Electrochemical, Biological, and Technological Properties of Anodized Titanium for Color Coded Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subtrate Material
2.2. Anodization Procedure
2.3. Surface Characterization and Color Assessment
2.4. Contact Angle Measurement
2.5. Electrochemical Testing
2.6. Biocompatibility Tests
3. Results
3.1. Surface Character and Color Assessment
3.2. Contact Angle Measurement
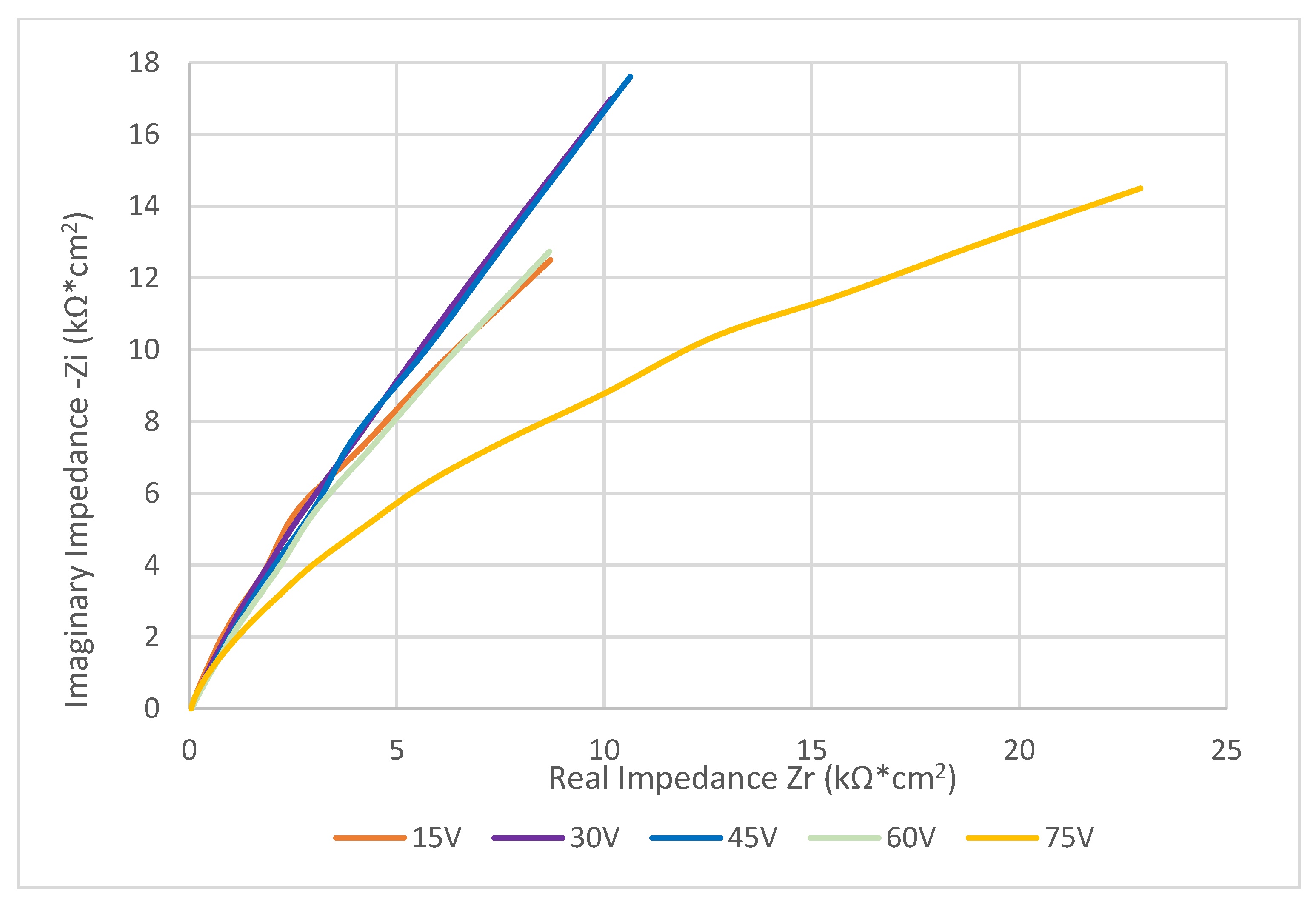
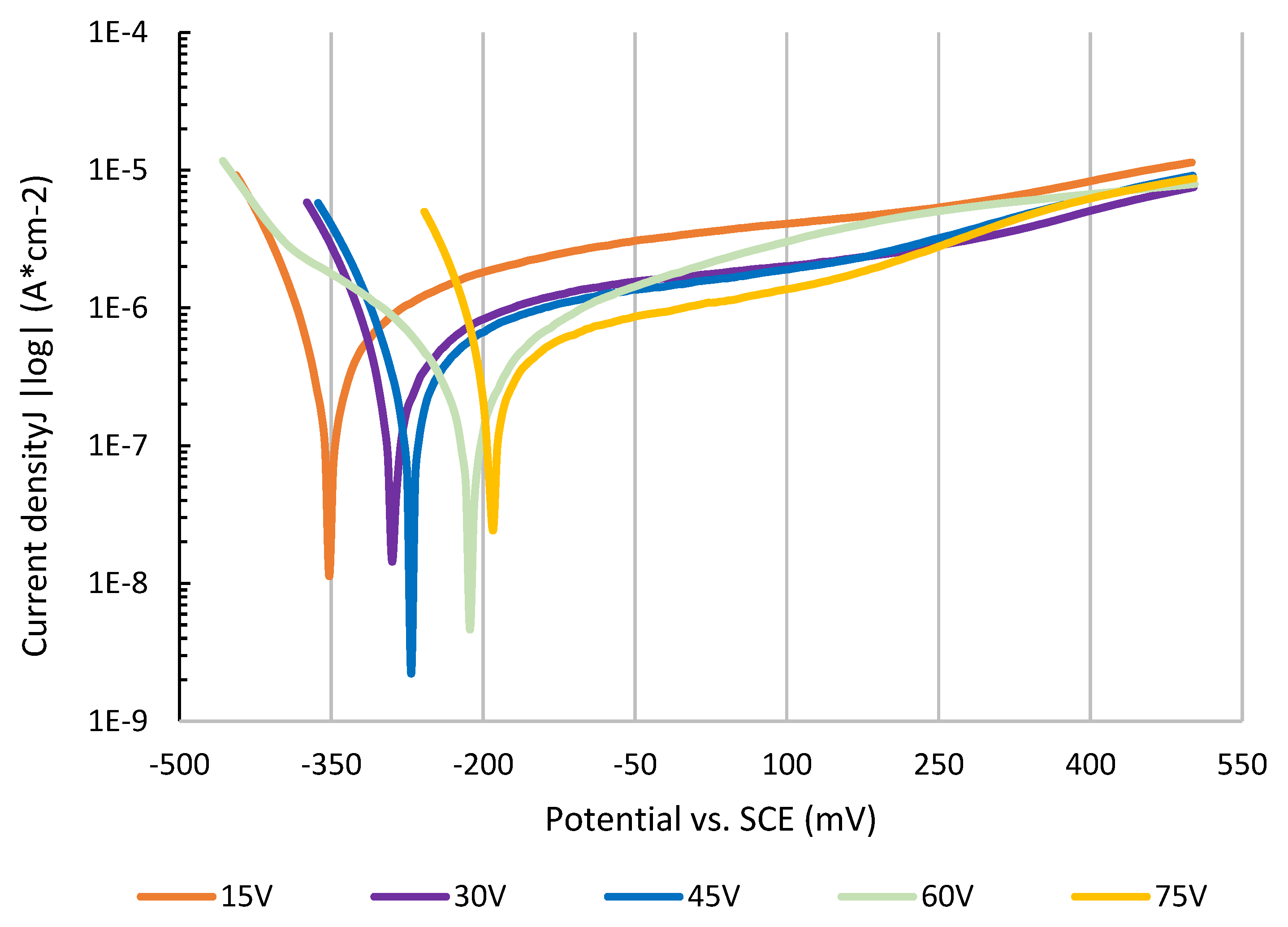
3.3. Electrochemical Testing
3.4. Biocompatibility and Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pruitt, L.A.; Chakravartula, A.M. Biocompatibility, sterilization, and materials selection for implant design. In Mechanics of Biomaterials; Cambridge University Press: Cambridge, UK, 2012; pp. 3–25. [Google Scholar] [CrossRef]
- Ross, A.P.; Webster, T.J. Anodizing color coded anodized Ti6Al4V medical devices for increasing bone cell functions. Int. J. Nanomed. 2013, 8, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Popovich, A.; Sufiiarov, V.; Polozov, I.; Borisov, E.; Masaylo, D. Producing hip implants of titanium alloys by additive manufacturing. Int. J. Bioprint. 2016, 946, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Napoli, G.; Di Schino, A.; Paura, M.; Vela, T. Colouring titanium alloys by anodic oxidation. Metalurgija 2018, 57, 111–113. [Google Scholar]
- Rodrigues, D.C.; Valderrama, P.; Wilson, T.G.; Palmer, K.; Thomas, A.; Sridhar, S.; Adapalli, A.; Burbano, M.; Wadhwani, C. Titanium corrosion mechanisms in the oral environment: A retrieval study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [Green Version]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The impact of implant-abutment connection on clinical outcomes and microbial colonization: A narrative review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef] [Green Version]
