Pd–Co-Based Electrodes for Hydrogen Production by Water Splitting in Acidic Media
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Deposit on Carbon Paper
3.2. The Array of Nanowires
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reijnders, L. Fuels for the future. J. Integr. Environ. Sci. 2009, 6, 279–294. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Sunseri, C.; Cocchiara, C.; Ganci, F.; Moncada, A.; Oliveri, R.L.; Patella, B.; Piazza, S.; Inguanta, R. Nanostructured electrochemical devices for sensing, energy conversion and storage. Chem. Eng. Trans. 2016, 47, 43–48. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Logroño, W.; Mohamed, A.; Hasan, H.A. Hydrogen Production Through Electrolysis. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer New York: New York, NY, USA, 2018; pp. 1–20. ISBN 978-1-4939-2493-6. [Google Scholar]
- Mac Dowell, N.; Sunny, N.; Brandon, N.; Herzog, H.; Ku, A.Y.; Maas, W.; Ramirez, A.; Reiner, D.M.; Sant, G.N.; Shah, N. The hydrogen economy: A pragmatic path forward. Joule 2021, 5, 2524–2529. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Santos, A.L.; Cebola, M.-J.; Santos, D.M.F. Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers. Energies 2021, 14, 3193. [Google Scholar] [CrossRef]
- Le Bideau, D.; Mandin, P.; Benbouzid, M.; Kim, M.; Sellier, M.; Ganci, F.; Inguanta, R. Eulerian Two-Fluid Model of Alkaline Water Electrolysis for Hydrogen Production. Energies 2020, 13, 3394. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Falcão, D.S.; Pinto, A.M.F.R. A review on PEM electrolyzer modelling: Guidelines for beginners. J. Clean. Prod. 2020, 261, 121184. [Google Scholar] [CrossRef]
- Campagna Zignani, S.; Faro, M.L.; Carbone, A.; Italiano, C.; Trocino, S.; Monforte, G.; Aricò, A.S. Performance and stability of a critical raw materials-free anion exchange membrane electrolysis cell. Electrochim. Acta 2022, 413, 140078. [Google Scholar] [CrossRef]
- Niaz, A.K.; Park, J.-Y.; Lim, H.-T. Operational parameters correlated with the long-term stability of anion exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2021, 46, 31550–31562. [Google Scholar] [CrossRef]
- Niaz, A.K.; Akhtar, A.; Park, J.-Y.; Lim, H.-T. Effects of the operation mode on the degradation behavior of anion exchange membrane water electrolyzers. J. Power Sources 2021, 481, 229093. [Google Scholar] [CrossRef]
- Niaz, A.K.; Lim, H.-T. Stability Tests on Anion Exchange Membrane Water Electrolyzer under On-Off Cycling with Continuous Solution Feeding. J. Electrochem. Sci. Technol. 2022, 13, 369–376. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Küngas, R.; Lin, T.-E.; Diethelm, S.; Maréchal, F.; Van Herle, J. Power-to-fuels via solid-oxide electrolyzer: Operating window and techno-economics. Renew. Sustain. Energy Rev. 2019, 110, 174–187. [Google Scholar] [CrossRef]
