Superior Comprehensive Mechanical Properties of a Low-Carbon Medium Manganese Steel for Replacing AISI 4330 Steel in the Oil and Gas Industry
Abstract
:1. Introduction
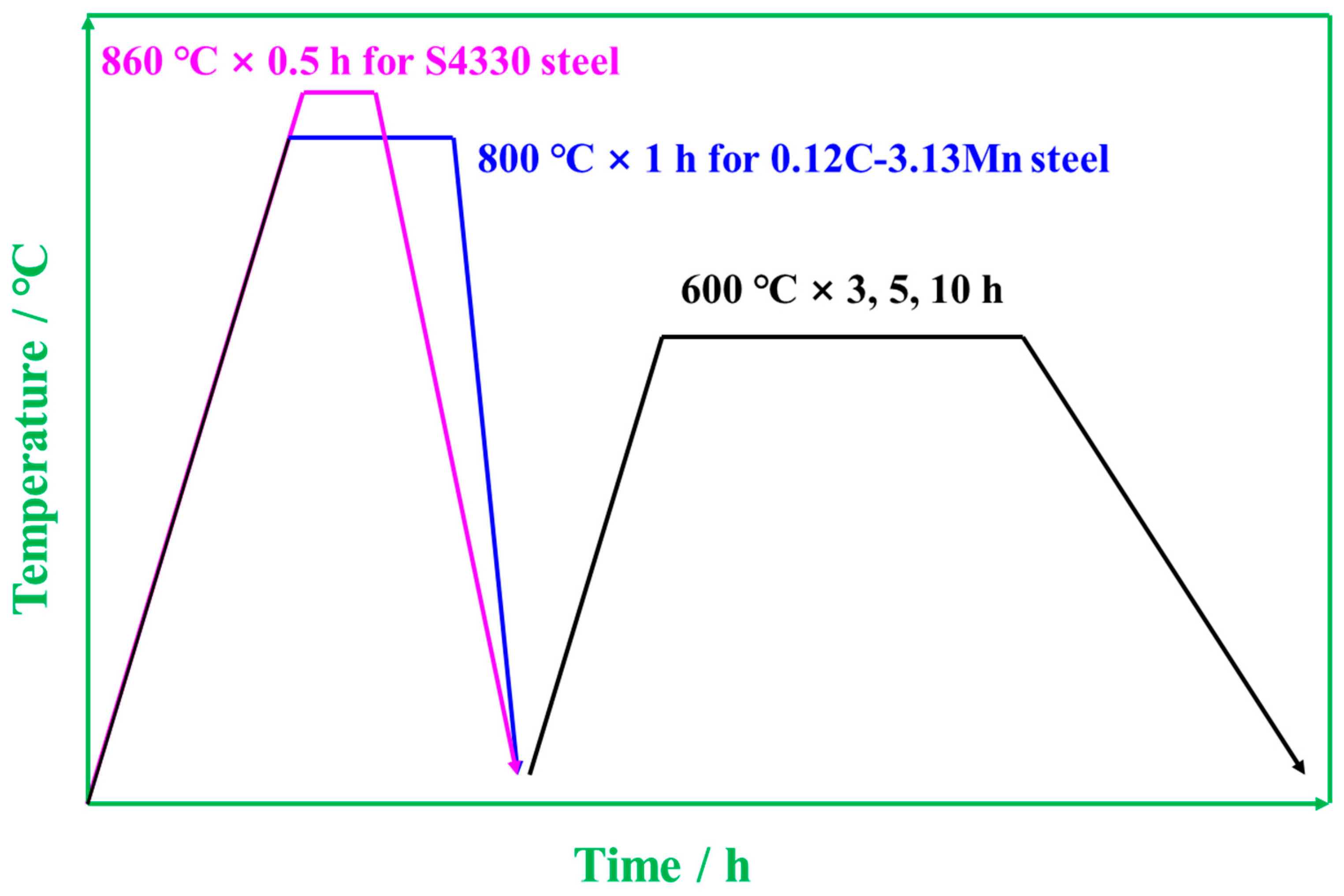
