The Effect of Temperatures on the Passivation Behavior of Q235 Steel in the Simulated Concrete Pore Solution
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Sample Preparation
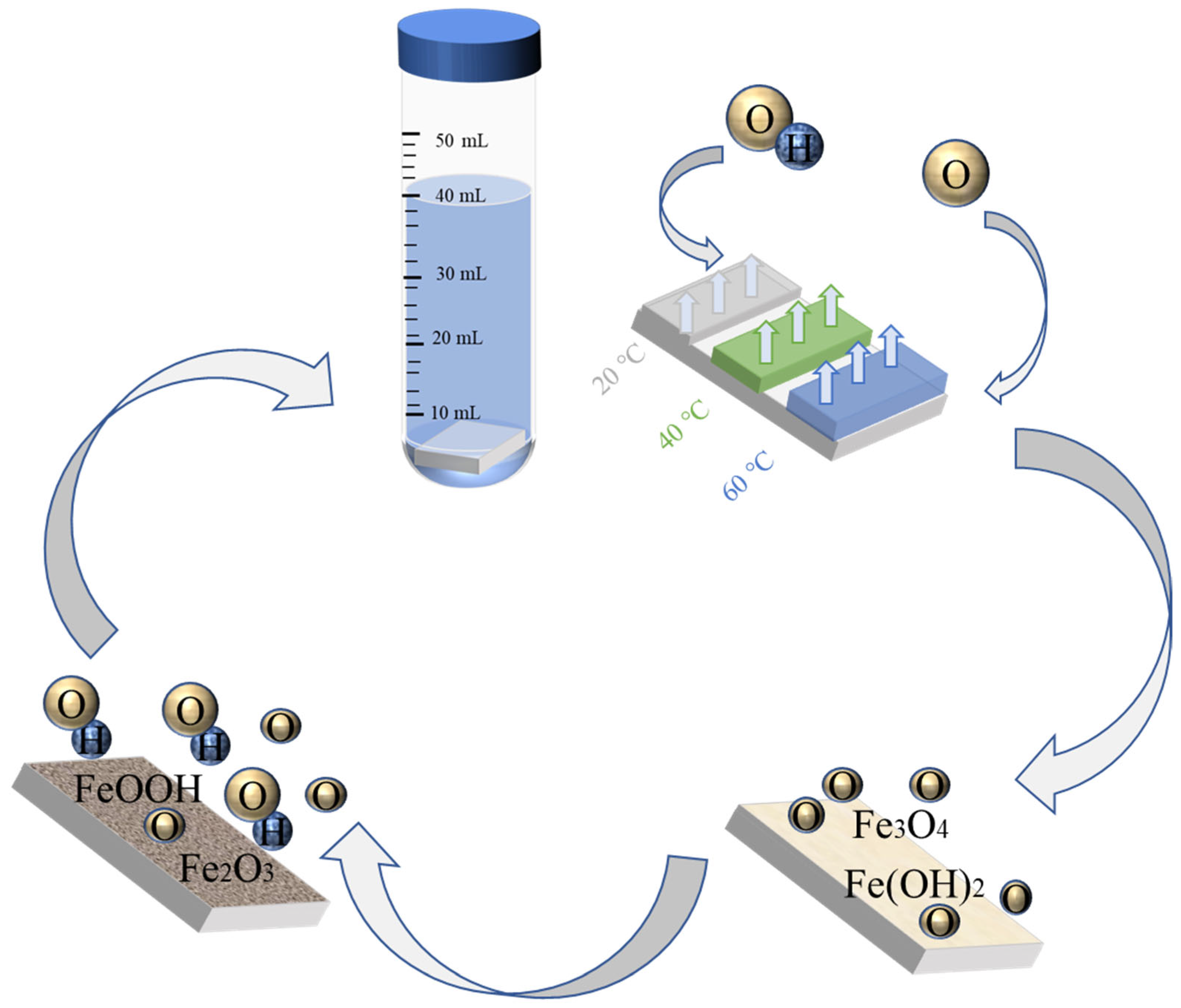
