Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy
Abstract
:1. Introduction
2. Importance of New Drugs Development in Rare Diseases Field
3. Development of Novel Drug Delivery Systems
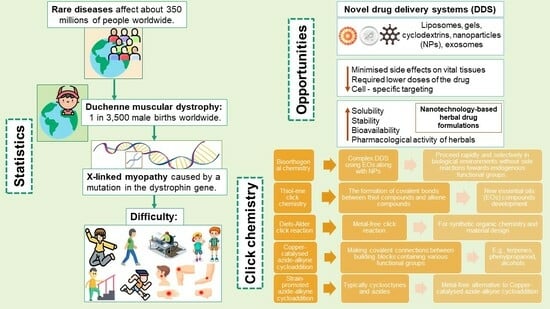
- Minimized side effects on vital tissues because of their ability to be specifically transported to the place of action [40].
- Opportunity to require lower doses of the drug because the accumulation of therapeutic compounds in the target site increases [40].
- Cell-specific targeting is achieved by attaching drugs to designed carriers (nanoparticles, liposomes, etc.). [40]
3.1. Importance of Novel Drug Delivery Systems Development for Herbal Medicine
3.1.1. Solubility and Delivery of EOs in Blood
3.1.2. EOs Constituents with NPs for Drug Delivery
3.2. Application of Click Chemistry for DMD and EOs Molecule Development
3.2.1. Polymer-Based Drug Delivery Systems
- Hydrogels. These are three-dimensional networks of polymers that can absorb water and swell, making them useful for delivering drugs in a controlled manner. Stimuli-responsive hydrogels, also called “smart hydrogels,” are attractive for modern drug formulations because they can release drugs in response to external stimuli [88,89].
- Polymeric Micelles: Polymeric micelles are nano-sized assemblies formed by amphiphilic block copolymers in dilute solution when the concentration of a polymer is increased. These self-assembling polymers can be used to encapsulate hydrophobic drugs and control their release profile in response to stimuli such as pH, enzymes, and temperature [90,91].
- Polymer conjugates: Polymer conjugates are a type of drug delivery system that involves the use of polymers to facilitate the controlled release of therapeutic agents [80,92]. Polymeric drug delivery systems have been developed using both natural and synthetic polymers. Natural polymers such as arginine, chitosan, dextrin, polysaccharides, poly (glycolic acid), poly (lactic acid), and hyaluronic acid have been used in these systems [93]. Synthetic polymers such as dendrimers and dendron-polymer conjugates have also been used to create nanosized drug delivery vehicles [94].
3.2.2. Thiol-Ene Click Chemistry
3.2.3. Diels-Alder Click (DA) Reaction
3.2.4. Copper-Catalysed Azide-Alkyne Cycloaddition (CuAAC)
- Terpenes: EOs such as limonene, pinene, and myrcene, which are rich in terpenes, have been used as the starting materials for the azide component of the reaction. Terpenes are a large and diverse class of natural products that are widely distributed in the plant kingdom [133]. One way that terpenes have been used in the CuAAC reaction is by derivatizing them with an azide-containing compound. This can be conducted by reacting the terpene with a reagent such as sodium azide or an azide-containing linker molecule. The resulting azide-derivatized terpene can then be reacted with an alkyne-containing compound in the presence of a copper catalyst to form the 1,4-disubstituted 1,2,3-triazole product [134]. Another way that terpenes have been used in the CuAAC reaction is by incorporating them directly into the alkyne component of the reaction. This can be conducted by synthesizing terpene-containing alkyne compounds, such as those with a terminal alkyne group, and then reacting these compounds with an azide-containing compound in the presence of a copper catalyst [124].
- Phenylpropanoids: EOs such as eugenol and thymol, which are rich in phenylpropanoids, have also been used as starting materials for the azide component of the reaction. Phenylpropanoids are a large class of naturally occurring compounds that are derived from phenylalanine and have a wide range of biological activities [135]. The CuAAC reaction is a widely used method for the efficient and selective formation of 1,4-disubstituted 1,2,3-triazoles. In the case of phenylpropanoids, researchers use this reaction to couple azide-functionalized phenylpropanoids with alkyne-functionalized phenylpropanoids, resulting in the formation of a 1,4-disubstituted 1,2,3-triazole product. This reaction typically requires the use of a copper (I) catalyst, such as copper (I) chloride, and is typically carried out in the presence of a ligand, such as 1,10-phenanthroline, to optimize the reaction conditions. The reaction is typically carried out in a polar solvent, such as dimethylformamide or dimethyl sulfoxide, at room temperature or slightly elevated temperatures [136,137].
