109. [Google Scholar] [CrossRef]
- Julián, B.; Corberán, R.; Cordoncillo, E.; Escribano, P.; Viana, B.; Sanchez, C. Synthesis and Optical Properties of Eu 3+ -Doped Inorganic–Organic Hybrid Materials Based on Siloxane Networks. J. Mater. Chem. 2004, 14, 3337–3343. [Google Scholar] [CrossRef]
- Julian, B.; Beltrán, H.; Cordoncillo, E.; Escribano, P.; Viana, B.; Sanchez, C. Influence of the Matrix in the Optical Response of Organic-Inorganic Hybrid Materials Doped with Europium(III). J. Sol-Gel Sci. Technol. 2003, 26, 977–980. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent Metal-Organic Frameworks and Their Composites: Potential Future Materials for Organic Light Emitting Displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.-Y. Photoluminescent Metal–Organic Frameworks and Their Application for Sensing Biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Pang, X.; Li, L.; Wei, Y.; Yu, X.; Li, Y. Novel Luminescent Lanthanide(III) Hybrid Materials: Fluorescence Sensing of Fluoride Ions and N, N-Dimethylformamide. Dalton Trans. 2018, 47, 11530–11538. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, E.B.; Kai, J.; Teotonio, E.E.S.; Malta, O.L.; Felinto, M.C.F.C.; Brito, H.F. Photoluminescent PMMA Polymer Films Doped with Eu3+-β-Diketonate Crown Ether Complex. J. Photochem. Photobiol. A Chem. 2013, 251, 154–159. [Google Scholar] [CrossRef]
- Łyszczek, R.; Vlasyuk, D.; Podkościelna, B.; Głuchowska, H.; Piramidowicz, R.; Jusza, A. A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates. Materials 2022, 15, 8826. [Google Scholar] [CrossRef]
- Jena, K.K.; Narayan, R.; Raju, K.V.S.N. Surface Functionalized Zinc Oxide (ZnO) Nanoparticle Filled Organic–Inorganic Hybrid Materials with Enhanced Thermo-Mechanical Properties. Prog. Org. Coat. 2015, 89, 82–90. [Google Scholar] [CrossRef]
- Qi, W.; Wu, L. Polyoxometalate/Polymer Hybrid Materials: Fabrication and Properties: Polyoxometalate/Polymer Hybrid Materials. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Puszka, A.; Podkościelna, B. Special Issue: Synthesis, Processing, Structure and Properties of Polymer Materials. Polymers 2022, 14, 4550. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Vlasyuk, D.; Łyszczek, R.; Mazur, L.; Pladzyk, A.; Hnatejko, Z.; Woźny, P. A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-Dicarboxylate Building Block and Lanthanide(III) Ions—Temperature Dependence Investigations. Molecules 2023, 28, 6360. [Google Scholar] [CrossRef]
- Song, Y.-J.; Wang, M.; Zhang, X.-Y.; Wu, J.-Y.; Zhang, T. Investigation on the Role of the Molecular Weight of Polyvinyl Pyrrolidone in the Shape Control of High-Yield Silver Nanospheres and Nanowires. Nanoscale Res. Lett. 2014, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Anasuya, K.V.; Veeraiah, M.K.; Hemalatha, P.; Manju, M. Synthesis and Characterisation of Poly (Vinylpyrrolidone)—Nickel (II) Complexes. J. Appl. Chem. 2014, 7, 61–66. [Google Scholar] [CrossRef]
