Phase Equilibria in Ternary System CsBr-AgBr-InBr3
Abstract
:1. Introduction
2. Experimental
2.1. Analysis Methods
2.2. Synthesis of Samples in Ternary System CsBr-AgBr-InBr3
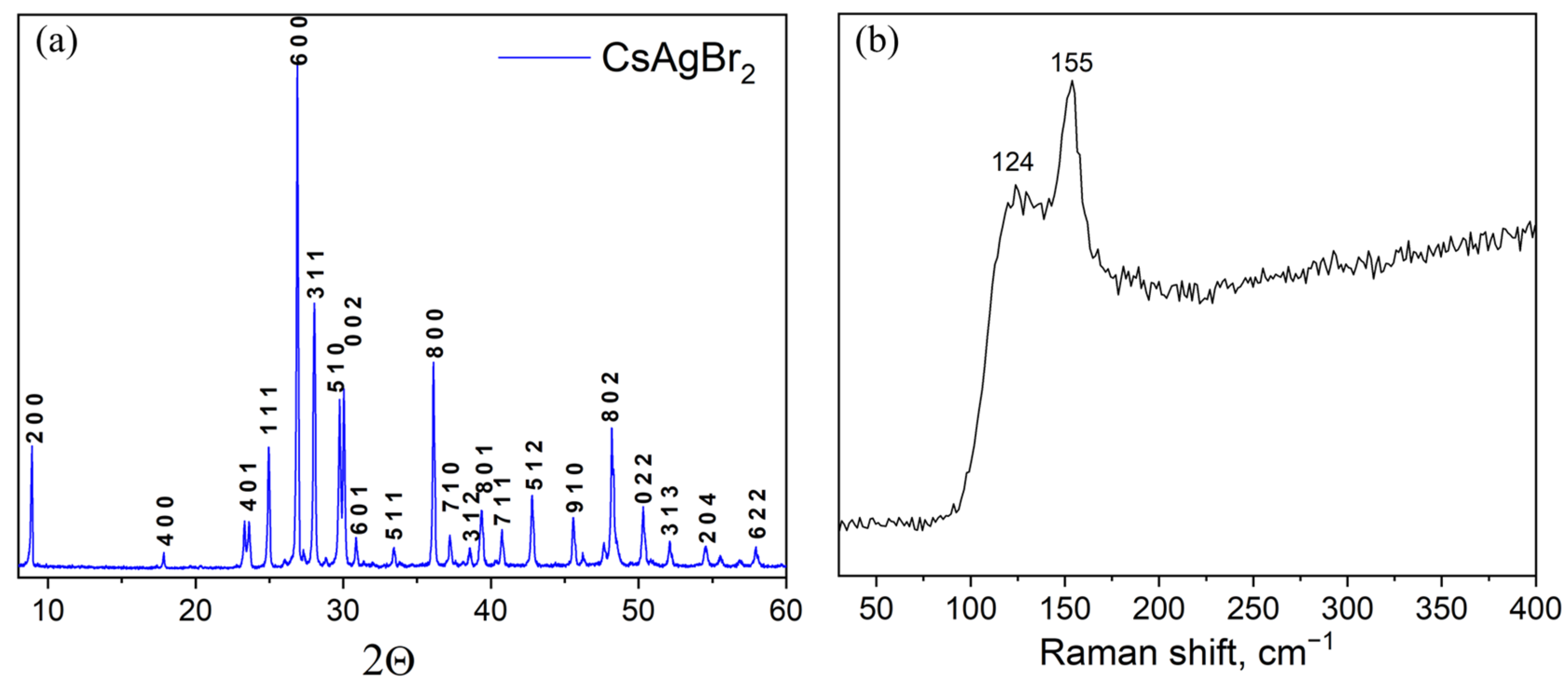
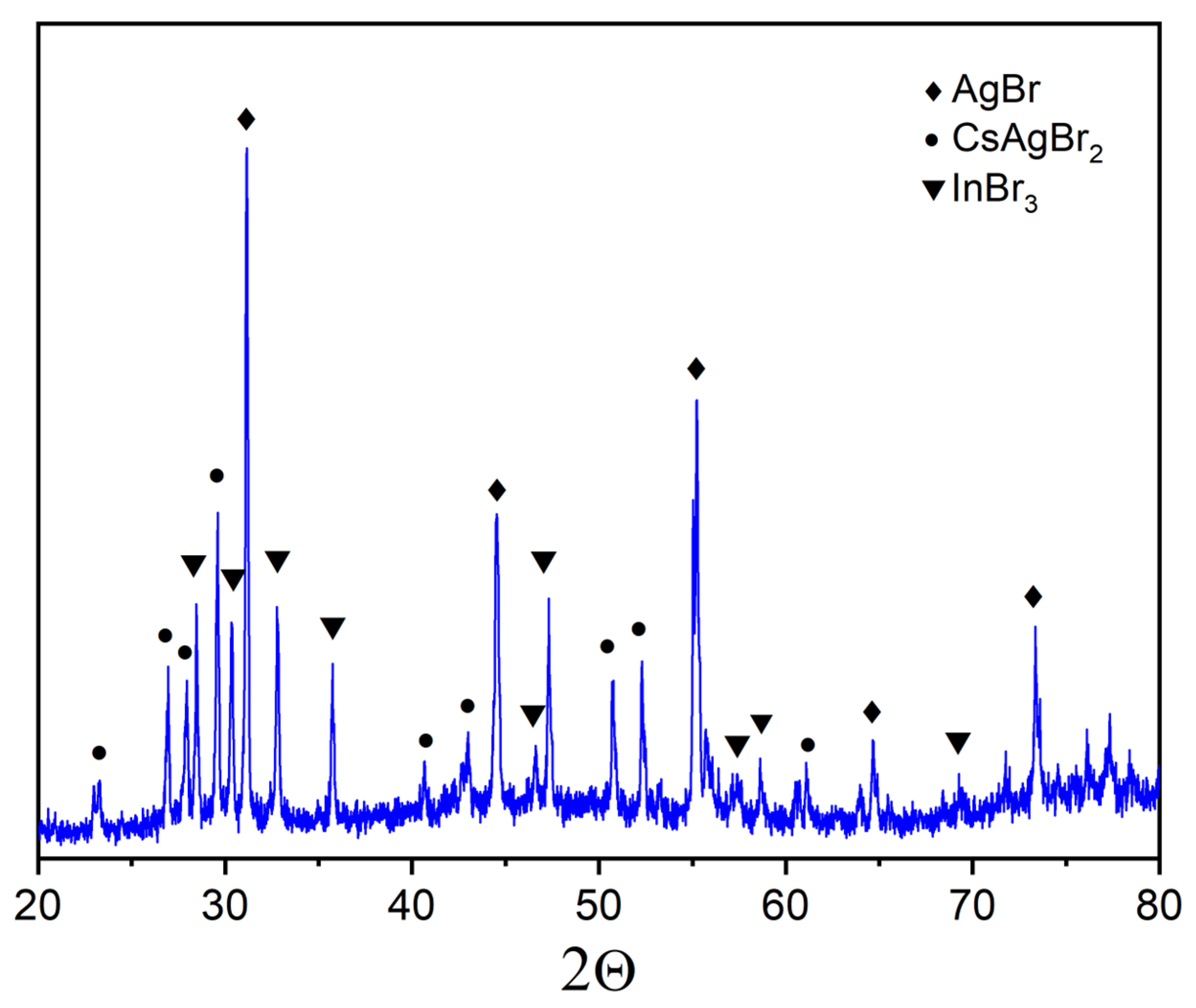
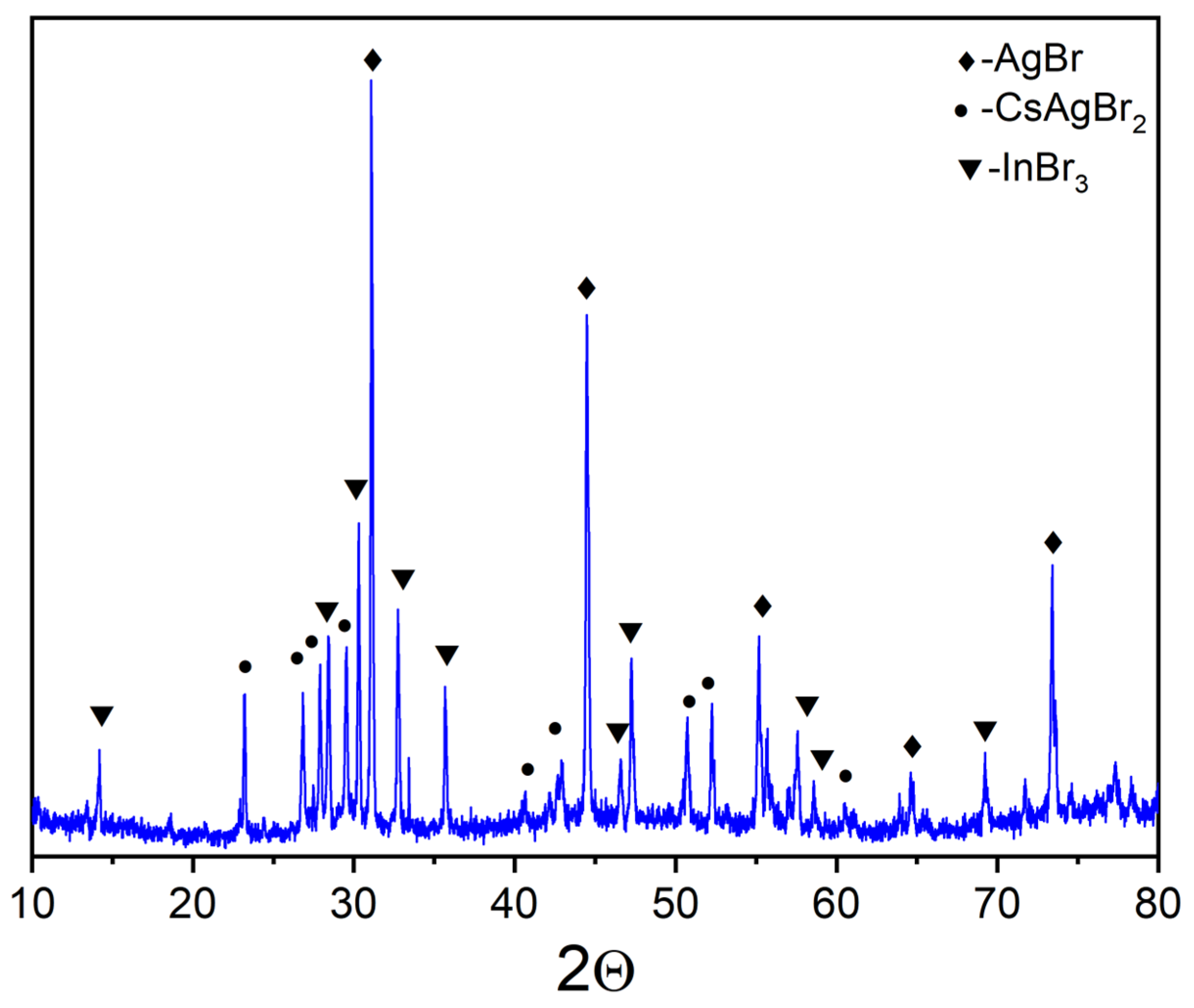
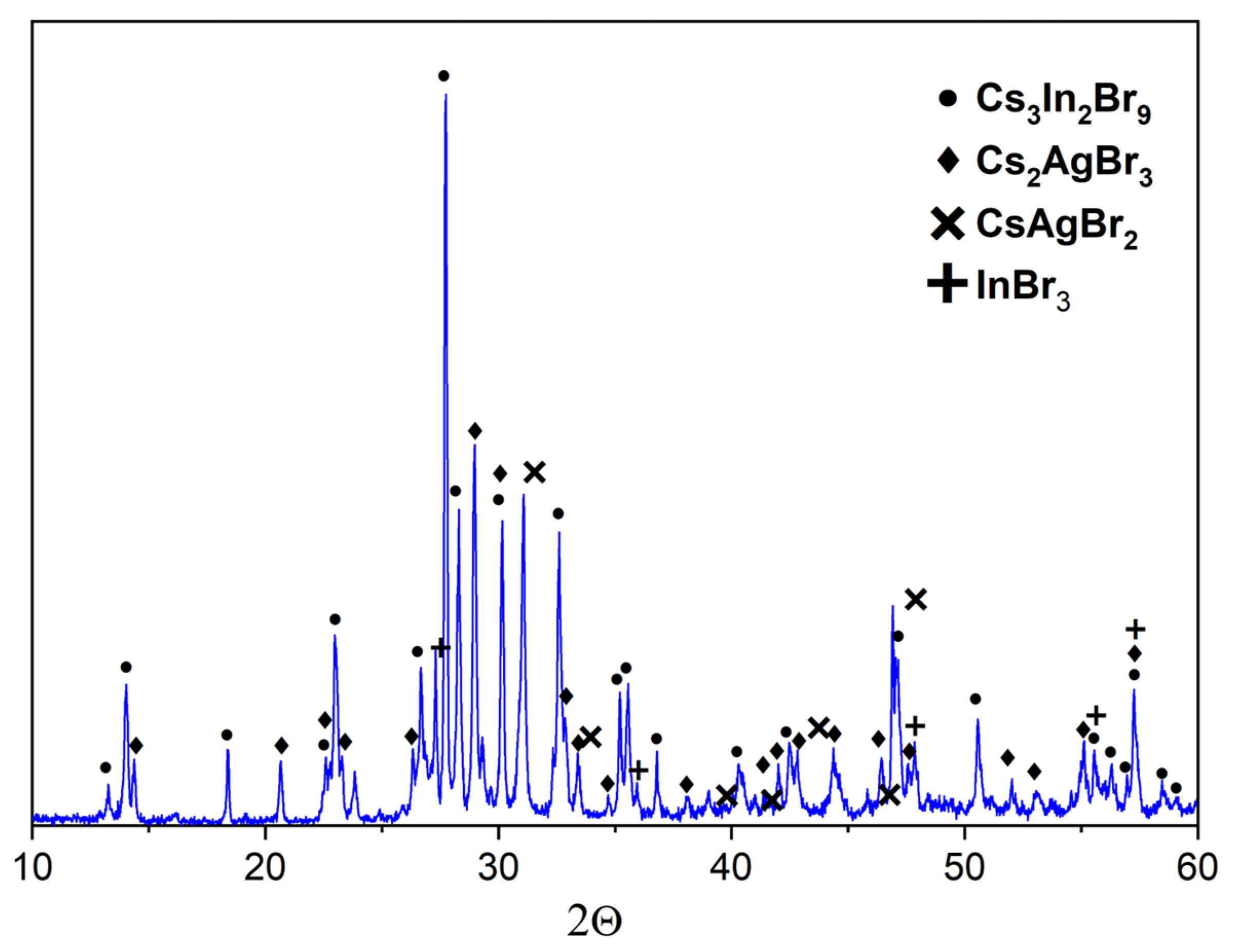