- Alcohols: EOs such as linalool, geraniol, and citronellol, which are rich in alcohols, have also been used as starting materials for the azide component of the reaction. These EOs are known for their pleasant fragrances and are widely used in the perfumery and cosmetic industries [138]. The functionalization of these alcohol-rich EOs can be carried out by various methods, such as through the use of different reagents. For example, to functionalize linalool with an azide group, researchers can use N-hydroxysuccinimide and aryl azides or alkyl azides to introduce the azide group. Similarly, to functionalize geraniol with the alkyne group, researchers can use terminal alkynes and palladium catalysts to introduce the alkyne group. These functionalized EOs can be used in CuAAC reactions with other functionalized EOs or other molecules to produce a range of products, such as 1,4-disubstituted 1,2,3-triazoles [134]. It is important to note that reaction conditions such as solvent, temperature, and the concentration of reactants and catalysts play a crucial role in the efficiency of the reaction. Researchers often optimize these conditions to achieve high yields and selectivity for their desired products [134].
3.2.5. Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duchenne Muscular Dystrophy. National Organization for Rare Disorders. Available online: https://rarediseases.org/rare-diseases/duchenne-muscular-dystrophy/ (accessed on 24 January 2023).
- Aartsma-Rus, A.; Ginjaar, I.B.; Bushby, K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J. Med. Genet. 2016, 53, 145–151. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; Campelj, D.G.; Hafner, P.; Gueven, N.; Fischer, D.; Rybalka, E. Standard of care versus new-wave corticosteroids in the treatment of Duchenne muscular dystrophy: Can we do better? Orphanet J. Rare Dis. 2021, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kong, X. Recent advances of glucocorticoids in the treatment of Duchenne muscular dystrophy (Review). Exp. Ther. Med. 2021, 21, 447. [Google Scholar] [CrossRef]
- De Souza, P.A.T.; Marques, M.J.; Lima, C.A.H.; Matheus, S.M.M. Effect of Citrus Aurantium L. Essential Oil on Muscle Regeneration in Mdx Mice. Int. J. Morphol. 2011, 29, 1357–1363. [Google Scholar] [CrossRef]
- Yamamoto, T.; Awano, H.; Zhang, Z.; Sakuma, M.; Kitaaki, S.; Matsumoto, M.; Nagai, M.; Sato, I.; Imanishi, T.; Hayashi, N.; et al. Cardiac Dysfunction in Duchenne Muscular Dystrophy Is Less Frequent in Patients with Mutations in the Dystrophin Dp116 Coding Region Than in Other Regions. Circulation. Genom. Precis. Med. 2018, 11, e001782. [Google Scholar] [CrossRef] [PubMed]
- Samdup, D.Z.; Smith, R.G.; Song, S., II. The Use of Complementary and Alternative Medicine in Children with Chronic Medical Conditions. Am. J. Phys. Med. Rehabil. 2006, 85, 842–846. [Google Scholar] [CrossRef]
- Woodman, K.G.; Coles, C.A.; Lamandé, S.R.; White, J.D. Nutraceuticals and Their Potential to Treat Duchenne Muscular Dystrophy: Separating the Credible from the Conjecture. Nutrients 2016, 8, 713. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, X.; Luo, H. The clinical effects of combining acupuncture and physical therapy with drugs in treating children with Duchenne muscular dystrophy. Chin. J. Phys. Med. Rehabil. 2015, 12, 446–448. [Google Scholar]
- Importance of Research into Rare Disease. Available online: https://www.news-medical.net/health/Importance-of-Research-into-Rare-Disease.aspx (accessed on 24 January 2023).
- Chung, C.C.Y.; Hong Kong Genome Project; Chu, A.T.W.; Chung, B.H.Y. Rare Disease Emerging as a Global Public Health Priority. Front. Public Health 2022, 10, 1028545. [Google Scholar] [CrossRef]
- Orphanet: About Rare Diseases. Available online: https://www.orpha.net/consor/cgi-bin/Education_AboutRareDiseases.php?lng=EN;%20https://www.mdpi.com/1660-4601/18/3/1022 (accessed on 24 January 2023).
- Smith, C.I.E.; Bergman, P.; Hagey, D.W. Estimating the number of diseases–the concept of rare, ultra-rare, and hyper-rare. iScience 2022, 25, 104698. [Google Scholar] [CrossRef]
- Nguengang Wakap, S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating Cumulative Point Prevalence of Rare Diseases: Analysis of the Orphanet Database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef]
- Garrison, S.; Kennedy, A.; Manetto, N.; Pariser, A.R.; Rutter, J.L.; Yang, G. The Economic Burden of Rare Diseases: Quantifying the Sizeable Collective Burden and Offering Solutions. Health Aff. Forefront 2022, 1, 987667. [Google Scholar] [CrossRef]
- Australian Government; Department of Health and Aged Care. What We’re Doing About Rare Diseases. Australian Government Department of Health and Aged Care. Available online: https://www.health.gov.au/topics/chronic-conditions/what-were-doing-about-chronic-conditions/what-were-doing-about-rare-diseases (accessed on 24 January 2023).