- Száraz, I.; Forsling, W. A Spectroscopic Study of the Solvation of 1-Vinyl-2-Pyrrolidone and Poly(1-Vinyl-2-Pyrrolidone) in Different Solvents. Polymer 2000, 41, 4831–4839. [Google Scholar] [CrossRef]
- Borodko, Y.; Sook Lee, H.; Hoon Joo, S.; Zhang, Y.; Somorjai, G. Spectroscopic Study of the Thermal Degradation of PVP-Capped Rh and Pt Nanoparticles in H2 and O2 Environments. J. Phys. Chem. C 2010, 114, 1117–1126. [Google Scholar] [CrossRef][Green Version]
- Wong, K.-L.; Bünzli, J.-C.G.; Tanner, P.A. Quantum Yield and Brightness. J. Lumin. 2020, 224, 117256. [Google Scholar] [CrossRef]
- Woźny, P.; Soler-Carracedo, K.; Stopikowska, N.; Martín, I.R.; Runowski, M. Structure-Dependent Luminescence of Eu3+-Doped Strontium Vanadates Synthesized with Different V: Sr Ratios—Application in WLEDs and Ultra-Sensitive Optical Thermometry. J. Mater. Chem. C 2023, 11, 4792–4807. [Google Scholar] [CrossRef]
- Cuan, J.; Yan, B. Cool-White Light Emitting Hybrid Materials of a Resin–Mesoporous Silica Composite Matrix Encapsulating Europium Polyoxometalates through an Ionic Liquid Linker. RSC Adv. 2013, 3, 20077. [Google Scholar] [CrossRef]
- Huang, X.; Zucchi, G.; Tran, J.; Pansu, R.B.; Brosseau, A.; Geffroy, B.; Nief, F. Visible-Emitting Hybrid Sol–Gel Materials Comprising Lanthanide Ions: Thin Film Behaviour and Potential Use as Phosphors for Solid-State Lighting. New J. Chem. 2014, 38, 5793–5800. [Google Scholar] [CrossRef]
- Hao, J.-N.; Yan, B. Photofunctional Host–Guest Hybrid Materials and Thin Films of Lanthanide Complexes Covalently Linked to Functionalized Zeolite A. Dalton Trans. 2014, 43, 2810–2818. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, H.; Yu, X.; Wang, T.; Geng, L.; Wang, Y.; Li, Y. Synthesis and Characterization of Novel Luminescent Europium(iii) Hybrid Materials with a Host Based on Titania–Mesoporous Silica or Alumina–Mesoporous Silica. RSC Adv. 2015, 5, 84790–84796. [Google Scholar] [CrossRef]
- Kłonkowski, A.M.; Szałkowska, I.; Pietraszkiewicz, M.; Hnatejko, Z.; Lis, S.; Klukowska, A.; Posset, U. Influence of Xerogel Matrices and Co-Ligands on Luminescence Parameters in Materials with an Europium(III) Cryptate. J. Non-Cryst. Solids 2005, 351, 2047–2056. [Google Scholar] [CrossRef]
- Kayaman-Apohan, N.; Demirci, R.; Cakir, M.; Gungor, A. UV-Curable Interpenetrating Polymer Networks Based on Acrylate/Vinylether Functionalized Urethane Oligomers. Radiat. Phys. Chem. 2005, 73, 254–262. [Google Scholar] [CrossRef]
