Fabrication of Cu/Al/Cu Laminated Composites Reinforced with Graphene by Hot Pressing and Evaluation of Their Electrical Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Methods of Sample Preparation
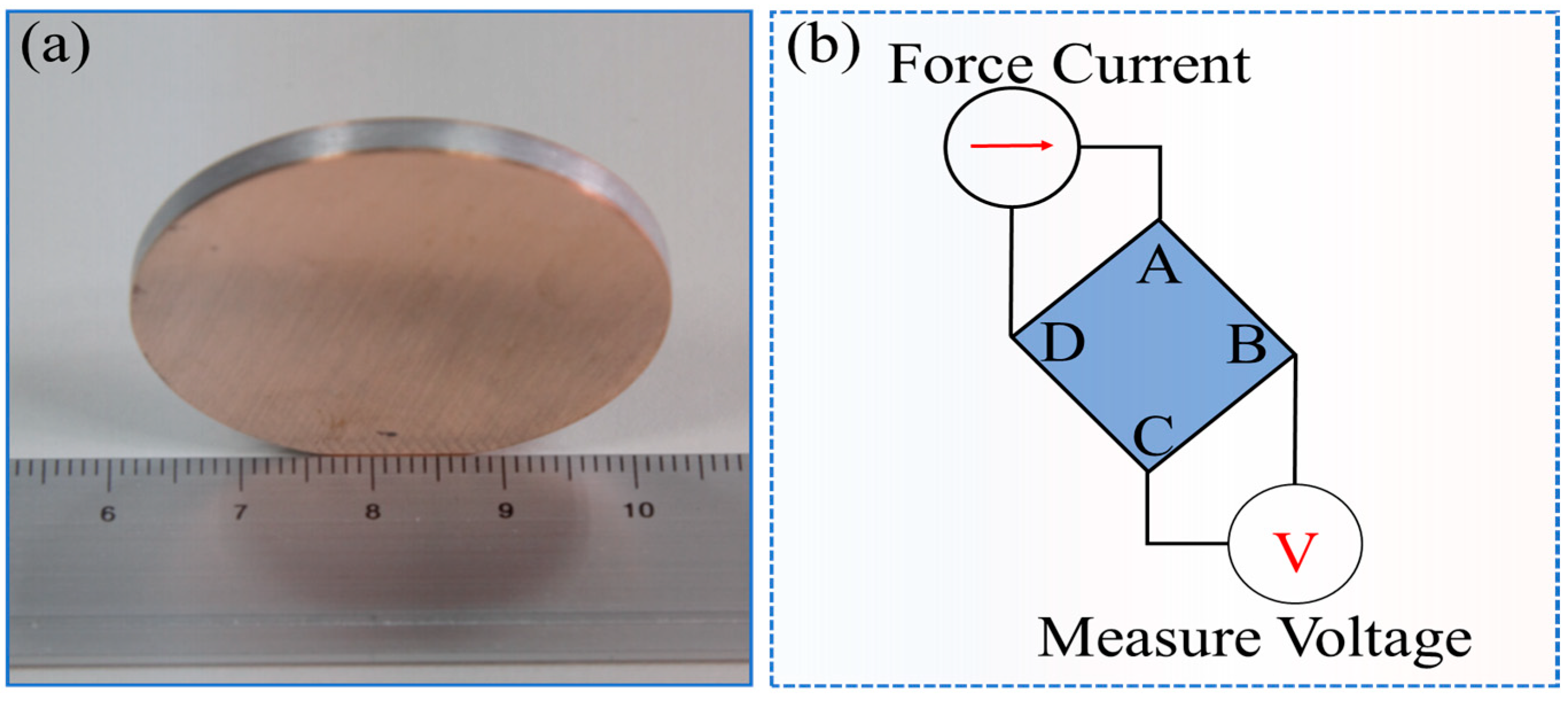
2.2.2. Characterization
3. Results and Discussion
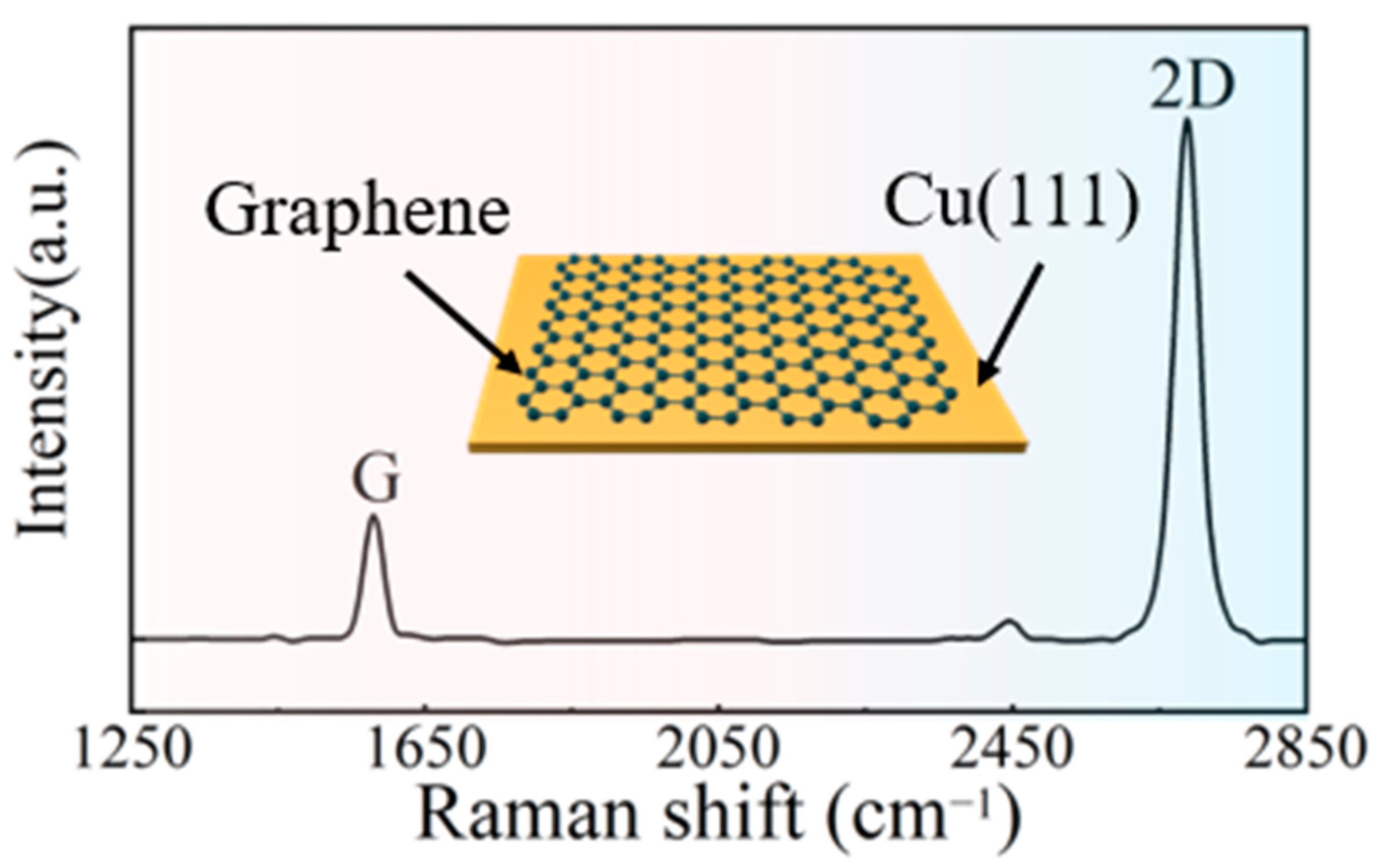
3.1. Cu Miller Indices and Graphene State
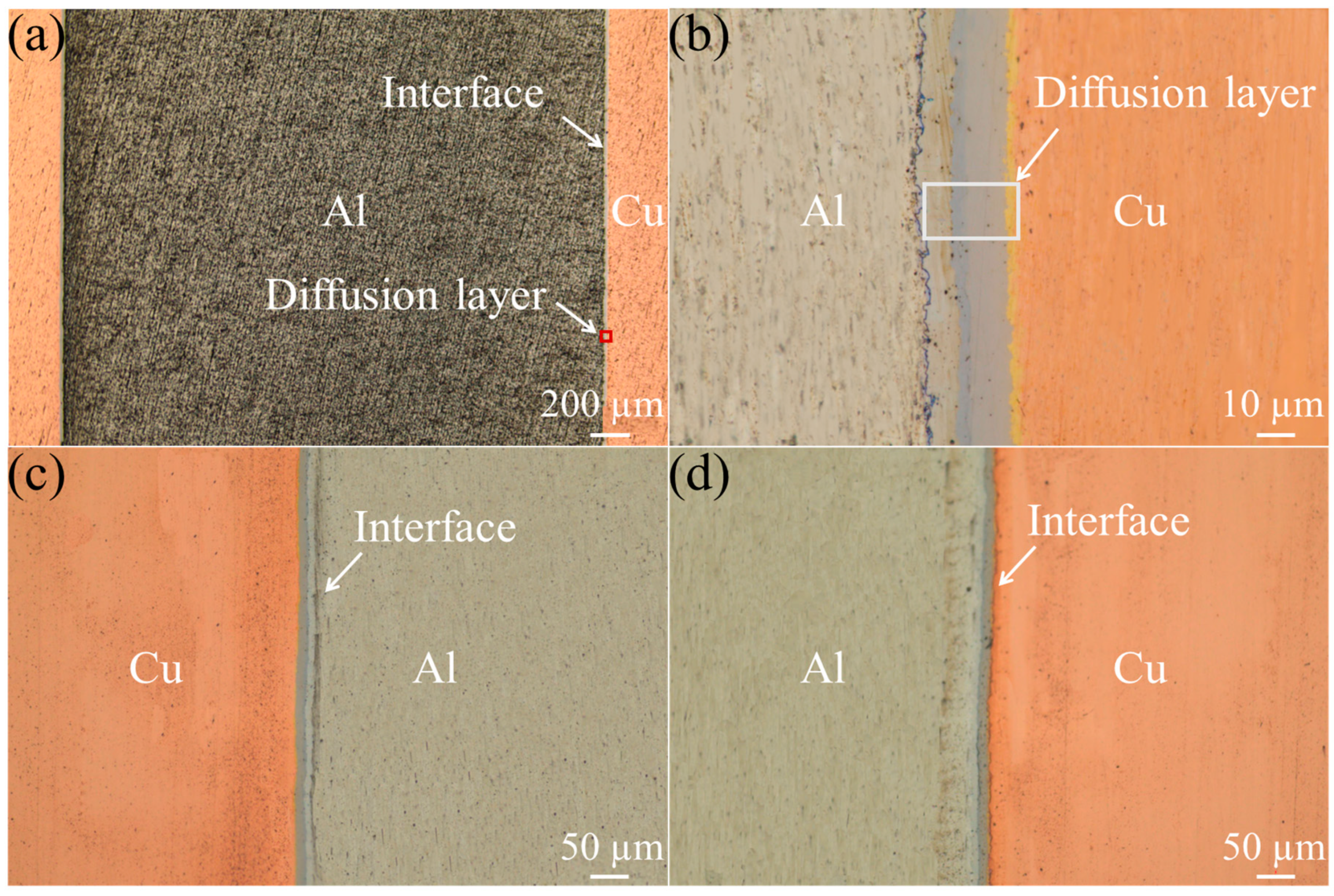
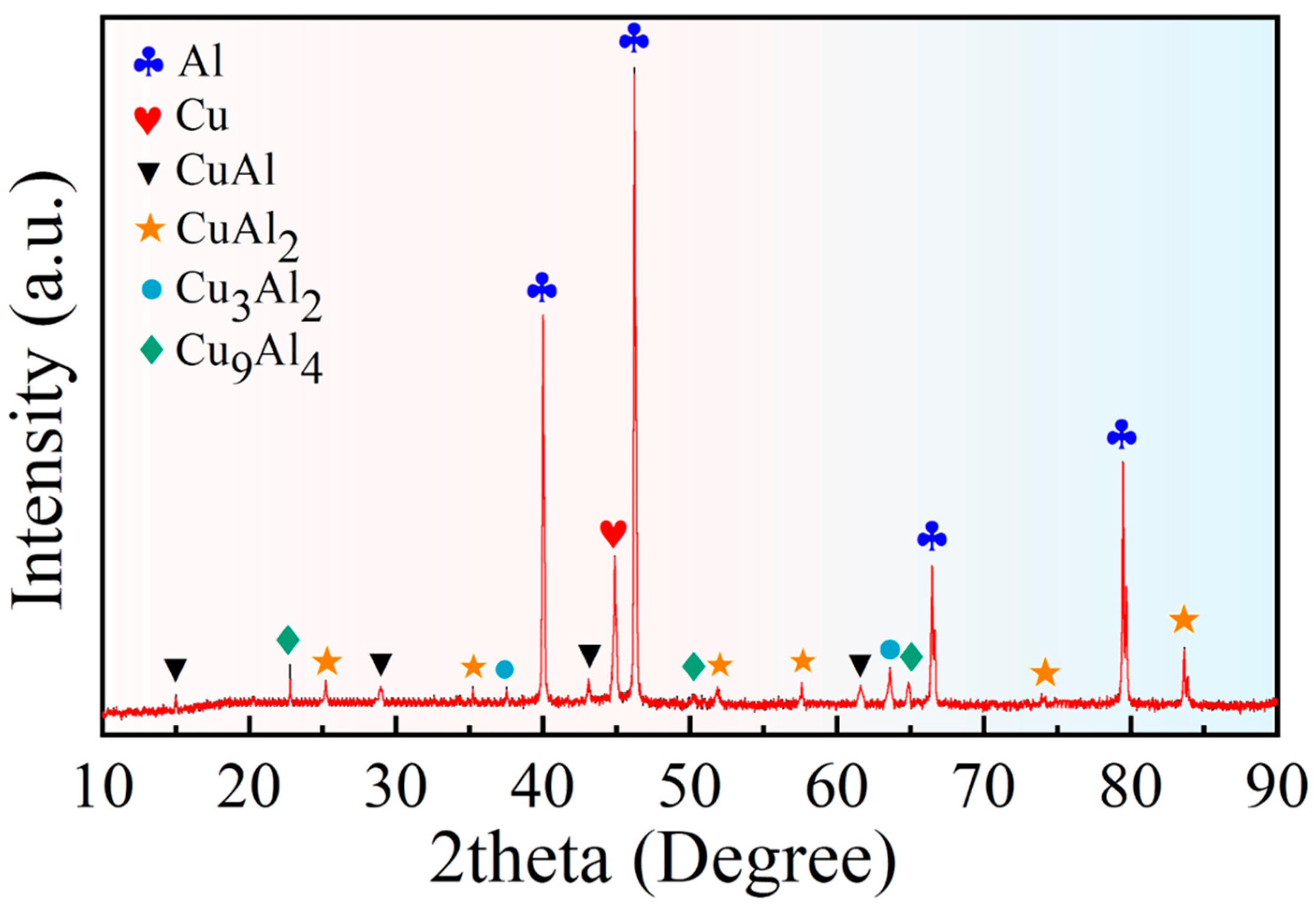
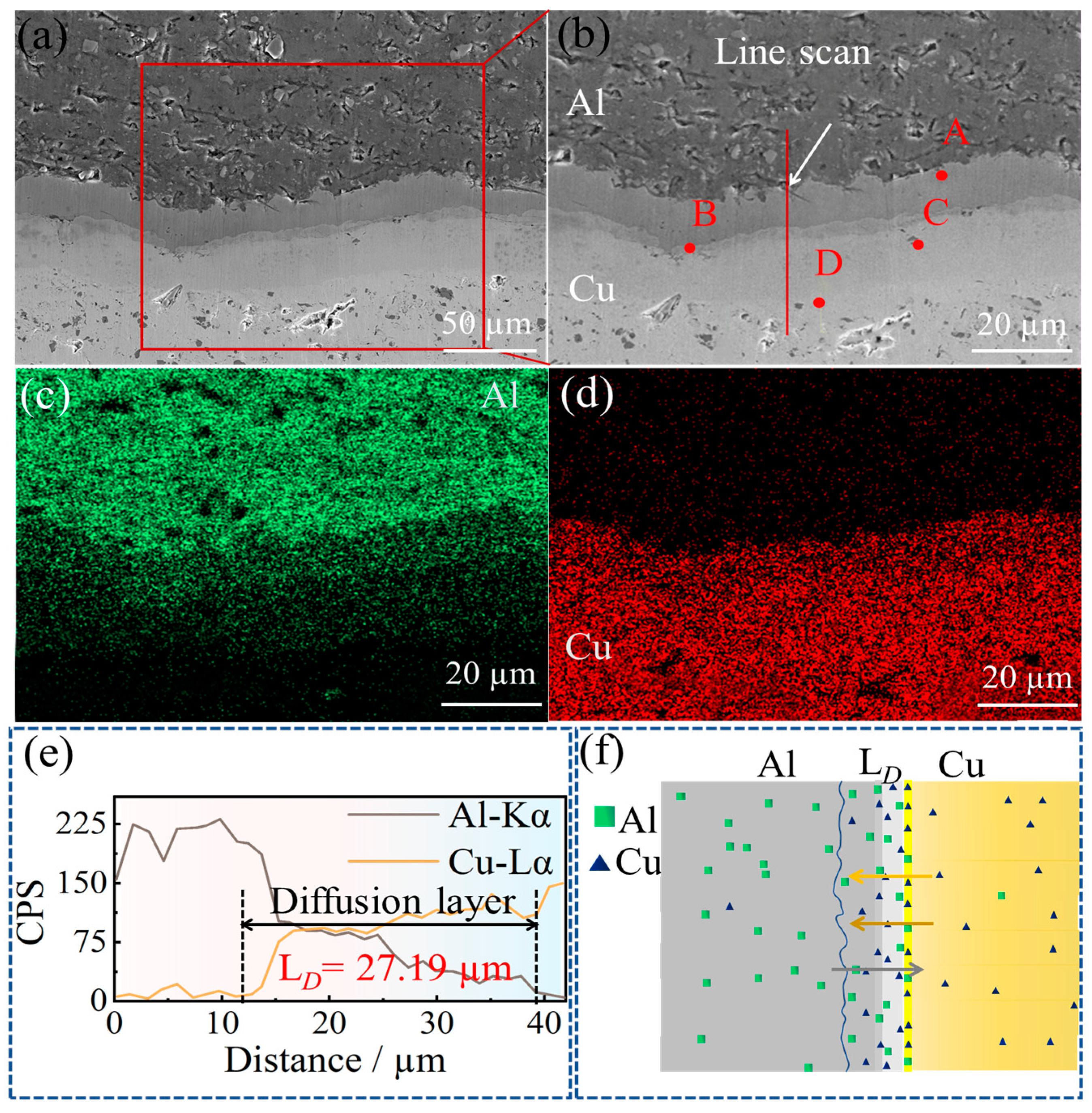
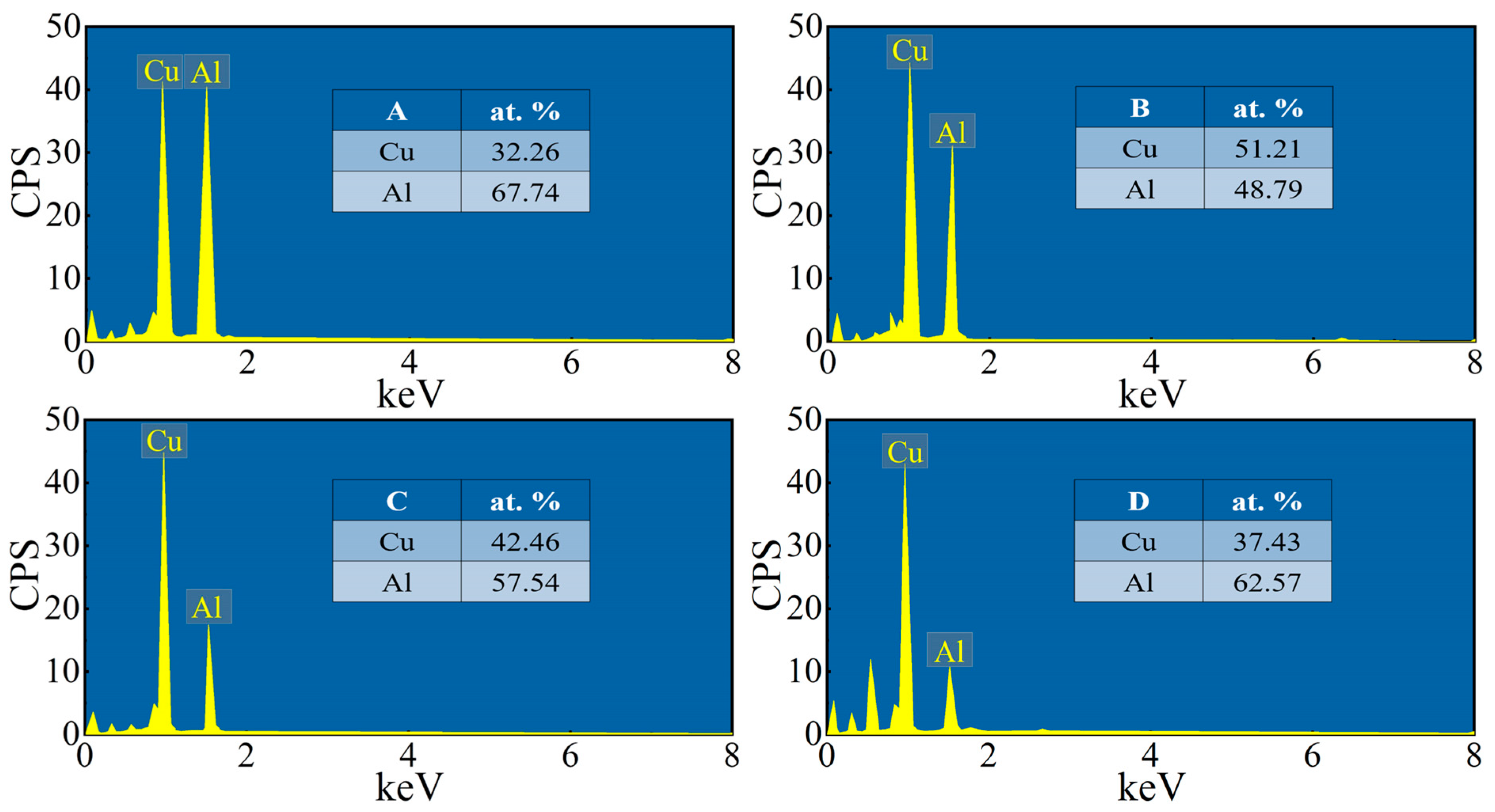
3.2. Microstructure of the Composite Interface
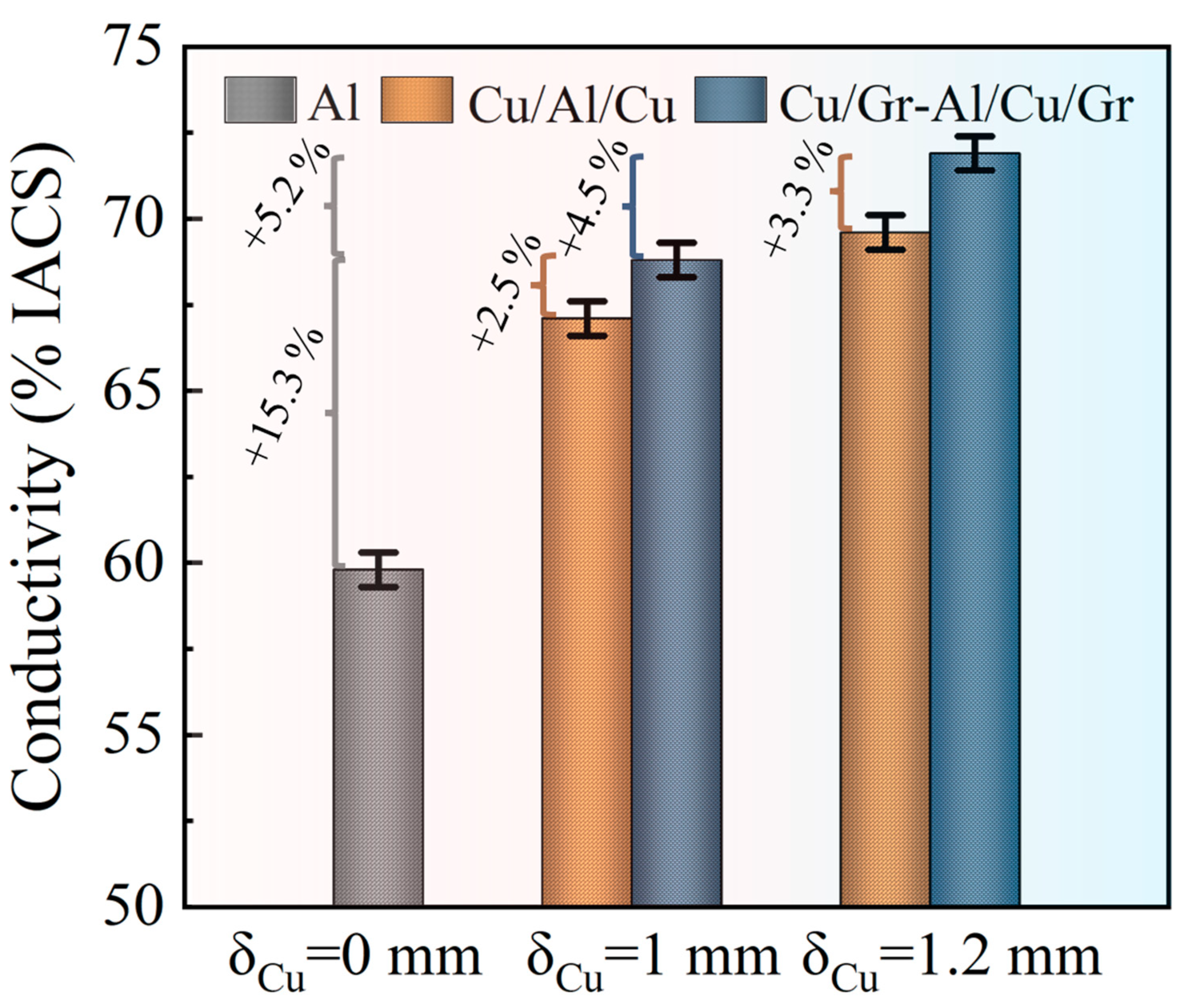
3.3. Electrical Conductivity
4. Conclusions
- The Cu(111)/Gr sample obtained by CVD was vacuum pressed twice at 900 °C and 530 °C. The Miller indices of the sample remained at the (111) crystal face, and the Cu block was still in the single crystal state;
- The Cu/Gr-Al-Cu/Gr laminated composites were successfully prepared by hot pressing for 1 h at a temperature of 530 °C and a pressure of 10 MPa at a heating rate of 10 °C/min. It was found that the laminated composites were well bonded without pores or cracks, there was an obvious diffusion layer at the interface bond, and the transition layer generated by the diffusion reaction of Cu and Al connected the