2. Materials and Methods
3. Results
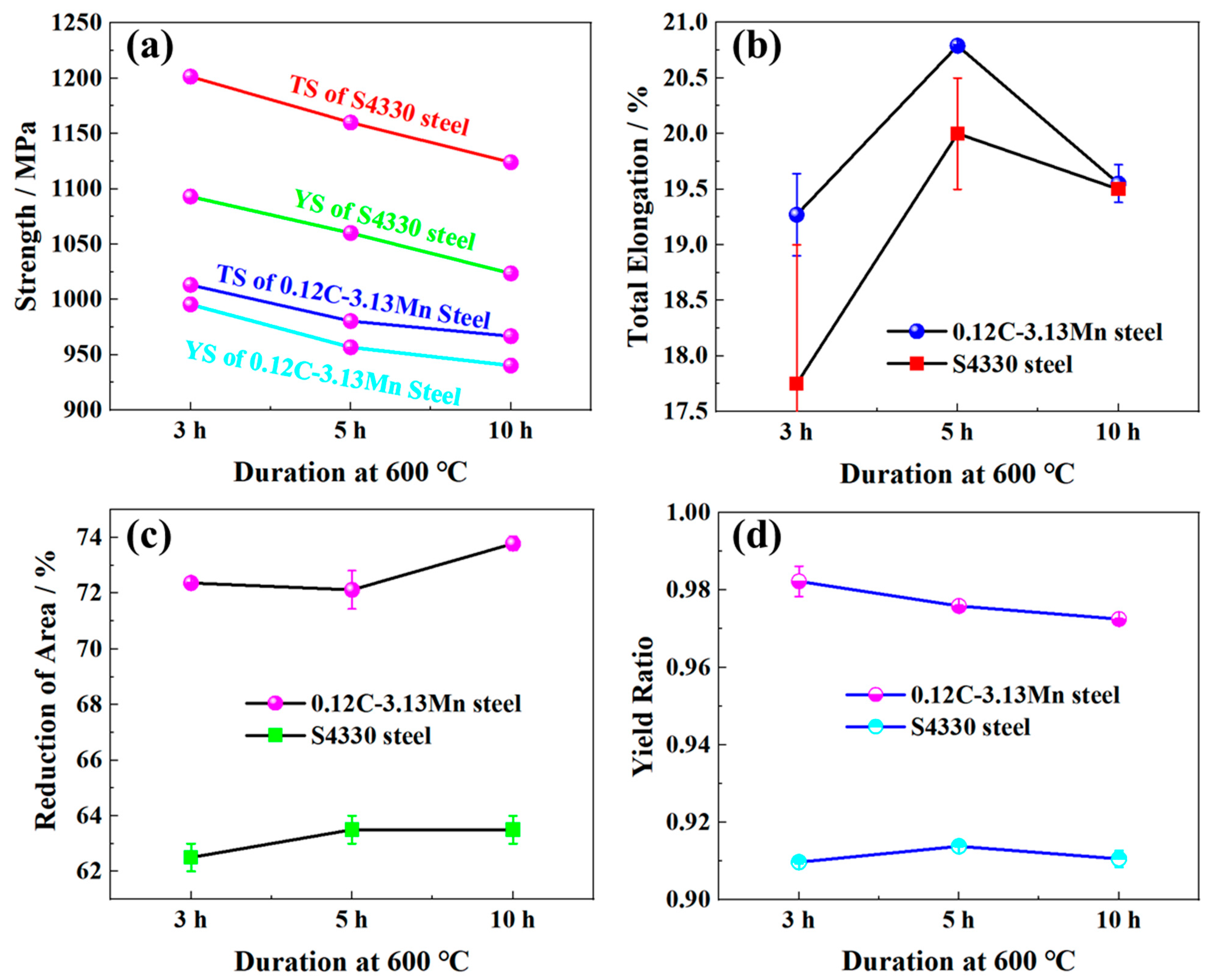
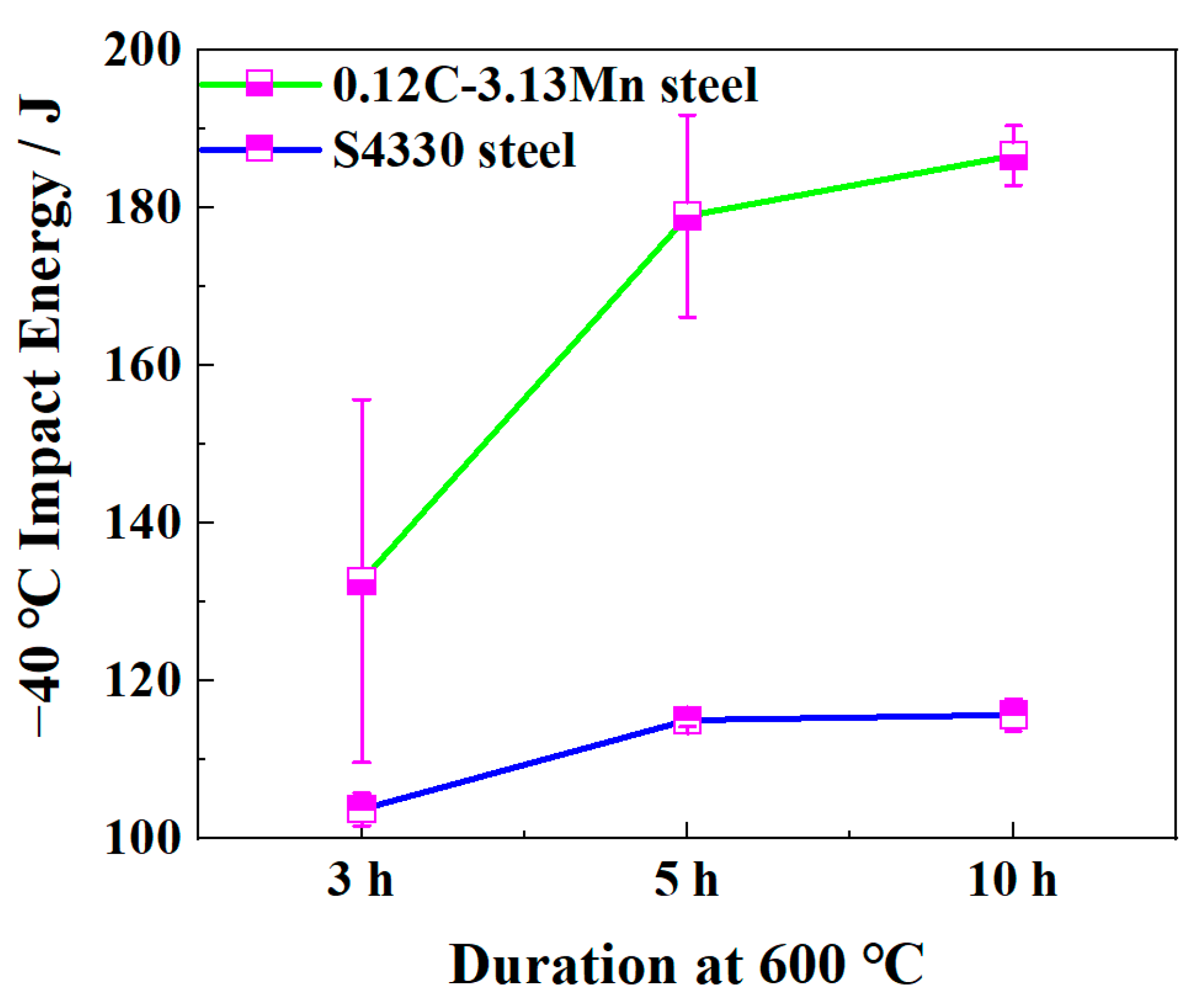
3.1. Mechanical Properties
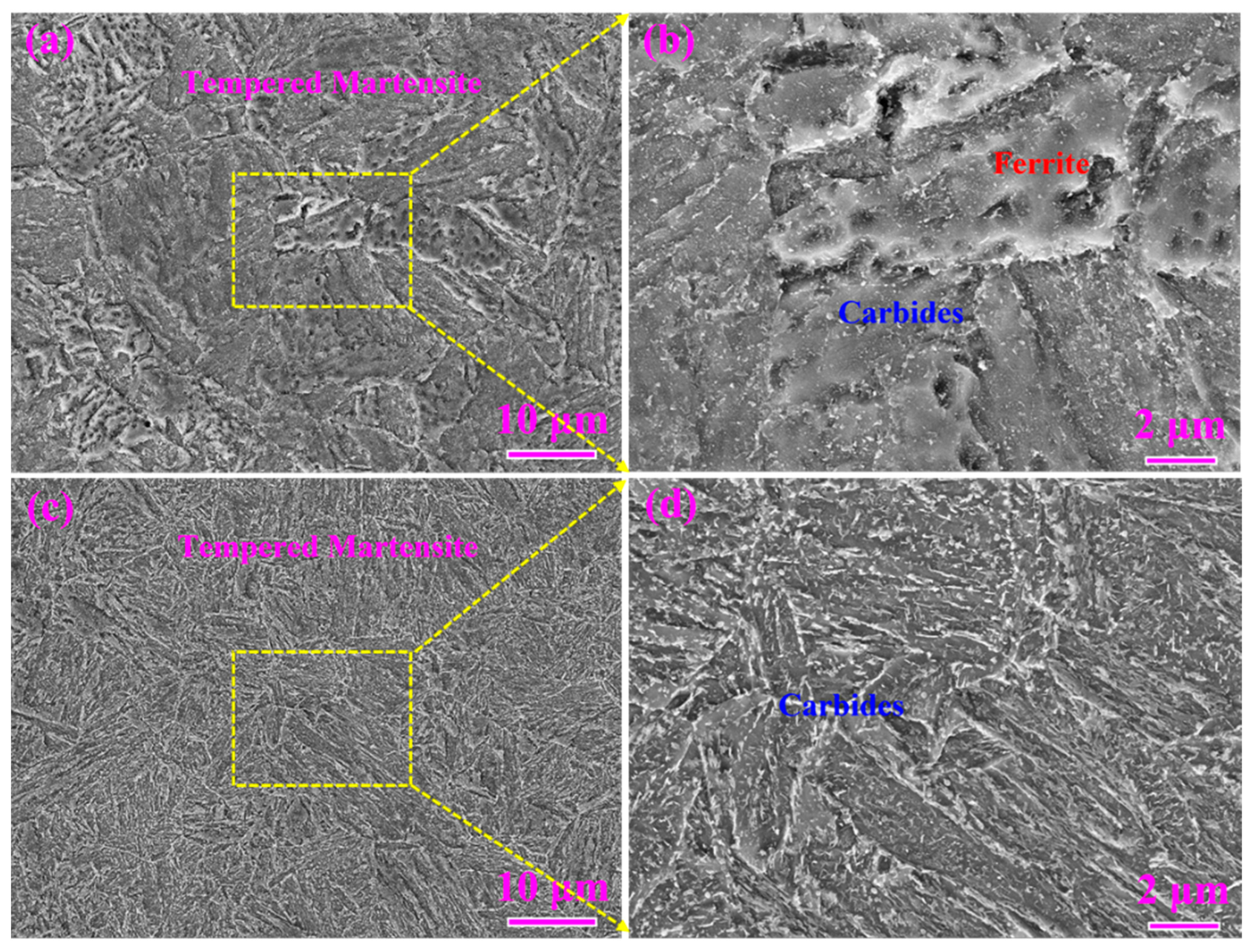
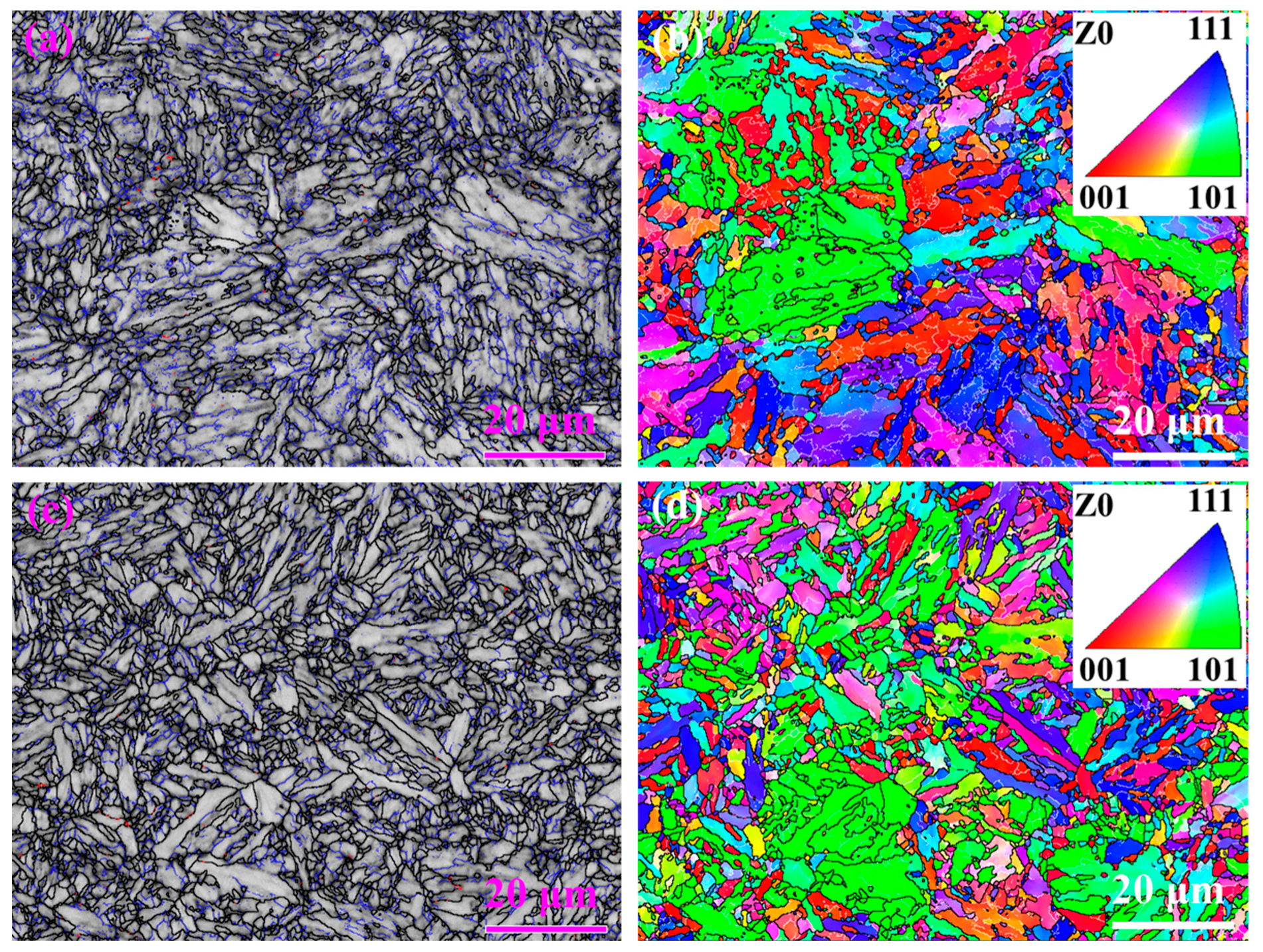