- Loría-Bastarrachea, M.I.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Vázquez-Torres, H.; Ávila-Ortega, A. A TG/FTIR Study on the Thermal Degradation of Poly(Vinyl Pyrrolidone). J. Therm. Anal. Calorim. 2011, 104, 737–742. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Using TGA/FTIR TGA/MS and Cone Calorimetry to Understand Thermal Degradation and Flame Retardancy Mechanism of Polycarbonate Filled with Solid Bisphenol A Bis(Diphenyl Phosphate) and Montmorillonite. Polym. Degrad. Stab. 2012, 97, 605–614. [Google Scholar] [CrossRef]
- Puszka, A. Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]





| Sample/% | 0.1% | 0.2% | 0.5% | 1% | 2% |
|---|---|---|---|---|---|
| Lifetime τ/μs | τ1 = 78.1 ± 0.69 τ2 = 447.3 ± 0.84 | τ1 = 93.42 ± 0.58 τ2 = 460.72 ± 0.78 | τ1 = 119.29 ± 0.62 τ2 = 605.69 ± 1.56 | τ1 = 143.57 ± 0.85 τ2 = 670.30 ± 1.95 | τ1 = 147.71 ± 1.06 τ2 = 535.87 ± 1.38 |
| 5.20 | 5.07 | 4.86 | 4.71 | 4.50 | |
| ϕ/% | 14.0 | 11.8 | 11.8 | 9.0 | 9.3 |
| Materials | Stage I | Stage II | Stage III | |||
|---|---|---|---|---|---|---|
| Temperature Range (°C) | Mass Loss (%) | Temperature Range (°C) | Mass Loss (%) | Temperature Range (°C) | Mass Loss (%) | |
| BPA.DA-NVP | 105–323 | 8.7 | 323–458 | 63.9 | 458–645 | 26.8 |
| BPA.DA-NVP@0.1%Eu2L3 | 117–321 | 10.9 | 321–459 | 62.2 | 459–610 | 27.1 |
| BPA.DA-NVP@0.2%Eu2L3 | 144–262 | 6.3 | 262–472 | 69.8 | 472–585 | 24.1 |
| BPA.DA-NVP@0.5%Eu2L3 | 122–256 | 8.8 | 256–445 | 62.2 | 445–563 | 29 |
| BPA.DA-NVP@1%Eu2L3 | 115–345 | 23.2 | 345–474 | 54.1 | 474–553 | 22.4 |
| BPA.DA-NVP@2%Eu2L3 | 90–355 | 37.8 | 355–453 | 36.2 | 453–545 | 25.8 |
| BPA.DA-NVP@1%Eu(NO3)3 | 128–334 | 10.8 | 334–468 | 61.9 | 468–564 | 27.3 |
| BPA.DA-NVP@1%H2L | 115–310 | 14.9 | 310–467 | 61.4 | 467–627 | 23.7 |
| Mass Loss (%) | Temperature (°C) for Materials BPA.DA-NVP with Different Content of Metal Complex and Substrates in Air/Nitrogen Atmosphere | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% Eu2L3 | 0.1% Eu2L3 | 0.2% Eu2L3 | 0.5% Eu2L3 | 1% Eu2L3 | 2% Eu2L3 | 1% Eu(NO3)3 | 1% H2L | |
| 1 | 144/106 | 137/145 | 165/164 | 140/155 | 137/145 | 112/123 | 153/161 | 126/140 |
| 5 | 251/223 | 227/238 | 238/239 | 203/226 | 216/211 | 179/179 | 240/246 | 189/200 |
| 20 | 396/398 | 384/391 | 379/384 | 331/356 | 325/321 | 285/278 | 382/385 | 361/376 |
| 50 | 422/430 | 423/420 | 418/420 | 416/413 | 409/406 | 397/392 | 415/409 | 421/420 |
| Name of Sample | E’onset (°C) | E’20 (GPa) | E’’max (°C) | tan δmax (°C) | tan δmax | FWHM (°C) |
|---|---|---|---|---|---|---|
| BPA.DA-NVP | 99.87 | 3.18 | 99.79 | 137.6 | 0.548 | 34.28 |
| BPA.DA-NVP@0.1%Eu2L3 | 89.36 | 3.52 | 80.55 | 132.21 | 0.515 | 35.28 |