- Kamlungsua, K.; Su, P.-C.; Chan, S.H. Hydrogen Generation Using Solid Oxide Electrolysis Cells. Fuel Cells 2020, 20, 644–649. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Xu, H.; Xuan, J. Advancing the multiscale understanding on solid oxide electrolysis cells via modelling approaches: A review. Renew. Sustain. Energy Rev. 2021, 141, 110863. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Bareiß, K.; de la Rua, C.; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Jurasz, J.; Canales, F.A.; Kies, A.; Guezgouz, M.; Beluco, A. A review on the complementarity of renewable energy sources: Concept, metrics, application and future research directions. Sol. Energy 2020, 195, 703–724. [Google Scholar] [CrossRef]
- Egeland-Eriksen, T.; Hajizadeh, A.; Sartori, S. Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. Hydrogen Energy 2021, 46, 31963–31983. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, P.; Lev, B.; Zhou, X. Balancing of supply and demand of renewable energy power system: A review and bibliometric analysis. Sustain. Futures 2020, 2, 100013. [Google Scholar] [CrossRef]
- Hiesl, A.; Ajanovic, A.; Haas, R. On current and future economics of electricity storage. Greenh. Gases Sci. Technol. 2020, 10, 1176–1192. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy storage systems: A review. Energy Storage Sav. 2022, 1, 166. [Google Scholar] [CrossRef]
- Lucas, T.R.; Ferreira, A.F.; Santos Pereira, R.B.; Alves, M. Hydrogen production from the WindFloat Atlantic offshore wind farm: A techno-economic analysis. Appl. Energy 2022, 310, 118481. [Google Scholar] [CrossRef]
- Calado, G.; Castro, R. Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 5561. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mehrpooya, M. A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 2018, 158, 632–655. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Ursúa, A.; Barrios, E.L.; Pascual, J.; San Martín, I.; Sanchis, P. Integration of commercial alkaline water electrolysers with renewable energies: Limitations and improvements. Int. J. Hydrogen Energy 2016, 41, 12852–12861. [Google Scholar] [CrossRef]
- Ursúa, A.; San Martín, I.; Barrios, E.L.; Sanchis, P. Stand-alone operation of an alkaline water electrolyser fed by wind and photovoltaic systems. Int. J. Hydrogen Energy 2013, 38, 14952–14967. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Kees, J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H.; Kolhe, M.L. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. [Google Scholar] [CrossRef]
- Bhalothia, D.; Krishnia, L.; Yang, S.-S.; Yan, C.; Hsiung, W.-H.; Wang, K.-W.; Chen, T.-Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hu, C.; Yu, S.; Yang, P.; Cheng, D.; Zhao, Z.-J.; Gong, J. Fabrication of bilayer Pd-Pt nanocages with sub-nanometer thin shells for enhanced hydrogen evolution reaction. Nano Res. 2019, 12, 2268–2274. [Google Scholar] [CrossRef]
- Tominaka, S.; Momma, T.; Osaka, T. Electrodeposited Pd-Co catalyst for direct methanol fuel cell electrodes: Preparation and characterization. Electrochim. Acta 2008, 53, 4679–4686. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Zhang, H.; Geng, H. A Nanoporous PdCo Alloy as a Highly Active Electrocatalyst for the Oxygen-Reduction Reaction and Formic Acid Electrooxidation. Chem. Asian J. 2013, 8, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Peter, S.C. An overview on Pd-based electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Elumalai, P.; Vasan, H.N.; Munichandraiah, N.; Shivashankar, S.A. Kinetics of hydrogen evolution on submicron size Co, Ni, Pd and Co–Ni alloy powder electrodes by d.c. polarization and a.c. impedance studies. J. Appl. Electrochem. 2002, 32, 1005–1010. [Google Scholar] [CrossRef]
