Mechanical and Hydration Characteristics of Stabilized Gold Mine Tailings Using a Sustainable Industrial Waste-Based Binder
Abstract
:1. Introduction
2. Materials
2.1. Industrial Wastes as a Binder Preparation
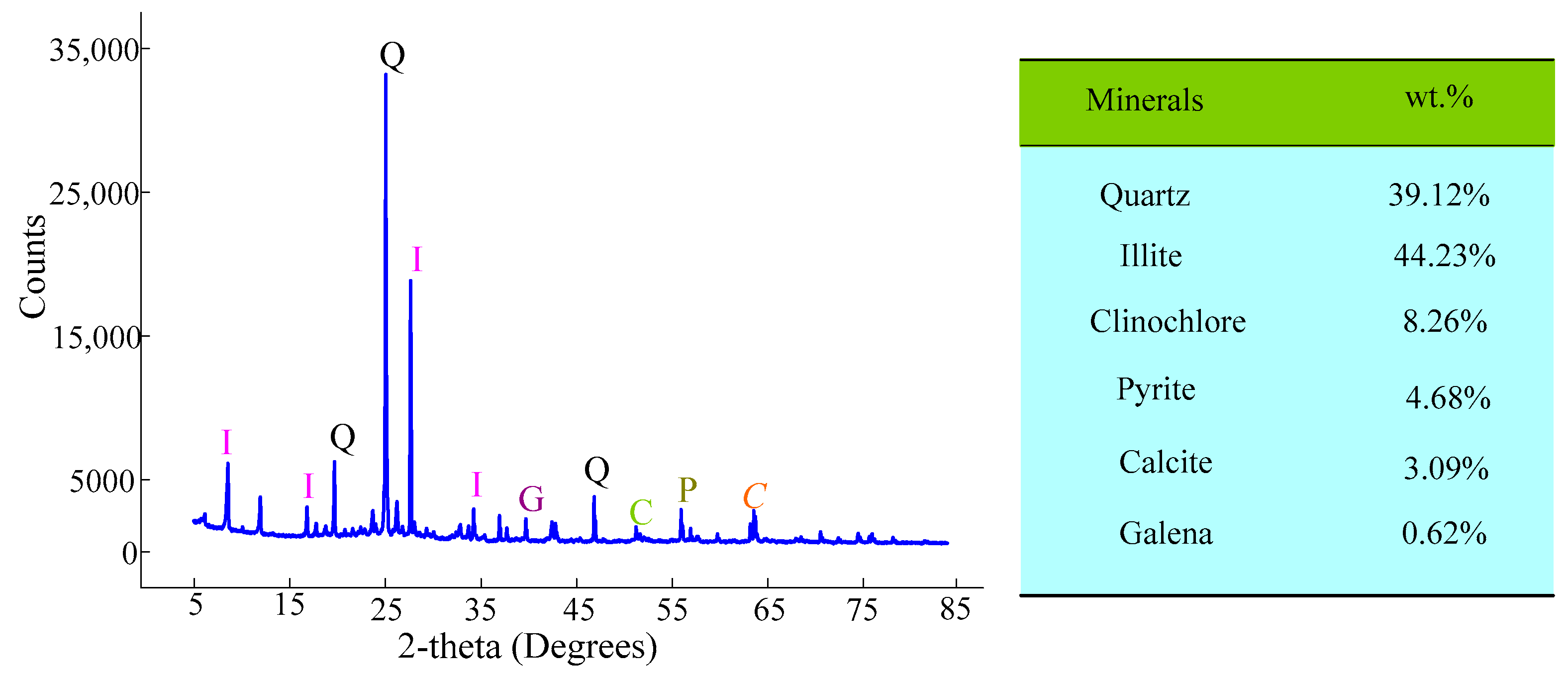
2.2. Gold Mine Tailings
3. Sample Preparation