| BPA.DA-NVP@0.2%Eu2L3 | 52.73 | 3.95 | 55.92 | 98.57 | 0.800 | 36.84 |
| BPA.DA-NVP@0.5%Eu2L3 | 31.45 | 3.56 | 32.73 | 61.09 1 73.98 2 | 0.748 1 0.763 2 | 43.68 |
| BPA.DA-NVP @1%Eu2L3 | 28.60 | 2.96 | 29.89 | 50.11 1 70.74 2 | 0.705 1 0.648 2 | 52.09 |
| BPA.DA-NVP@1%Eu(NO3)3 | 85.58 | 3.94 | 86.51 | 134.05 | 0.492 | 42.24 |
| BPA.DA-NVP@1%H2L | 38.88 | 3.37 | 41.46 | 70.21 1 99.44 2 | 0.437 1 0.578 2 | 56.30 |
| Sample | Stress at Break (MPa) | Relative Elongate at Break (%) | Young’s Modulus (GPa) |
|---|---|---|---|
| Avg | Avg | Avg | |
| BPA.DA-NVP | 111.5 ± 0.5 | 3.48 ± 0.05 | 3.66 ± 0.08 |
| BPA.DA-NVP@0.1%Eu2L3 | 104.8 ± 4.8 | 3.05 ± 0.14 | 3.79 ± 0.09 |
| BPA.DA-NVP@0.2%Eu2L3 | 107.76 ± 4.47 | 3.34 ± 0.36 | 3.90 ± 0.29 |
| BPA.DA-NVP@0.5%Eu2L3 | 75.77 ± 4.91 | 3.95 ± 0.40 | 2.98 ± 0.18 |
| BPA.DA-NVP@1%Eu2L3 | 14.55 ± 0.26 | >7 * ± 1.0 | 0.34 ± 0.02 |
| BPA.DA-NVP@1%Eu(NO3)3 | 97.17 ± 1.52 | 2.67 ± 0.22 | 3.81 ± 0.08 |
| BPA.DA-NVP@1%H2L | 61.31 ± 3.92 | 3.75 ± 0.34 | 2.48 ± 0.30 |
| Sample | Hardness (°Sh) (D Scale) | |
|---|---|---|
| Samples before DMA | Samples after DMA | |
| BPA.DA-NVP | 81.7 ± 1.1 | 83.0 ± 1.1 |
| BPA.DA-NVP@0.1%Eu2L3 | 81.0 ± 0.4 | 83.2 ± 1.5 |
| BPA.DA-NVP@0.2%Eu2L3 | 80.7 ± 1.3 | 80.7 ± 1.0 |
| BPA.DA-NVP@0.5%Eu2L3 | 59.5 ± 1.5 | 59.2 ± 1.5 |
| BPA.DA-NVP@1%Eu2L3 | 42.0 ± 1.5 | 44.2 ± 0.8 |
| BPA.DA-NVP@2%Eu2L3 | 19.8 ± 1.9 | - * |
| BPA.DA-NVP@1%Eu(NO3)3 | 81.0 ± 0.3 | 84.2 ± 1.5 |
| BPA.DA-NVP@1%H2L | 77.8 ± 0.7 | 76.0 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasyuk, D.; Łyszczek, R.; Podkościelna, B.; Puszka, A.; Hnatejko, Z.; Stankevič, M.; Głuchowska, H. Luminescent Hybrid BPA.DA-NVP@Eu2L3 Materials: In Situ Synthesis, Spectroscopic, Thermal, and Mechanical Characterization. Materials 2023, 16, 6509. https://doi.org/10.3390/ma16196509
Vlasyuk D, Łyszczek R, Podkościelna B, Puszka A, Hnatejko Z, Stankevič M, Głuchowska H. Luminescent Hybrid BPA.DA-NVP@Eu2L3 Materials: In Situ Synthesis, Spectroscopic, Thermal, and Mechanical Characterization. Materials. 2023; 16(19):6509. https://doi.org/10.3390/ma16196509
Chicago/Turabian StyleVlasyuk, Dmytro, Renata Łyszczek, Beata Podkościelna, Andrzej Puszka, Zbigniew Hnatejko, Marek Stankevič, and Halina Głuchowska. 2023. "Luminescent Hybrid BPA.DA-NVP@Eu2L3 Materials: In Situ Synthesis, Spectroscopic, Thermal, and Mechanical Characterization" Materials 16, no. 19: 6509. https://doi.org/10.3390/ma16196509
APA StyleVlasyuk, D., Łyszczek, R., Podkościelna, B., Puszka, A., Hnatejko, Z., Stankevič, M., & Głuchowska, H. (2023). Luminescent Hybrid BPA.DA-NVP@Eu2L3 Materials: In Situ Synthesis, Spectroscopic, Thermal, and Mechanical Characterization. Materials, 16(19), 6509. https://doi.org/10.3390/ma16196509