- Rare Diseases. Available online: https://health.ec.europa.eu/non-communicable-diseases/steering-group/rare-diseases_en (accessed on 24 January 2023).
- Lumaka, A.; Carstens, N.; Devriendt, K.; Krause, A.; Kulohoma, B.; Kumuthini, J.; Mubungu, G.; Mukisa, J.; Nel, M.; Olanrewaju, T.O.; et al. Increasing African Genomic Data Generation and Sharing to Resolve Rare and Undiagnosed Diseases in Africa: A Call-to-Action by the H3Africa Rare Diseases Working Group. Orphanet J. Rare Dis. 2022, 17, 230. [Google Scholar] [CrossRef]
- Rare Diseases in the Americas. Wilson Center. Available online: https://www.wilsoncenter.org/article/infographic-rare-diseases-americas (accessed on 24 January 2023).
- Suffering in Silence: Rare Disease Patients in Asia-Pacific. Available online: https://www.cslbehring.com/vita/2020/suffering-in-silence-rare-disease-patients-in-asiapacific (accessed on 24 January 2023).
- Government of Canada. Canadians affected by rare diseases and disorders: Improving access to treatment. Available online: https://publications.gc.ca/site/eng/9.869064/publication.html (accessed on 24 January 2023).
- Bruckner-Tuderman, L. Epidemiology of Rare Diseases Is Important. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 783–784. [Google Scholar] [CrossRef]
- Kanagawa, M.; Toda, T. The Genetic and Molecular Basis of Muscular Dystrophy: Roles of Cell–Matrix Linkage in the Pathogenesis. J. Hum. Genet. 2006, 51, 915–926. [Google Scholar] [CrossRef]
- Sun, C.; Shen, L.; Zhang, Z.; Xie, X. Therapeutic Strategies for Duchenne Muscular Dystrophy: An Update. Genes 2020, 11, 837. [Google Scholar] [CrossRef]
- Nowak, K.J.; Davies, K.E. Duchenne muscular dystrophy and dystrophin: Pathogenesis and opportunities for treatment. EMBO Rep. 2004, 5, 872–876. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne Muscular Dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef]
- Elangkovan, N.; Dickson, G. Gene Therapy for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8 (Suppl. S2), S303–S316. [Google Scholar] [CrossRef] [PubMed]
- Signs & Symptoms. Parent Project Muscular Dystrophy. Available online: https://www.parentprojectmd.org/about-duchenne/is-it-duchenne/signs-and-symptoms/ (accessed on 24 January 2023).
- Erkut, E.; Yokota, T. CRISPR Therapeutics for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2022, 23, 1832. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V.; Pavlakis, S. Duchenne Muscular Dystrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Muscular Dystrophy–Symptoms and Causes. Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/symptoms-causes/syc-20375388 (accessed on 24 January 2023).
- Alghamdi, F.; Al-Tawari, A.; Alrohaif, H.; Alshuaibi, W.; Mansour, H.; Aartsma-Rus, A.; Mégarbané, A. Case Report: The Genetic Diagnosis of Duchenne Muscular Dystrophy in the Middle East. Front. Pediatr. 2021, 9, 859930. [Google Scholar] [CrossRef]
- What Is Duchenne? Parent Project Muscular Dystrophy. Available online: https://www.parentprojectmd.org/about-duchenne/what-is-duchenne/ (accessed on 24 January 2023).