4. Apparatus and Test Procedure
5. Results and Analysis
5.1. Mechanical Properties of Gold Tailings Blended with Binder
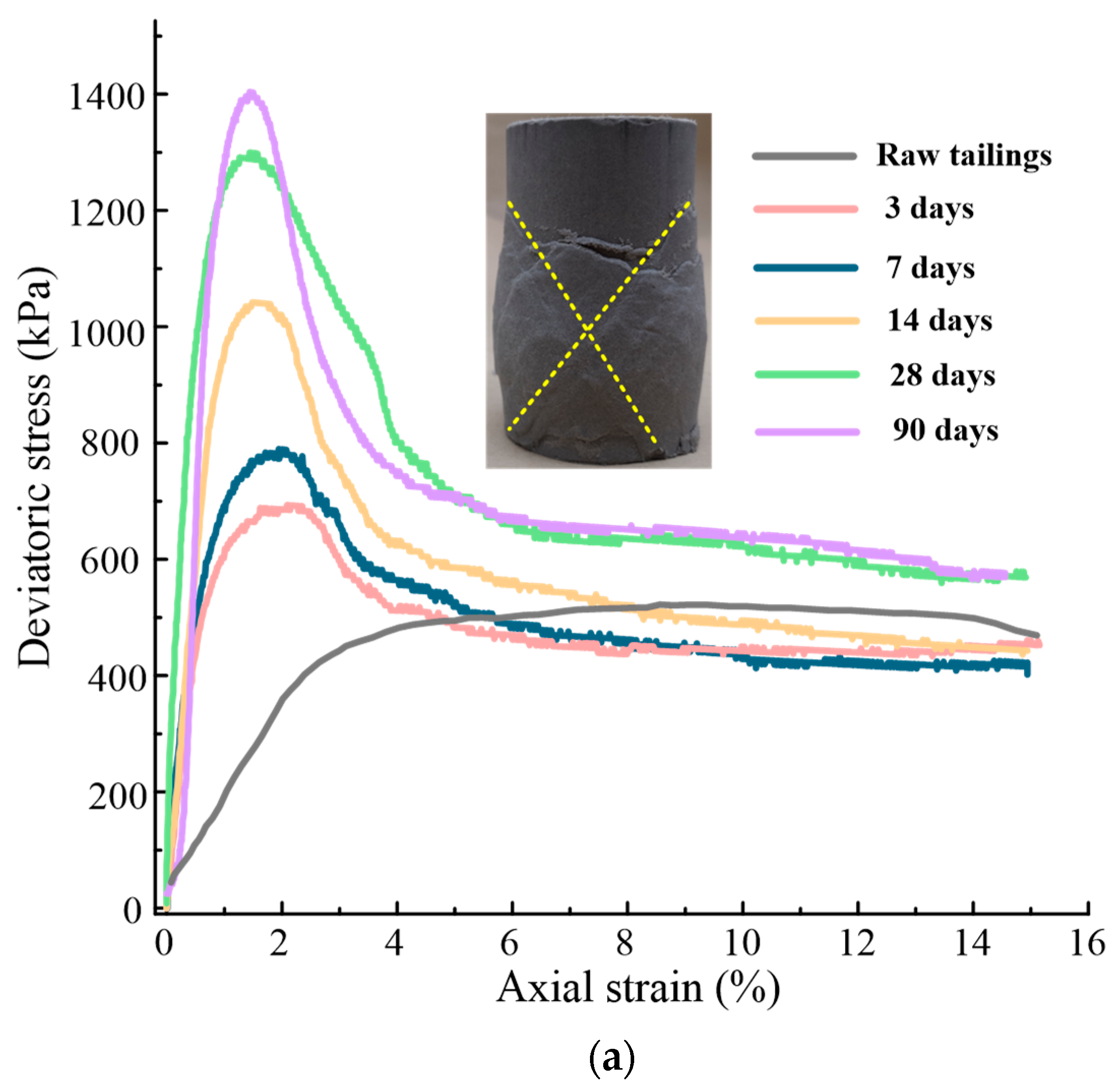
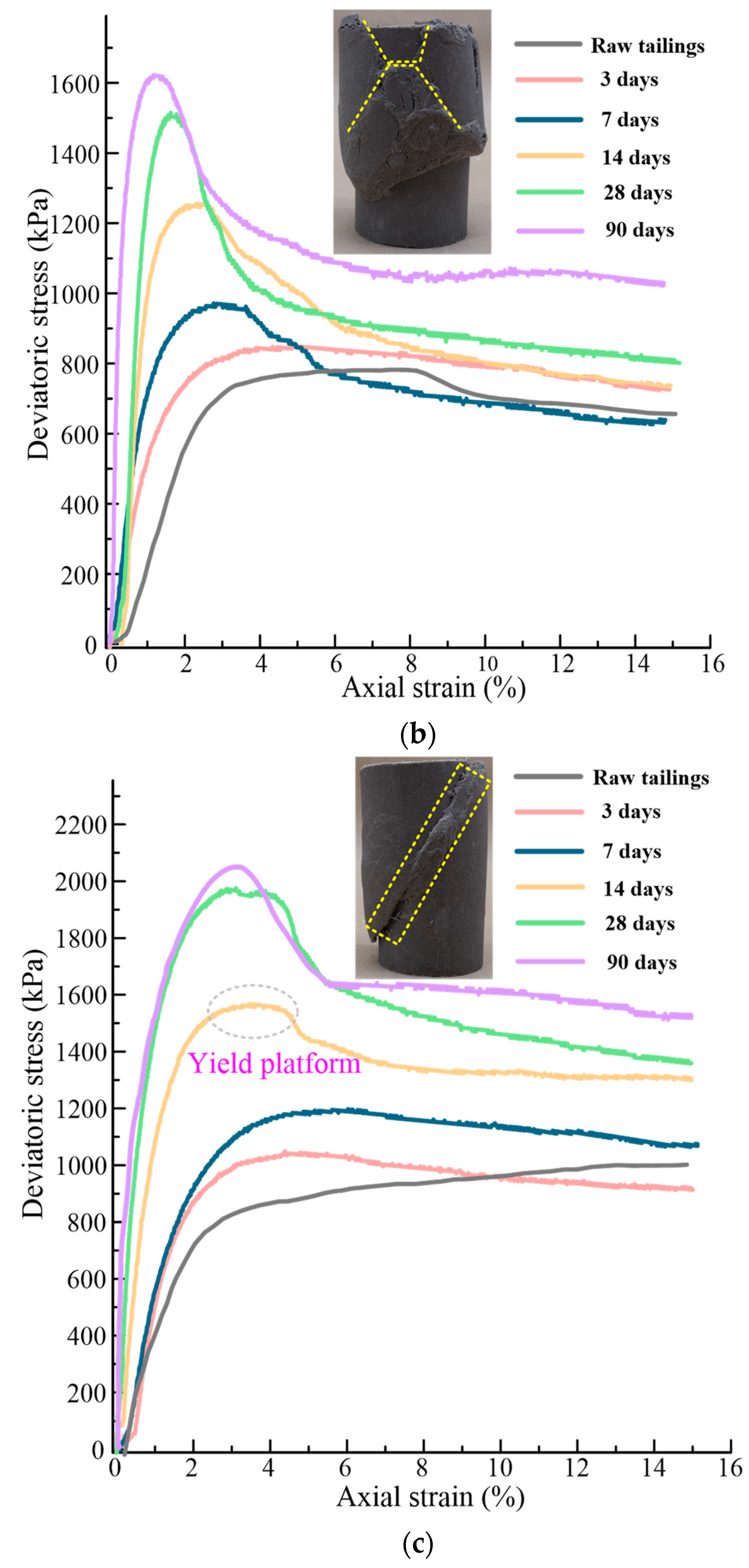
5.1.1. Stress-Strain and Deformation Characteristics
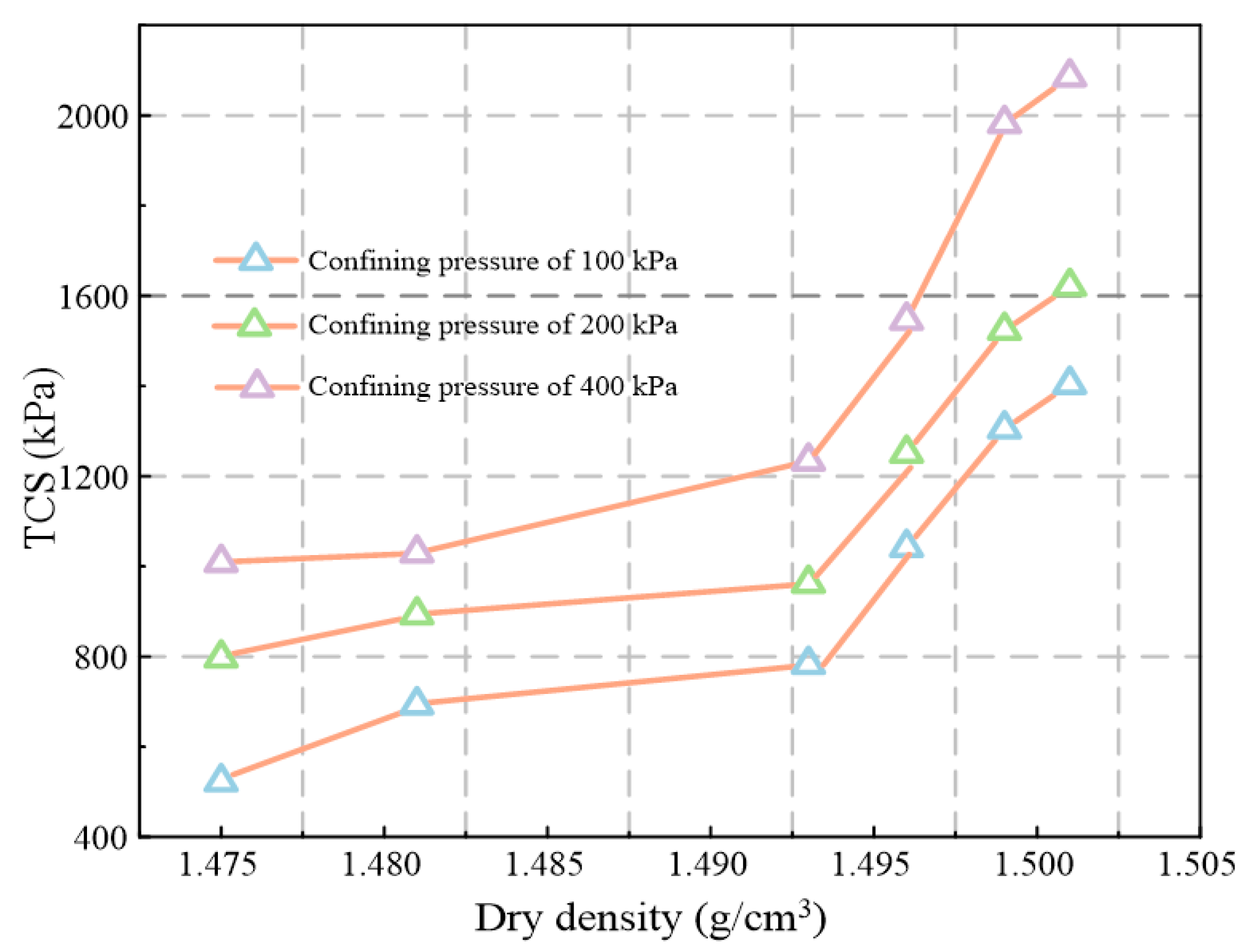
