Smart PEG-Block-PLA/PLA Nanosystems: Impact of the Characteristics of the Polymer Blend on the Redox Responsiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
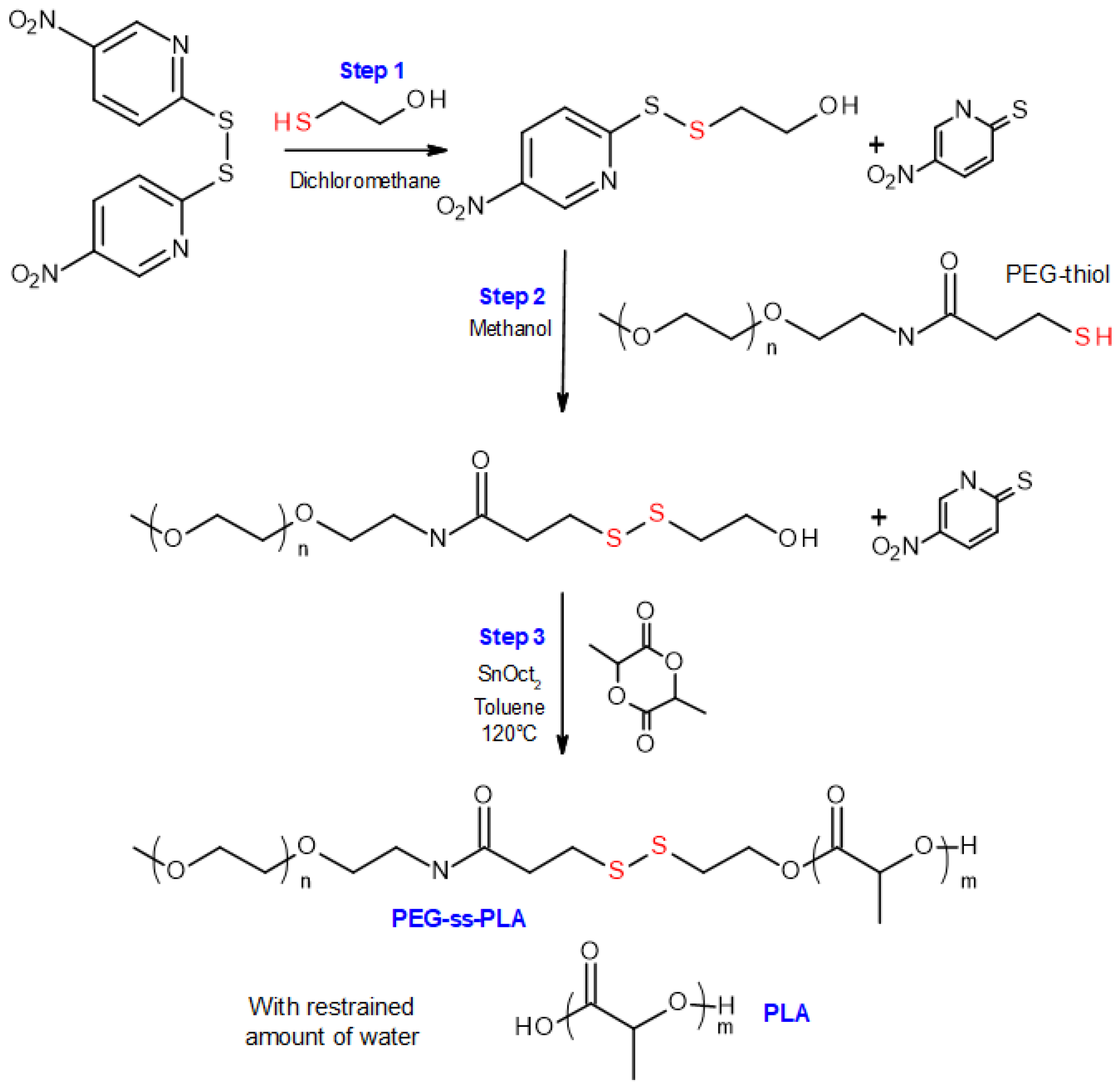
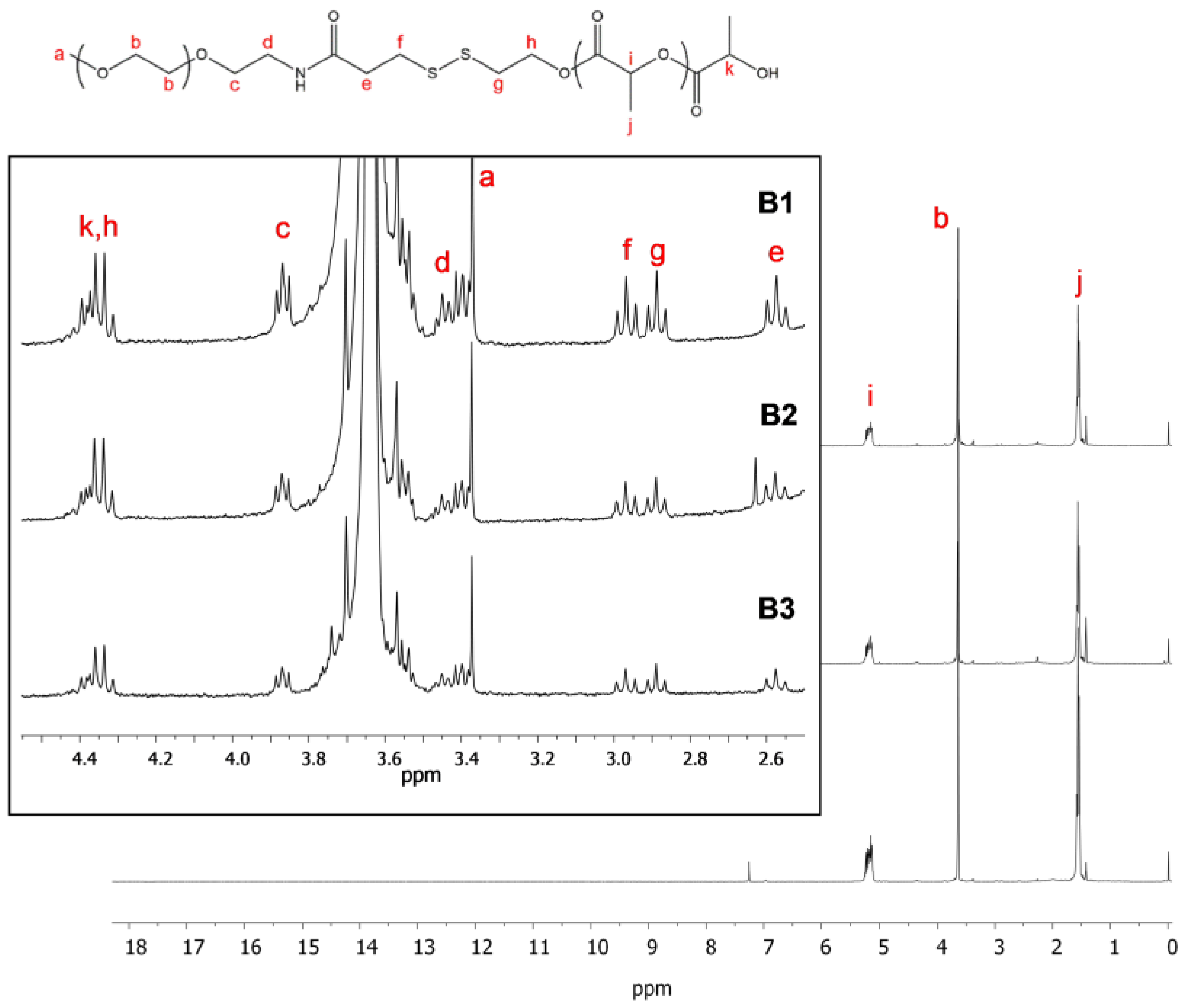
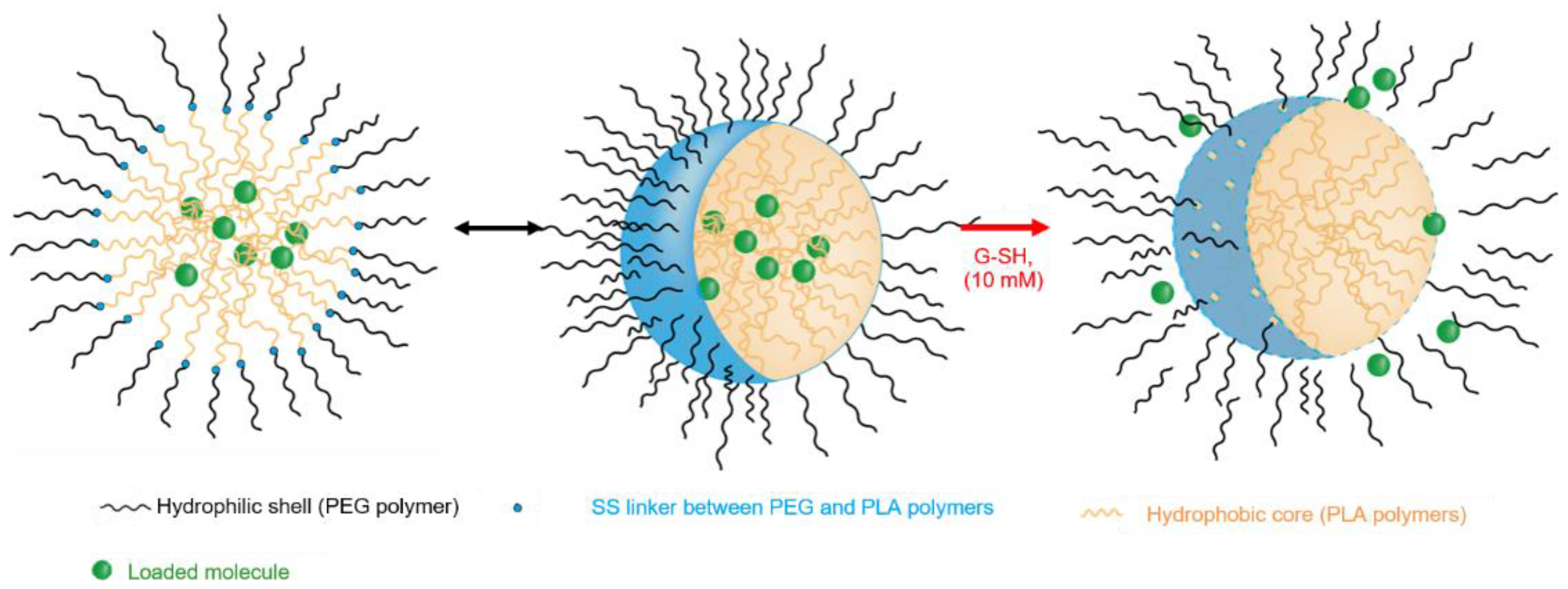
2.2. Synthesis and Characterization of PEG-b-PLA/PLA Redox-Responsive Blend
2.3. Preparation and Characterization of Polymer Nanocarriers
nanocarriers/Initial weight of Nile red placed in the reaction medium
2.4. Response to the Redox Stimulus of Copolymer-Based Nanocarriers
t)/Fluorescence intensity t0 × 100
diluted nitric acid solution × 100
3. Results
3.1. Synthesis and Characterization of Redox-Responsive PEG-Block-PLA/PLA Blends
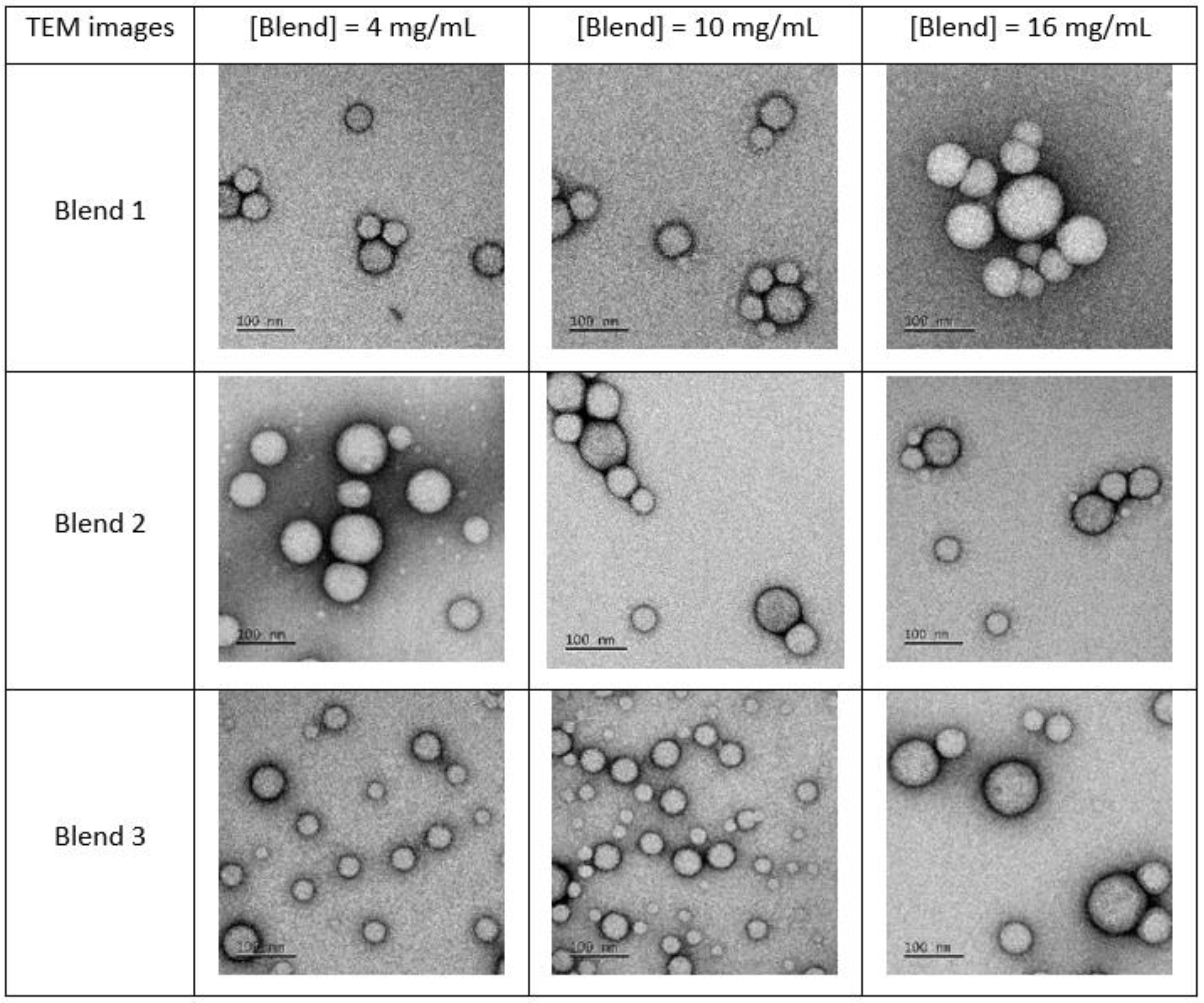
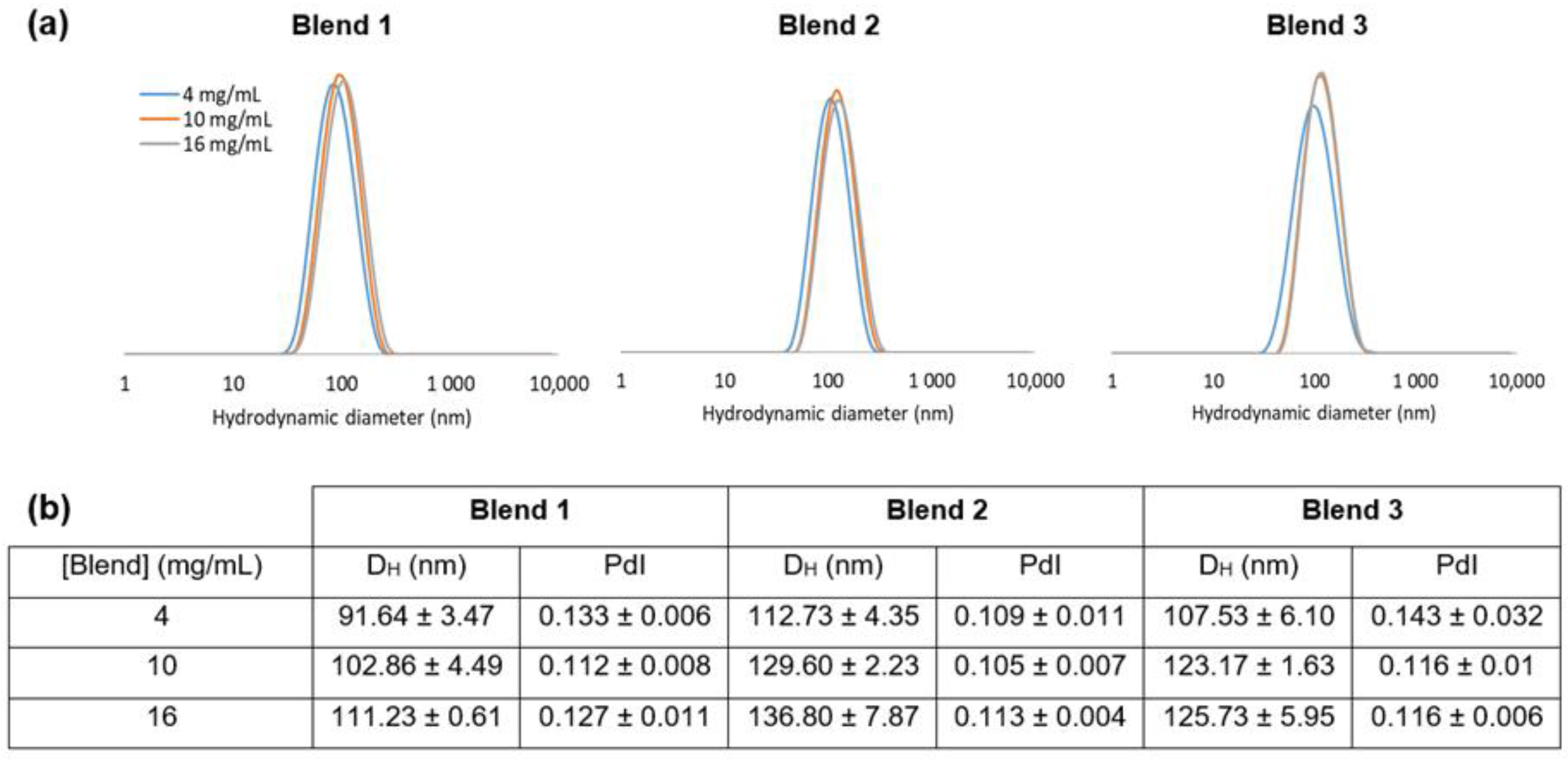
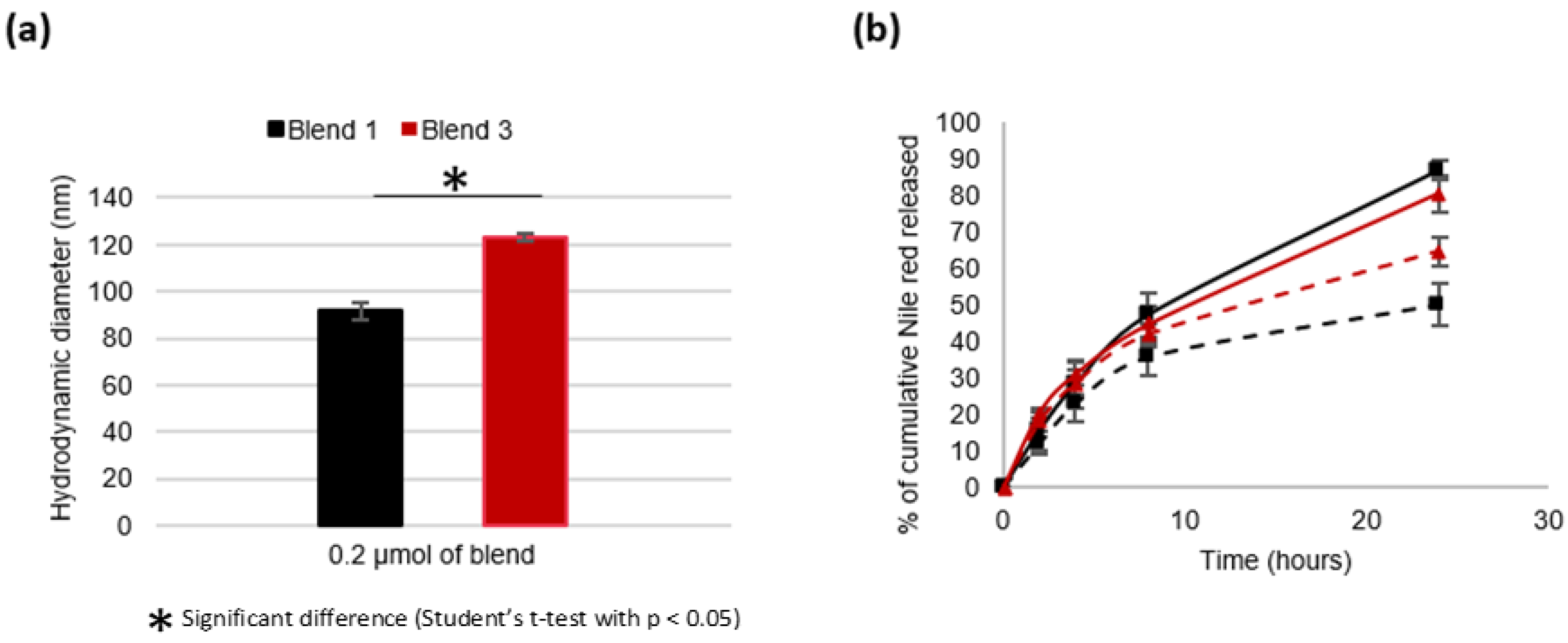
3.2. Nanocarriers Physicochemical Characteristics
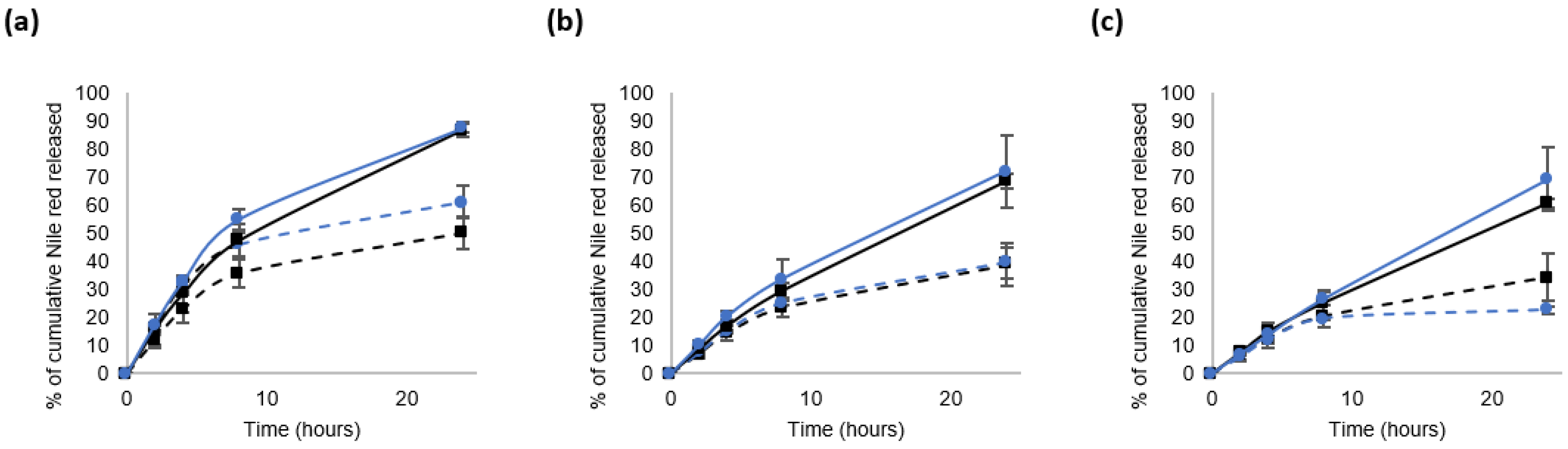
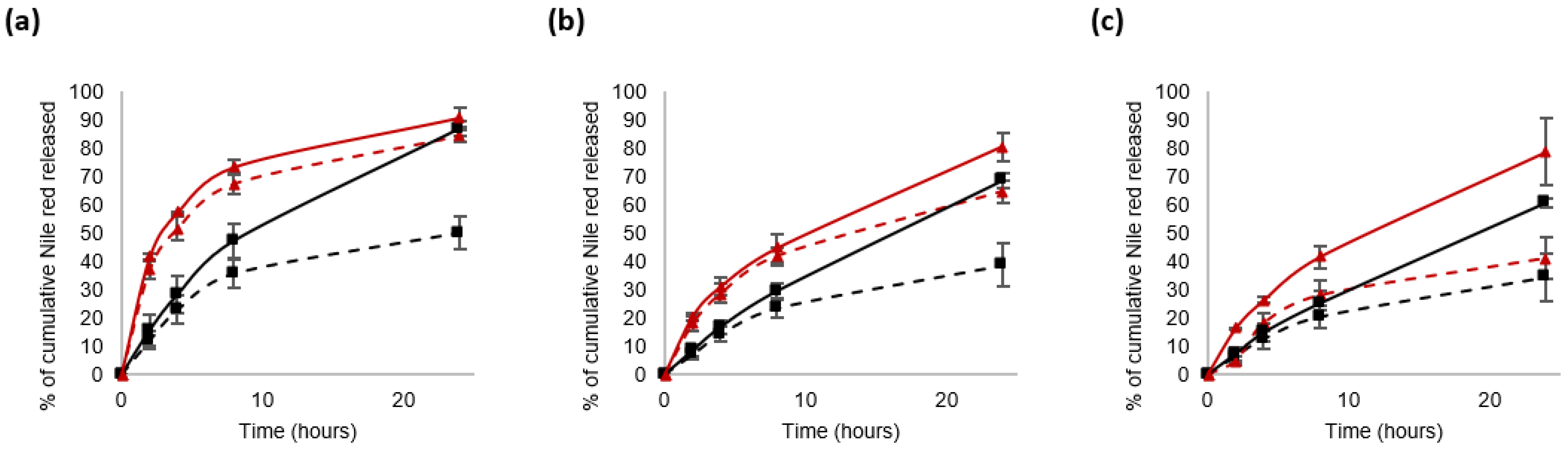
3.3. Nile Red Release from the Nanocarriers in a Reductive Medium
3.3.1. Impact of the mPEG-SS-PLA/PLA Ratio on the Physico-Chemical Characteristics of NCs
3.3.2. Impact of the Molecular Weight of PLA Blocks on the Physico-Chemical Characteristics of the Nanocarriers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salahpour-Anarjan, F.; Nezhad-Mokhtari, P.; Akbarzadeh, A. Smart Drug Delivery Systems. In Modeling and Control of Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–44. [Google Scholar] [CrossRef]