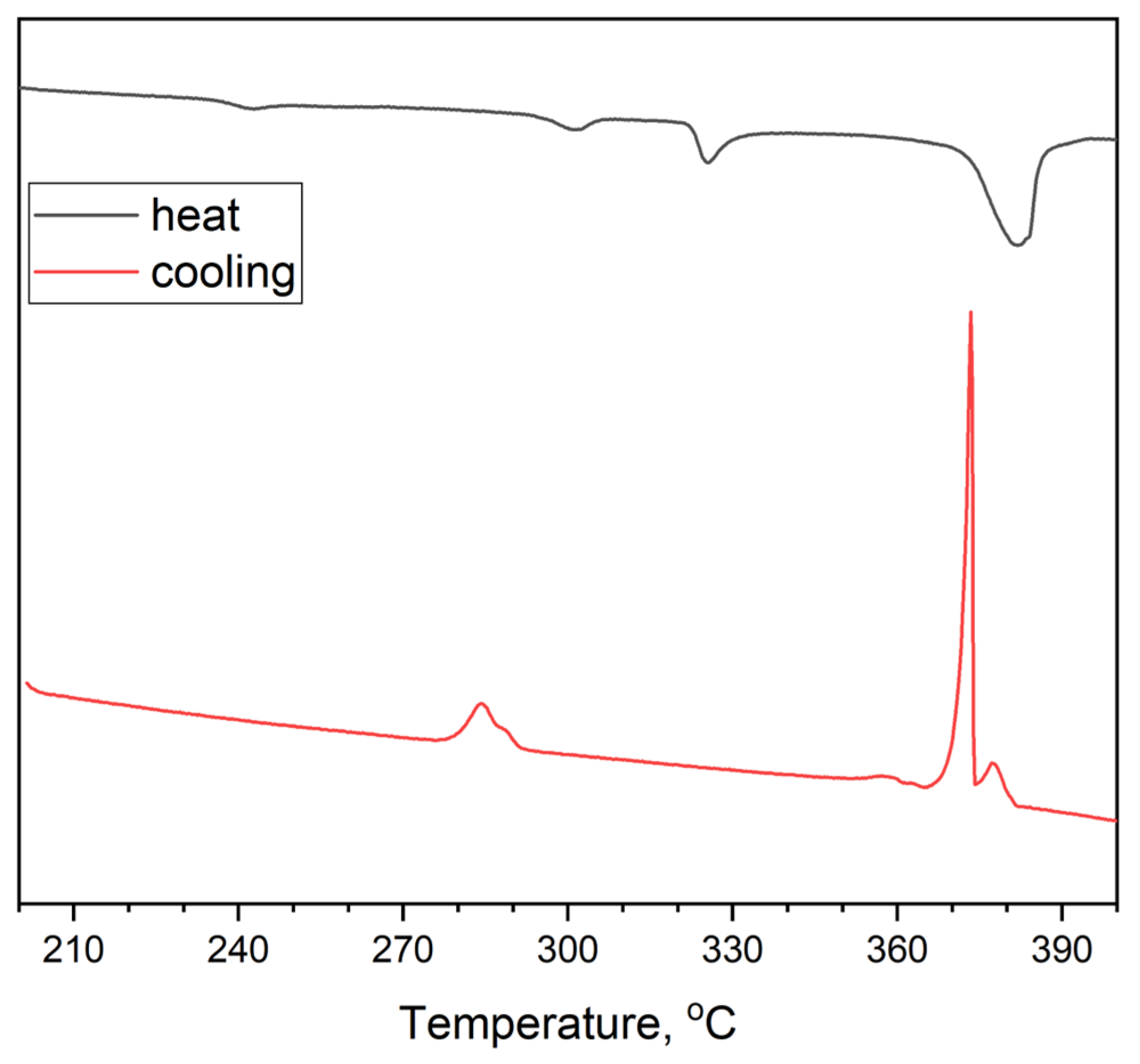
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirotkin, O.S.; Sirotkin, R.O.; Shibaev, P.B. Effect of the character of homo- and heteronuclear chemical bond on the intermolecular interaction energy and properties of halogens and hydrogen halides. Russ. J. Inorg. Chem. 2011, 56, 1104–1108. [Google Scholar] [CrossRef]
- Umedov, S.T.; Grigorieva, A.V.; Lepnev, S.L.; Knotko, A.V.; Koji, N.; Shinichi, O.; Shevelkov, A.V. Indium Doping of Lead-Free Perovskite Cs2SnI6. Front. Chem. 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Miyasaka, T.; Shirai, Y. Novel photoelectrochemical cell with mesoscopic electrodes sensitized by lead-halide compounds (2). In Proceedings of the 210th ECS Meeting, Cancun, Mexico, 29 October–3 November 2006. Abstract #397. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with effciency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Renewable Energy Laboratory. Best Research Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev220630.pdf (accessed on 30 June 2022).