5.1.2. Strength Characteristics
5.1.3. Strength and Deformation Parameters
5.2. Microstructural Analysis
5.2.1. Particle Characteristics of Solidified/Stabilized Gold Tailings
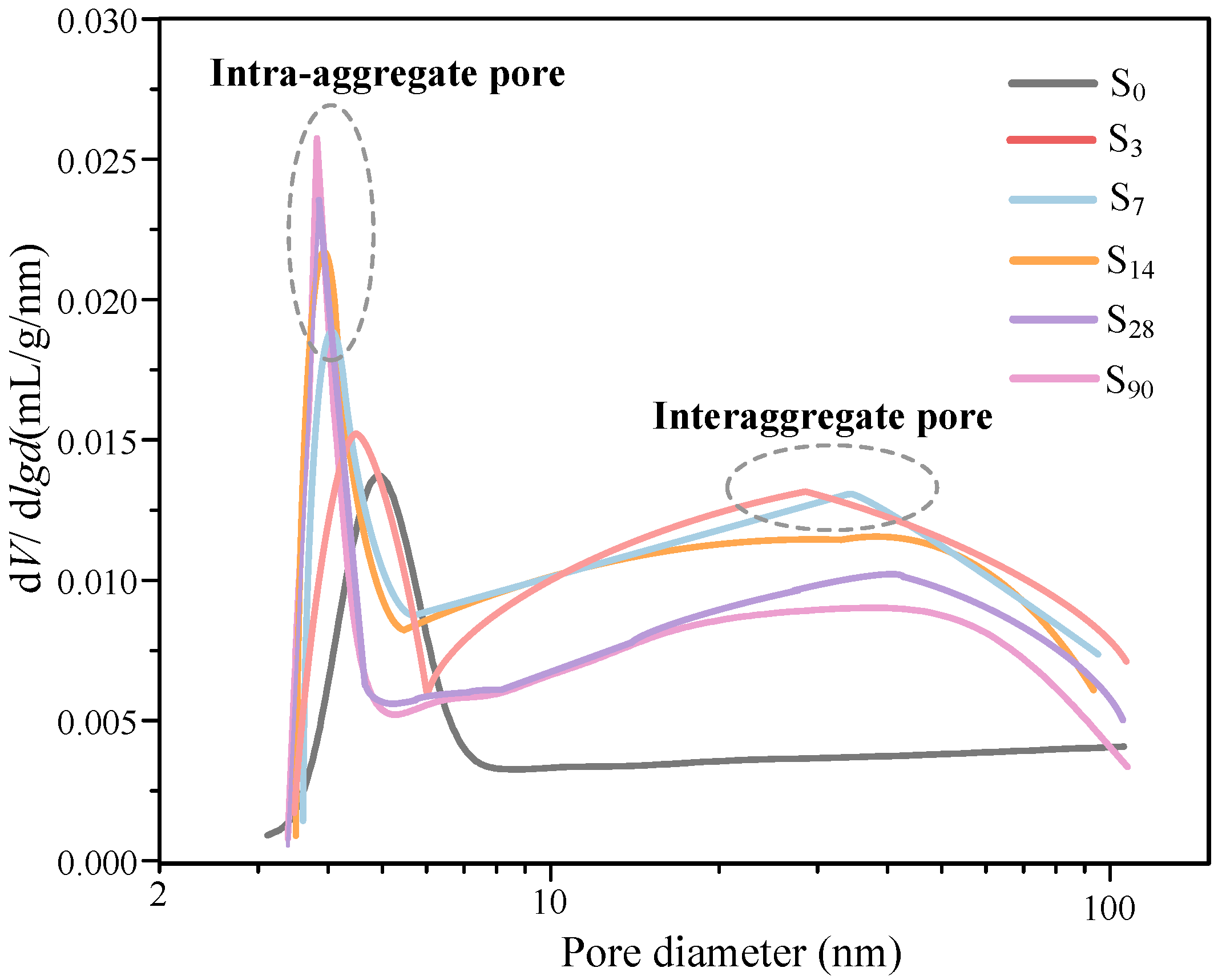
5.2.2. Pore-Size Distribution
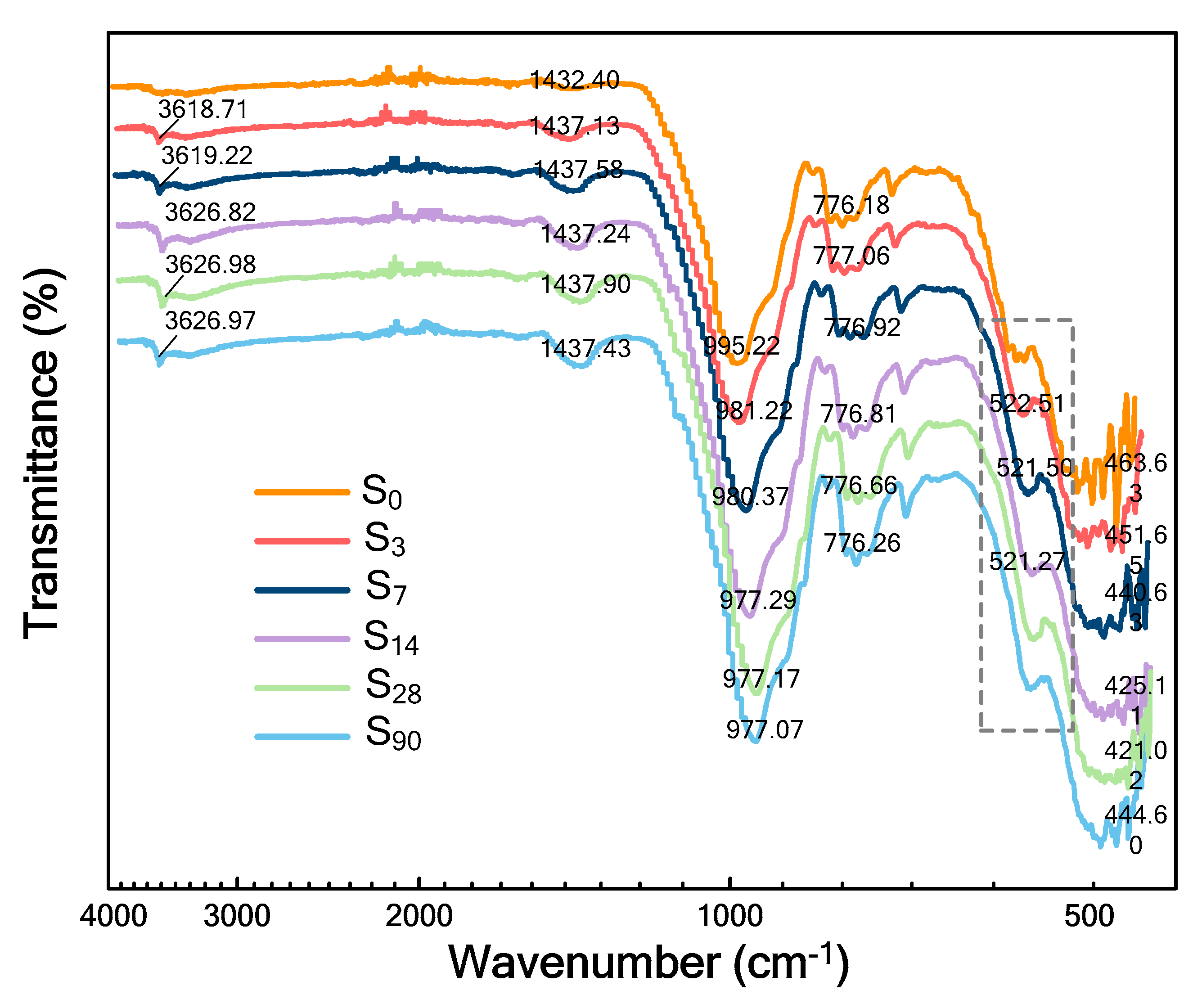