- Happi Mbakam, C.; Lamothe, G.; Tremblay, J.P. Therapeutic Strategies for Dystrophin Replacement in Duchenne Muscular Dystrophy. Front. Med. 2022, 9, 859930. [Google Scholar] [CrossRef]
- Dudley, R.W.; Lu, Y.; Gilbert, R.; Matecki, S.; Nalbantoglu, J.; Petrof, B.J.; Karpati, G. Sustained improvement of muscle function one year after full-length dystrophin gene transfer into mdx mice by a gutted helper-dependent adenoviral vector. Hum. Gene Ther. 2004, 15, 145–156. [Google Scholar] [CrossRef]
- Hoffman, E.P. The Discovery of Dystrophin, the Protein Product of the Duchenne Muscular Dystrophy Gene. FEBS J. 2020, 287, 3879–3887. [Google Scholar] [CrossRef]
- Rajput, B.S.; Chakrabarti, S.K.; Dongare, V.S.; Ramirez, C.M.; Deb, K.D. Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Duchenne Muscular Dystrophy: Safety and Feasibility Study in India. J. Stem Cells 2015, 10, 141–156. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.A.; Vader, P. Extracellular Vesicle-Based Hybrid Systems for Advanced Drug Delivery. Pharmaceutics 2022, 14, 267. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Kayser, O.; Lemke, A.; Hernandez-Trejo, N. The Impact of Nanobiotechnology on the Development of New Drug Delivery Systems. Curr. Pharm. Biotechnol. 2005, 6, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Abdullah, M.A. Novel Drug Delivery Systems Based on Silver Nanoparticles, Hyaluronic Acid, Lipid Nanoparticles and Liposomes for Cancer Treatment. Appl. Nanosci. 2022, 12, 3071–3096. [Google Scholar] [CrossRef]
- Ishida, O.; Maruyama, K.; Tanahashi, H.; Iwatsuru, M.; Sasaki, K.; Eriguchi, M.; Yanagie, H. Liposomes Bearing Polyethyleneglycol-Coupled Transferrin with Intracellular Targeting Property to the Solid Tumors in Vivo. Pharm. Res. 2001, 18, 1042–1048. [Google Scholar] [CrossRef]
- Derksen, J.T.P.; Scherphof, G.L. An Improved Method for the Covalent Coupling of Proteins to Liposomes. Biochim. Biophys. Acta (BBA)-Biomembr. 1985, 814, 151–155. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.-M.; Wang, D. Click Chemistry, a Powerful Tool for Pharmaceutical Sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef]
- Cavalli, S.; Tipton, A.R.; Overhand, M.; Kros, A. The Chemical Modification of Liposome Surfaces via a Copper-Mediated [3 + 2] Azide-Alkyne Cycloaddition Monitored by a Colorimetric Assay. Chem. Commun. 2006, 30, 3193–3195. [Google Scholar] [CrossRef]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the Role of Mesoporous Silica Nanoparticle in the Development of Novel Drug Delivery Systems. Drug Deliv. Transl. Res. 2022, 12, 105–123. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.-G.; Qiao, H. Technology Insight: Plant-Derived Vesicles—How Far from the Clinical Biotherapeutics and Therapeutic Drug Carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.K.; Jain, N.; Valli, K.S. Importance of Novel Drug Delivery Systems in Herbal Medicines. Pharmacogn. Rev. 2010, 4, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Achalla, V.P.K.; Narwaria, A.; Sharma, S.; Jaswanth Gowda, B.H.; Kesharwani, P. Microparticulate and Nanotechnology Mediated Drug Delivery System for the Delivery of Herbal Extracts. J. Biomater. Sci. Polym. Ed. 2022, 33, 1531–1554. [Google Scholar] [CrossRef] [PubMed]
- Kambale, E.K.; Quetin-Leclercq, J.; Memvanga, P.B.; Beloqui, A. An Overview of Herbal-Based Antidiabetic Drug Delivery Systems: Focus on Lipid- and Inorganic-Based Nanoformulations. Pharmaceutics 2022, 14, 2135. [Google Scholar] [CrossRef]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal Nanomedicines: Recent Advancements, Challenges, Opportunities and Regulatory Overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Gupta, R.; Wadhera, G.; Kumar, P. Modern Herbal Nanogels: Formulation, Delivery Methods, and Applications. Gels 2022, 8, 97. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.L.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Oral Delivery of Zedoary Essential Oil: Formulation and Bioavailability Studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef]
- Shakeel, F.; Alam, P.; Anwer, M.K.; Alanazi, S.A.; Alsarra, I.A.; Alqarni, M.H. Wound Healing Evaluation of Self-Nanoemulsifying Drug Delivery System Containing Piper Cubeba Essential Oil. 3 Biotech 2019, 9, 82. [Google Scholar] [CrossRef]