- Carinci, F.; Lauritano, D.; Cura, F.; Lopez, M.A.; Bassi, M.A.; Confalone, L.; Pezzetti, F. Prevention of bacterial leakage at implant-Abutment connection level: An in vitro study of the efficacy of three different implant systems. J. Biol. Regul. Homeost. Agents 2016, 30, 69–73. [Google Scholar]
- Lopez, M.A.; Bassi, M.A.; Confalone, L.; Gaudio, R.M.; Lombardo, L.; Lauritano, D. The influence of “conical plus octagonal” internal connection on implant survival and success rate: A retrospective study of 66 fixtures. J. Biol. Regul. Homeost. Agents 2016, 30, 49–54. [Google Scholar]
- Costa, R.C.; Abdo, V.L.; Mendes, P.H.C.; Mota-Veloso, I.; Bertolini, M.; Mathew, M.T.; Barāo, V.A.R.; Souza, J.G.S. Microbial Corrosion in Titanium-Based Dental Implants: How Tiny Bacteria Can Create a Big Problem? J. Bio-Tribo-Corros. 2021, 7, 136. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Donatus, U.; Thompson, G.E.; Skeldon, P. Corrosion behaviour of the anodic oxide film on commercially pure titanium in NaCl environment. Int. J. Electrochem. Sci. 2014, 9, 3558–3573. [Google Scholar]
- Do, M.T.; Gauquelin, N.; Nguyen, M.D.; Wang, J.; Verbeeck, J.; Blom, F.; Koster, G.; Houwman, E.P.; Rijnders, G. Interfacial dielectric layer as an origin of polarization fatigue in ferroelectric capacitors. Sci. Rep. 2020, 10, 7310. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Pedeferri, M.P. Effect of anodic oxidation parameters on the titanium oxides formation. Corros. Sci. 2007, 49, 939–948. [Google Scholar] [CrossRef]
- Yan, Z.M.; Guo, T.W.; Pan, H.B.; Yu, J.J. Influences of electrolyzing voltage on chromatics of anodized titanium dentures. Mater. Trans. 2002, 43, 3142–3145. [Google Scholar] [CrossRef] [Green Version]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure commercial titanium color anodizing and corrosion resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. In Recent Advances in Arthroplasty; InTechOpen: London, UK, 2012. [Google Scholar]
- ASTM Standard B348; Standard Specification for Titanium and Titanium Alloy. ASTM Book of Standards; ASTM: West Conshohocken, PA, USA, 2013.