composites. The total thickness of the diffusion layer was found to be 27.19 µm by EDS spotting, line scan, surface scan, and the intermediate phases from the Al side to the Cu side are CuAl2, CuAl, Cu3Al2, and Cu9Al4, in that order;
- The Cu/Gr-Al-Cu/Gr laminated composites prepared by the hot pressing method were able to exploit the high carrier mobility of graphene to improve the electrical conductivity of the composites and the thickness of the Cu(111)/Gr layer from 1 mm to 1.2 mm; the electrical conductivity of the Cu/Gr-Al-Cu/Gr increased by 4.5%, while the increase in the thickness of the Cu(111) layer was from 1 mm to 1.2 mm, and the Cu/Al/Cu conductivity increased by only 3.6%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunčická, L.; Kocich, R.; Dvořák, K.; Macháčková, A. Rotary swaged laminated Cu-Al composites: Effect of structure on residual stress and mechanical and electric properties. Mater. Sci. Eng. A 2019, 742, 743–750. [Google Scholar] [CrossRef]
- Wu, K.; Chang, H.; Maawad, E.; Gan, W.; Brokmeier, H.; Zheng, M. Microstructure and mechanical properties of the Mg/Al laminated composite fabricated by accumulative roll bonding (ARB). Mater. Sci. Eng. A 2010, 527, 3073–3078. [Google Scholar] [CrossRef]
