Determination of the Ni(II) Ions Sorption Mechanism on Dowex PSR2 and Dowex PSR3 Ion Exchangers Based on Spectroscopic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sorbate and Sorbents Characteristics and Calculations
2.2. FTIR Spectroscopy
2.3. XPS Spectroscopy
2.4. CHN Analysis
3. Results and Discussion
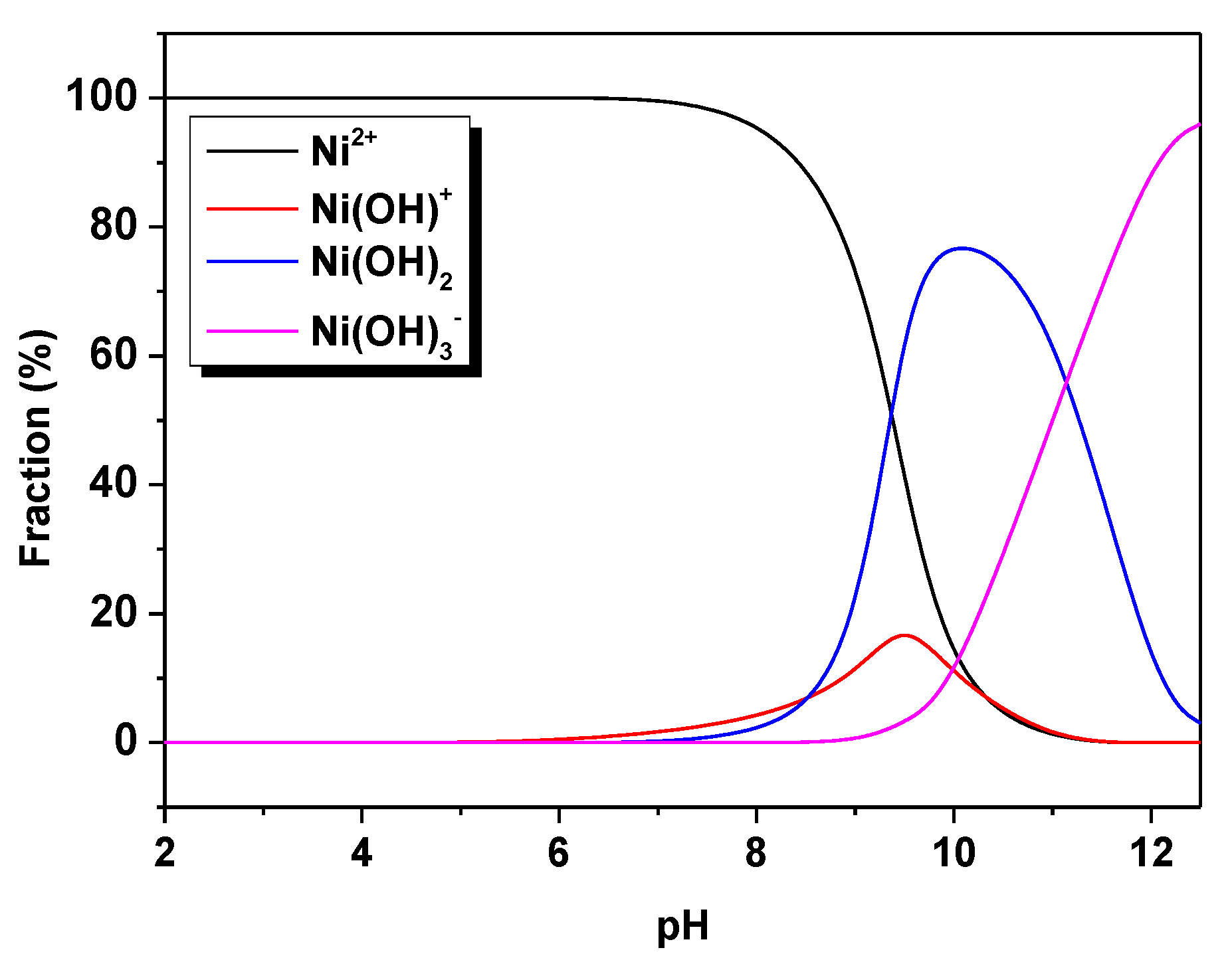
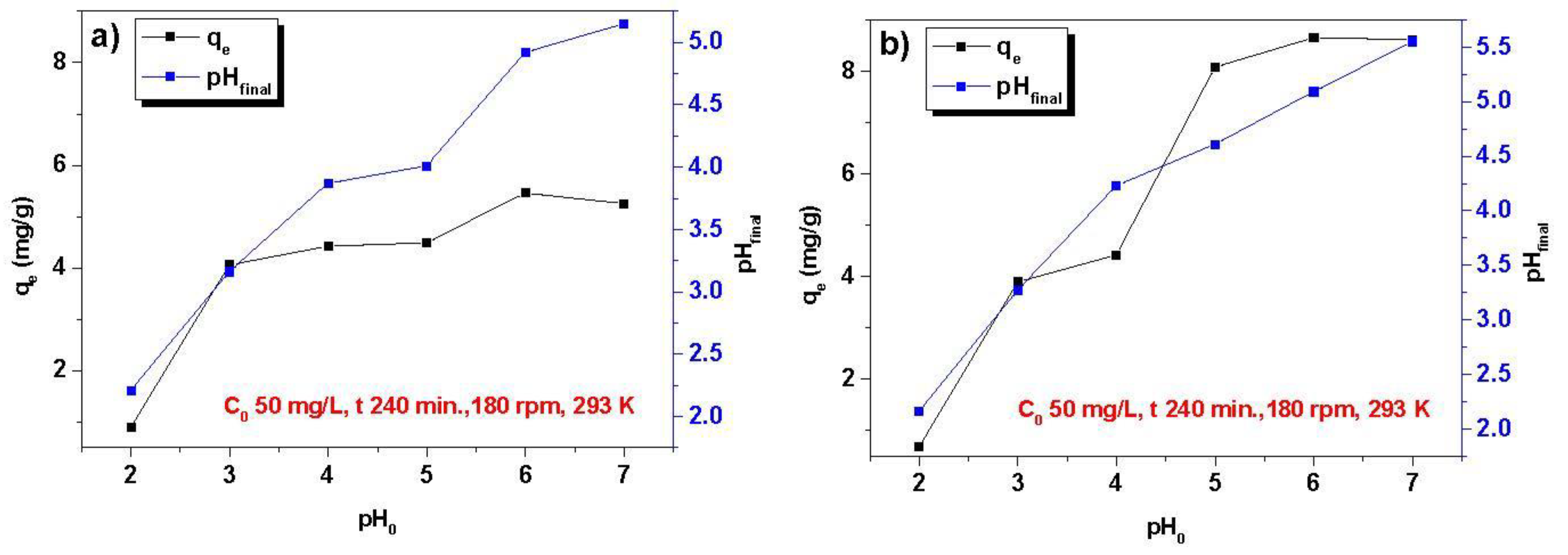