- ASTM D6762-18; ASTM Standard Test Method for Determining the Hiding Power of Paint by Visual Evaluation of Spray Applied Coatings 1. ASTM: West Conshohocken, PA, USA, 2015.
- Kohonen, O.; Parkkinen, J.; Jääskeläinen, T. Databases for spectral color science. Color Res. Appl. 2006, 31, 381–390. [Google Scholar] [CrossRef]
- Kam, D.H.; Bhattacharya, S.; Mazumder, J. Control of the wetting properties of an AISI 316L stainless steel surface by femtosecond laser-induced surface modification. J. Micromech. Microeng. 2012, 22, 105019. [Google Scholar] [CrossRef]
- Calvimontes, A. The Measurement of the Surface Energy of Solids by Sessile Drop Accelerometry. Microgravity Sci. Technol. 2018, 30, 277–293. [Google Scholar] [CrossRef]
- Mohd Shafiee, M.A.; Muhamad Asri, M.A.; Syed Alwi, S.S. Review on the in vitro cytotoxicity assessment in accordance to the international organization for standardization (ISO). Malays. J. Med. Health Sci. 2021, 17, 261–269. [Google Scholar]
- Diamanti, M.V.; Pozzi, P.; Randone, F.; Del Curto, B.; Pedeferri, M.P. Robust anodic colouring of titanium: Effect of electrolyte and colour durability. Mater. Des. 2016, 90, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Itagaki, M.; Suzuki, S.; Shitanda, I.; Watanabe, K. Electrochemical impedance and complex capacitance to interpret electrochemical capacitor. Electrochemistry 2007, 75, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470041406. [Google Scholar]
- Yeh, T.K.; Wu, P.I.; Tsai, C.H. Corrosion of ZrO2 treated type 304 stainless steels in high temperature pure water with various amounts of hydrogen peroxide. Prog. Nucl. Energy 2012, 57, 62–70. [Google Scholar] [CrossRef]
- Papavinasam, S. Pitting corrosion. In Trends in Oil and Gas Corrosion Research and Technologies: Production and Transmission; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081012192. [Google Scholar]
- International Organization for Standardization Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009; pp. 1–52.