- Wang, S.; Xie, L.; Liu, Y.; Yang, Q.; Jia, W.; Zhao, D.; Zhao, X. Study on the Preparation and Activity of Intelligent Response Poly(Lactic-Co-Glycolic Acid)-Ss-Polyethylene Glycol Copolymer Micelles. J. Biomater. Appl. 2022, 37, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, P.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Bio-Based ABA Triblock Copolymers with Central Degradable Moieties. Eur. Polym. J. 2021, 147, 110321. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, X.; Yu, Y.; Li, W.; Huang, H.; Zhang, W.; Hu, Z.; Li, Z. Reduction-sensitive Carrier Containing Disulfide Bond Based on Pluronic F68 with Cholesterol for Drug Delivery. Polym. Int. 2021, 70, 107–115. [Google Scholar] [CrossRef]
- Kim, S.; Wittek, K.I.; Lee, Y. Synthesis of Poly(Disulfide)s with Narrow Molecular Weight Distributions via Lactone Ring-Opening Polymerization. Chem. Sci. 2020, 11, 4882–4886. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ding, J.; He, C.; Cao, Y.; Xu, W.; Chen, X. Disulfide Cross-Linked Polyurethane Micelles as a Reduction-Triggered Drug Delivery System for Cancer Therapy. Adv. Healthc. Mater. 2014, 3, 752–760. [Google Scholar] [CrossRef]