- Tayyebi, M.; Eghbali, B. Study on the microstructure and mechanical properties of multilayer Cu/Ni composite processed by accumulative roll bonding. Mater. Sci. Eng. A 2013, 559, 759–764. [Google Scholar] [CrossRef]
- Abbas, M.; Rasheed, M. Solid State Reaction Synthesis and Characterization of Cu Doped TiO2 Nanomaterials; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; pp. 12059–12063. [Google Scholar]
- Mozaffari, A.; Manesh, H.D.; Janghorban, K. Evaluation of mechanical properties and structure of multilayered Al/Ni composites produced by accumulative roll bonding (ARB) process. J. Alloy. Compd. 2010, 489, 103–109. [Google Scholar] [CrossRef]
- Ghalandari, L.; Mahdavian, M.; Reihanian, M.; Mahmoudiniya, M. Production of Al/Sn multilayer composite by accumulative roll bonding (ARB): A study of microstructure and mechanical properties. Mater. Sci. Eng. A 2016, 661, 179–186. [Google Scholar] [CrossRef]
- Sun, Y.; Tsuji, N.; Fujii, H.; Li, F. Cu/Zr nanoscaled multi-stacks fabricated by accumulative roll bonding. J. Alloy. Compd. 2010, 504, S443–S447. [Google Scholar] [CrossRef]
- Motevalli, P.D.; Eghbali, B. Microstructure and mechanical properties of Tri-metal Al/Ti/Mg laminated composite processed by accumulative roll bonding. Mater. Sci. Eng. A 2015, 628, 135–142. [Google Scholar] [CrossRef]