- Martín, A.J.; Chaparro, A.M.; Daza, L. Electrochemical quartz crystal microbalance study of the electrodeposition of Co, Pt and Pt–Co alloy. J. Power Sources 2007, 169, 65–70. [Google Scholar] [CrossRef]
- Conway, B.E.; Jerkiewicz, G. Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’ for cathodic H2 evolution kinetics. Electrochim. Acta 2000, 45, 4075–4083. [Google Scholar] [CrossRef]
- Zabinski, P.; Mech, K.; Banbur-Pawlowska, S.; Kowalik, R. Modification of Electrodeposited Co-Pd Alloy Catalysts By Superimposed High Magnetic Field. ECS Meet. Abstr. 2015, MA2015-02, 907. [Google Scholar] [CrossRef]
- Li, L.; Ji, Y.; Luo, X.; Geng, S.; Fang, M.; Pi, Y.; Li, Y.; Huang, X.; Shao, Q. Compressive Strain in N-Doped Palladium/Amorphous-Cobalt (II) Interface Facilitates Alkaline Hydrogen Evolution. Small 2021, 17, 2103798. [Google Scholar] [CrossRef]
- Ganci, F.; Lombardo, S.; Sunseri, C.; Inguanta, R. Nanostructured electrodes for hydrogen production in alkaline electrolyzer. Renew. Energy 2018, 123, 117–124. [Google Scholar] [CrossRef]
- Battaglia, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Fabrication and characterization of nanostructured Ni–IrO2 electrodes for water electrolysis. Int. J. Hydrogen Energy 2014, 39, 16797–16805. [Google Scholar] [CrossRef]
- Cirone, J.; Dondapati, J.S.; Chen, A. Design of bimetallic nickel-iron quantum dots with tunable compositions for enhanced electrochemical water splitting. Electrochim. Acta 2021, 392, 139016. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Fabrication and characterization of metal and metal oxide nanostructures grown by metal displacement deposition into anodic alumina membranes. Chem. Eng. Trans. 2011, 24, 199–204. [Google Scholar] [CrossRef]
- Patella, B.; Piazza, S.; Sunseri, C.; Inguanta, R. Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes. Appl. Sci. 2022, 12, 869. [Google Scholar] [CrossRef]
- Ganci, F.; Baguet, T.; Aiello, G.; Cusumano, V.; Mandin, P.; Sunseri, C.; Inguanta, R. Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers. Energies 2019, 12, 3669. [Google Scholar] [CrossRef]
- Du, L.; Feng, D.; Xing, X.; Fu, Y.; Fonseca, L.F.; Yang, D. Palladium/cobalt nanowires with improved hydrogen sensing stability at ultra-low temperatures. Nanoscale 2019, 11, 21074–21080. [Google Scholar] [CrossRef]
- Ganci, F.; Patella, B.; Cannata, E.; Cusumano, V.; Aiello, G.; Sunseri, C.; Mandin, P.; Inguanta, R. Ni alloy nanowires as high efficiency electrode materials for alkaline electrolysers. Int. J. Hydrogen Energy 2021, 46, 35777–35789. [Google Scholar] [CrossRef]
- Degaga, G.D.; Kaur, S.; Pandey, R.; Jaszczak, J.A. First-Principles Study of a MoS2-PbS van der Waals Heterostructure Inspired by Naturally Occurring Merelaniite. Materials 2021, 14, 1649. [Google Scholar] [CrossRef]