- Xu, C.; Iqbal, S.; Shen, S.; Luo, Y.; Yang, X.; Wang, J. Development of “CLAN” Nanomedicine for Nucleic Acid Therapeutics. Small 2019, 15, 1900055. [Google Scholar] [CrossRef]
- Laredj-Bourezg, F.; Bolzinger, M.-A.; Pelletier, J.; Valour, J.-P.; Rovère, M.-R.; Smatti, B.; Chevalier, Y. Skin Delivery by Block Copolymer Nanoparticles (Block Copolymer Micelles). Int. J. Pharm. 2015, 496, 1034–1046. [Google Scholar] [CrossRef]
- Sohail, M.; Yu, B.; Sun, Z.; Liu, J.; Li, Y.; Zhao, F.; Chen, D.; Yang, X.; Xu, H. Complex Polymeric Nanomicelles Co-Delivering Doxorubicin and Dimethoxycurcumin for Cancer Chemotherapy. Drug Deliv. 2022, 29, 1523–1535. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Al-Joufi, F.; Anwar, M.; Attia, M.F.; El-Bana, M.A. Curcumin-Loaded PLA-PEG Copolymer Nanoparticles for Treatment of Liver Inflammation in Streptozotocin-Induced Diabetic Rats. Colloids Surf. B Biointerfaces 2019, 177, 389–398. [Google Scholar] [CrossRef]
- Mundel, R.; Thakur, T.; Chatterjee, M. Emerging Uses of PLA–PEG Copolymer in Cancer Drug Delivery. 3 Biotech 2022, 12, 41. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Lee, J.S.; Hwang, S.J.; Lee, D.S.; Kim, S.C.; Kim, D.J. Formation of Poly(Ethylene Glycol)-Poly(ε-Caprolactone) Nanoparticles via Nanoprecipitation. Macromol. Res. 2009, 17, 72–78. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, S.-S. Methoxy Poly(Ethylene Glycol)-Poly(Lactide) (MPEG-PLA) Nanoparticles for Controlled Delivery of Anticancer Drugs. Biomaterials 2004, 25, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
- Känkänen, V.; Seitsonen, J.; Tuovinen, H.; Ruokolainen, J.; Hirvonen, J.; Balasubramanian, V.; Santos, H.A. Evaluation of the Effects of Nanoprecipitation Process Parameters on the Size and Morphology of Poly(Ethylene Oxide)-Block-Polycaprolactone Nanostructures. Int. J. Pharm. 2020, 590, 119900. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, S.-S. Nanoparticles of Poly(D,L-Lactide)/Methoxy Poly(Ethylene Glycol)-Poly(D,L-Lactide) Blends for Controlled Release of Paclitaxel. J. Biomed. Mater. Res. 2006, 78A, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, T.; Pappalardo, D.; Finne-Wistrand, A. Redox-Responsive Disulfide Cross-Linked PLA–PEG Nanoparticles. Macromolecules 2017, 50, 7052–7061. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, L.; He, C.; Liu, B.; Xu, Y.; Zhu, Z.; Shao, Z.; Gong, B.; Shen, Y.-M. Redox-Responsive Micelles Self-Assembled from Dynamic Covalent Block Copolymers for Intracellular Drug Delivery. Acta Biomater. 