- Liu, Z.J.; Zhong, X.; Walton, J.; Thompson, G.E. Anodic Film Growth of Titanium Oxide Using the 3-Electrode Electrochemical Technique: Effects of Oxygen Evolution and Morphological Characterizations. J. Electrochem. Soc. 2016, 163, E75–E82. [Google Scholar] [CrossRef] [Green Version]
- Diamanti, M.V.; Del Curto, B.; Masconale, V.; Passaro, C.; Pedeferri, M.P. Anodic coloring of titanium and its alloy for jewels production. Color Res. Appl. 2012, 37, 384–390. [Google Scholar] [CrossRef]
- Napoli, G.; Zitelli, C.; Corapi, D.; Di Schino, A. Titanium alloys anodic oxidation: Effect of experimental parameters on surface colouring. Mater. Sci. Forum 2018, 941, 730–734. [Google Scholar] [CrossRef]
- ASTM D1524; International United States Standard Test Method for ASTM Color of Petroleum Products (ASTM Color Scale). ASTM: West Conshohocken, PA, USA, 2011.
- Vera, M.L.; Avalos, M.C.; Rosenberger, M.R.; Bolmaro, R.E.; Schvezov, C.E.; Ares, A.E. Evaluation of the influence of texture and microstructure of titanium substrates on TiO2anodic coatings at 60 V. Mater. Charact. 2017, 131, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, E.D.S.; Moura de Souza Soares, F.; Nunes, L.F.; Santana, A.I.C.; de Biasi, R.S.; Elias, C.N. Comparison of the wettability and corrosion resistance of two biomedical Ti alloys free of toxic elements with those of the commercial ASTM F136 (Ti–6Al–4V) alloy. J. Mater. Res. Technol. 2020, 9, 16329–16338. [Google Scholar] [CrossRef]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci. Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef]