- Quach, H.; Le, T.-V.; Nguyen, T.-T.; Nguyen, P.; Nguyen, C.K.; Dang, L.H. Nano-Lipids Based on Ginger Oil and Lecithin as a Potential Drug Delivery System. Pharmaceutics 2022, 14, 1654. [Google Scholar] [CrossRef] [PubMed]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical Characterization and Biological Evaluation of Origanum Vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef]
- Caliskan, U.K.; Karakus, M.M. Essential Oils as Skin Permeation Boosters and Their Predicted Effect Mechanisms. J. Dermatol. Ski. Sci. 2020, 2, 24–30. [Google Scholar]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, S.B.; Akere, A.M.; Orege, J.I.; Ejeromeghene, O.; Orege, O.B.; Akolade, J.O. Polymeric Nanoparticles for Enhanced Delivery and Improved Bioactivity of Essential Oils. Heliyon 2023, 9, e16543. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of Essential Oils as Efficient Tools against Antimicrobial Resistance: A Review of the Type and Physical-Chemical Properties of the Delivery Systems and Applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- De Andrade, S.F.; Rijo, P.; Rocha, C.; Zhu, L.; Rodrigues, L.M. Characterizing the Mechanism of Action of Essential Oils on Skin Homeostasis—Data from Sonographic Imaging, Epidermal Water Dynamics, and Skin Biomechanics. Cosmetics 2021, 8, 36. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Karavasili, C.; Wahane, A.; Freitas, D.; Booz, K.; Lee, D.T.H.; Hua, T.; Scala, S.; Lopes, A.; Hess, K.; et al. Development of oil-based gels as versatile drug delivery systems for pediatric applications. Sci. Adv. 2022, 8, 8478. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of Nanoparticles from Natural Lipids for Topical Delivery of Thymol: Investigation of Its Anti-Inflammatory Properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Mei, Z.; Wu, Q.; Hu, S.; Li, X.; Yang, X. Triptolide Loaded Solid Lipid Nanoparticle Hydrogel for Topical Application. Drug Dev. Ind. Pharm. 2005, 31, 161–168. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Cossetin, L.F.; Dalla Lana, D.F.; Monteiro, S.G. Achyrocline Satureioides Essential Oil Loaded in Nanocapsules Ameliorate the Antioxidant/Oxidant Status in Heart of Rats Infected with Trypanosoma Evansi. Microb. Pathog. 2017, 105, 30–36. [Google Scholar] [CrossRef]
- Scinto, S.L.; Bilodeau, D.A.; Hincapie, R.; Lee, W.; Nguyen, S.S.; Xu, M.; Am Ende, C.W.; Finn, M.G.; Lang, K.; Lin, Q.; et al. Bioorthogonal Chemistry. Nat. Rev. Methods Primers 2021, 1, 30. [Google Scholar] [CrossRef]
- Dos Santos Morais, R.; Delalande, O.; Pérez, J.; Mias-Lucquin, D.; Lagarrigue, M.; Martel, A.; Molza, A.-E.; Chéron, A.; Raguénès-Nicol, C.; Chenuel, T.; et al. Human Dystrophin Structural Changes upon Binding to Anionic Membrane Lipids. Biophys. J. 2018, 115, 1231–1239. [Google Scholar] [CrossRef]
- Zhao, J.; Kodippili, K.; Yue, Y.; Hakim, C.H.; Wasala, L.; Pan, X.; Zhang, K.; Yang, N.N.; Duan, D.; Lai, Y. Dystrophin Contains Multiple Independent Membrane-Binding Domains. Hum. Mol. Genet. 2016, 25, 3647–3653. [Google Scholar] [CrossRef]
- You, Y.; Deng, Q.; Wang, Y.; Sang, Y.; Li, G.; Pu, F.; Ren, J.; Qu, X. DNA-based platform for efficient and precisely targeted bioorthogonal catalysis in living systems. Nat. Commun. 2022, 13, 1459. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Meghani, N.M.; Amin, H.H.; Lee, B.-J. Mechanistic Applications of Click Chemistry for Pharmaceutical Drug Discovery and Drug Delivery. Drug Discov. Today 2017, 22, 1604–1619. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P. Significance of Polymers in Drug Delivery System. J. Pharmacovigil. 2015, 3, e127. [Google Scholar] [CrossRef]
- Han, Q.; Fu, H.; Chu, X.; Wen, R.; Zhang, M.; You, T.; Fu, P.; Qin, J.; Cui, T. Research Advances in Treatment Methods and Drug Development for Rare Diseases. Front. Pharmacol. 2022, 13, 971541. [Google Scholar] [CrossRef]