2015, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Van Gheluwe, L.; Buchy, E.; Chourpa, I.; Munnier, E. Three-Step Synthesis of a Redox-Responsive Blend of PEG–Block–PLA and PLA and Application to the Nanoencapsulation of Retinol. Polymers 2020, 12, 2350. [Google Scholar] [CrossRef] [PubMed]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical Aspects of Preparing PEG-PLGA Nanoparticles as Carrier for Chemotherapeutic agents by Nanoprecipitation Method. Int. J. Pharm. 2017, 533, 275–284. [Google Scholar] [CrossRef]
- Hornig, S.; Heinze, T.; Becer, C.R.; Schubert, U.S. Synthetic Polymeric Nanoparticles by Nanoprecipitation. J. Mater. Chem. 2009, 19, 3838. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Discher, D.E.; Ahmed, F. POLYMERSOMES. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Tayarani-Najaran, Z.; Minbashi, E.; Alibolandi, M.; Hosseini, J.; Sepahi, S.; Kamali, H.; Hadizadeh, F. Docetaxel-Loaded Mixed Micelles and Polymersomes Composed of Poly (Caprolactone)-Poly (Ethylene Glycol) (PEG-PCL) and Poly (Lactic Acid)-Poly (Ethylene Glycol) (PEG-PLA): Preparation and In-Vitro Characterization. Iran. J. Pharm. Res. 2019, 18, 142–155. [Google Scholar] [PubMed]
- Lalloz, A.; Bolzinger, M.-A.; Briançon, S.; Faivre, J.; Rabanel, J.-M.; Ac, A.G.; Hildgen, P.; Banquy, X. Subtle and Unexpected Role of PEG in Tuning the Penetration Mechanisms of PLA-Based Nano-Formulations into Intact and Impaired Skin. Int. J. Pharm. 