2.3. Environment
2.4. Electrochemical Test
2.5. Microscopic Morphology Observation
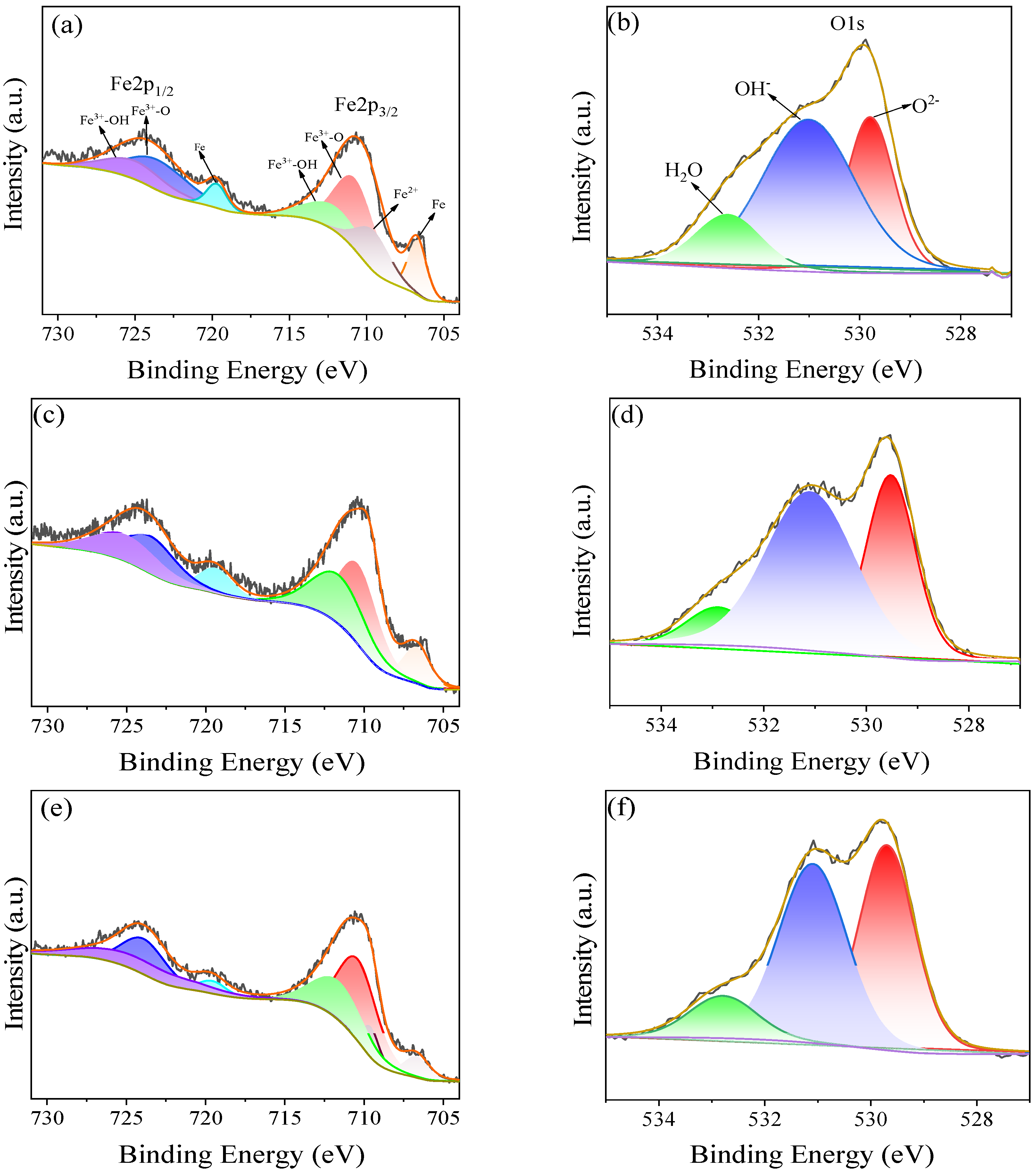
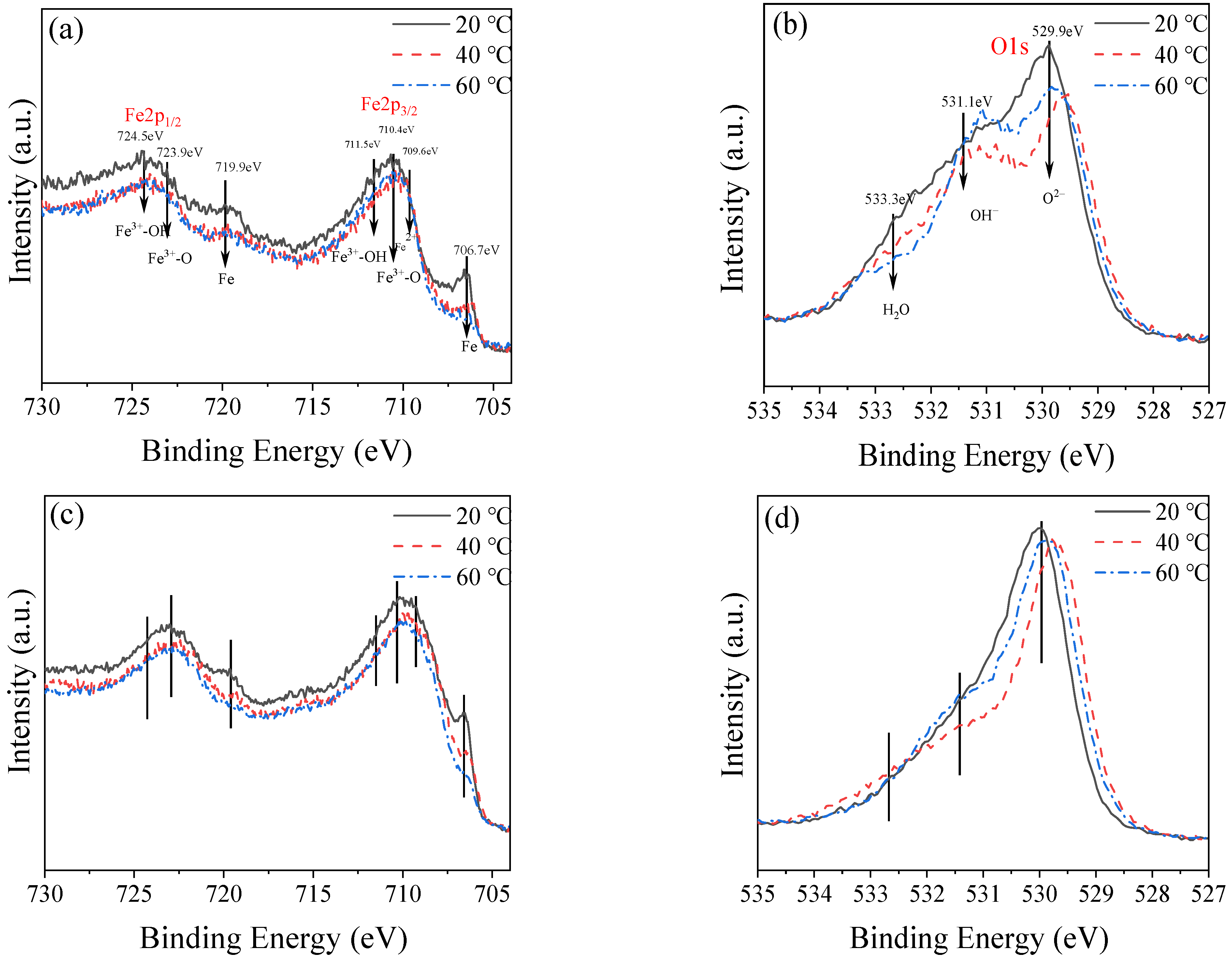
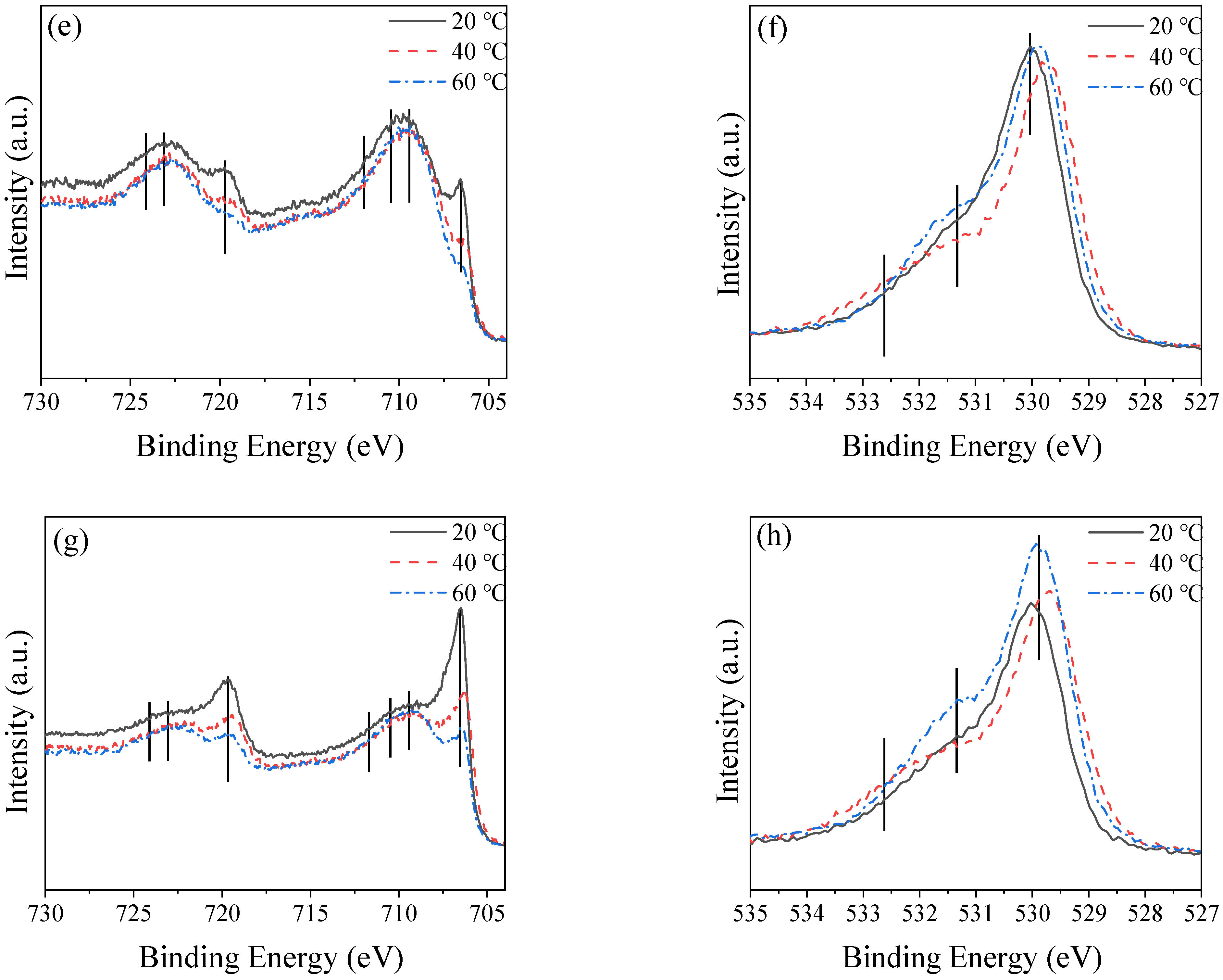
2.6. Composition of Passive Film