5.3. FTIR Analysis
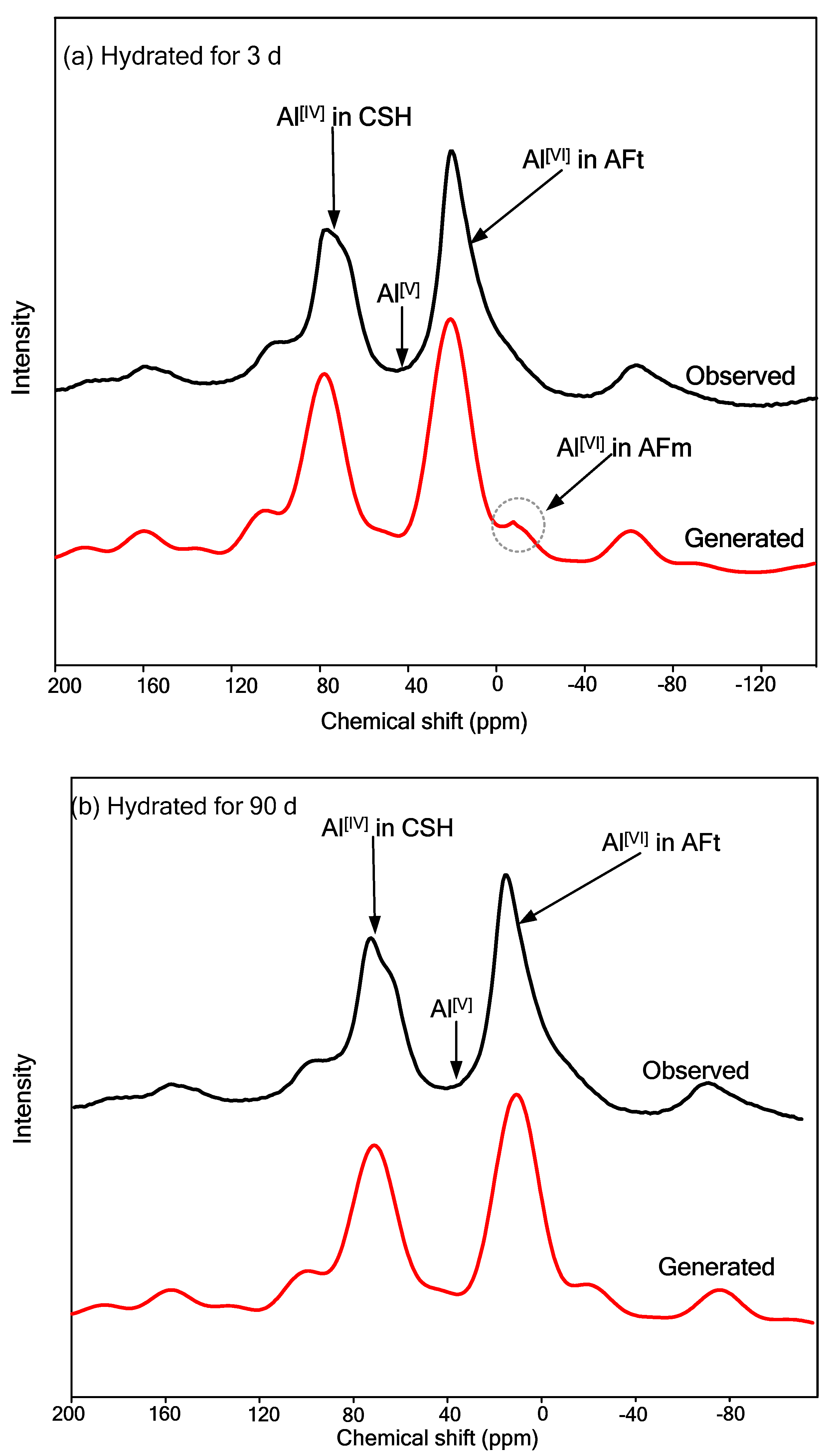
5.4. 27Al MAS-NMR Analysis
6. Conclusions
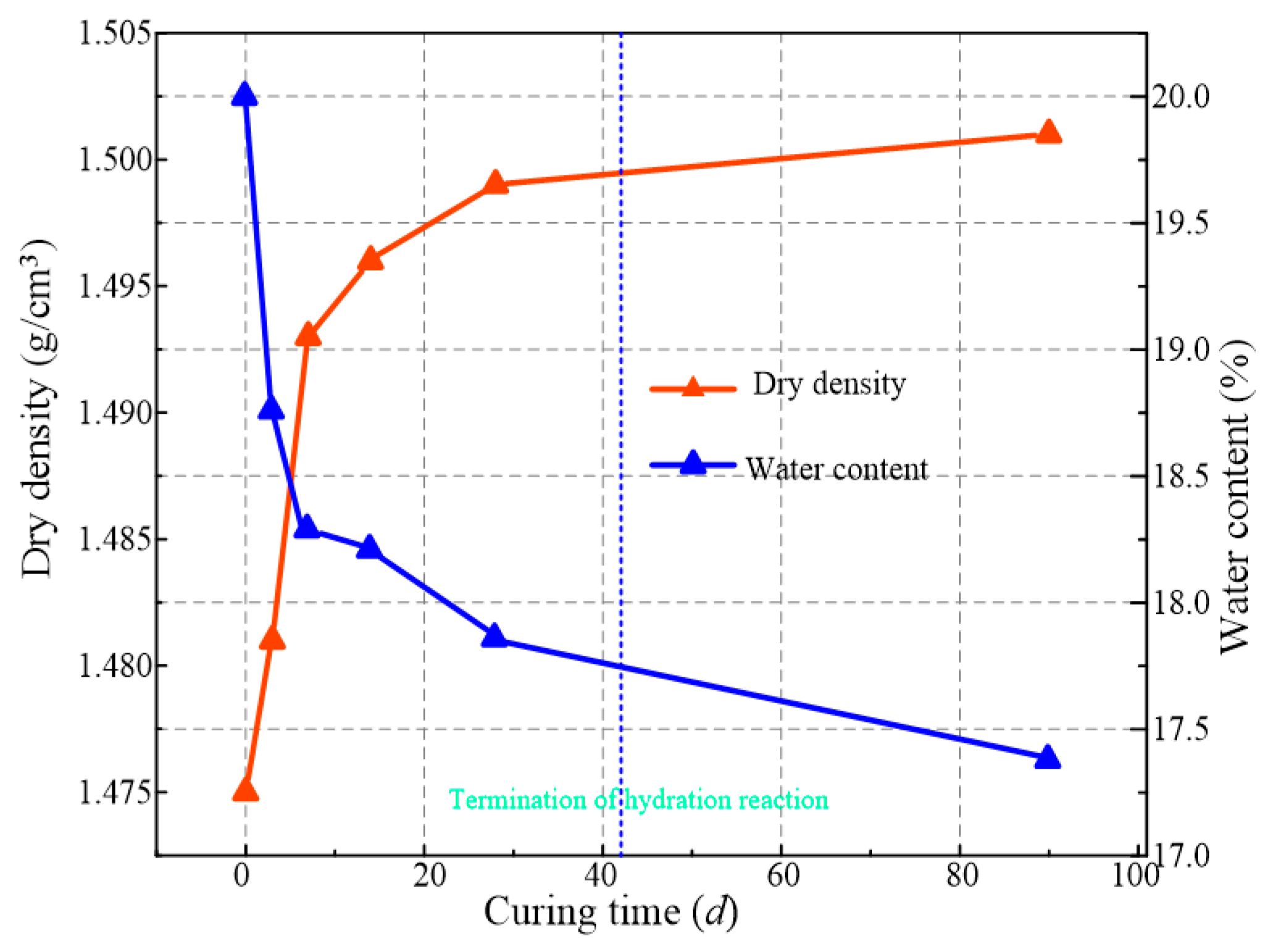
- All the solidified/stabilized gold tailings show strain softening. A local shear band is observed in these samples due to the change of particles and pores distribution. The curing period of 42 d is considered the end time of the hydration reaction. The strength parameters and secant modulus of all solidified/stabilized samples increase with increasing curing period, whereas the breaking strain decreases with it.