- Ganci, F.; Buccheri, B.; Patella, B.; Cannata, E.; Aiello, G.; Mandin, P.; Inguanta, R. Electrodeposited nickel–zinc alloy nanostructured electrodes for alkaline electrolyzer. Int. J. Hydrogen Energy 2021, 47, 11302–11315. [Google Scholar] [CrossRef]
- Serôdio Costa, B.F.; Arias-Serrano, B.I.; Yaremchenko, A.A. Development of Ni-Sr(V,Ti)O3-δ Fuel Electrodes for Solid Oxide Fuel Cells. Materials 2021, 15, 278. [Google Scholar] [CrossRef]
- Buccheri, B.; Ganci, F.; Patella, B.; Aiello, G.; Mandin, P.; Inguanta, R. Ni-Fe alloy nanostructured electrodes for water splitting in alkaline electrolyser. Electrochim. Acta 2021, 388, 138588. [Google Scholar] [CrossRef]
- Ganci, F.; Cusumano, V.; Livreri, P.; Aiello, G.; Sunseri, C.; Inguanta, R. Nanostructured Ni–Co alloy electrodes for both hydrogen and oxygen evolution reaction in alkaline electrolyzer. Int. J. Hydrogen Energy 2021, 46, 10082–10092. [Google Scholar] [CrossRef]
- Ding, D.; Huang, J.; Deng, X.; Fu, K. Recent Advances and Perspectives of Nanostructured Amorphous Alloys in Electrochemical Water Electrolysis. Energy Fuels 2021, 35, 15472–15488. [Google Scholar] [CrossRef]
- Kumar, A.; Mohammadi, M.M.; Swihart, M.T. Synthesis, growth mechanisms, and applications of palladium-based nanowires and other one-dimensional nanostructures. Nanoscale 2019, 11, 19058–19085. [Google Scholar] [CrossRef] [PubMed]
- Kelaidis, N.; Zervos, M.; Lathiotakis, N.N.; Chroneos, A.; Tanasă, E.; Vasile, E. Vapor–liquid–solid growth and properties of one dimensional PbO and PbO/SnO2 nanowires. Mater. Adv. 2022, 3, 1695–1702. [Google Scholar] [CrossRef]
- Oliveri, R.L.; Insinga, M.G.; Pisana, S.; Patella, B.; Aiello, G.; Inguanta, R. High-Performance Lead-Acid Batteries Enabled by Pb and PbO2 Nanostructured Electrodes: Effect of Operating Temperature. Appl. Sci. 2021, 11, 6357. [Google Scholar] [CrossRef]
- Insinga, M.G.; Oliveri, R.L.; Sunseri, C.; Inguanta, R. Template electrodeposition and characterization of nanostructured Pb as a negative electrode for lead-acid battery. J. Power Sources 2019, 413, 107–116. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C.; Cino, A.; Di Dio, V.; La Cascia, D.; Miceli, R.; Rando, C.; Zizzo, G. An electrochemical route towards the fabrication of nanostructured semiconductor solar cells. In Proceedings of the SPEEDAM 2010, Pisa, Italy, 14–16 June 2010; pp. 1166–1171. [Google Scholar] [CrossRef]
- Patella, B.; Inguanta, R.; Piazza, S.; Sunseri, C. Nanowire ordered arrays for electrochemical sensing of H2O2. Chem. Eng. Trans. 2016, 47, 19–24. [Google Scholar] [CrossRef]
- O’Sullivan, B.; O’Sullivan, S.; Narayan, T.; Shao, H.; Patella, B.; Seymour, I.; Inguanta, R.; O’Riordan, A. A Direct Comparison of 2D versus 3D Diffusion Analysis at Nanowire Electrodes: A Finite Element Analysis and Experimental Study. Electrochim. Acta 2022, 408, 139890. [Google Scholar] [CrossRef]
- Patella, B.; Piazza, S.; Sunseri, C.; Inguanta, R. NiO thin film for mercury detection in water by square wave anodic stripping voltammetry. Chem. Eng. Trans. 2017, 60, 1–6. [Google Scholar] [CrossRef]