3. Results and Discussion
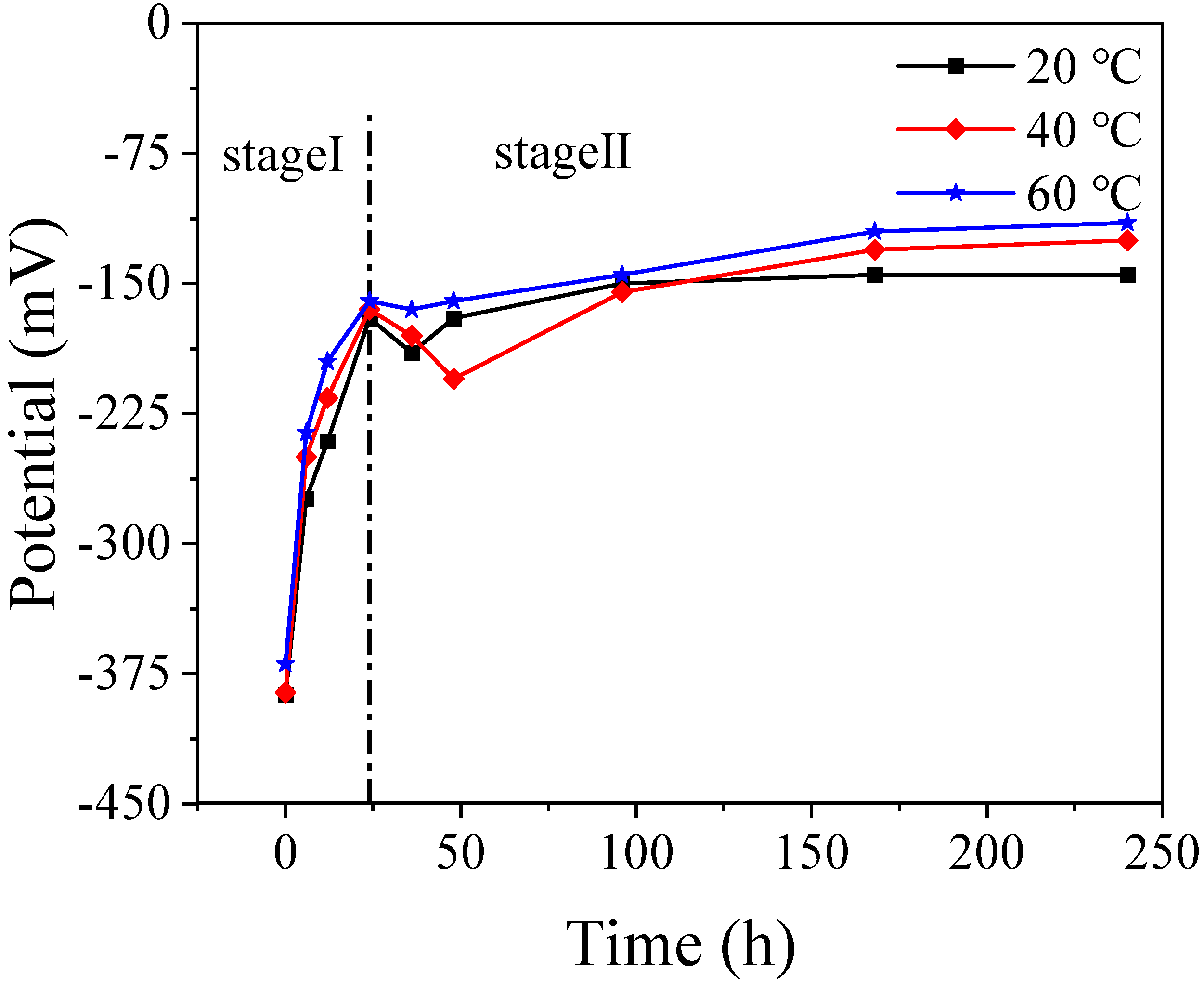
3.1. Corrosion Potential Curve
3.2. Characteristics of Passive Film
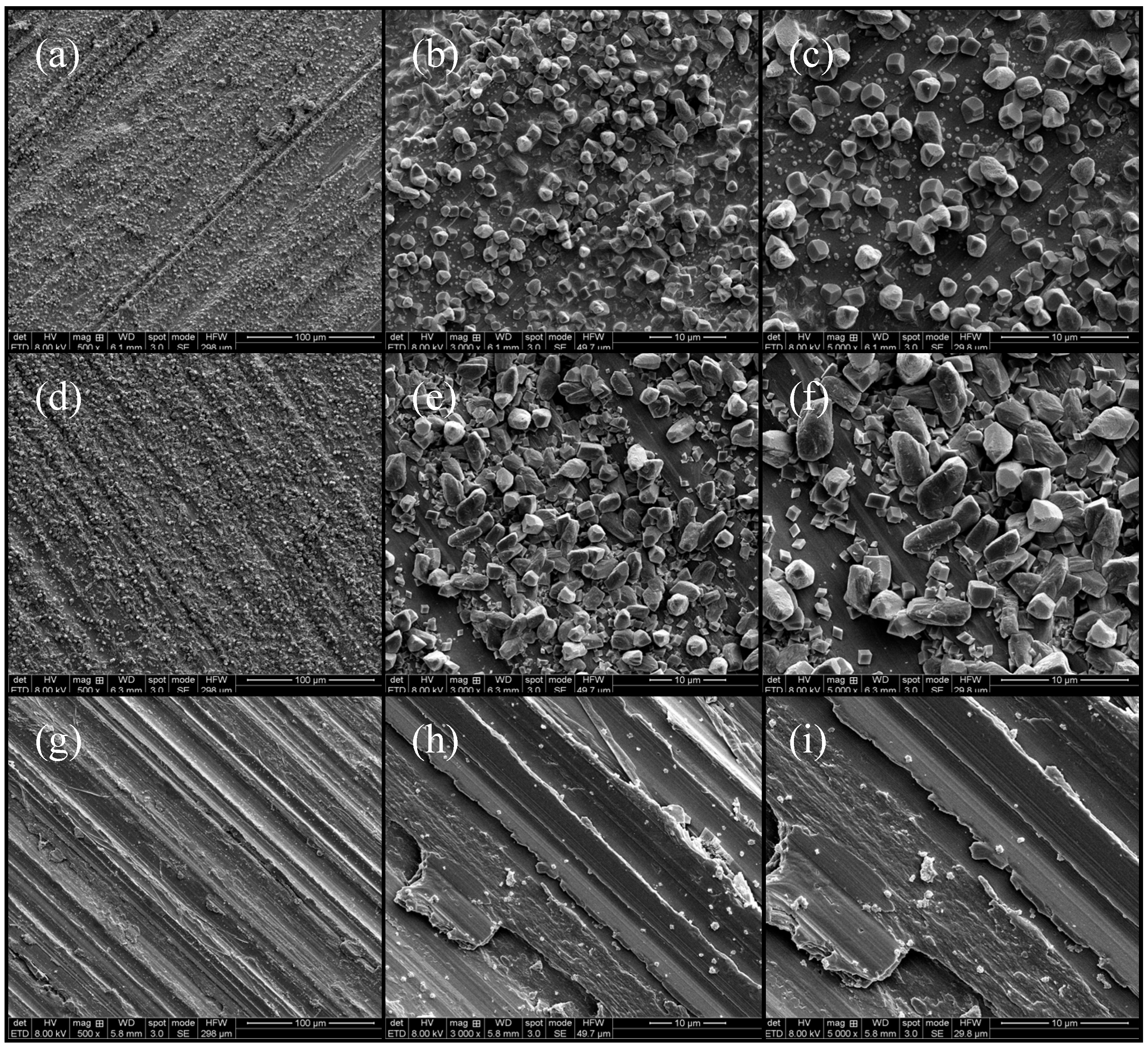
3.2.1. Characteristics of Passive Film on the Surface