- Rogachev, S.; Andreev, V.; Yusupov, V.; Bondareva, S.; Khatkevich, V.; Nikolaev, E. Effect of Rotary Forging on Microstructure Evolution and Mechanical Properties of Aluminum Alloy/Copper Bimetallic Material. Met. Mater. Int. 2022, 28, 1038–1046. [Google Scholar] [CrossRef]
- Shayanpoor, A.; Ashtiani, H.R. Microstructural and mechanical investigations of powder reinforced interface layer of hot extruded Al/Cu bimetallic composite rods. J. Manuf. Process. 2022, 77, 313–328. [Google Scholar] [CrossRef]
- Xing, B.-H.; Huang, T.; Song, K.-X.; Xu, L.-J.; Xiang, N.; Chen, X.-W.; Chen, F.-X. Effect of electric current on formability and microstructure evolution of Cu/Al laminated composite. J. Mater. Res. Technol. 2022, 21, 1128–1140. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Zhao, D.; Liu, Y.; Jiang, Z. Effects of annealing temperature on interface microstructure and element diffusion of ultra-thin Cu/Al composite sheets. Mater. Lett. 2022, 322, 132491. [Google Scholar] [CrossRef]
- Kocich, R.; Kunčická, L.; Davis, C.F.; Lowe, T.C.; Szurman, I.; Macháčková, A. Deformation behavior of multilayered Al-Cu clad composite during cold-swaging. Mater. Des. 2016, 90, 379–388. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R. Optimizing electric conductivity of innovative Al-Cu laminated composites via thermomechanical treatment. Mater. Des. 2022, 215, 110441. [Google Scholar] [CrossRef]