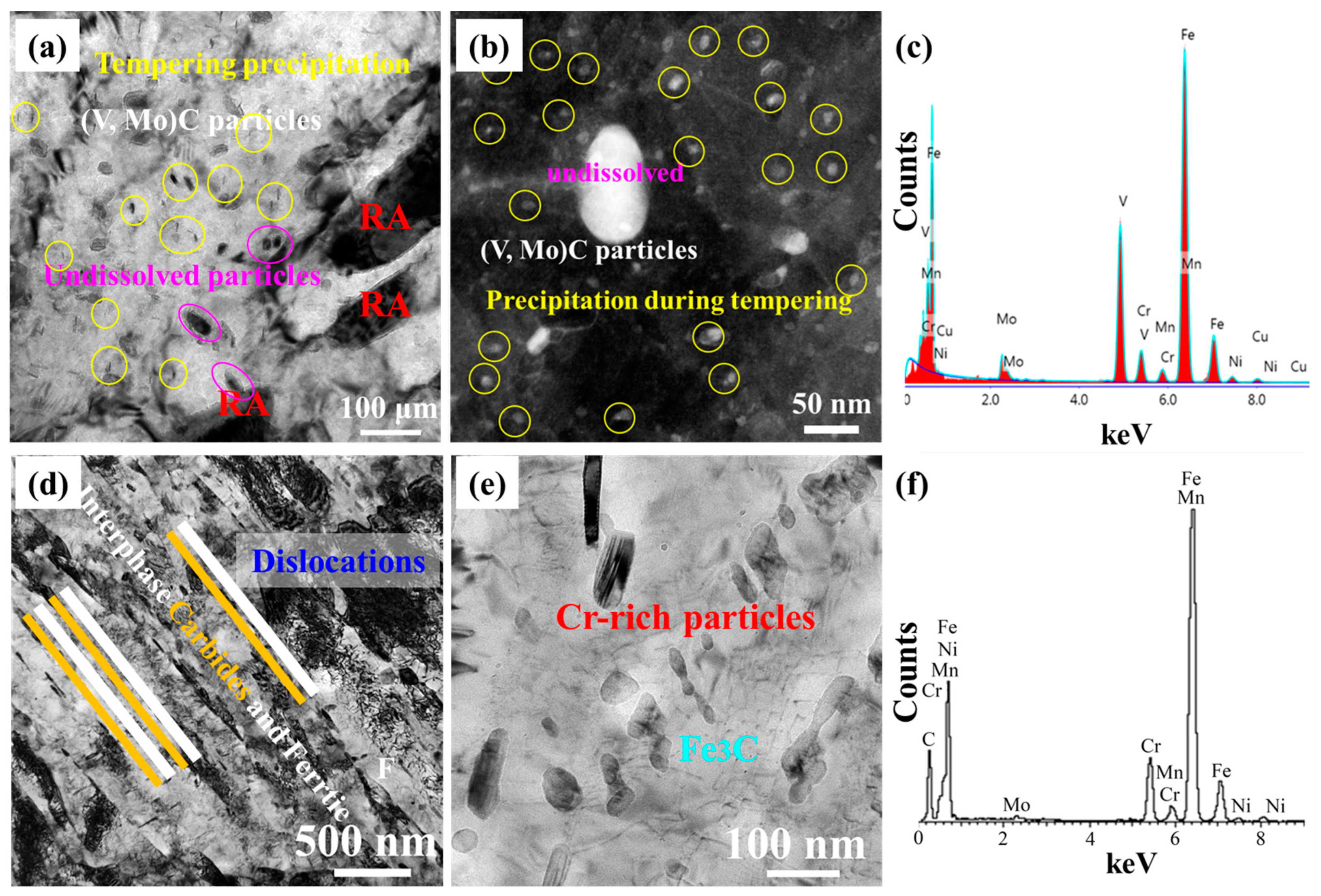
3.2. Microstructure Characterization
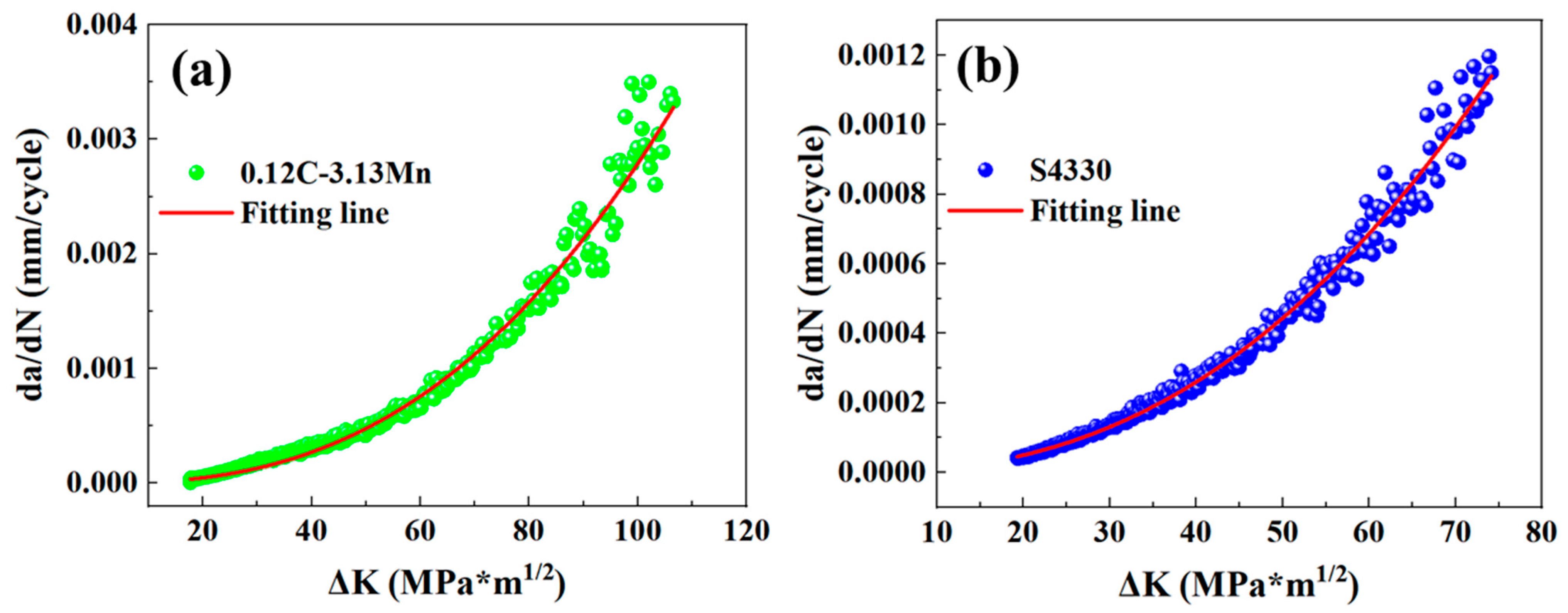
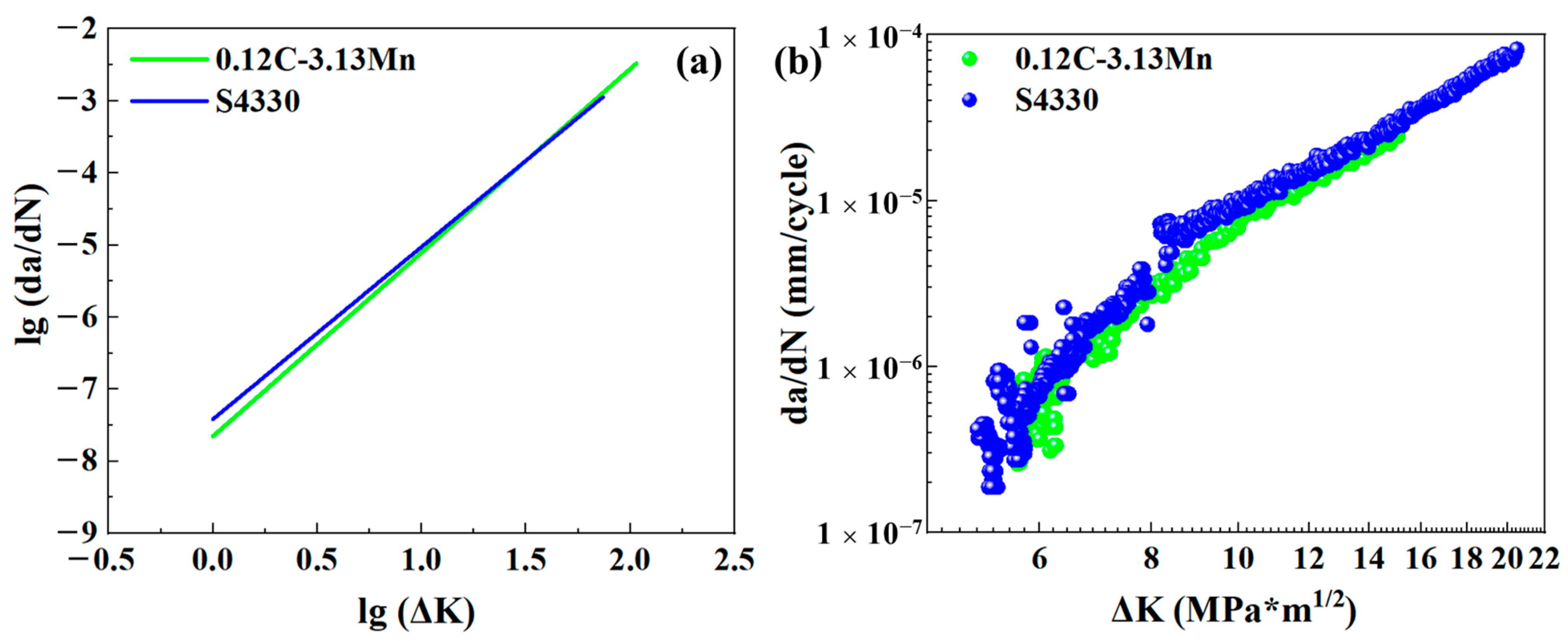
3.3. Fatigue Crack Growth Rate
4. Discussions