3.2.2. Characteristics of Passive Film at Different Depths
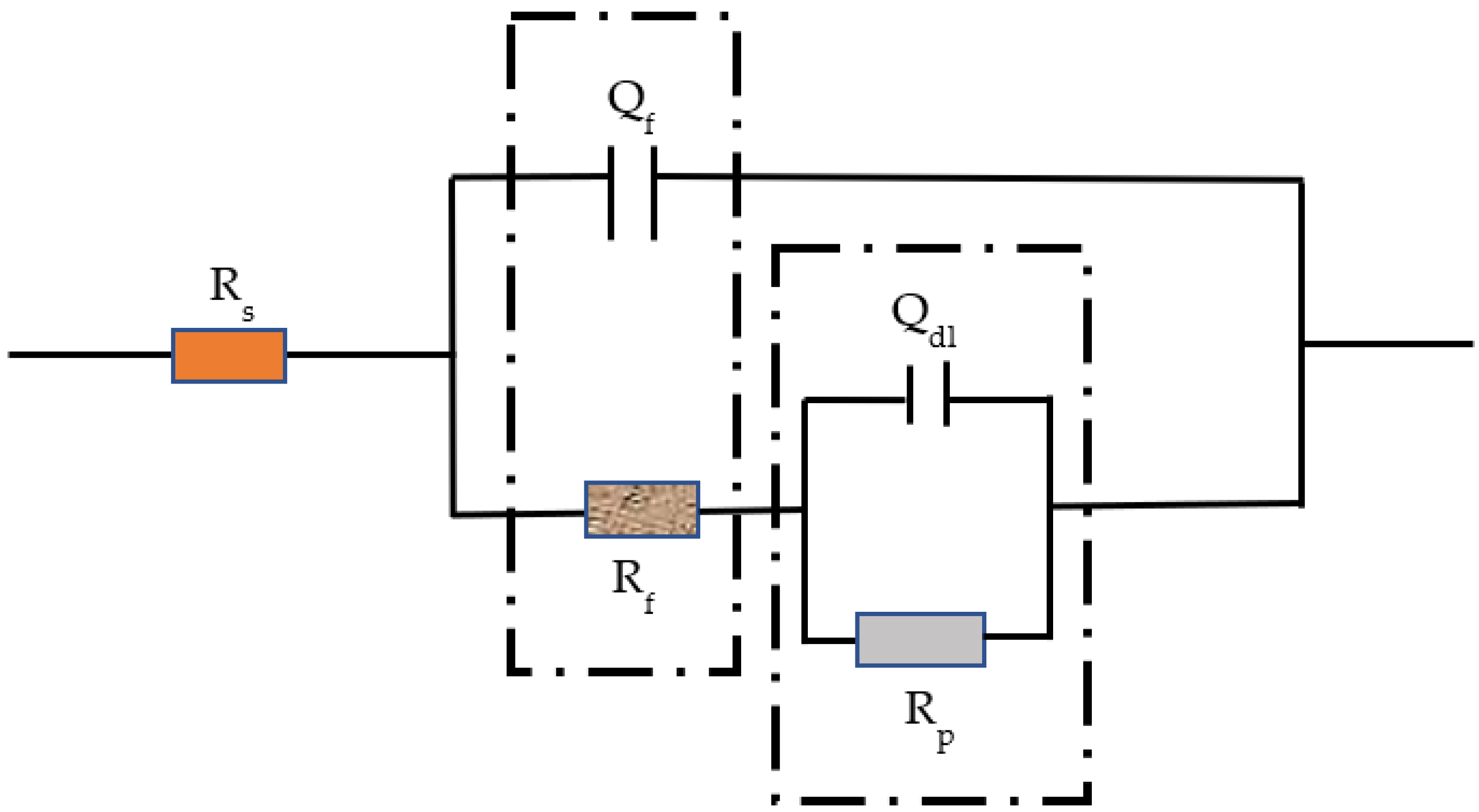
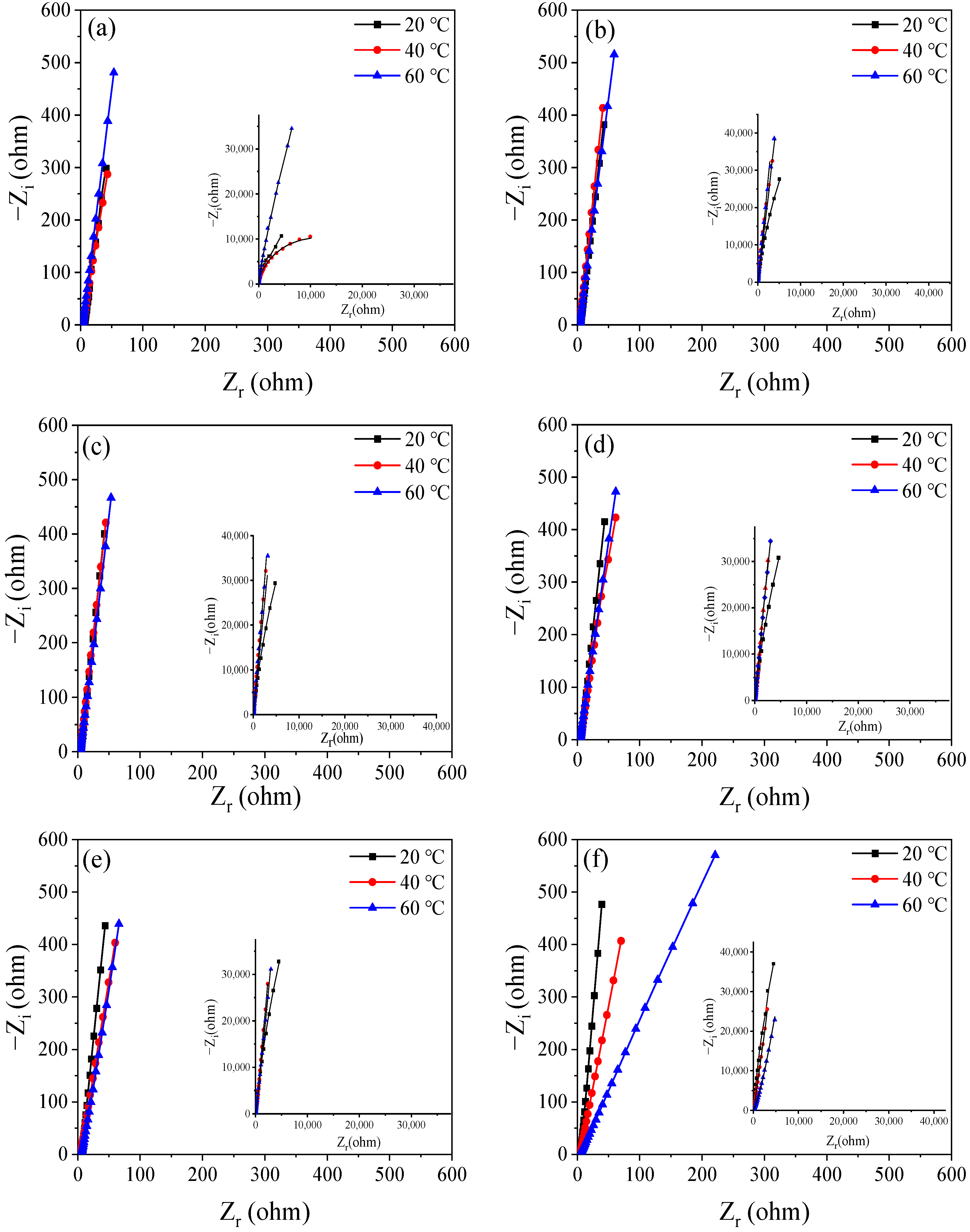
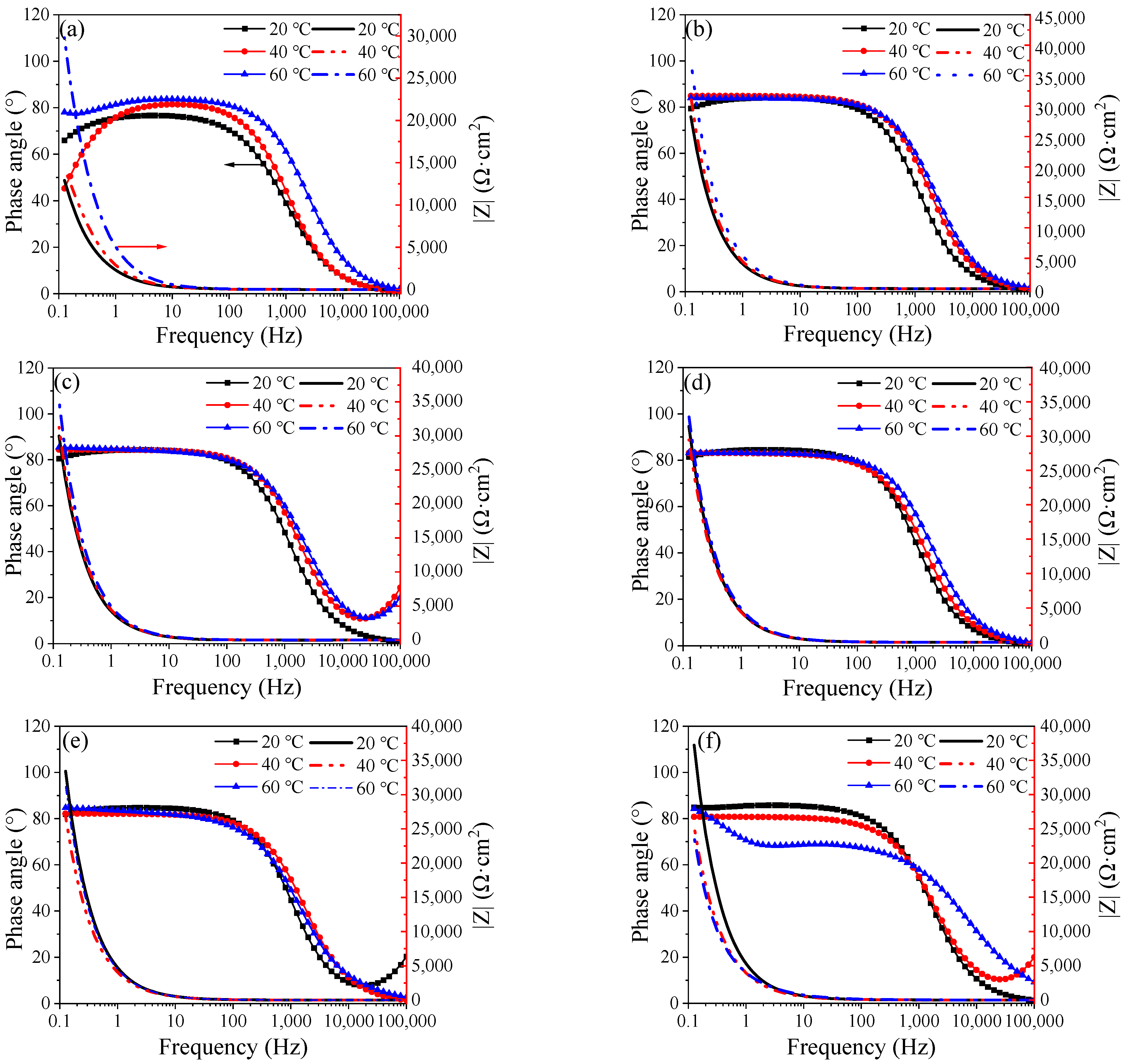
3.3. EIS Evolution of Q235 Steel during Passivation