2019, 563, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, F.; Cheng, R.; Zhong, Z. PH-Sensitive Degradable Polymersomes for Triggered Release of Anticancer Drugs: A Comparative Study with Micelles. J. Control. Release 2010, 142, 40–46. [Google Scholar] [CrossRef]
- Ren, J.; Yu, X.; Ren, T.; Hong, H. Preparation and Characterization of Fenofibrate-Loaded PLA–PEG Microspheres. J. Mater. Sci. Mater. Med. 2007, 18, 1481–1487. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Composition on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Ghasemi, R.; Abdollahi, M.; Emamgholi Zadeh, E.; Khodabakhshi, K.; Badeli, A.; Bagheri, H.; Hosseinkhani, S. MPEG-PLA and PLA-PEG-PLA Nanoparticles as New Carriers for Delivery of Recombinant Human Growth Hormone (RhGH). Sci. Rep. 2018, 8, 9854. [Google Scholar] [CrossRef]
- Andima, M.; Costabile, G.; Isert, L.; Ndakala, A.; Derese, S.; Merkel, O. Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity. Pharmaceutics 2018, 10, 232. [Google Scholar] [CrossRef]
- Duncan, S.A.; Dixit, S.; Sahu, R.; Martin, D.; Baganizi, D.R.; Nyairo, E.; Villinger, F.; Singh, S.R.; Dennis, V.A. Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles. Nanomaterials 2019, 9, 1074. [Google Scholar] [CrossRef]