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Cho, H.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Chu, L.; Hu, R.; Liu, W.; Ma, Y.; Zhang, R.; Yang, J.; Li, X. Screen printing large-area organometal halide perovskite thin films for efficient photodetectors. Mater. Res. Bull. 2018, 98, 322. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, B.; Zheng, K.; Yang, S.; Li, Y.; Deng, W.; He, R. Formamidinium lead bromide (FAPbBr3) perovskite microcrystals for sensitive and fast photodetectors. Nano-Micro Lett. 2018, 10, 43. [Google Scholar] [CrossRef]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 75, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Babayigit, A.; Thanh, D.D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar]
- Conings, B.; Drijkoningen, J.; Gauguelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef] [Green Version]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Wilks, R.G.; Bar, M. Perovskite solar cells: Danger from within. Nat. Energy 2017, 2, 16204. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Yang, J.-H.; Fu, Y.; Yang, D.; Xu, Q.; Yu, L.; Wei, S.-H.; Zhang, L. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 2017, 139, 2630–2638. [Google Scholar] [CrossRef] [Green Version]
- Filip, M.R.; Hillman, S.; Haghighirad, A.A.; Snaith, H.J.; Giustino, F. Band gaps of the lead-free halide double perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from theory and experiment. J. Phys. Chem. Lett. 2016, 7, 2579–2585. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Ostrikov, K.K.; Wasalathilake, K.C.; Yan, C.; Wang, H.; Tesfamichael, T. Insight into lead-free organic-inorganic hybrid perovskites for photovoltaics and optoelectronics: A first-principles study. Org. Electron. 2018, 59, 99–106. [Google Scholar] [CrossRef]