- Lapovok, R.; Berner, A.; Qi, Y.; Xu, C.; Rabkin, E.; Beygelzimer, Y. The effect of a small copper addition on the electrical conductivity of aluminum. Adv. Eng. Mater. 2020, 22, 2000058. [Google Scholar] [CrossRef]
- Kocich, R.; Kunčická, L.; Král, P.; Strunz, P. Characterization of innovative rotary swaged Cu-Al clad composite wire conductors. Mater. Des. 2018, 160, 828–835. [Google Scholar] [CrossRef]
- Han, Y.-q.; Ben, L.-h.; Yao, J.-j.; Feng, S.-w.; Wu, C.-j. Investigation on the interface of Cu/Al couples during isothermal heating. Int. J. Miner. Metall. Mater. 2015, 22, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Chen, Y.-q.; Li, L.; Hu, H.-d.; Zhu, Z.-a. Microstructure and properties of Al/Cu bimetal in liquid–solid compound casting process. Trans. Nonferrous Met. Soc. China 2016, 26, 1555–1563. [Google Scholar]
- Shamaila, Y.; Ahmad, B.A.; Adnan, A. Study of Carbon Nanbotubes and Boron Nanotubes Using Degree Based Topological Indices. Polycycl. Aromat. Compd. 2022, 42, 7724–7737. [Google Scholar]