- The micro-structural analysis of waste-based binder solidified/stabilized gold tailings helps explain the effect of hydration on the microscopic mechanism of strength enhancement. The adsorption capacity of the particle surface of solidified/stabilized materials weakens with the increase of curing time. A bimodal pore-size distribution characteristic was found in solidified/stabilized gold tailings samples.
- The hydration products formed at room temperature are grossly C–S–H gel and AFt (ettringite). They facilitate the formation of micro-aggregates, thus providing better mechanical strength to the solidified/stabilized sample. From 27Al MAS-NMR analysis results, the formation of tetrahedral-coordinated aluminum in C–S–H gel and hexahedral-coordinated aluminum in AFm could be observed in hydrated samples. This novel industrial waste-based binder is proven to be economical, reliable, and environmentally friendly and can broaden the applications of tailings in engineering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, M.; Li, Y.; Yu, B.; Wei, W.; Liu, L.; Xue, Q. Low-carbon treatment of zinc contaminated iron tailings using high-calcium geopolymer: Influence of wet-dry cycle coupled with acid attack. J. Clean. Prod. 2022, 338, 130636. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, C.; Guo, X.; Ma, C.; Ma, L.; Gan, S. Influence of particle size scale on mechanical properties of tailings under high pressure. Proc. Inst. Civ. Eng.-Geotech. Eng. 2022, 175, 200–213. [Google Scholar] [CrossRef]
- Liu, W.S.; Guo, M.N.; Liu, C.; Yuan, M.; Chen, X.T.; Huot, H.; Zhao, C.M.; Tang, Y.T.; Morel, J.L.; Qiu, R.L. Water, sediment and agricultural soil contamination from an ion-adsorption rare earth mining area. Chemosphere 2019, 216, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Li, X.; Zhang, N.; Yuan, J.; Wang, X.; Zhang, Y.; Chen, F.; Zhang, Y. Preparation and microstructural characterization of a novel 3D printable building material composed of copper tailings and iron tailings. Constr. Build. Mater. 2020, 249, 118779. [Google Scholar] [CrossRef]
- Young, G.; Yang, M. Preparation and characterization of Portland cement clinker from iron ore tailings. Constr. Build. Mater. 2019, 197, 152–156. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Sun, Y.; Li, Y. Innovative utilization of refractory iron ore via suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 352, 16–24. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Forbes, E.; Verster, I.; Jokovic, V.; Awatey, B.; Parbhakar-Fox, A. Review on advances in mineral processing technologies suitable for critical metal recovery from mining and processing wastes. Clean. Eng. Technol. 2022, 7, 100451. [Google Scholar] [CrossRef]
- Li, N.; Lv, S.; Wang, W.; Guo, J.; Jiang, P.; Liu, Y. Experimental investigations on the mechanical behavior of iron tailings powder with compound admixture of cement and nano-clay. Constr. Build. Mater. 2020, 254, 119259. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, Z.; Ma, C.; Ma, L.; Li, X. Influence of clay mineral content on mechanical properties and microfabric of tailings. Sci. Rep. 2022, 12, 10700. [Google Scholar] [CrossRef]
- Gou, M.; Zhou, L.; Then, N.W.Y. Utilization of tailings in cement and concrete: A review. Sci. Eng. Compos. Mater. 2019, 26, 449–464. [Google Scholar] [CrossRef]
- Ince, C. Reusing gold-mine tailings in cement mortars: Mechanical properties and socio-economic developments for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2019, 238, 117871. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, S.; Wang, Y.; Chen, X. Can carbon emission trading scheme achieve energy conservation and emission reduction? Evidence from the industrial sector in China. Energy Econ. 2020, 85, 104590. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, F.; Ling, X.; Lin, B. Effects of freeze-thaw cycles on mechanical properties of polypropylene fiber and cement stabilized clay. Cold Reg. Sci. Technol. 2018, 154, 155–165. [Google Scholar] [CrossRef]
- Hou, Y.; Li, P.; Wang, J. Review of chemical stabilizing agents for improving the physical and mechanical properties of loess. Bull. Eng. Geol. Environ. 2021, 80, 9201–9215. [Google Scholar] [CrossRef]
- Çevik, A.; Alzeebaree, R.; Humur, G.; Niş, A.; Gülşan, M.E. Effect of nano-silica on the chemical durability and mechanical performance of fly ash based geopolymer concrete. Ceram. Int. 2018, 44, 12253–12264. [Google Scholar] [CrossRef]
- Du, Y.J.; Yu, B.W.; Liu, K.; Jiang, N.J.; Liu, M.D. Physical, hydraulic, and mechanical properties of clayey soil stabilized by lightweight alkali-activated slag geopolymer. J. Mater. Civ. Eng. 2017, 29, 04016217. [Google Scholar] [CrossRef] [Green Version]
- Kurtoglu, A.E.; Alzeebaree, R.; Aljumaili, O.; Nis, A.; Gulsan, M.E.; Humur, G.; Cevik, A. Mechanical and durability properties of fly ash and slag based geopolymer concrete. Adv. Concr. Constr. 2018, 6, 345. [Google Scholar]
- Zhao, X.; Yang, B.; Li, Y.; Tang, D.; Xu, K.; Li, D. Application of biochar in the remediation of contaminated soil with high concentration of lead and zinc. Adv. Civ. Eng. 2021, 2021, 6630982. [Google Scholar] [CrossRef]
- Wei, M.; Li, Y.; Yu, B.; Liu, L.; Xue, Q.; Du, Y. Assessment of semi-dynamic leaching characteristics of lead and zinc from stabilized contaminated soil using sustainable phosphate-based binder after carbonation. J. Clean. Prod. 2022, 332, 130126. [Google Scholar] [CrossRef]
- Xiao, H.; Yao, K.; Liu, Y.; Goh, S.H.; Lee, F.H. Bender element measurement of small strain shear modulus of cement-treated marine clay–Effect of test setup and methodology. Constr. Build. Mater. 2018, 172, 433–447. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, C.; Li, Y.; Yang, C. Solidification/stabilization of gold ore tailings powder using sustainable waste-based composite geopolymer. Eng. Geol. 2022, 309, 106793. [Google Scholar] [CrossRef]
- ASTM D2850-15; Standard Specification for Unconsolidated-Undrained Triaxial Compression Test on Cohesive Soils. ASTM International: West Conshohocken, PA, USA, 2015.