- Verma, S.; Khosla, A.; Arya, S. Performance of Electrochemically Synthesized Nickel-Zinc and Nickel-Iron (Ni–Zn//Ni–Fe) Nanowires as Battery Type Supercapacitor. J. Electrochem. Soc. 2020, 167, 120527. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, V.; Khosla, A.; Kumar, S.; Arya, S. High performance asymmetric supercapacitor based on vertical nanowire arrays of a novel Ni@Co–Fe LDH core@shell as negative and Ni(OH)2 as positive electrode. Nanotechnology 2020, 31, 245401. [Google Scholar] [CrossRef]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.P.; Wang, Z.L. Low-Cost High-Performance Solid-State Asymmetric Supercapacitors Based on MnO2 Nanowires and Fe2O3 Nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, R.L.; Patella, B.; Di Pisa, F.; Mangione, A.; Aiello, G.; Inguanta, R. Fabrication of CZTSe/CIGS Nanowire Arrays by One-Step Electrodeposition for Solar-Cell Application. Materials 2021, 14, 2778. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Sarkar, K.J.; Banerji, P. Fabrication and studies on Si/InP core-shell nanowire based solar cell using etched Si nanowire arrays. Sol. Energy Mater. Sol. Cells 2020, 204, 110217. [Google Scholar] [CrossRef]
- Yue, G.; Lin, Y.; Wen, X.; Wang, L.; Peng, D. SnS homojunction nanowire-based solar cells. J. Mater. Chem. 2012, 22, 16437. [Google Scholar] [CrossRef]
- Bertero, E.; Manzano, C.V.; Bürki, G.; Philippe, L. Stainless steel-like FeCrNi nanostructures via electrodeposition into AAO templates using a mixed-solvent Cr(III)-based electrolyte. Mater. Des. 2020, 190, 108559. [Google Scholar] [CrossRef]
- Pendergast, A.D.; Glasscott, M.W.; Renault, C.; Dick, J.E. One-step electrodeposition of ligand-free PdPt alloy nanoparticles from water droplets: Controlling size, coverage, and elemental stoichiometry. Electrochem. Commun. 2019, 98, 1–5. [Google Scholar] [CrossRef]
- Inguanta, R.; Rinaldo, E.; Piazza, S.; Sunseri, C. Lead Nanowires for Microaccumulators Obtained Through Indirect Electrochemical Template Deposition. Electrochem. Solid-State Lett. 2010, 13, K1–K4. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Livreri, P.; Piazza, S.; Sunseri, C. Ruthenium Oxide Nanotubes Via Template Electrosynthesis. Curr. Nanosci. 2011, 7, 210–218. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, B.; Shao, Z.; Yang, D.; Ming, P.; Li, B.; Zhang, C. Mechanism and Model for Optimizing Polytetrafluoroethylene Distribution to Improve the Electrical and Thermal Conductivity of Treated Carbon Fiber Paper in Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 14207–14220. [Google Scholar] [CrossRef]
- Yu, S.; Hao, J.; Li, J.; Zhang, L. Effect of distribution of polytetrafluoroethylene on durability of gas diffusion backing in proton exchange membrane fuel cell. Mater. Res. Bull. 2020, 122, 110684. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. 1. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; DE GRUYTER: Berlin, Germany, 2015; pp. 1–30. ISBN 978-3-11-041704-3. [Google Scholar]
- Ungár, T.; Gubicza, J.; Ribárik, G.; Pantea, C.; Zerda, T.W. Microstructure of carbon blacks determined by X-ray diffraction profile analysis. Carbon 2002, 40, 929–937. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys Principles and Practice; Elsevier Science: Saint Louis, MO, USA, 2014; ISBN 978-1-4832-2311-7. [Google Scholar]