3.4. Reaction Rate
3.5. Micro-Structure
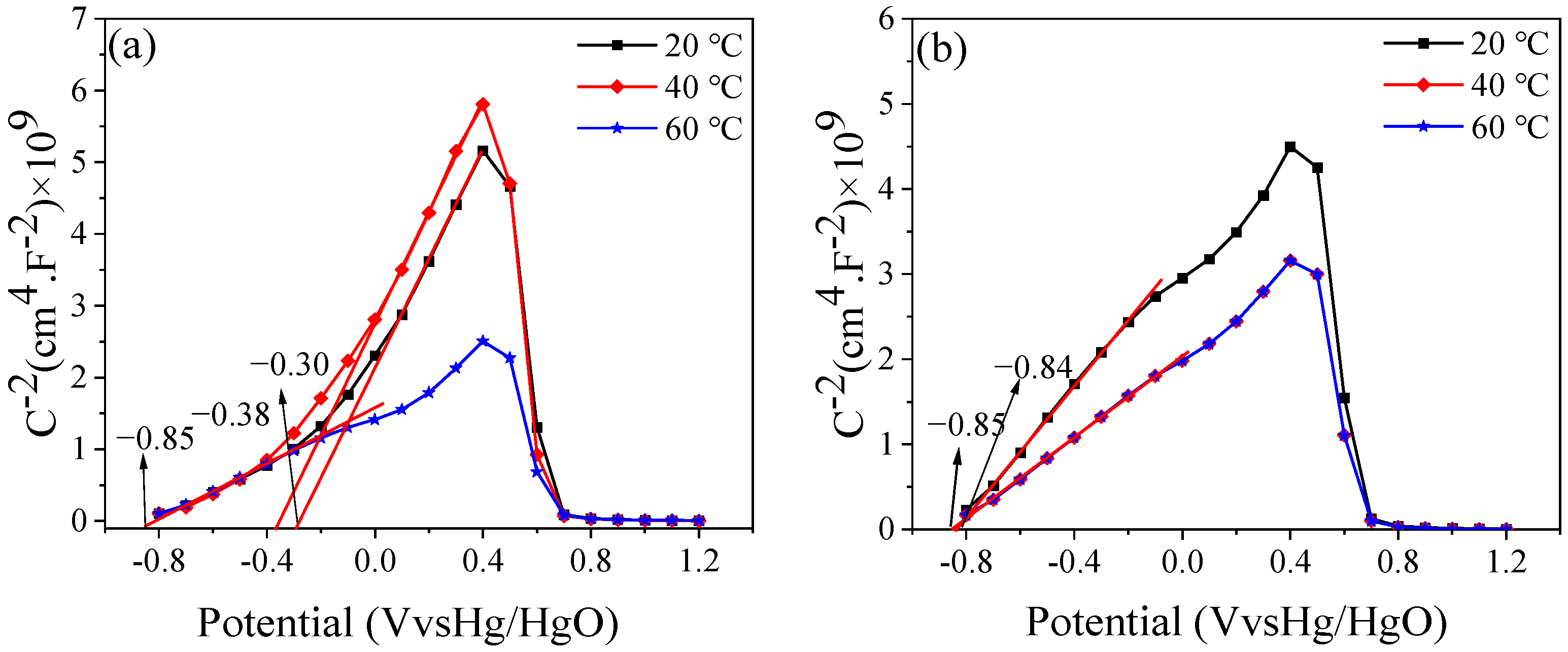
3.6. Mott–Schottky Curve
3.7. Passivation Mechanism
4. Conclusions
- (1)
- The passivation process was divided into a rapid passivation phase before 24 h and a stable passivation phase after 24 h. Among them, the early passivation rate was faster at 40 °C and 60 °C, indicating that the increase in temperature accelerated the formation of the passive film.