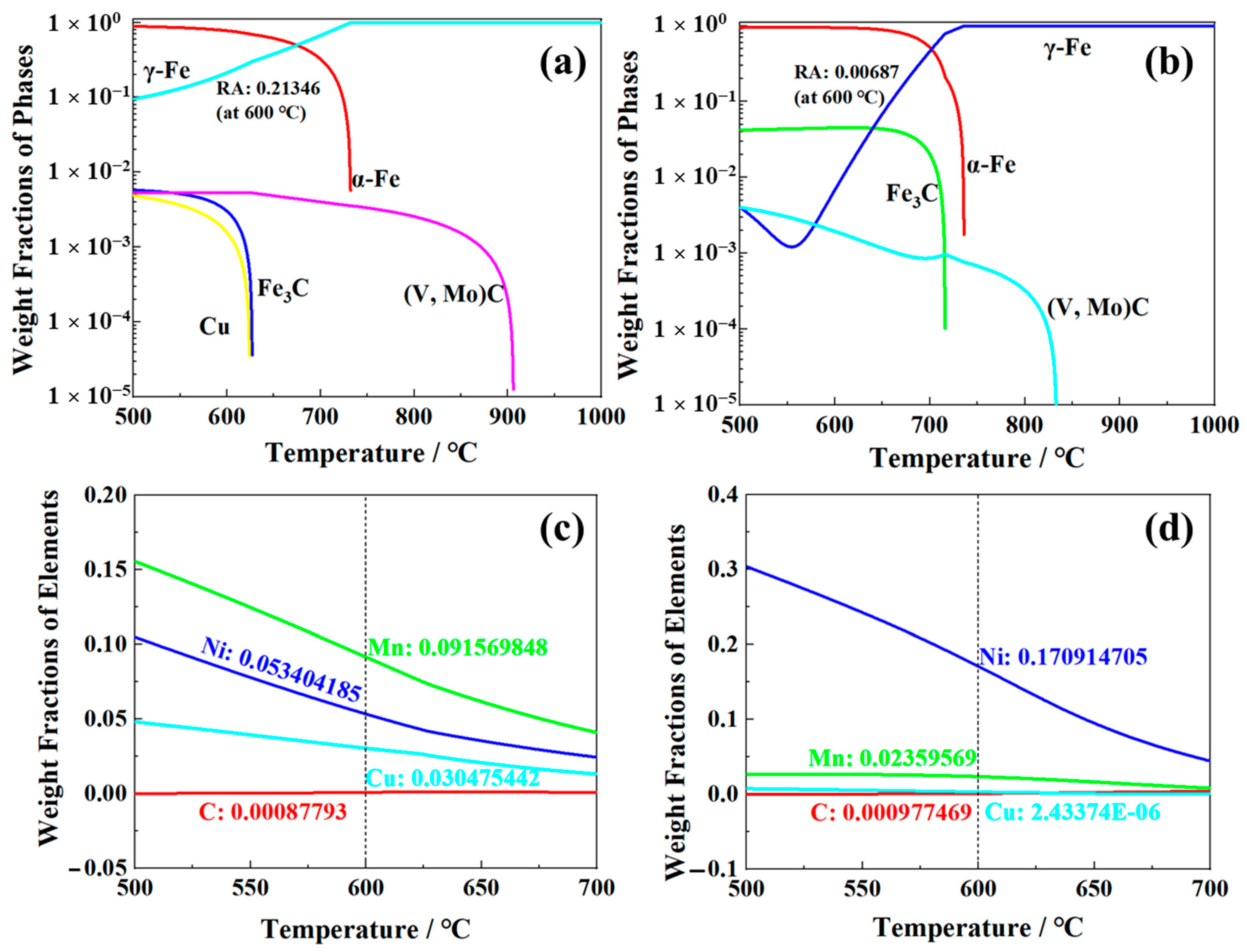
4.1. “Medium Manganese Design” for Optimizing the RA Transformation
4.2. Precipitation Strengthening and Toughening Mechanism Analysis
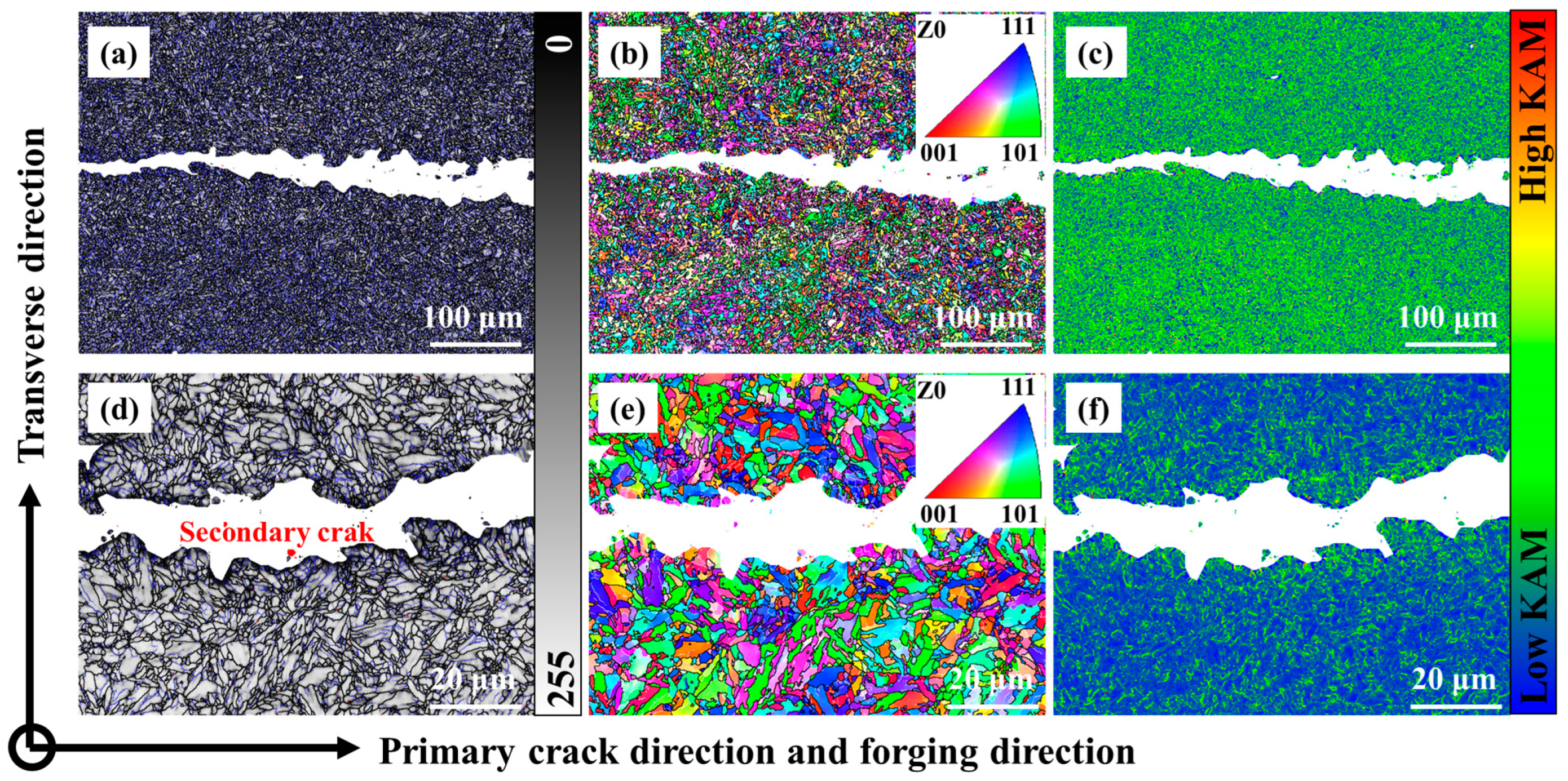
4.3. Fatigue Mechanism Analysis
5. Conclusions
- (1)
- The yield strength of 0.12C-3.13Mn steel at 957 MPa was slightly lower than that of S4330 steel at 1060 MPa when subjected to the same tempering process (600 °C for 5 h), but the ductility of the former was superior to that of the latter. The main reason for the lower strength was the low carbon content, dislocation density, and larger effective grain size.