- Schlesinger, M.; Paunovic, M. (Eds.) Modern Electroplating, 5th ed.; The Electrochemical Society Series; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-16778-6. [Google Scholar]
- Tominaka, S.; Osaka, T. Nanoporous PdCo Catalyst for Microfuel Cells: Electrodeposition and Dealloying. Adv. Phys. Chem. 2011, 2011, 821916. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, W.; Zhang, H.; Du, Y.; Yang, P.; Wang, C.; Xu, J. Facile template-free synthesis of pine needle-like Pd micro/nano-leaves and their associated electro-catalytic activities toward oxidation of formic acid. Nanoscale Res. Lett. 2011, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh Kakhki, R. A review to recent developments in modification of carbon fiber electrodes. Arab. J. Chem. 2019, 12, 1783–1794. [Google Scholar] [CrossRef]
- Liu, C.; Sun, C.; Gao, Y.; Lan, W.; Chen, S. Improving the Electrochemical Properties of Carbon Paper as Cathodes for Microfluidic Fuel Cells by the Electrochemical Activation in Different Solutions. ACS Omega 2021, 6, 19153–19161. [Google Scholar] [CrossRef] [PubMed]
- International Centre for Diffraction Data. Power Diffraction File; International Centre for Diffraction Data: Newtown Square, PA, USA, 2007. [Google Scholar]
- West, A.R. Solid State Chemistry and Its Applications; Wiley: Chichester, UK, 1984; ISBN 978-0-471-90377-2. [Google Scholar]
- Schiavi, P.G.; Altimari, P.; Pagnanelli, F.; Moscardini, E.; Toro, L. Synthesis of cobalt nanoparticles by electrodeposition onto aluminium foils. Chem. Eng. Trans. 2015, 43, 673–678. [Google Scholar] [CrossRef]
- Jiang, S.P.; Tseung, A.C.C. Reactive Deposition of Cobalt Electrodes: II. Role of Bubbling Oxygen. J. Electrochem. Soc. 1990, 137, 3381–3386. [Google Scholar] [CrossRef]
- Rahimi, S.A.; Norouzi, P.; Ganjali, M.R. One-step cathodic electrodeposition of a cobalt hydroxide–graphene nanocomposite and its use as a high performance supercapacitor electrode material. RSC Adv. 2018, 8, 26818–26827. [Google Scholar] [CrossRef]
- Kan, S.; Sachan, M.; Kirchhoff, J.; Majetich, S.A. Crystallographic alignment of nanoparticles during self-assembly. IEEE Trans. Magn. 2005, 41, 3370–3372. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, J.; Yuan, J.; Rong, S.; Ou, P.; Chen, X.; Zhang, B.; Ke, Q.; Zhu, Z. Solvothermal synthesis and characterization of porous co microspheres. Mater. Tehnol. 2021, 55, 663–666. [Google Scholar] [CrossRef]
- Tong, G.; Yuan, J.; Wu, W.; Hu, Q.; Qian, H.; Li, L.; Shen, J. Flower-like Co superstructures: Morphology and phase evolution mechanism and novel microwave electromagnetic characteristics. CrystEngComm 2012, 14, 2071. [Google Scholar] [CrossRef]
- Zhong, M.; Li, L.; Zhao, K.; He, F.; Su, B.; Wang, D. PdCo alloys@N-doped porous carbon supported on reduced graphene oxide as a highly efficient electrocatalyst for hydrogen evolution reaction. J. Mater. Sci. 2021, 56, 14222–14233. [Google Scholar] [CrossRef]
- Yun, M.; Ahmed, M.S.; Jeon, S. Thiolated graphene oxide-supported palladium cobalt alloyed nanoparticles as high performance electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 293, 380–387. [Google Scholar] [CrossRef]