- Krawczyk, J.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of Spontaneous and Water-Based Passivation on Components and Parameters of Ti6Al4V (ELI Grade) Surface Tension and Its Wettability by an Aqueous Solution of Sucrose Ester Surfactants. Molecules 2021, 27, 179. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Shim, I.K.; Chung, H.J.; Jung, M.R.; Nam, S.Y.; Lee, S.Y.; Lee, H.; Heo, S.J.; Lee, S.J. Biofunctional porous anodized titanium implants for enhanced bone regeneration. J. Biomed. Mater. Res. Part A 2013, 102, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Ehlinger, M.; Adam, P.; Simon, P.; Bonnomet, F. Technical difficulties in hardware removal in titanium compression plates with locking screws. Orthop. Traumatol. Surg. Res. 2009, 95, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavana, H.; Neumann, A.W. Recent progress in the determination of solid surface tensions from contact angles. Adv. Colloid Interface Sci. 2007, 132, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- De Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Pajkossy, T.; Jurczakowski, R. Electrochemical impedance spectroscopy in interfacial studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Chulkin, P.; Data, P. Electrochemical Impedance Spectroscopy as a Tool for Electrochemical Rate Constant Estimation. J. Vis. Exp. 2018, 140, e56611. [Google Scholar] [CrossRef] [Green Version]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. In situ synthesis of polyaniline-camphorsulfonate particles in an epoxy matrix for corrosion protection of mild steel in NaCl solution. Corros. Sci. 2014, 85, 204–214. [Google Scholar] [CrossRef]