- (2)
- Under different temperature conditions, the passive film products are not the same, and more Fe2O3 is produced with an increase in temperature—which is the main reason for the temperature increases—and the passive film is better and thicker.
- (3)
- After passivation, the semiconductor properties of the passive films formed are the same. At a temperature of 20 °C, the capacitance of the passive film after passivation is significantly smaller than that at 40 and 60 °C. As the temperature increases, better passivated films form.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, S.; Ji, H.; Zhu, F.; Pei, W.; Xiao, W.; Tang, X. Research on the Corrosion Behavior of Q235 Pipeline Steel in an Atmospheric Environment through Experiment. Materials 2022, 15, 6502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J. Corrosion resistance of carbon steel in alkaline concrete pore solutions containing phytate and chloride ions. Corros. Sci. 2022, 205, 110451. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Qian, H.; Luo, Z.; Gao, Q. Corrosion behavior of Q235 steel by synergistic action of high concentration Cl− and complex scale in mixed salt flooding. Vacuum 2022, 204, 111365. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, D.; Wen, B.; Luo, D. Concrete protective layer cracking caused by non-uniform corrosion of reinforcements. Materials 2019, 12, 4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Li, S.; Fu, C.; Dong, Z.; Zhang, X.; Jin, N.; Xia, T. Macrocell corrosion of steel in concrete under carbonation, internal chloride admixing and accelerated chloride penetration conditions. Materials 2021, 14, 7691. [Google Scholar] [CrossRef] [PubMed]
- Elsener, B.; Fantauzzi, M.; Rossi, A. Stainless steels: Passive film composition, pitting potentials, and critical chloride content in concrete. Mater. Corros. 2020, 71, 797–807. [Google Scholar] [CrossRef]
- Li, B.; Huan, Y.; Zhang, W. Passivation and corrosion behavior of P355 carbon steel in simulated concrete pore solution at pH 12.5 to 14. Int. J. Electrochem. Sci. 2017, 12, 10402–10420. [Google Scholar] [CrossRef]
- Mikimoto, T.; Sasaki, H. Microstructure-Dependent corrosion and passivation of iron with a high carbon content in weak alkaline solution. Constr. Build. Mater. 2021, 269, 121297. [Google Scholar]
- Wu, T.; Lu, X.; Chen, Z.; Feng, X.; Zhang, Y.; Chen, X.; Zhuang, N. Effect of alternating temperature on passivation characteristics of carbon steel in chloride-free saturated Ca (OH) 2 solution. Corros. Sci. 2022, 198, 110102. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.F. Passive film growth on carbon steel and its nanoscale features at various passivating potentials. Appl. Surf. Sci. 2017, 396, 144–153. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, X.; Wang, Z.; Yu, Y.; Chen, L.; Du, Y.; Dong, L. Corrosion Behavior and Passive Film Composition of Alloy 825 in High Temperature and High H2S-CO2 Containing Environment. Front. Mater. 2021, 8, 728898. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Zhang, L. Effect of high temperature on the corrosion behavior and passive film composition of 316 L stainless steel in high H2S-Containing environments. Corros. Sci. 2020, 174, 108844. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, J.; Zhang, G.H.; Fan, X.H.; Zhang, L. Effect of temperature on the passive film structure and corrosion performance of CoCrFeMoNi high-Entropy alloy. Corros. Sci. 2022, 208, 110661. [Google Scholar] [CrossRef]
- Du, F.; Jin, Z.; Xiong, C.; Yu, Y.; Fan, J. Effects of transverse crack on chloride ions diffusion and steel bars corrosion behavior in concrete under electric acceleration. Materials 2019, 12, 2481. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Tian, Y.; Zhao, R.; Jin, N.; Jin, X.; Tian, Z.; Yan, D.; Ye, H. Passivation and Depassivation of HPB335 Carbon Steel in Simulated Concrete Pore Solution. Int. J. Electrochem. Sci. 2020, 15, 6488–6507. [Google Scholar] [CrossRef]
- Shi, J.; Li, M.; Wu, M.; Ming, J. Role of red mud in natural passivation and chloride-Induced depassivation of reinforcing steels in alkaline concrete pore solutions. Corros. Sci. 2021, 190, 109669. [Google Scholar] [CrossRef]
- Chen, Z.; Nong, Y.; Chen, Y.; Chen, J.; Yu, B. Study on the adsorption of OH− and Ca(OH)2 on Fe (100) surface and their effect on passivation of steel bar: Experiments and DFT modelling. Corros. Sci. 2020, 174, 108804. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, X.; Du, Y.; Chen, J.; Yu, B. Comprehensive properties of passive film formed in SCP of alkali-activated concrete. Constr. Build. Mater. 2022, 319, 126142. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, X.; Yang, H. Effects of pH and Cl− Content on Degradation Process of Pre-Passivated Stainless Steels in an Alkaline Solution. J. Electrochem. Soc. 2022, 169, 031506. [Google Scholar] [CrossRef]