- Kaushik, P.; Kaur, G.; Ram Chaudhary, G.; Batra, U. Cleaner way for overall water splitting reaction by using palladium and cobalt based nanocomposites prepared from mixed metallosurfactants. Appl. Surf. Sci. 2021, 556, 149769. [Google Scholar] [CrossRef]
- Bera, P.; Seenivasan, H.; Rajam, K.S.; William Grips, V.K. Characterization of amorphous Co–P alloy coatings electrodeposited with pulse current using gluconate bath. Appl. Surf. Sci. 2012, 258, 9544–9553. [Google Scholar] [CrossRef]
- Orue, I.; García-Arribas, A.; Fdez-Gubieda, M.L.; Herreros, J.; Plazaola, F.; Barandiarán, J.M. Observation of a Strong Short Range Order in Co Rich Amorphous Alloys Prepared by Different Methods. J. Phys. IV France 1997, 7, C2-995–C2-996. [Google Scholar] [CrossRef]
- Luo, Z.; Song, K.; Li, G.; Yang, L. Hydrogen Evolution Ability of Selected Pure Metals and Galvanic Corrosion Behavior between the Metals and Magnesium. J. Electrochem. Sci. Technol. 2020, 11, 323–329. [Google Scholar] [CrossRef]
- Patel, P.P.; Hanumantha, P.J.; Datta, M.K.; Velikokhatnyi, O.I.; Hong, D.; Poston, J.A.; Manivannan, A.; Kumta, P.N. Cobalt based nanostructured alloys: Versatile high performance robust hydrogen evolution reaction electro-catalysts for electrolytic and photo-electrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 17049–17062. [Google Scholar] [CrossRef]
- Chen, J.; Xia, G.; Jiang, P.; Yang, Y.; Li, R.; Shi, R.; Su, J.; Chen, Q. Active and Durable Hydrogen Evolution Reaction Catalyst Derived from Pd-Doped Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2016, 8, 13378–13383. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Yang, M.; Zhao, S.; Li, Z.; Miao, J.; Gao, Z.-D.; Gao, Y.; Song, Y.-Y. Tuning the surface segregation composition of a PdCo alloy by the atmosphere for increasing electrocatalytic activity. Sustain. Energy Fuels 2020, 4, 380–386. [Google Scholar] [CrossRef]


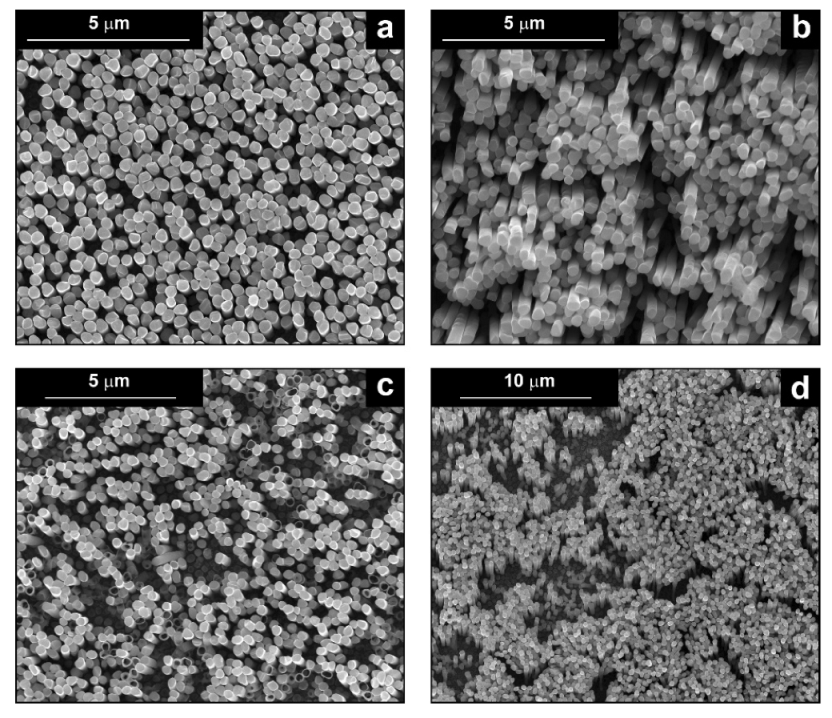
| Sample | FESEM | EDS | XRD |
|---|---|---|---|
| Carbon Paper |  | 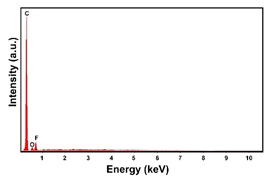 |  |
| Pd (−15.6 mA cm−2, 3600 s) | 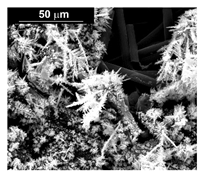 | 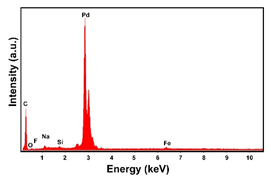 | 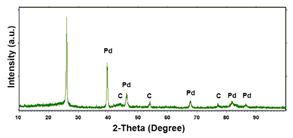 |
| Pd (−1.56 mA cm−2, 3600 s) | 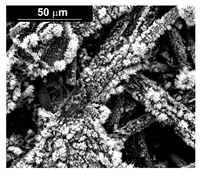 | 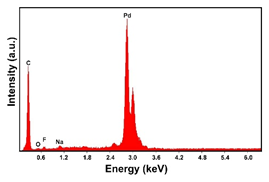 | |
| Co (−15.6 mA cm−2, 3600 s) | 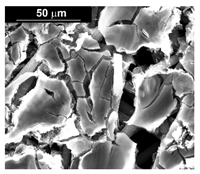 | 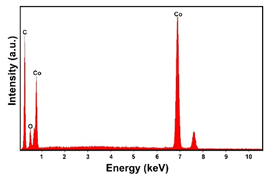 | 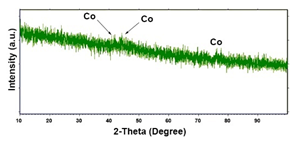 |
| Co (−1.56 mA cm−2, 3600 s) | 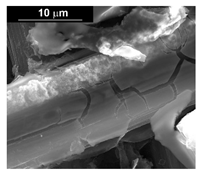 | 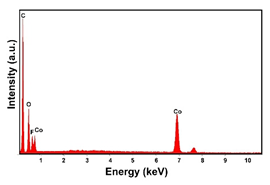 |
| Sample | FESEM | EDS | XRD |
|---|---|---|---|
| Pd–Co (−1.56 mA cm−2 0.035 M Pd2+, 0.0069 M Co2+, 60 s) | 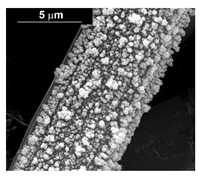 | 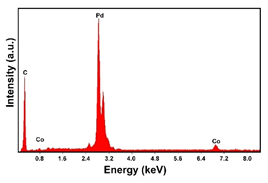 | |
| Pd–Co (−15.6 mA cm−2 0.035 M Pd2+, 0.0069 M Co2+, 300 s) | 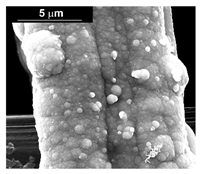 | 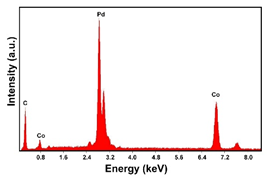 | 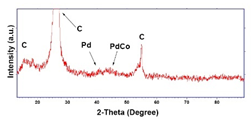 |