- Hlinka, J.; Lasek, S.; Faisal, N. Corrosion properties of anodized titanium. Acta Metall. Slovaca 2017, 23, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson, W.; Petrini, D.; Edström, K.; Björefors, F.; Nyholm, L. Corrosion resistances and passivation of powder metallurgical and conventionally cast 316L and 2205 stainless steels. Corros. Sci. 2013, 67, 268–280. [Google Scholar] [CrossRef]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Corrosion in titanium dental implants/prostheses—A review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Fujii, M.; Seri, O. Polarization curve and its analysis of titanium in a mixture solution of hydrogen peroxide and ammonia. Keikinzoku/J. Jpn. Inst. Light Met. 2016, 66, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Hiromoto, S. Corrosion of metallic biomaterials. In Metals for Biomedical Devices; Woodhead Publishing: Southen, UK, 2010; pp. 99–121. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, M.; Baby, S.; Menon, K.V. Spinal fixation device: A 6-year postimplantation study. J. Biomater. Appl. 2003, 18, 109–121. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J.; et al. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef]
- Syam, S.; Wu, C.J.; Lan, W.C.; Ou, K.L.; Huang, B.H.; Lin, Y.Y.; Saito, T.; Tsai, H.Y.; Chuo, Y.C.; Yen, M.L.; et al. The potential of a surface-modified titanium implant with tetrapeptide for osseointegration enhancement. Appl. Sci. 2021, 11, 2616. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.A.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F.J. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Chandar, S.; Kotian, R.; Madhyastha, P.; Kabekkodu, S.; Rao, P. In vitro evaluation of cytotoxicity and corrosion behavior of commercially pure titanium and Ti-6Al-4V alloy for dental implants. J. Indian Prosthodont. Soc. 2017, 17, 35–40. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef]