- Saralidze, K.; Koole, L.H.; Knetsch, M.L.W. Polymeric Microspheres for Medical Applications. Materials 2010, 3, 3537–3564. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Korah, L.V.; Anilkumar, G.; Thomas, S. 5–Hydrogels, DNA, and RNA Polypeptides for the Preparation of Biomaterials. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 85–104. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels–Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Jin, L.; Qi, R.Q.; Ma, T. PH-Responsive Polymeric Micelles Self-Assembled from Amphiphilic Copolymer Modified with Lipid Used as Doxorubicin Delivery Carriers. R. Soc. Open Sci. 2018, 5, 171654. [Google Scholar] [CrossRef] [PubMed]
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-Polymer Platforms into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef]
- Eras, A.; Castillo, D.; Suárez, M.; Vispo, N.S.; Albericio, F.; Rodriguez, H. Chemical Conjugation in Drug Delivery Systems. Front. Chem. 2022, 10, 889083. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Bolu, B.S.; Sanyal, R.; Sanyal, A. Drug Delivery Systems from Self-Assembly of Dendron-Polymer Conjugates †. Molecules 2018, 23, 1570. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Verma, S.; Kumar, S. Microsphere-A Novel Drug Delivery System. Res. Chron. Health Sci. 2019, 5, 5–14. [Google Scholar]
- Sailaja, A.K. Review on Microspheres as a Drug Delivery Carrier. Int. J. Adv. Pharm. 2017, 6, 96–102. [Google Scholar]
- Sharma, M.; Dev, S.; Kumar, M.; Shukla, A. Microspheres as Suitable Drug Carrier in Sustained Release Drug Delivery: An Overview. Asian J. Pharm. Pharmacol. 2018, 4, 102–108. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.-Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming Limitations in Nanoparticle Drug Delivery: Triggered, Intravascular Release to Improve Drug Penetration into Tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Cocarta, A.I. Smart Macroporous IPN Hydrogels Responsive to PH, Temperature, and Ionic Strength: Synthesis, Characterization, and Evaluation of Controlled Release of Drugs. ACS Appl. Mater. Interfaces 2016, 8, 12018–12030. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional Polymeric Micelles for Delivery of Drugs and SiRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R. Polymeric Micelles and Their Properties. J. Nanomed. Nanotechnol. 2017, 8, 433. [Google Scholar] [CrossRef]
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric Micelles for Anticancer Drug Delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef]
- Williams, J.H.; Sirsi, S.R.; Latta, D.R.; Lutz, G.J. Induction of Dystrophin Expression by Exon Skipping in Mdx Mice Following Intramuscular Injection of Antisense Oligonucleotides Complexed with PEG-PEI Copolymers. Mol. Ther. 2006, 14, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Schwartz, A.K.; Farrell, R.A.; Garrell, R.L. Thiol–Ene Click Reaction as a General Route to Functional Trialkoxysilanes for Surface Coating Applications. J. Am. Chem. Soc. 2011, 133, 11026–11029. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.D.; Scanlan, E.M. Applications of Thiol-Ene Chemistry for Peptide Science. Front. Chem. 2020, 8, 583272. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Stimuli Responsive Self-Healing Polymers: Gels, Elastomers and Membranes. Polym. Chem. 2017, 8, 6464–6484. [Google Scholar] [CrossRef]
- El Azab, I.H.; Elkanzi, N.A.A. Design, Synthesis, and Antimicrobial Evaluation of New Annelated Pyrimido [2,1-c][1,2,4]Triazolo[3,4-f][1,2,4]Triazines. Molecules 2020, 25, 1339. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Sun, H.; Cui, C.; Zhang, L.; Jiang, Y.; Wu, Y.; Wang, Y.; Li, J.; Sumerlin, B.S.; et al. Thiol–Ene Click Chemistry: A Biocompatible Way for Orthogonal Bioconjugation of Colloidal Nanoparticles. Chem. Sci. 2017, 8, 6182–6187. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. The Thiol-Ene (Click) Reaction for the Synthesis of Plant Oil Derived Polymers. Eur. J. Lipid Sci. Technol. 2013, 115, 41–54. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97(B), 438–453. [Google Scholar] [CrossRef]
- Oluwasanmi, A.; Hoskins, C. Potential Use of the Diels-Alder Reaction in Biomedical and Nanomedicine Applications. Int. J. Pharm. 2021, 604, 120727. [Google Scholar] [CrossRef] [PubMed]