- Jones, R.F.; Speer, H.L.; Kury, W. Studies on the Growth of the Red Alga Porphyridium Cruentum. Physiol. Plant. 1963, 16, 636–643. [Google Scholar] [CrossRef]
- Smith, C.V.; Jones, D.P.; Guenthner, T.M.; Lash, L.H.; Lauterburg, B.H. Compartmentation of Glutathione: Implications for the Study of Toxicity and Disease. Toxicol. Appl. Pharmacol. 1996, 140, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Nie, T.; Fang, Y.; Huang, H.; Wu, J. Poly(Disulfide)s: From Synthesis to Drug Delivery. Biomacromolecules 2022, 23, 1–19. [Google Scholar] [CrossRef]
- Klaikherd, A.; Nagamani, C.; Thayumanavan, S. Multi-Stimuli Sensitive Amphiphilic Block Copolymer Assemblies. J. Am. Chem. Soc. 2009, 131, 4830–4838. [Google Scholar] [CrossRef]
- Greenspan, P.; Fowler, S. Spectrofluorometric Studies of the Lipid Probe, Nile Red. J. Lipid Res. 1985, 26, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Goyal, A.K.; Gupta, P.N.; Khatri, K.; Mishra, N.; Mehta, A.; Mangal, S.; Vyas, S.P. Synthesis, Characterization and Evaluation of Novel Triblock Copolymer Based Nanoparticles for Vaccine Delivery against Hepatitis B. J. Control. Release 2009, 136, 161–169. [Google Scholar] [CrossRef]
| No. Blend | mPEG-SS-PLA Molecular Weight (kDa) | PLA Molecular Weight (kDa) | Molar Ratio mPEG-SS-PLA/PLA | PEG Content (fPEG) in mPEG-SS-PLA | PEG Content (fPEG) in Blend | Representative Diagram of the Blends * |