- Bi, Q.; Li, C.H.; Chen, J.X. Effect of fine particle content on mechanical properties of tailings under high confining pressure. Arab. J. Geosci. 2021, 14, 942. [Google Scholar] [CrossRef]
- Torres-Cruz, L.A.; Santamarina, J.C. The critical state line of nonplastic tailings. Can. Geotech. J. 2020, 57, 1508–1517. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Q.; Li, B.; Zhao, X. Experimental analysis of shear band formation in plane strain tests on Shanghai silty clay. Bull. Eng. Geol. Environ. 2013, 72, 107–114. [Google Scholar] [CrossRef]
- Liu, C.; Roddatis, V.; Kenesei, P.; Maaß, R. Shear-band thickness and shear-band cavities in a Zr-based metallic glass. Acta Mater. 2017, 140, 206–216. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Chen, Y.; Liang, J.; Zhang, L.; Fong, H. A surface treatment technique of electrochemical oxidation to simultaneously improve the interfacial bonding strength and the tensile strength of PAN-based carbon fibers. Mater. Chem. Phys. 2010, 122, 548–555. [Google Scholar] [CrossRef]
- Massinas, S.A.; Sakellariou, M.G. Closed-form solution for plastic zone formation around a circular tunnel in half-space obeying Mohr–Coulomb criterion. Geotechnique 2009, 59, 691–701. [Google Scholar] [CrossRef]
- Meng, F.; Zhou, H.; Zhang, C.; Xu, R.; Lu, J. Evaluation methodology of brittleness of rock based on post-peak stress–strain curves. Rock Mech. Rock Eng. 2015, 48, 1787–1805. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Li, N.; Tao, F.; Yao, K. Characterisation of nano magnesia–cement-reinforced seashore soft soil by direct-shear test. Mar. Georesources Geotechnol. 2019, 37, 989–998. [Google Scholar] [CrossRef]
- Wei, L.; Chai, S.; Guo, Q.; Wang, P.; Li, F. Mechanical properties and stabilizing mechanism of stabilized saline soils with four stabilizers. Bull. Eng. Geol. Environ. 2020, 79, 5341–5354. [Google Scholar] [CrossRef]
- Dong, S.; Wang, L.; Gao, X.; Zhu, W.; Wang, Z.; Ma, Z.; Gao, C. Freeze casting of novel porous silicate cement supports using tert-butyl alcohol-water binary crystals as template: Microstructure, strength and permeability. J. Membr. Sci. 2017, 541, 143–152. [Google Scholar] [CrossRef]
- Vivekh, P.; Kumja, M.; Bui, D.T.; Chua, K.J. Recent developments in solid desiccant coated heat exchangers–A review. Appl. Energy 2018, 229, 778–803. [Google Scholar] [CrossRef]
- Frías, M.; Savastano, H.; Villar, E.; de Rojas, M.I.S.; Santos, S. Characterization and properties of blended cement matrices containing activated bamboo leaf wastes. Cem. Concr. Compos. 2012, 34, 1019–1023. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Sun, H.; Li, L. Pozzolanic behaviour of compound-activated red mud-coal gangue mixture. Cem. Concr. Res. 2011, 41, 270–278. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, S.Y.; Miron, G.D.; Collings, I.E.; L’Hôpital, E.; Skibsted, J.; Winnefeld, F.; Scrivener, K.; Lothenbach, B. Effect of alkali hydroxide on calcium silicate hydrate (CSH). Cem. Concr. Res. 2022, 151, 106636. [Google Scholar] [CrossRef]
- Pekarek, R.T.; Affolter, A.; Baranowski, L.L.; Coyle, J.; Hou, T.; Sivonxay, E.; Smith, B.A.; McAuliffe, R.D.; Persson, K.A.; Key, B.; et al. Intrinsic chemical reactivity of solid-electrolyte interphase components in silicon–lithium alloy anode batteries probed by FTIR spectroscopy. J. Mater. Chem. A 2020, 8, 7897–7906. [Google Scholar] [CrossRef]
- Gülşan, M.E.; Alzeebaree, R.; Rasheed, A.A.; Niş, A.; Kurtoğlu, A.E. Development of fly ash/slag based self-compacting geopolymer concrete using nano-silica and steel fiber. Constr. Build. Mater. 2019, 211, 271–283. [Google Scholar] [CrossRef]
- Alzeebaree, R.; Çevik, A.; Nematollahi, B.; Sanjayan, J.; Mohammedameen, A.; Gülşan, M.E. Mechanical properties and durability of unconfined and confined geopolymer concrete with fiber reinforced polymers exposed to sulfuric acid. Constr. Build. Mater. 2019, 215, 1015–1032. [Google Scholar] [CrossRef]