- Lligadas, G.; Ronda, J.; Galià, M.; Cadiz, V. Monomers and Polymers from Plant Oils via Click Chemistry Reactions. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2124. [Google Scholar] [CrossRef]
- Gheneim, R.; Perez-Berumen, C.; Gandini, A. Diels−Alder Reactions with Novel Polymeric Dienes and Dienophiles: Synthesis of Reversibly Cross-Linked Elastomers. Macromolecules 2002, 35, 7246–7253. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder Reaction in Polymer Chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Pérez, Y.A.; Urista, C.M.; Martínez, J.I.; Nava, M.D.C.D.; Rodríguez, F.A.R. Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules. Polymers 2016, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- González-Lainez, M.; Gallegos, M.; Munarriz, J.; Azpiroz, R.; Passarelli, V.; Jiménez, M.V.; Pérez-Torrente, J.J. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) by Functionalized NHC-Based Polynuclear Catalysts: Scope and Mechanistic Insights. Organometallics 2022, 41, 2154–2169. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(i) Acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Zhu, L.; Brassard, C.J.; Zhang, X.; Guha, P.M.; Clark, R.J. On the Mechanism of Copper(I)-Catalyzed Azide–Alkyne Cycloaddition. Chem. Rec. 2016, 16, 1501–1517. [Google Scholar] [CrossRef]
- Li, H.; Aneja, R.; Chaiken, I. Click Chemistry in Peptide-Based Drug Design. Molecules 2013, 18, 9797–9817. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Adzima, B.; Baker, N.; Bowman, C. Photopolymerization Reactions Using the Photoinitiated Copper (I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) Reaction. Adv. Mater. 2013, 25, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, M.; Wafer, C.; Morin, J.F. Recent advances in click chemistry applied to dendrimer synthesis. Molecules 2015, 20, 9263–9294. [Google Scholar] [CrossRef] [PubMed]
- Giofrè, S.V.; Tiecco, M.; Ferlazzo, A.; Romeo, R.; Ciancaleoni, G.; Germani, R.; Iannazzo, D. Base-Free Copper-Catalyzed Azide-Alkyne Click Cycloadditions (CuAAc) in Natural Deep Eutectic Solvents as Green and Catalytic Reaction Media. Eur. J. Org. Chem. 2021, 2021, 4777–4789. [Google Scholar] [CrossRef]
- Mann, V.R.; Powers, A.S.; Tilley, D.C.; Sack, J.T.; Cohen, B.E. Azide–Alkyne Click Conjugation on Quantum Dots by Selective Copper Coordination. ACS Nano 2018, 12, 4469–4477. [Google Scholar] [CrossRef]
- Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction (CuAAC) Runs in Batch with P7 Loaded with Cu(I) or Cu(II) Salts. Available online: https://www.researchgate.net/figure/Copper-catalyzed-azide-alkyne-cycloaddition-reaction-CuAAC-runs-in-batch-with-P7-loaded_tbl1_340698972 (accessed on 24 January 2023).
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-Catalysed Azide–Alkyne Cycloadditions (CuAAC): An Update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Aflak, N.; El Mouchtari, E.M.; Ben El Ayouchia, H.; Anane, H.; Rafqah, S.; Julve, M.; Stiriba, S.-E. Copper-on-Magnetically Activated Carbon-Catalyzed Azide-Alkyne Click Cycloaddition in Water. Catalysts 2022, 12, 1244. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Cunha Lima, J.A.D.; De Farias Silva, J.; Santos, C.S.; Caiana, R.R.A.; De Moraes, M.M.; Da Câmara, C.A.G.; Freitas, J.C.R. Synthesis of New 1,4-Disubstituted 1,2,3-Triazoles Using the CuAAC Reaction and Determination of Their Antioxidant Activities. Acad. Bras. Cienc. 2021, 93, e20201672. [Google Scholar] [CrossRef] [PubMed]
- Israr, M.; Ye, C.; Muhammad, M.T.; Li, Y.; Bao, H. Copper(I)-Catalyzed Tandem Reaction: Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from Alkyl Diacyl Peroxides, Azidotrimethylsilane, and Alkynes. Beilstein J. Org. Chem. 2018, 14, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fong, D.; Meichsner, E.; Adronov, A. A Survey of Strain-Promoted Azide–Alkyne Cycloaddition in Polymer Chemistry. Chem. A Eur. J. 2021, 27, 5057–5073. [Google Scholar] [CrossRef]
- Dommerholt, J.; Rutjes, F.P.J.T.; van Delft, F.L. Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. Top Curr. Chem. 2016, 374, 16. [Google Scholar] [CrossRef]
- Cu–Free Click Chemistry (SPAAC). Click Chemistry Tools. Available online: https://clickchemistrytools.com/cu-free-click-chemistry-spaac/ (accessed on 24 January 2023).