|---|---|---|---|---|---|---|
| 1 | 8 kDa | 4 kDa | 1:2 | 63% | 31% |  |
| 2 | 10 kDa | 5 kDa | 1:3 | 50% | 20% |  |
| 3 | 16 kDa | 11 kDa | 1:2 | 31% | 13% | 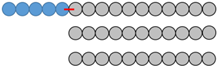 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Gheluwe, L.; David, S.; Buchy, E.; Chourpa, I.; Munnier, E. Smart PEG-Block-PLA/PLA Nanosystems: Impact of the Characteristics of the Polymer Blend on the Redox Responsiveness. Materials 2023, 16, 539. https://doi.org/10.3390/ma16020539
Van Gheluwe L, David S, Buchy E, Chourpa I, Munnier E. Smart PEG-Block-PLA/PLA Nanosystems: Impact of the Characteristics of the Polymer Blend on the Redox Responsiveness. Materials. 2023; 16(2):539. https://doi.org/10.3390/ma16020539
Chicago/Turabian StyleVan Gheluwe, Louise, Stephanie David, Eric Buchy, Igor Chourpa, and Emilie Munnier. 2023. "Smart PEG-Block-PLA/PLA Nanosystems: Impact of the Characteristics of the Polymer Blend on the Redox Responsiveness" Materials 16, no. 2: 539. https://doi.org/10.3390/ma16020539
APA StyleVan Gheluwe, L., David, S., Buchy, E., Chourpa, I., & Munnier, E. (2023). Smart PEG-Block-PLA/PLA Nanosystems: Impact of the Characteristics of the Polymer Blend on the Redox Responsiveness. Materials, 16(2), 539. https://doi.org/10.3390/ma16020539