- Filip, M.R.; Giustino, F. Computational screening of homovalent lead substitution in organic-inorganic halide perovskites. J. Phys. Chem. C 2015, 120, 166–173. [Google Scholar] [CrossRef]
- Körbel, S.; Marques, M.A.; Botti, S. Stability and electronic properties of new inorganic perovskites from high-throughput ab initio calculations. J. Mater. Chem. C 2016, 4, 3157–3167. [Google Scholar] [CrossRef]
- Volonakis, G.; Haghighirad, A.A.; Milot, R.L.; Sio, W.H.; Filip, M.R.; Wenger, B.; Johnston, M.B.; Herz, L.M.; Snaith, H.J.; Giustino, F. Cs2InAgCl6: A New Lead-Free Halide Double Perovskite with Direct Band Gap. J. Phys. Chem. Lett. 2017, 8, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Wu, T.; Li, Y.; Jiang, L.; Deng, W.; Han, K. Combining theory and experiment in the design of a lead-free ((CH3NH3)2AgBiI6) double perovskite. New J. Chem. 2017, 41, 9598–9601. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Li, S.; Liu, J.; Guo, Y.; Niu, G.; Yao, L.; Fu, Y.; Gao, L.; Dong, Q.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Du, K.; Meng, W.; Mitzi, D.; Yan, Y. Chemical origin of the stability difference between Cu(I)- and Ag(I)-based halide double perovskites. Angew. Chem. Int. Ed. 2017, 56, 12107–12111. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, Y.; Zhang, M.; Shi, J.; Cai, W. Promising Lead-Free Double-Perovskite Photovoltaic Materials Cs2MM′Br6 (M = Cu, Ag, and Au; M′ = Ga, In, Sb, and Bi) with an Ideal Band Gap and High Power Conversion Efficiency. J. Phys. Chem. C 2021, 125, 21160–21168. [Google Scholar]
- Menedjhi, A.; Bouarissa, N.; Saib, S.; Bauamama, K. Halide double perovskite Cs2AgInBr6 for photovoltaic’s applications: Optical properties and stability. Optik 2021, 243, 167198. [Google Scholar] [CrossRef]