- Kanematu, N.; Shibata, K.I.; Kurenuma, S.; Watanabe, K.; Yamagami, A.; Nishio, Y.; Fujii, T. Cytotoxicity of oxide anodized titanium alloy evaluated by cell and organic culture study. Gifu Shika Gakkai Zasshi J. Gifu Dent. Soc. 1990, 17, 583–591. [Google Scholar]
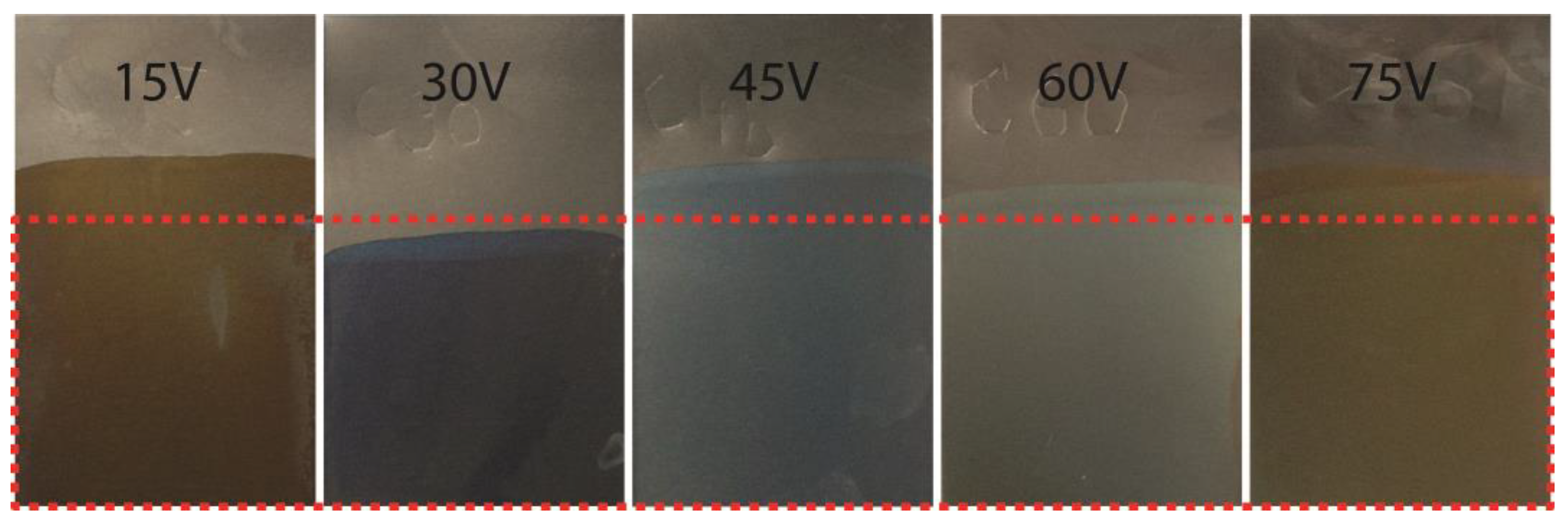
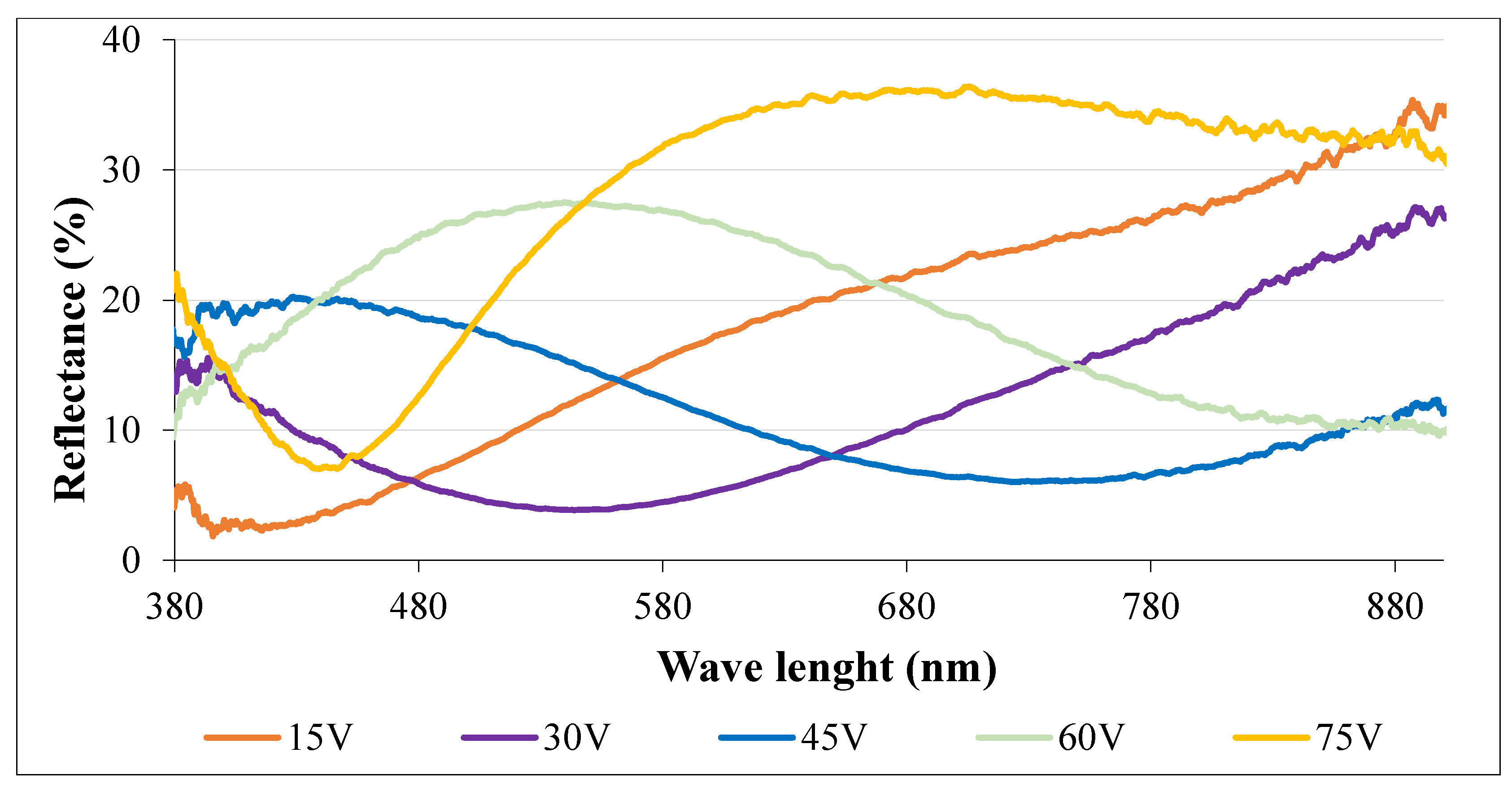
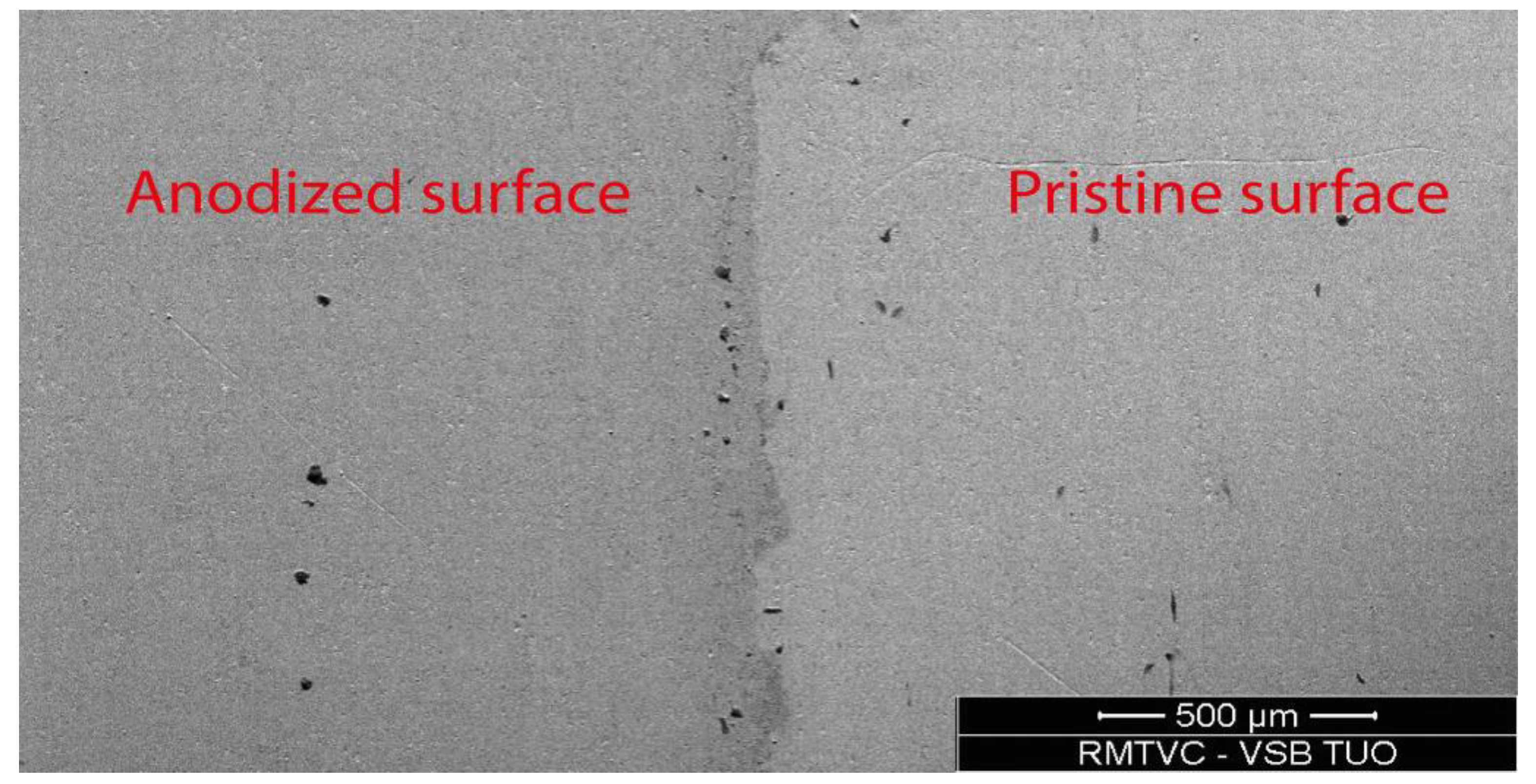
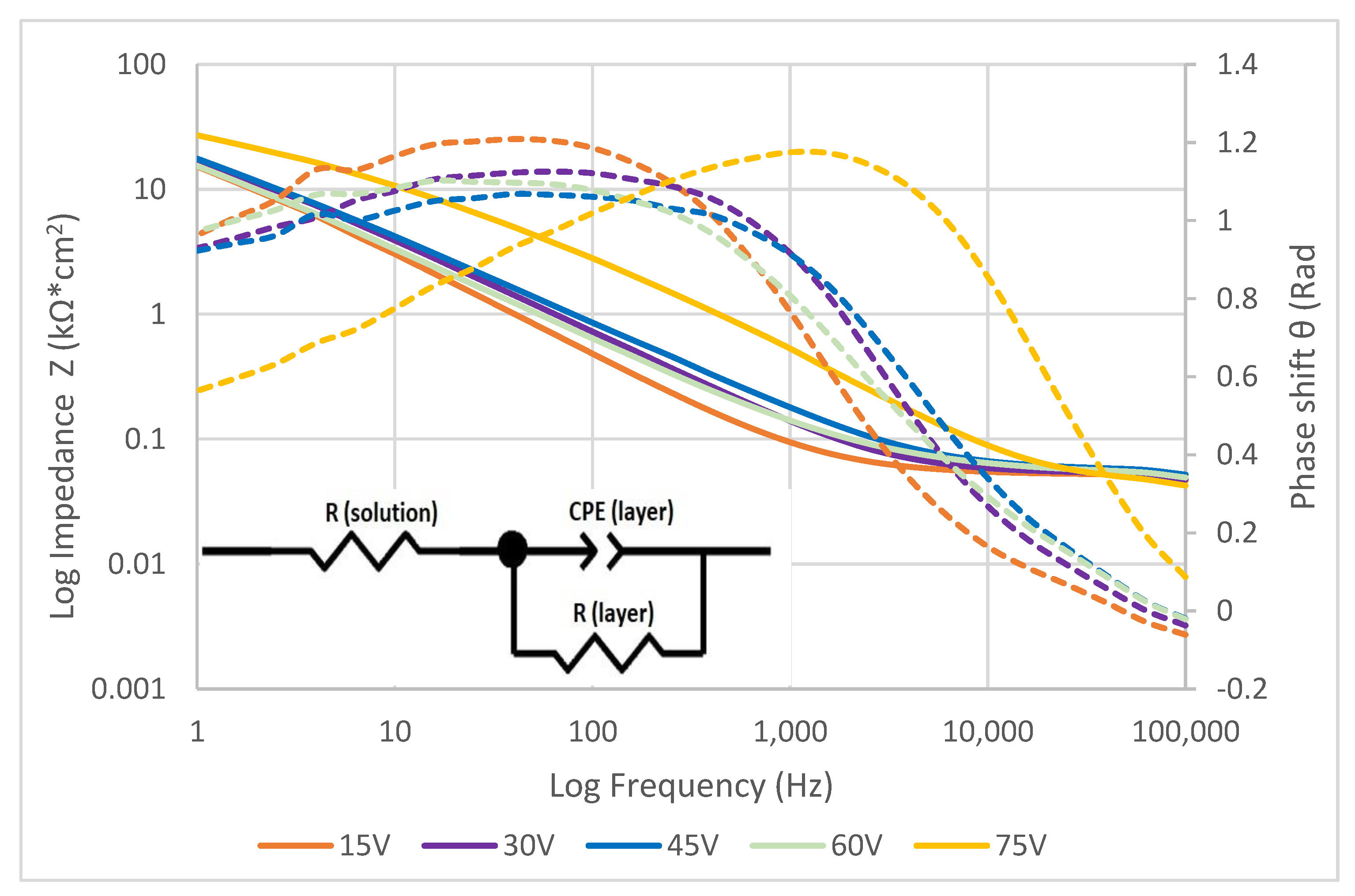
| Voltage (V) | RAL (Name) | RAL (Code) |
|---|---|---|
| 15 | Clay brown | 8003 |
| 30 | Steel blue | 5011 |
| 45 | Sky blue | 5015 |
| 60 | Pale green | 6019 |
| 75 | Ochre yellow | 1024 |
| Anodization Voltage (V) | Contact Angle (°) | Surface Energy (mJ/m2) |
|---|---|---|
| 15 | 81 ± 2 | 35.0 |
| 30 | 82 ± 4 | 34.1 |
| 45 | 80 ± 2 | 35.3 |
| 60 | 81 ± 4 | 34.8 |
| 75 | 82 ± 3 | 34.1 |
| Anodization Voltage (V) | Resistance of Solution (Ω) | Resistance of Layer (Ω·cm2) | P | n | Capacitance of Layer (F·cm2) |
|---|---|---|---|---|---|
| 15 | 48.59 | 4.32 × 104 | 1.17 × 10−5 | 0.81 | 1.00 × 10−5 |
| 30 | 48.411 | 5.08 × 104 | 1.12 × 10−5 | 0.76 | 9.33 × 10−6 |
| 45 | 51.581 | 6.12 × 104 | 1.19 × 10−5 | 0.72 | 1.05 × 10−5 |
| 60 | 50.435 | 6.93 × 104 | 1.44 × 10−5 | 0.74 | 1.44 × 10−5 |
| 75 | 36.086 | 2.93 × 104 | 2.54 × 10−6 | 0.76 | 1.11 × 10−6 |
| Voltage (V) | Ecorr vs. SCE (mV) | Corr. Rate (nm/year) | Polar. Resistance (kΩ·cm2) |
|---|---|---|---|