- Rasheed, M.; Shihab, S.; Sabah, O.W. An Investigation of the Structural, Electrical and Optical Properties of Graphene-Oxide Thin Films Using Different Solvents; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; p. 012052. [Google Scholar]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J.; et al. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Niu, M.; Yang, F.; Jia, Y.; Cheng, Y. Ultrafast electro-thermal responsive heating film fabricated from graphene modified conductive materials. Eng. Sci. 2019, 8, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Liu, Z.; Xu, J.; Zong, Y. Electrodeposition-based fabrication of graphene/copper composites with excellent overall properties. J. Alloy. Compd. 2022, 924, 166610. [Google Scholar] [CrossRef]
- Dong, L.; Chen, W.; Zheng, C.; Deng, N. Microstructure and properties characterization of tungsten–copper composite materials doped with graphene. J. Alloy. Compd. 2017, 695, 1637–1646. [Google Scholar] [CrossRef]
- Cao, M.; Xiong, D.-B.; Tan, Z.; Ji, G.; Amin-Ahmadi, B.; Guo, Q.; Fan, G.; Guo, C.; Li, Z.; Zhang, D. Aligning graphene in bulk copper: Nacre-inspired nanolaminated architecture coupled with in-situ processing for enhanced mechanical properties and high electrical conductivity. Carbon 2017, 117, 65–74. [Google Scholar] [CrossRef]
- Chen, F.; Ying, J.; Wang, Y.; Du, S.; Liu, Z.; Huang, Q. Effects of graphene content on the microstructure and properties of copper matrix composites. Carbon 2016, 96, 836–842. [Google Scholar] [CrossRef]