- (2)
- The −40 °C impact energy of 0.12C-3.13Mn steel reached 179 J, which was obviously higher than that of S4330 steel (about 115 J), resulting from the lower dislocation density and the presence of 5.3% RA.
- (3)
- The (V, Mo)C and Cu-rich particles jointly provided a high precipitation strengthening, leading to a higher yield ratio.
- (4)
- The 0.12C-3.13Mn steel showed a higher capacity to inhibit crack initiation than the S4330 steel due to the higher 5.23 MPa*m1/2. The FCGR of 0.12C-3.13Mn steel increased more rapidly than S4330 steel, and it was higher than that of S4330 steel at the end of the second stage. However, the FCGR of 0.12C-3.13Mn steel finished at 0.00330 mm/cycle, which was much higher than the latter at 0.00114 mm/cycle. The high tolerance of FCG in 0.12C-3.13Mn steel was due to the presence of RA and secondary cracks.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Alloying: Understanding the Basics; ASM International: Almere, The Netherlands, 2001. [Google Scholar]
- Parker, E.R.; Zackay, V.F. Microstructural features affecting fracture toughness of high strength steels. Eng. Fract. Mech. 1975, 7, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Chun, S. The effect of thermal cycling treatments on the microstructures and mechanical properties of AISI 4330 steel. J. Korean Inst. Met. 1981, 19, 208–217. [Google Scholar]
- Kiani, M.; Ishak, J.; Walker, R.; Babaeidarabad, S. Numerical Modeling and Experimental Investigation of M-4330 Low Alloy and 17–4 PH Stainless Steels Low Cycle Fatigue Behavior. Exp. Tech. 2018, 42, 93–104. [Google Scholar] [CrossRef]
- Bitterlin, M.; Shahriari, D.; Lapierre-Boire, L.-P.; Morin, J.-B.; Jahazi, M. Hot deformation behavior of a nickel-modified AISI 4330 steel. ISIJ Int. 2018, 58, 1711–1720. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.N.; Dikshit, S.; Malakondaiah, G.; Rao, P.R. On mixed upper bainite-martensite in an AISI 4330 steel exhibiting an uncommonly improved strength-toughness combination. Scr. Metall. Et Mater. 1990, 24, 1323–1328. [Google Scholar] [CrossRef]
- Saxena, V.K.; Malakondaiah, G.; Radhakrishnan, V.; Rao, P.R. On the fatigue crack growth resistance of upper bainite-martensite in an AISI 4330 steel. Scr. Metall. Et Mater. 1993, 28, 1257–1260. [Google Scholar] [CrossRef]
- Mangonon, P.L. Hardenabilities of vanadium-modified 4330 low alloy constructional steels. J. Heat Treat. 1980, 1, 47–60. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.; Shang, C.; Wang, Y.; He, X. Influence of carbon content and microstructure on corrosion behaviour of low alloy steels in a Cl− containing environment. Corros. Sci. 2009, 51, 242–251. [Google Scholar] [CrossRef]
- Van Zwieten, A.C.T.M.; Bulloch, J.H. Some considerations on the toughness properties of ferritic stainless steels—A brief review. Int. J. Press. Vessel. Pip. 1993, 56, 1–31. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, F.; Wang, Y.; Wang, Q.; Huang, M.; Wang, Y.; Chen, C.; Zhang, F. Making composite steel higher strength and higher ductility via introducing carbon diffusion strategy. Mater. Res. Lett. 2021, 9, 391–397. [Google Scholar] [CrossRef]
- Yang, F.; Luo, H.; Hu, C.; Pu, E.; Dong, H. Effects of intercritical annealing process on microstructures and tensile properties of cold-rolled 7Mn steel. Mater. Sci. Eng. A 2017, 685, 115–122. [Google Scholar] [CrossRef]
- Luo, H.; Shi, J.; Wang, C.; Cao, W.; Sun, X.; Dong, H. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5Mn steel. Acta Mater. 2011, 59, 4002–4014. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, G.; Zhang, K.; Sun, X.; Liang, X.; Yong, Q. Effect of Nb microalloying on microstructure evolution and mechanical properties in low carbon medium manganese steel. Mater. Sci. Eng. A 2021, 824, 141813. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, X.; Zhang, M.; Li, Z.; Ye, X.; Zhu, Z.; Huang, Z.; Yong, Q. Kinetics of (Ti, V, Mo) C precipitated in γ/α matrix of Ti-V-Mo complex microalloyed steel. Acta Metall. Sin. 2018, 54, 1122–1130. [Google Scholar]
- Ju, Y.; Goodall, A.; Strangwood, M.; Davis, C. Characterisation of precipitation and carbide coarsening in low carbon low alloy Q&T steels during the early stages of tempering. Mater. Sci. Eng. A 2018, 738, 174–189. [Google Scholar]
- Yang, K.; Ren, Y. Nickel-free austenitic stainless steels for medical applications. Sci. Technol. Adv. Mater. 2010, 11, 014105. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, G.; Sun, X. Different roles of reversed austenite, athermal martensite and tempered martensite on low-temperature toughness in ultra-low carbon medium Mn steel. Mater. Lett. 2021, 297, 129958. [Google Scholar] [CrossRef]
- Li, Z.; Misra, R.; Cai, Z.; Li, H.; Ding, H. Mechanical properties and deformation behavior in hot-rolled 0.2 C-1.5/3Al-8.5 Mn-Fe TRIP steel: The discontinuous TRIP effect. Mater. Sci. Eng. A 2016, 673, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Speich, G.R.; Leslie, W.C. Tempering of steel. Metall. Trans. 1972, 3, 1043–1054. [Google Scholar] [CrossRef]
- Wang, F.; Ye, X.; Ren, S.; Zhang, K.; Liang, X.; Liu, G. Effect of Intercritical Annealing Time on Microstructure Evolution and Mechanical Properties of Low Carbon Medium Manganese Steel Subjected to Multi-Step Heat Treatment Process. Materials 2022, 15, 2425. [Google Scholar] [CrossRef]
- Ungár, T.; Dragomir, I.; Révész, Á.; Borbély, A. The contrast factors of dislocations in cubic crystals: The dislocation model of strain anisotropy in practice. J. Appl. Crystallogr. 1999, 32, 992–1002. [Google Scholar] [CrossRef] [Green Version]