- Müller, C.M.; Pejcic, B.; Esteban, L.; Piane, C.D.; Raven, M.; Mizaikoff, B. Infrared attenuated total reflectance spectroscopy: An innovative strategy for analyzing mineral components in energy relevant systems. Sci. Rep. 2014, 4, 6764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Liu, X.; Sun, H.; Li, L. Evaluation of blends bauxite-calcination-method red mud with other industrial wastes as a cementitious material: Properties and hydration characteristics. J. Hazard. Mater. 2011, 185, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Yang, J.; Hou, D.; Zhang, G. Insight on the mechanism of sulfate attacking on the cement paste with granulated blast furnace slag: An experimental and molecular dynamics study. Constr. Build. Mater. 2018, 169, 601–611. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]




| Chemicals | FA | GBFS | CCR | MK | SH | PG |
|---|---|---|---|---|---|---|
| Content (%) | 10.53 | 57.89 | 7.37 | 15.79 | 3.16 | 5.26 |
| Test Parameters | Material | Test Standards | |||||
|---|---|---|---|---|---|---|---|
| FA | GBFS | CCR | MK | SH | PG | ||
| Specific gravity, Gs | 2.71 | 3.13 | 2.11 | 2.39 | 2.22 | 2.54 | ASTM D854-14 |
| Specific surface areas (m2/g) | 4.13 | 3.58 | 2.32 | 10.23 | 1.51 | 8.24 | China GB/T 8074-2008 |
| pH | 10.34 | 11.06 | 12.37 | 6.38 | 14.0 | 7.0 | ASTM D4972-18 |
| Particle size distribution (%) | ASTM D422-63 | ||||||
| <0.005 mm | 12.36 | 13.65 | 10.21 | 39.75 | 0 | 20.36 | |
| 0.005–0.075 mm | 80.05 | 69.46 | 51.92 | 60.25 | 20.13 | 79.64 | |
| 0.075–0.5 mm | 7.59 | 16.89 | 37.87 | 0 | 79.87 | 0 | |
| Test Parameters | Value | Test Standards |
|---|---|---|
| Specific gravity, Gs | 2.78 | ASTM D854-14 |
| Maximum dry density (g/cm3) | 1.67 | ASTM D698-12 |
| Minimum dry density (g/cm3) | 1.31 | |
| Optimum water content (%) | 19.7 | |
| Liquid limit, wL (%) | 39.23 | ASTM D4318-10 |
| Plasticity limit, wP (%) | 20.71 | |
| Initial water content (%) | 15.9 | ASTM D2216-19 |
| Soil pH | 8.56 | ASTM D4972-18 |
| Soil classification | CL | ASTM D2487-17 |
| Particle size distribution (%) | ||
| Sand particle (>75 µm) | 1.2 | ASTM D422-63 ASTM D2487-17 |
| Silt particle (5–75 µm) | 78.2 | |
| Clay particle (<5 µm) | 20.6 | |
| Particle size d50 (µm) | 15 | |
| Uniformity coefficient, Cu | 10.01 | |
| Curvature coefficient, Cc | 1.23 |
| Major oxide (%) | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Na2O | K2O | SO3 | |
|---|---|---|---|---|---|---|---|---|---|
| Materials | |||||||||
| GMTs | 59.72 | 19.06 | 4.34 | 5.04 | 3.78 | -- | 4.44 | 1.73 | |
| FA | 49.52 | 16.68 | 34.91 | 0.84 | 6.67 | 0.39 | 0.61 | 2.14 | |
| GFBS | 30.85 | 14.82 | 41.23 | 0.63 | 8.52 | 2.0 | 5.7 | 1.9 | |
| CCR | 2.83 | 2.24 | 68.89 | 0.25 | 0.18 | 0.13 | 0.21 | 0.78 | |
| MK | 52.23 | 44.01 | 0.32 | 0.71 | 0.31 | 0.23 | 0.76 | 0.38 | |
| SH | -- | -- | -- | -- | -- | 99.0 | -- | -- | |
| PG | -- | -- | 41.2 | -- | -- | -- | -- | 58.8 | |
| Confining Pressure (kPa) | s(1) | a(2) | xc(2) | w(2) | σ(3) | R2(4) |
|---|---|---|---|---|---|---|
| 100 | 1438.79 | −15.01 | −23.37 | 22.17 | 34.43 | 0.990 |
| 200 | 1645.59 | −8.59 | −5.52 | 15.29 | 30.90 | 0.991 |
| 400 | 2110.91 | −10.68 | −1.88 | 13.43 | 31.59 | 0.995 |
| Curing Periods (d) | Strength Parameters | Deformation Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Cohesive Strength (kPa) | Internal Friction Angle (°) | Secant Modulus (MPa) | Breaking Strain (%) | |||||
| σ = 100 kPa | σ = 200 kPa | σ = 400 kPa | σ = 100 kPa | σ = 200 kPa | σ = 400 kPa | |||
| 0 | 142.59 | 21.16 | 5.62 | 9.55 | 6.73 | 9.29 | 8.34 | 15 |
| 3 | 218.14 | 20.14 | 31.47 | 31.54 | 35.51 | 2.24 | 3.71 | 3.85 |
| 7 | 205.49 | 25.18 | 34.99 | 40.11 | 35.24 | 2.13 | 2.71 | 4.23 |
| 14 | 274.65 | 26.89 | 70.05 | 58.85 | 46.49 | 1.58 | 2.25 | 2.7 |
| 28 | 300.25 | 31.64 | 72.61 | 87.05 | 60.43 | 1.31 | 2.12 | 3.14 |
| 90 | 323.43 | 32.18 | 103.33 | 133.65 | 68.97 | 1.43 | 1.18 | 2.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Hu, S.; Zhang, C.; Zhou, T.; Hua, G.; Li, Y.; Lv, X. Mechanical and Hydration Characteristics of Stabilized Gold Mine Tailings Using a Sustainable Industrial Waste-Based Binder. Materials 2023, 16, 634. https://doi.org/10.3390/ma16020634
Pan Z, Hu S, Zhang C, Zhou T, Hua G, Li Y, Lv X. Mechanical and Hydration Characteristics of Stabilized Gold Mine Tailings Using a Sustainable Industrial Waste-Based Binder. Materials. 2023; 16(2):634. https://doi.org/10.3390/ma16020634
Chicago/Turabian StylePan, Zhenkai, Shaohua Hu, Chao Zhang, Tong Zhou, Guowei Hua, Yuan Li, and Xiaolin Lv. 2023. "Mechanical and Hydration Characteristics of Stabilized Gold Mine Tailings Using a Sustainable Industrial Waste-Based Binder" Materials 16, no. 2: 634. https://doi.org/10.3390/ma16020634