- Ensslin, F.; Dreyer, H.Z. Über einige Salze des Indiums. I. Anorg. Allg. Chem. 1942, 249, 119–132. [Google Scholar] [CrossRef]
- Ensslin, F.; Ziemeck, B.; DeSchaepdryver, L.Z. Zur Chemie des Indiums. VI. Die Löslichkeit von Indium(III)-chlorid, Indium(III)-bromid und Indium(III)-jodid im Wasser bei verschiedenen Temperaturen. Anorg. Chem. 1947, 254, 293–296. [Google Scholar] [CrossRef]
- Du, K.; Meng, W.; Wang, X.; Yan, Y.; Mitzi, D. Bandgap Engineering of Lead-Free Double Perovskite Cs2AgBiBr6 through Trivalent Metal Alloying. Angew. Chem. Int. Ed. 2017, 56, 8158–8162. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Peng, H.; Zheng, Z.; Chen, Z.; Tan, S.; Chen, X.; Luo, Y.; Su, Z.; Luo, J.; Liang, G. Single-Source Vapor-Deposited Cs2AgBiBr6 Thin Films for Lead-Free Perovskite Solar Cells. Nanomaterials 2019, 9, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyalikov, K.S. Doklady Akad. Nauk USSR. Dokl. Chem. 1949, 65, 17. [Google Scholar]
- Meyer, G.Z. Zur Kenntnis der Chloro- und Bromo-Indate (III). A3In2Cl9 (A = Cs, Rb, In, Tl) und Cs3In2Br9−xClx (x = 0, 3, 6, 7, 8). Anorg. Allg. Chem. 1978, 445, 140–146. [Google Scholar] [CrossRef]
- Dudareva, A.G.; Bogatov, Y.E.; Ivanov-Emin, B.N.; Fedorov, P.I. Study of the interaction of indium tribromide with cesium bromide. J. Inorg. Chem. 1971, XVI, 2586. [Google Scholar]
- Zhou, L.; Liao, J.-F.; Huang, Z.-G.; Wei, J.-H.; Wang, X.-D.; Li, W.-G.; Su, C.-Y. Highly Red Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. IR and Raman Spectra of Inorganic and Coordination Compounds; Mir: Moscow, Russia, 1991; p. 537. [Google Scholar]
- Waters, D.N.; Basak, B. Vibrational spectra of dihalogeno-anions of copper(I) and silver(I) in solution in tri-n-butyl phosphate. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 2733–2735. [Google Scholar] [CrossRef]