- Mundra, S.; Provis, J.L. Mechanisms of passivation and chloride-induced corrosion of mild steel in sulfide-Containing alkaline solutions. J. Mater. Sci. 2021, 56, 14783–14802. [Google Scholar] [CrossRef]
- Wei, J.; Chen, R.; Huang, W.; Bian, X.; Chen, B. Effect of endogenous chloride ion content and mineral admixtures on the passivation behavior of reinforcement embedded in sea-Sand ultra-High-Performance concrete matrix. Constr. Build. Mater. 2022, 321, 126402. [Google Scholar] [CrossRef]
- You, N.; Shi, J.; Zhang, Y. Electrochemical performance of low-Alloy steel and low-Carbon steel immersed in the SCPs of alkali-activated slag/steel slag pastes in the presence of chlorides. Corros. Sci. 2022, 205, 110438. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, H.; Hu, X.; Lu, J.; Poon, C.S.; Li, W. Comparative studies on passivation and corrosion behaviors of two types of steel bars in simulated concrete pore solution. Constr. Build. Mater. 2021, 266, 120971. [Google Scholar] [CrossRef]
- Deus, J.M.; Freire, L.; Montemor, M.F.; Nóvoa, X.R. The corrosion potential of stainless steel rebars in concrete: Temperature effect. Corros. Sci. 2012, 65, 556–560. [Google Scholar] [CrossRef]
| C | Mn | Si | S | P |
|---|---|---|---|---|
| 0.20% | 1.4% | 0.35% | 0.045% | 0.045% |
| 20 °C | 40 °C | 60 °C | |
|---|---|---|---|
| Fe0 | 1.27 | 1.31 | 0.82 |
| Fe2+ | 1.15 | 0.57 | 0.50 |
| Fe3+ | 6.15 | 6.84 | 7.72 |
| H2O | 6.21 | 3.74 | 4.44 |
| OH− | 24.75 | 22.64 | 18.87 |
| O2− | 13.11 | 14.23 | 17.66 |
| T (°C) | Time (h) | Rs (Ω·cm2) | Qf (μF−1 cm2 sn) | n | Rct (Ω·cm2) | Qp (μF−1 cm2 sn) | n | Rp × 105 (Ω·cm2) | χ2 (×10−3) |
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 0.5 | 5.844 | 0.887 | 0.86 | 6.21 | 0.36 | 0.79 | 0.77 | 2.92 |
| 6 | 5.166 | 0.475 | 0.94 | 3.13 | 0.21 | 0.83 | 1.50 | 5.19 | |
| 12 | 5.198 | 0.144 | 1.00 | 5.18 | 0.41 | 0.94 | 3.69 | 9.43 | |
| 24 | 5.468 | 0.110 | 1.00 | 6.18 | 0.39 | 0.95 | 4.59 | 7.12 | |
| 36 | 5.876 | 0.116 | 1.00 | 6.50 | 0.38 | 0.94 | 5.39 | 2.20 | |
| 48 | 5.162 | 0.108 | 0.99 | 6.40 | 0.37 | 0.95 | 6.16 | 1.23 | |
| 96 | 5.222 | 0.339 | 0.95 | 2.44 | 0.72 | 0.78 | 8.09 | 7.16 | |
| 168 | 5.767 | 0.334 | 0.95 | 2.13 | 0.97 | 0.95 | 8.45 | 2.85 | |
| 240 | 4.657 | 0.331 | 0.95 | 2.50 | 1.11 | 0.88 | 8.75 | 5.93 | |
| 40 °C | 0.5 | 4.546 | 0.609 | 0.93 | 1.64 | 0.52 | 0.63 | 1.03 | 2.56 |
| 6 | 3.624 | 0.397 | 0.94 | 3.97 | 0.14 | 0.74 | 3.11 | 1.46 | |
| 12 | 3.762 | 0.401 | 0.94 | 8.99 | 0.48 | 0.82 | 5.86 | 1.17 | |
| 24 | 5.276 | 0.421 | 0.92 | 5.22 | 0.10 | 0.86 | 6.42 | 1.01 | |
| 36 | 4.491 | 0.444 | 0.92 | 6.24 | 0.11 | 0.84 | 5.46 | 6.40 | |
| 48 | 4.302 | 0.408 | 0.99 | 6.41 | 0.37 | 0.85 | 6.16 | 1.23 | |
| 96 | 3.693 | 0.470 | 0.91 | 6.13 | 0.86 | 0.86 | 5.60 | 2.31 | |
| 168 | 4.107 | 0.469 | 0.90 | 5.09 | 0.10 | 0.85 | 3.53 | 2.47 | |
| 240 | 4.275 | 0.489 | 0.90 | 5.57 | 0.37 | 0.83 | 3.26 | 7.99 | |
| 60 °C | 0.5 | 3.474 | 0.353 | 0.93 | 1.24 | 0.98 | 0.86 | 2.62 | 1.96 |
| 6 | 3.965 | 0.333 | 0.93 | 2.13 | 0.56 | 0.82 | 5.36 | 1.89 | |
| 12 | 3.682 | 0.356 | 1.00 | 3.72 | 0.34 | 0.93 | 6.49 | 2.59 | |
| 24 | 4.363 | 0.377 | 0.92 | 7.79 | 0.83 | 0.87 | 7.69 | 1.08 | |
| 36 | 4.098 | 0.415 | 0.92 | 3.52 | 0.93 | 0.82 | 7.46 | 1.21 | |
| 48 | 5.710 | 0.415 | 0.91 | 1.64 | 0.36 | 0.80 | 7.32 | 9.79 | |
| 96 | 4.598 | 0.470 | 0.90 | 1.78 | 0.47 | 0.87 | 6.21 | 5.65 | |
| 168 | 4.544 | 0.503 | 0.94 | 1.03 | 0.81 | 0.86 | 5.48 | 2.31 | |
| 240 | 4.394 | 0.566 | 0.97 | 1.38 | 0.97 | 0.73 | 5.32 | 1.09 |
| T (°C) | lnk | 1/T (1/K × 10−3) | Ea. (KJ/mol) |
|---|---|---|---|
| 20 | −1.8262 | 3.41 | 28.35 |
| 40 | −0.4947 | 3.19 | 26.52 |
| 60 | −0.2151 | 3.00 | 24.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Jin, Z.; Zhang, X.; Qian, L.; Zhou, Z. The Effect of Temperatures on the Passivation Behavior of Q235 Steel in the Simulated Concrete Pore Solution. Materials 2023, 16, 588. https://doi.org/10.3390/ma16020588
Jiang H, Jin Z, Zhang X, Qian L, Zhou Z. The Effect of Temperatures on the Passivation Behavior of Q235 Steel in the Simulated Concrete Pore Solution. Materials. 2023; 16(2):588. https://doi.org/10.3390/ma16020588
Chicago/Turabian StyleJiang, Haosen, Zuquan Jin, Xiaoying Zhang, Lixing Qian, and Zhaoliang Zhou. 2023. "The Effect of Temperatures on the Passivation Behavior of Q235 Steel in the Simulated Concrete Pore Solution" Materials 16, no. 2: 588. https://doi.org/10.3390/ma16020588
APA StyleJiang, H., Jin, Z., Zhang, X., Qian, L., & Zhou, Z. (2023). The Effect of Temperatures on the Passivation Behavior of Q235 Steel in the Simulated Concrete Pore Solution. Materials, 16(2), 588. https://doi.org/10.3390/ma16020588