- Thomas, J.D.; Cui, H.; North, P.J.; Hofer, T.; Rader, C.; Burke, T.R. Application of Strain-Promoted Azide-Alkyne Cycloaddition and Tetrazine Ligation to Targeted Fc-Drug Conjugates. Bioconjug. Chem. 2012, 23, 2007–2013. [Google Scholar] [CrossRef]
- Ornelas, C.; Broichhagen, J.; Weck, M. Strain-Promoted Alkyne Azide Cycloaddition for the Functionalization of Poly(Amide)-Based Dendrons and Dendrimers. J. Am. Chem. Soc. 2010, 132, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Singha, G.; Majeeda, A.S.; Singha, R.; Georgea, N.; Singhb, G.; Guptab, S.; Singh, H.; Kaur, G.; Singh, J. CuAAC ensembled 1,2,3-triazole linked nanogels for targeted drug delivery: A review. RSC Adv. 2023, 13, 2912–2936. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Zhou, H.; She, P.; Zhang, P.; Cao, Y.; Zhang, X. A Novel PH-Sensitive Polymeric Prodrug Was Prepared by SPAAC Click Chemistry for Intracellular Delivery of Doxorubicin and Evaluation of Its Anti-Cancer Activity in Vitro. J. Drug Deliv. Sci. Technol. 2019, 53, 101130. [Google Scholar] [CrossRef]



| Essential Oil | Drug Delivery Systems | Results | Reference |
|---|---|---|---|
| Zedoary (Curcuma zedoaria) oil | Self-nanoemulsifying drug delivery system |
| [56] |
| Cubeb (Piper cubeba) oil | Self-nanoemulsifying drug delivery system |
| [57] |
| Ginger (Zingiber officinale) oil | Ginger EO was used along with lecithin (GL), cholesterol, and span 80 to fabricate nano-lipids (GL nano-lipids) using a thin-film method. |
| [58] |
| Oregano (Origanum vulgare) oil | Polymeric micelles drug delivery system as a possible non-invasive approach for the management of skin tags |
| [59] |
| Bitter orange (Citrus aurantium) oil | Anti-inflammatory drug delivery system in Duchenne muscular dystrophy patients |
| [5] |
| Terpene | Method of Encapsulation | Importance | In Vivo Results | Reference |
|---|---|---|---|---|
| Thymol | Nanostructured Lipid Carriers (NLCs) | Thymol is an antimicrobial, antioxidant, and antiseptic compound with potential applications in wound healing and inflammation management. | Anti-inflammatory effects and improved healing in psoriasis were shown while using the cutaneous acute inflammation model induced by croton oil in BALB/c mice. | [71] |
| Triptolide | Solid Lipid NPs (SLNs) | The anti-inflammatory effect is over two-fold higher than that of conventional topological (TP) hydrogel. | Efficient transdermal delivery and anti-inflammatory activity while using the carrageenan induced edema mouse model. | [72] |
| Achyrocline satureioides EO | Achyrocline satureioides loaded nanocapsules (AS-NC) treatment | Natural compounds with antioxidant and free radical scavenging properties, such as Achyrocline satureioides EO loaded in nanocapsules (AS-NC), may be an important approach to reducing cardiac damage. | Protected against oxidative stress in the cardiac tissue of Wister’s rats. | [73] |
| Type of Polymer-Based Drug Delivery Systems | Characteristics | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Microspheres | Small polymer particles that encapsulate drugs | Improved efficacy and reduced toxicity [95]. | Limited targeting capabilities (microspheres tend to migrate away from the injection site and lead to potential risks such as embolism) [96]. | Sustained release applications [97]. |
| NPs | Similar to microspheres but smaller (typically around 100 nm) | Improves the solubility and bioavailability of drugs [98]. | Nanoparticles may not release drugs in concentrations high enough to result in cell death [99]. | Targeting specific cells or tissues in the body [100]. |
| Hydrogels | 3D networks of polymers that absorb water and swell | Stimuli responsiveness, biocompatibility, high drug loading, and dependence of swellability on diffusivity of water [101]. | Limited targeting capabilities (hydrogels are relatively insensitive and inadequate for low concentration samples, usually limited at around 0.1–0.2 mg/mL) [102]. | Release drugs in response to changes in temperature, pH, or other stimuli [103,104]. |
| Polymeric Micelles | Spherical structures formed by the self-assembly of amphiphilic polymers | Solubilize hydrophobic drugs and improve bioavailability [105]. | Slow dissociation rates, which can limit the duration of drug release [106]. | Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin [107]. |
| Polymer Conjugates | Drug covalently attached to a polymer | Improves pharmacokinetics and pharmacodynamics [80]. | Low drug loading due to conjugation at the end [108]. | Polymer-drug conjugates have a range of therapeutic applications [108]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspute, G.; Arunagiri, B.D.; Alexander, R.; Ramanavicius, A.; Samukaite-Bubniene, U. Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy. Materials 2023, 16, 6537. https://doi.org/10.3390/ma16196537
Kaspute G, Arunagiri BD, Alexander R, Ramanavicius A, Samukaite-Bubniene U. Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy. Materials. 2023; 16(19):6537. https://doi.org/10.3390/ma16196537
Chicago/Turabian StyleKaspute, Greta, Bharani Dharan Arunagiri, Rakshana Alexander, Arunas Ramanavicius, and Urte Samukaite-Bubniene. 2023. "Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy" Materials 16, no. 19: 6537. https://doi.org/10.3390/ma16196537
APA StyleKaspute, G., Arunagiri, B. D., Alexander, R., Ramanavicius, A., & Samukaite-Bubniene, U. (2023). Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy. Materials, 16(19), 6537. https://doi.org/10.3390/ma16196537