| 15 | −356 | 2.1 | 63 |
| 30 | −278 | 1.1 | 79 |
| 45 | −256 | 1.5 | 85 |
| 60 | −221 | 1 | 82 |
| 75 | −194 | 0.7 | 35 |
| Cell Viability (%) | ||
|---|---|---|
| Sample | Extract Test | Contact Test |
| Ti-15V | 99.7 ± 4.3 | 99.2 ± 3.1 |
| Ti-30V | 99.2 ± 3.8 | 99.5 ± 3.6 |
| Ti-45V | 100.0 ± 0.9 | 99.4 ± 3.1 |
| Ti-50V | 100.0 ± 2.1 | 99.7 ± 4.0 |
| Ti-75V | 99.5 ± 3.3 | 99.5 ± 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlinka, J.; Dostalova, K.; Cabanova, K.; Madeja, R.; Frydrysek, K.; Koutecky, J.; Rybkova, Z.; Malachova, K.; Umezawa, O. Electrochemical, Biological, and Technological Properties of Anodized Titanium for Color Coded Implants. Materials 2023, 16, 632. https://doi.org/10.3390/ma16020632
Hlinka J, Dostalova K, Cabanova K, Madeja R, Frydrysek K, Koutecky J, Rybkova Z, Malachova K, Umezawa O. Electrochemical, Biological, and Technological Properties of Anodized Titanium for Color Coded Implants. Materials. 2023; 16(2):632. https://doi.org/10.3390/ma16020632
Chicago/Turabian StyleHlinka, Josef, Kamila Dostalova, Kristina Cabanova, Roman Madeja, Karel Frydrysek, Jan Koutecky, Zuzana Rybkova, Katerina Malachova, and Osamu Umezawa. 2023. "Electrochemical, Biological, and Technological Properties of Anodized Titanium for Color Coded Implants" Materials 16, no. 2: 632. https://doi.org/10.3390/ma16020632
APA StyleHlinka, J., Dostalova, K., Cabanova, K., Madeja, R., Frydrysek, K., Koutecky, J., Rybkova, Z., Malachova, K., & Umezawa, O. (2023). Electrochemical, Biological, and Technological Properties of Anodized Titanium for Color Coded Implants. Materials, 16(2), 632. https://doi.org/10.3390/ma16020632