| Pd–Co (−1.56 mA cm−2 0.035 M Pd2+, 0.17 M Co2+, 60 s) | 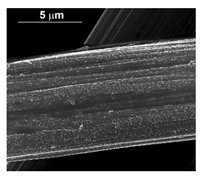 | 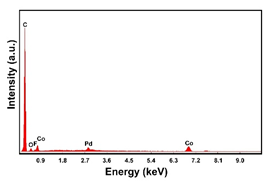 | 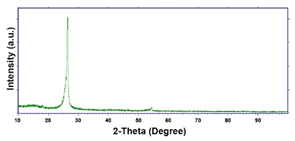 |
| Pd–Co (−15.6 mA cm−2 0.035 M Pd2+, 0.17 M Co2+, 60 s) |  | 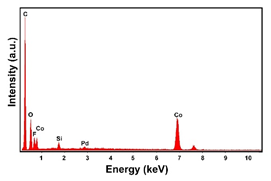 | |
| Pd–Co (−156 mA cm−2 0.035 M Pd2+, 0.17 M Co2+, 300 s) | 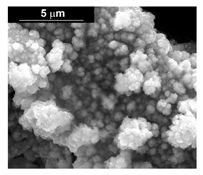 | 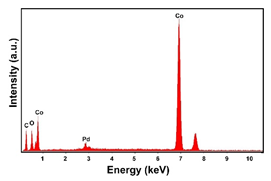 | 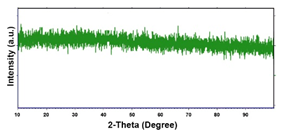 |
| Pd–Co (−156 mA cm−2 0.035 M Pd2+, 0.17 M Co2+, 300 s) | 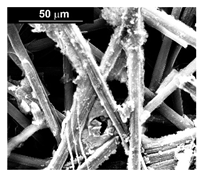 | 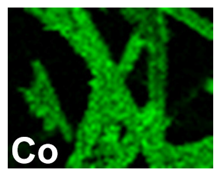 | 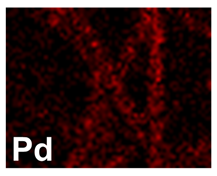 |
| Deposition Time, s | Pd–Co−1.56 mAcm−2 | Pd–Co−15.6 mA cm−2 | ||
|---|---|---|---|---|
| Pd% | i−0.8V(NHE) mAcm−2 | Pd% | i−0.8V(NHE) mAcm−2 | |
| 1800 | 40.6 ± 1.4 | −242 ± 8 | 56.1 ± 1.2 | −238 ± 10 |
| 900 | 65.8 ± 1.1 | −233 ± 10 | 56.6 ± 1.6 | −220 ± 12 |
| 180 | 75.7 ± 1.2 | −391 ± 9 | 91.1 ± 1.4 | −261 ± 7 |
| 60 | 80.6 ± 1.6 | −415 ± 13 | 96.5 ± 1.2 | −372 ± 8 |
| Deposition Time, s | −1.56 mAcm−2 | −15.6 mAcm−2 | −156 mAcm−2 |
|---|---|---|---|
| 1800 | 16.9 ± 1.2 | 8.4 ± 1.1 | 5.1 ± 1.1 |
| 900 | 27.1 ± 1.1 | 9.8 ± 1.2 | 7.5 ± 1.2 |
| 180 | 65.2 ± 1.2 | 30.2 ± 1.2 | 47.9 ± 1.4 |
| 60 | 72.1 ± 1.1 | 45.5 ± 1.3 | 82.2 ± 1.2 |
| Deposition Time, s | Pd | Co | ||
|---|---|---|---|---|
| −1.56 mAcm−2 | −15.6 mAcm−2 | −1.56 mAcm−2 | −15.6 mAcm−2 | |
| 3600 | −382 ± 12 | −412 ± 15 | −80 ± 6 | −281 ± 9 |
| 1800 | −288 ± 11 | −313 ± 12 | −65 ± 4 | −80 ± 6 |
| 900 | −264 ± 10 | −295 ± 10 | −56 ± 4 | −71 ± 4 |
| 60 | −199 ± 6 | −265 ± 9 | −43 ± 4 | −62 ± 6 |
| Sample | Test Conditions | Potential for HER | Reference |
|---|---|---|---|
| Pd–Co@CN | 0.5 M H2SO4 −36 mAcm−2 | −0.1 V/NHE | [106] |
| Pd–Co alloy on carbon cloth | 0.5 M H2SO4 −60 mAcm−2 | −0.075 V/NHE | [107] |
| Pd–Co@N–C/rGO | 0.5 M H2SO4 −10 mAcm−2 | −0.158 V/NHE | [99] |
| Pd–Co on Carbon Paper | 2 M H2SO4 −50 mAcm−2 | −0.12 V/NHE | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patella, B.; Zanca, C.; Ganci, F.; Carbone, S.; Bonafede, F.; Aiello, G.; Miceli, R.; Pellitteri, F.; Mandin, P.; Inguanta, R. Pd–Co-Based Electrodes for Hydrogen Production by Water Splitting in Acidic Media. Materials 2023, 16, 474. https://doi.org/10.3390/ma16020474
Patella B, Zanca C, Ganci F, Carbone S, Bonafede F, Aiello G, Miceli R, Pellitteri F, Mandin P, Inguanta R. Pd–Co-Based Electrodes for Hydrogen Production by Water Splitting in Acidic Media. Materials. 2023; 16(2):474. https://doi.org/10.3390/ma16020474
Chicago/Turabian StylePatella, Bernardo, Claudio Zanca, Fabrizio Ganci, Sonia Carbone, Francesco Bonafede, Giuseppe Aiello, Rosario Miceli, Filippo Pellitteri, Philippe Mandin, and Rosalinda Inguanta. 2023. "Pd–Co-Based Electrodes for Hydrogen Production by Water Splitting in Acidic Media" Materials 16, no. 2: 474. https://doi.org/10.3390/ma16020474
APA StylePatella, B., Zanca, C., Ganci, F., Carbone, S., Bonafede, F., Aiello, G., Miceli, R., Pellitteri, F., Mandin, P., & Inguanta, R. (2023). Pd–Co-Based Electrodes for Hydrogen Production by Water Splitting in Acidic Media. Materials, 16(2), 474. https://doi.org/10.3390/ma16020474