- Cong, C.; Yu, T.; Ni, Z.; Liu, L.; Shen, Z.; Huang, W. Fabrication of graphene nanodisk arrays using nanosphere lithography. J. Phys. Chem. C 2009, 113, 6529–6532. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, B.-h.; Chen, G.-h.; Wang, R.-m.; Miao, C.-h.; Zheng, Z.-x.; Tang, W.-m. Formation and growth of Cu–Al IMCs and their effect on electrical property of electroplated Cu/Al laminar composites. Trans. Nonferrous Met. Soc. China 2016, 26, 3283–3291. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hwang, W.-S. Effect of annealing on the interfacial structure of aluminum-copper joints. Mater. Trans. 2007, 48, 0706110009. [Google Scholar] [CrossRef] [Green Version]
- Yihui, G. Mathematical Analysis of van der Pauw’s Method for Measuring Resistivity; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2022; Volume 2321. [Google Scholar]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Yoon, T.; Jin, S.H.; Lee, J.; Kim, T.S.; Hong, S.H.; Jeon, S. Enhanced mechanical properties of graphene/copper nanocomposites using a molecular-level mixing process. Adv. Mater. 2013, 25, 6724–6729. [Google Scholar] [CrossRef]
- Yoon, T.; Shin, W.C.; Kim, T.Y.; Mun, J.H.; Kim, T.-S.; Cho, B.J. Direct measurement of adhesion energy of monolayer graphene as-grown on copper and its application to renewable transfer process. Nano Lett. 2012, 12, 1448–1452. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Zhang, R.; Xu, Q.; Kong, X.; Sun, W.; Fu, Y.; Wu, M.; Liu, K. Fabrication of Cu/Al/Cu Laminated Composites Reinforced with Graphene by Hot Pressing and Evaluation of Their Electrical Conductivity. Materials 2023, 16, 622. https://doi.org/10.3390/ma16020622
Zheng H, Zhang R, Xu Q, Kong X, Sun W, Fu Y, Wu M, Liu K. Fabrication of Cu/Al/Cu Laminated Composites Reinforced with Graphene by Hot Pressing and Evaluation of Their Electrical Conductivity. Materials. 2023; 16(2):622. https://doi.org/10.3390/ma16020622
Chicago/Turabian StyleZheng, Hang, Ruixiang Zhang, Qin Xu, Xiangqing Kong, Wanting Sun, Ying Fu, Muhong Wu, and Kaihui Liu. 2023. "Fabrication of Cu/Al/Cu Laminated Composites Reinforced with Graphene by Hot Pressing and Evaluation of Their Electrical Conductivity" Materials 16, no. 2: 622. https://doi.org/10.3390/ma16020622
APA StyleZheng, H., Zhang, R., Xu, Q., Kong, X., Sun, W., Fu, Y., Wu, M., & Liu, K. (2023). Fabrication of Cu/Al/Cu Laminated Composites Reinforced with Graphene by Hot Pressing and Evaluation of Their Electrical Conductivity. Materials, 16(2), 622. https://doi.org/10.3390/ma16020622