- HajyAkbary, F.; Sietsma, J.; Böttger, A.J.; Santofimia, M.J. An improved X-ray diffraction analysis method to characterize dislocation density in lath martensitic structures. Mater. Sci. Eng. A 2015, 639, 208–218. [Google Scholar] [CrossRef]
- Bigerelle, M.; Iost, A. Bootstrap analysis of FCGR, application to the Paris relationship and to lifetime prediction. Int. J. Fatigue 1999, 21, 299–307. [Google Scholar] [CrossRef]
- Amsterdam, E.; Wiegman, J.; Nawijn, M.; De Hosson, J.T.M. The effect of crack length and maximum stress on the fatigue crack growth rates of engineering alloys. Int. J. Fatigue 2022, 161, 106919. [Google Scholar] [CrossRef]
- Liang, X.; Fu, H.; Cui, M.; Liu, G. Effect of intercritical tempering temperature on microstructure evolution and mechanical properties of high strength and toughness medium manganese steel. Materials 2022, 15, 2162. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.; Sun, G.; Xie, H.; Misra, R.D.K. The determining role of reversed austenite in enhancing toughness of a novel ultra-low carbon medium manganese high strength steel. Scr. Mater. 2015, 104, 87–90. [Google Scholar] [CrossRef]
- Guo, Q.; Yen, H.-W.; Luo, H.; Ringer, S.P. On the mechanism of Mn partitioning during intercritical annealing in medium Mn steels. Acta Mater. 2022, 225, 117601. [Google Scholar] [CrossRef]
- Varanasi, R.S.; Gault, B.; Ponge, D. Effect of Nb micro-alloying on austenite nucleation and growth in a medium manganese steel during intercritical annealing. Acta Mater. 2022, 229, 117786. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Zhang, T.; Dorin, T.; Sun, X. Superior low temperature toughness in a newly designed low Mn and low Ni high strength steel. Mater. Sci. Eng. A 2021, 825, 141899. [Google Scholar] [CrossRef]
- Rong, X.; Hu, B.; Guo, H.; Enomoto, M.; Shang, C. Influence of cold rolling on the stability of retained austenite and mechanical properties of a Cu bearing low carbon low manganese steel. Mater. Sci. Eng. A 2022, 850, 143455. [Google Scholar] [CrossRef]
- Capdevila, C.; FG, C.; De Andrés, C.G. Determination of Ms temperature in steels: A Bayesian neural network model. ISIJ Int. 2002, 42, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-N.; Chang, L.-W.; Hsu, C.-A.; Yang, J.-R.; Chang, H.-Y.; Wang, S.-H.; Chen, H.-R. Microstructural variation in fatigued interphase arrayed nano-precipitated Ti-microalloyed steel. J. Mater. Res. Technol. 2021, 15, 2393–2404. [Google Scholar] [CrossRef]
- Russell, K.C.; Brown, L.M. A dispersion strengthening model based on differing elastic moduli applied to the iron-copper system. Acta Metall. 1972, 20, 969–974. [Google Scholar] [CrossRef]
- Nakashima, K.; Futamura, Y.; Tsuchiyama, T.; Takaki, S. Interaction between dislocation and copper particles in Fe-Cu alloys. ISIJ Int. 2002, 42, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Timokhina, I.; Pereloma, E. Clustering, nano-scale precipitation and strengthening of steels. Prog. Mater. Sci. 2021, 118, 100764. [Google Scholar] [CrossRef]
- Ma, X.; Miao, C.; Langelier, B.; Subramanian, S. Suppression of strain-induced precipitation of NbC by epitaxial growth of NbC on pre-existing TiN in Nb-Ti microalloyed steel. Mater. Des. 2017, 132, 244–249. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, Y.; Wang, L. The interaction between ε-copper and dislocation in a high copper 17-4PH steel. Mater. Sci. Eng. A 2019, 756, 319–327. [Google Scholar] [CrossRef]
- Zurob, H.; Hutchinson, C.; Brechet, Y.; Purdy, G. Modeling recrystallization of microalloyed austenite: Effect of coupling recovery, precipitation and recrystallization. Acta Mater. 2002, 50, 3077–3094. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Shi, J.; Hui, W.; Dong, H. Effect of microstructural refinement on the toughness of low carbon martensitic steel. Scr. Mater. 2008, 58, 492–495. [Google Scholar] [CrossRef]
- Barbosa, L.H.S.; Modenesi, P.J.; Godefroid, L.B.; Arias, A.R. Fatigue crack growth rates on the weld metal of high heat input submerged arc welding. Int. J. Fatigue 2019, 119, 43–51. [Google Scholar] [CrossRef]
- Cabrilo, A.; Sedmak, A.; Burzic, Z.; Perkovic, S. Fracture mechanics and fatigue crack propagation in armor steel welds. Eng. Fail. Anal. 2019, 106, 104155. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Kou, S.Q.; Jiang, L.G.; Lindqvist, P.A. Effects of loading rate on rock fracture: Fracture characteristics and energy partitioning. Int. J. Rock Mech. Min. Sci. 2000, 37, 745–762. [Google Scholar] [CrossRef]




| Samples | C | Si | Mn | Cr | Ni | Mo | V | Cu |
|---|---|---|---|---|---|---|---|---|
| 0.12C-3.13Mn | 0.12 | 0.20 | 3.13 | 1.01 | 1.90 | 0.41 | 0.29 | 1.0 |
| S4330 | 0.33 | 0.25 | 0.52 | 1.07 | 2.95 | 0.45 | 0.05 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Liu, G.; Liang, X.; Tong, S. Superior Comprehensive Mechanical Properties of a Low-Carbon Medium Manganese Steel for Replacing AISI 4330 Steel in the Oil and Gas Industry. Materials 2023, 16, 490. https://doi.org/10.3390/ma16020490
Sun X, Liu G, Liang X, Tong S. Superior Comprehensive Mechanical Properties of a Low-Carbon Medium Manganese Steel for Replacing AISI 4330 Steel in the Oil and Gas Industry. Materials. 2023; 16(2):490. https://doi.org/10.3390/ma16020490
Chicago/Turabian StyleSun, Xinjun, Gang Liu, Xiaokai Liang, and Shuai Tong. 2023. "Superior Comprehensive Mechanical Properties of a Low-Carbon Medium Manganese Steel for Replacing AISI 4330 Steel in the Oil and Gas Industry" Materials 16, no. 2: 490. https://doi.org/10.3390/ma16020490