- Dudareva, A.G.; Bogatov, Y.E.; Konstandia, A.; Ivanov-Emin, B.N.; Fedorov, P.I. Physicochemical study of the interaction of indium tribromide with silver, zinc and lead(II) bromides in a melt. Chem. Chem. Technol. 1971, XIV, 1299. [Google Scholar]

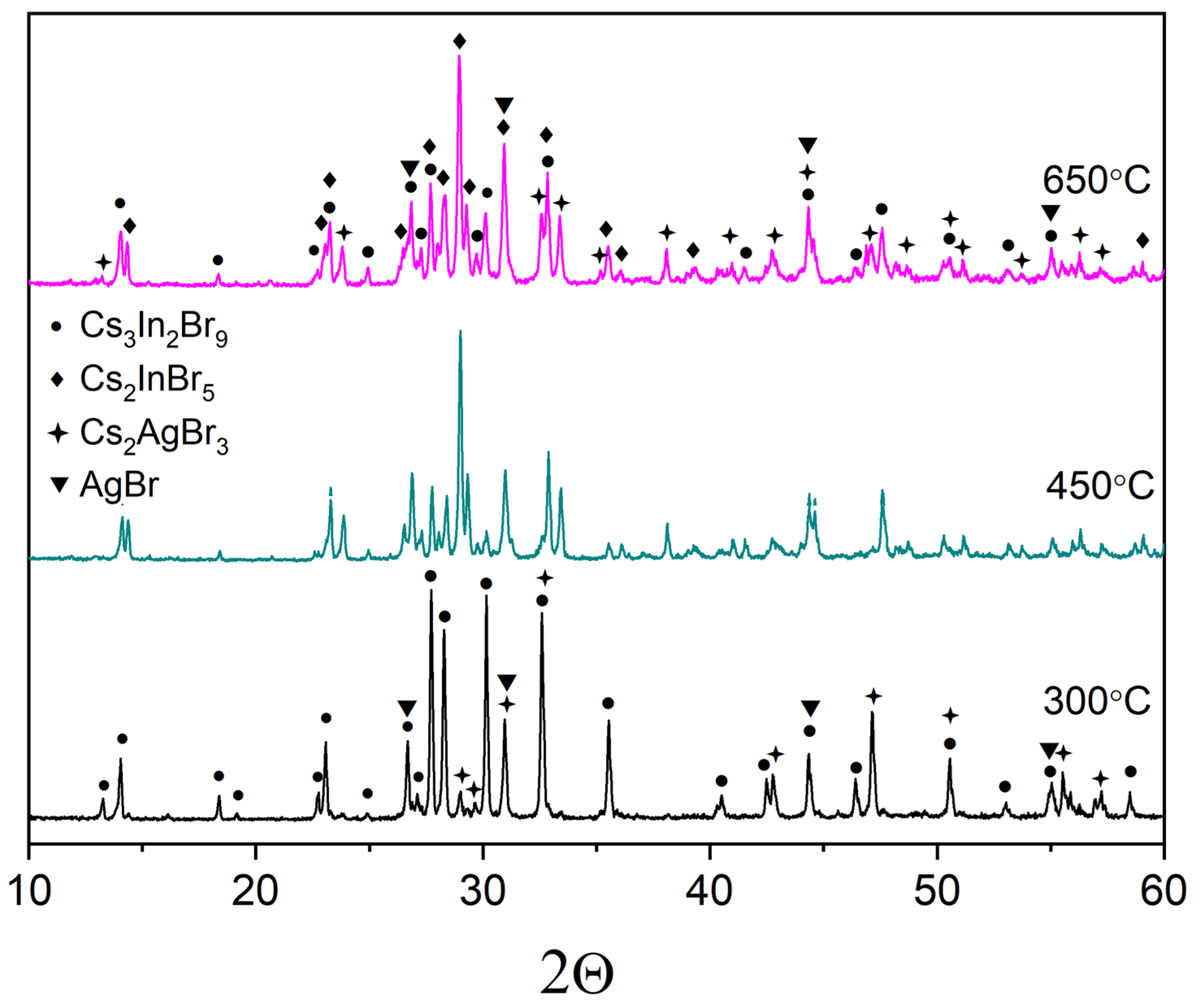
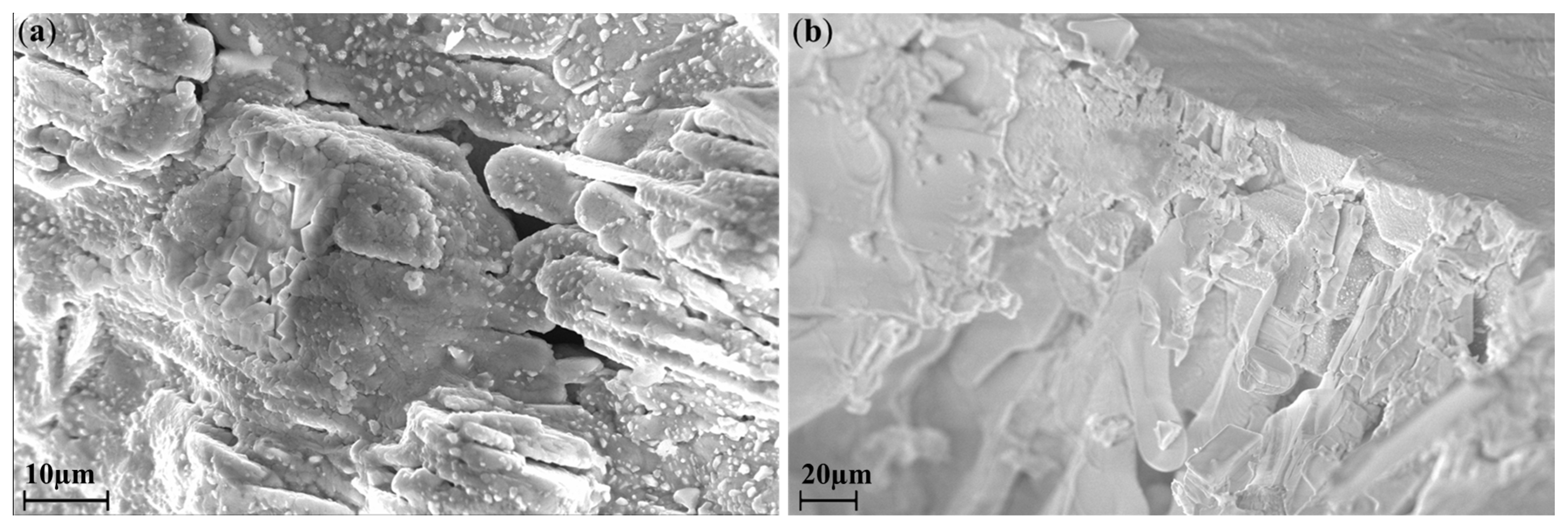
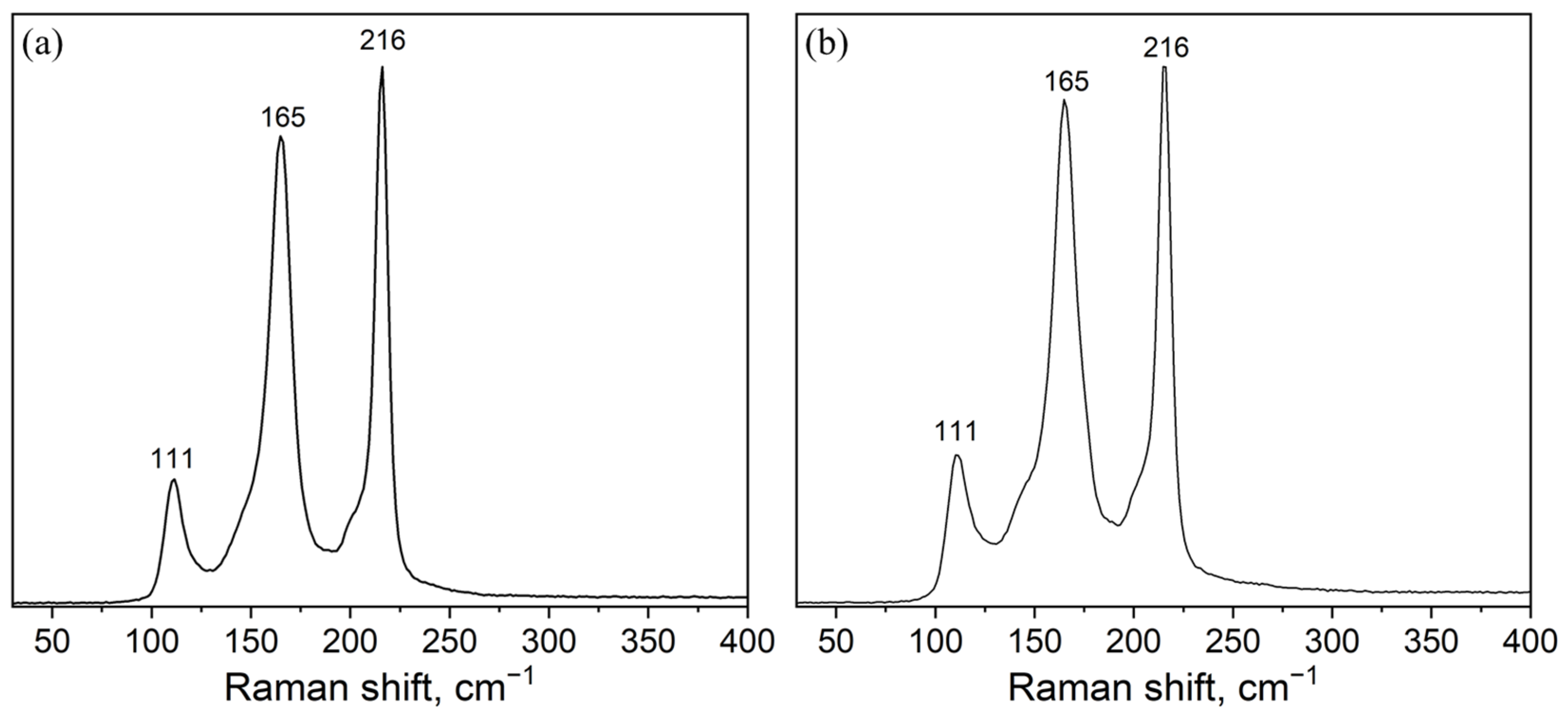
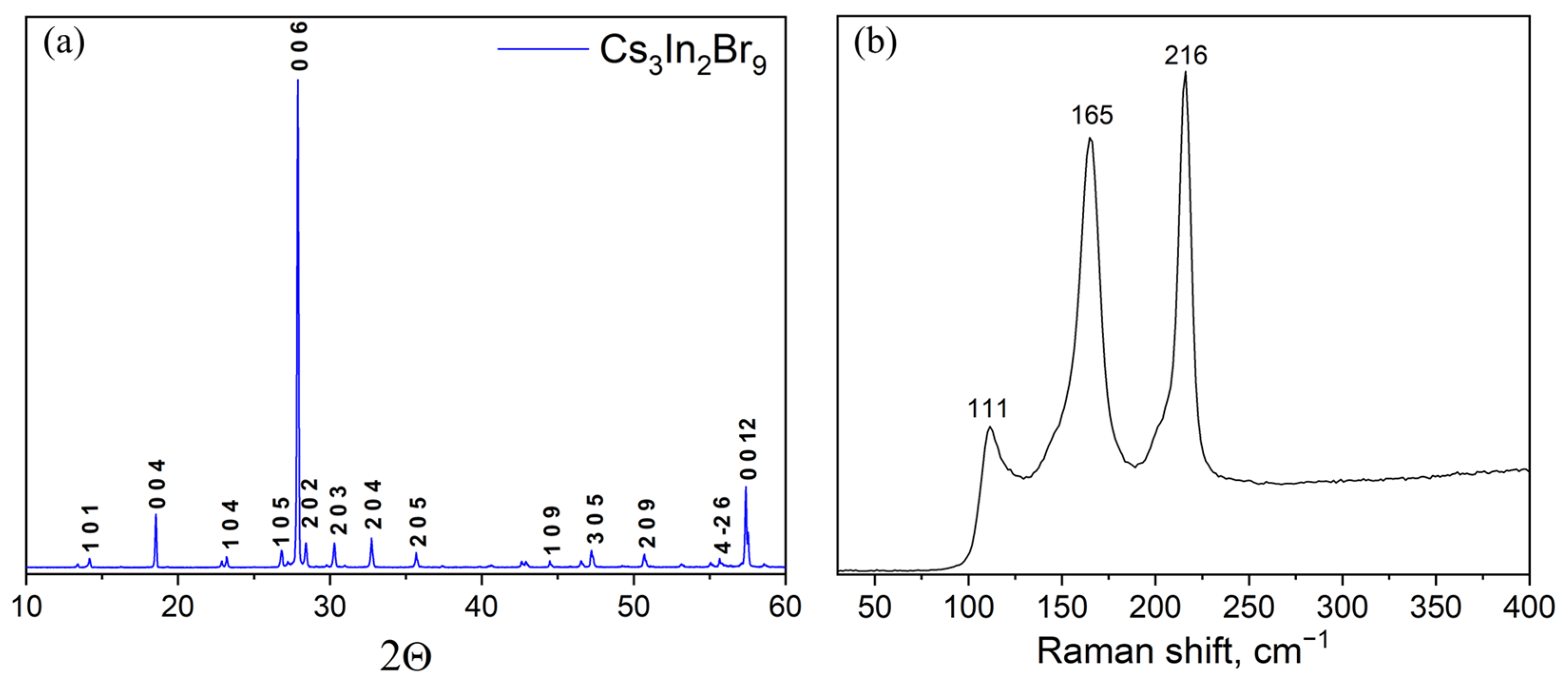
| Sample | Mole Fraction of Precursors | Weight of Precursors per 1 g. of Product | ||||
|---|---|---|---|---|---|---|
| n (CsBr) | n (AgBr) | n (InBr3) | m (CsBr) | m (AgBr) | m (InBr3) | |
| Point 1 | 0.5 | 0.25 | 0.25 | 0.44 | 0.19 | 0.37 |
| Point 2 | 0.5 | 0.5 | - | 0.531 | 0.469 | - |
| Point 3 | 0.6 | - | 0.4 | 0.474 | - | 0.526 |
| Point 4 | 0.375 | 0.375 | 0.251 | 0.334 | 0.295 | 0.372 |
| Point 5 | 0.546 | 0.273 | 0.181 | 0.501 | 0.221 | 0.278 |
| Point 6 | 0.4 | 0.4 | 0.2 | 0.368 | 0.325 | 0.307 |
| Synthesis Temperature, °C | Cs | Ag | In | Br | Cation Ratio Ag/In |
|---|---|---|---|---|---|
| T = 300 °C (solid-phase synthesis) | 25 | 13 | 11 | 51 | 1.17 |
| T = 650 °C (crystallization from the melt) | 25 | 12 | 11 | 52 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamilov, R.K.; Yuldoshev, J.Z.; Knotko, A.V.; Grigorieva, A.V. Phase Equilibria in Ternary System CsBr-AgBr-InBr3. Materials 2023, 16, 559. https://doi.org/10.3390/ma16020559
Kamilov RK, Yuldoshev JZ, Knotko AV, Grigorieva AV. Phase Equilibria in Ternary System CsBr-AgBr-InBr3. Materials. 2023; 16(2):559. https://doi.org/10.3390/ma16020559
Chicago/Turabian StyleKamilov, Rustam K., Jahongir Z. Yuldoshev, Alexander V. Knotko, and Anastasia V. Grigorieva. 2023. "Phase Equilibria in Ternary System CsBr-AgBr-InBr3" Materials 16, no. 2: 559. https://doi.org/10.3390/ma16020559