3.1. Optimization of the Sorption Process
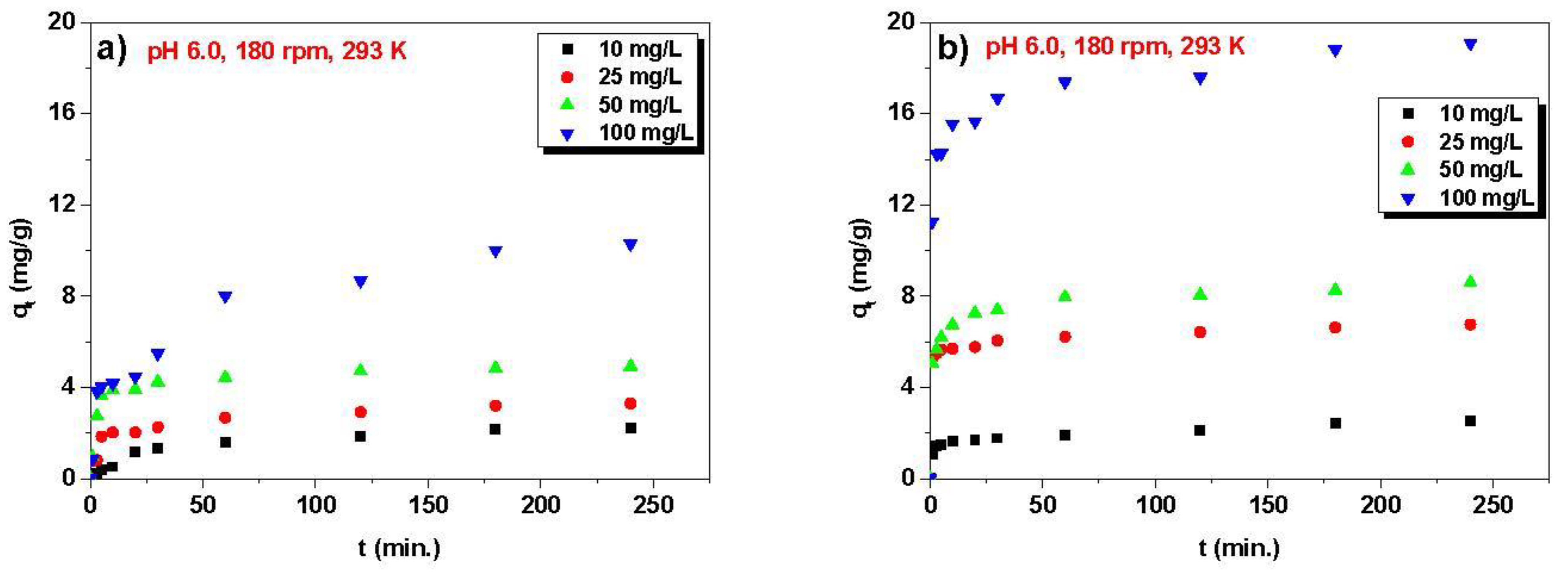
3.2. Kinetic, Isotherm, and Thermodynamic Parameters
3.3. Physicochemical Analyses
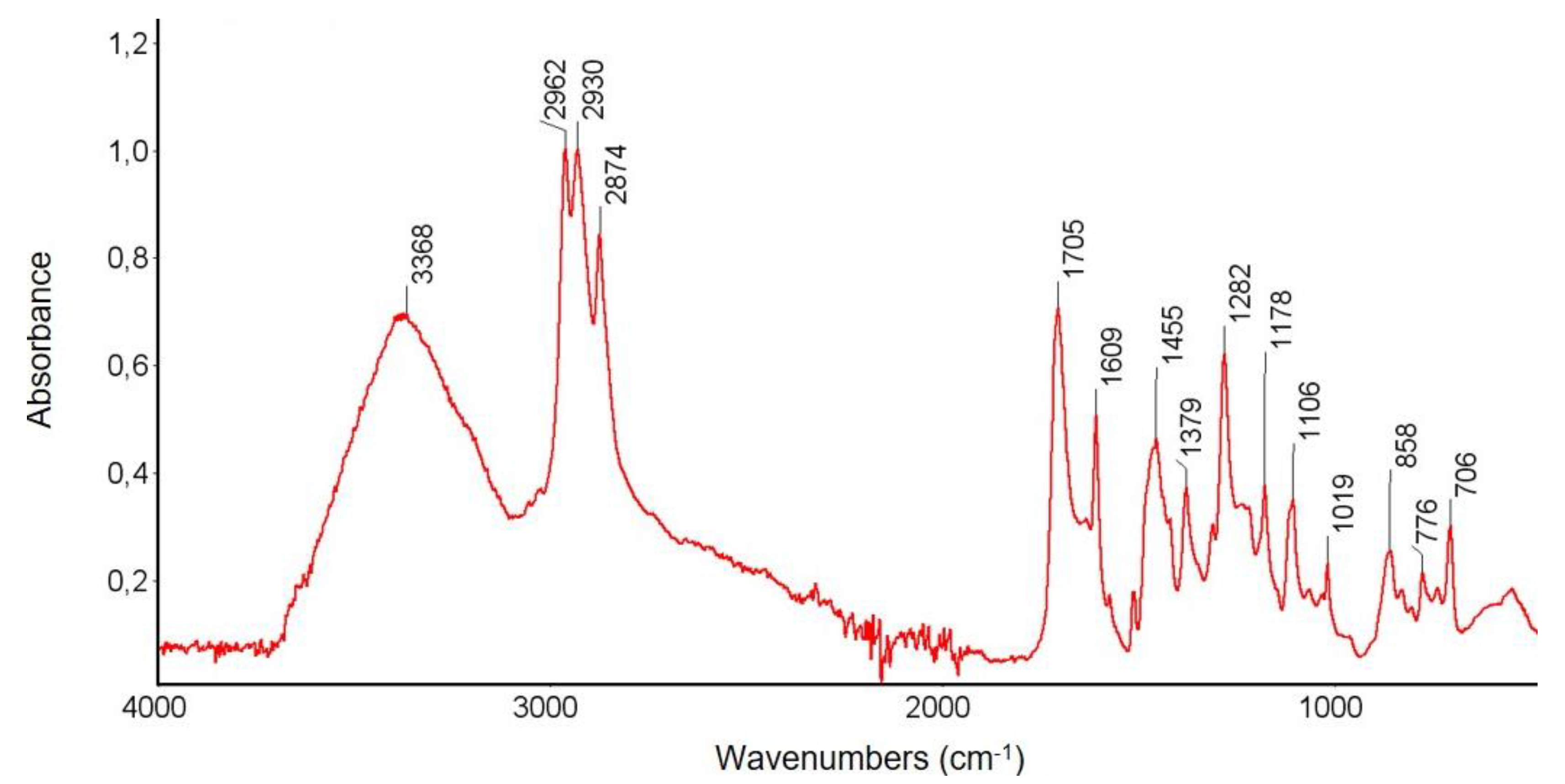
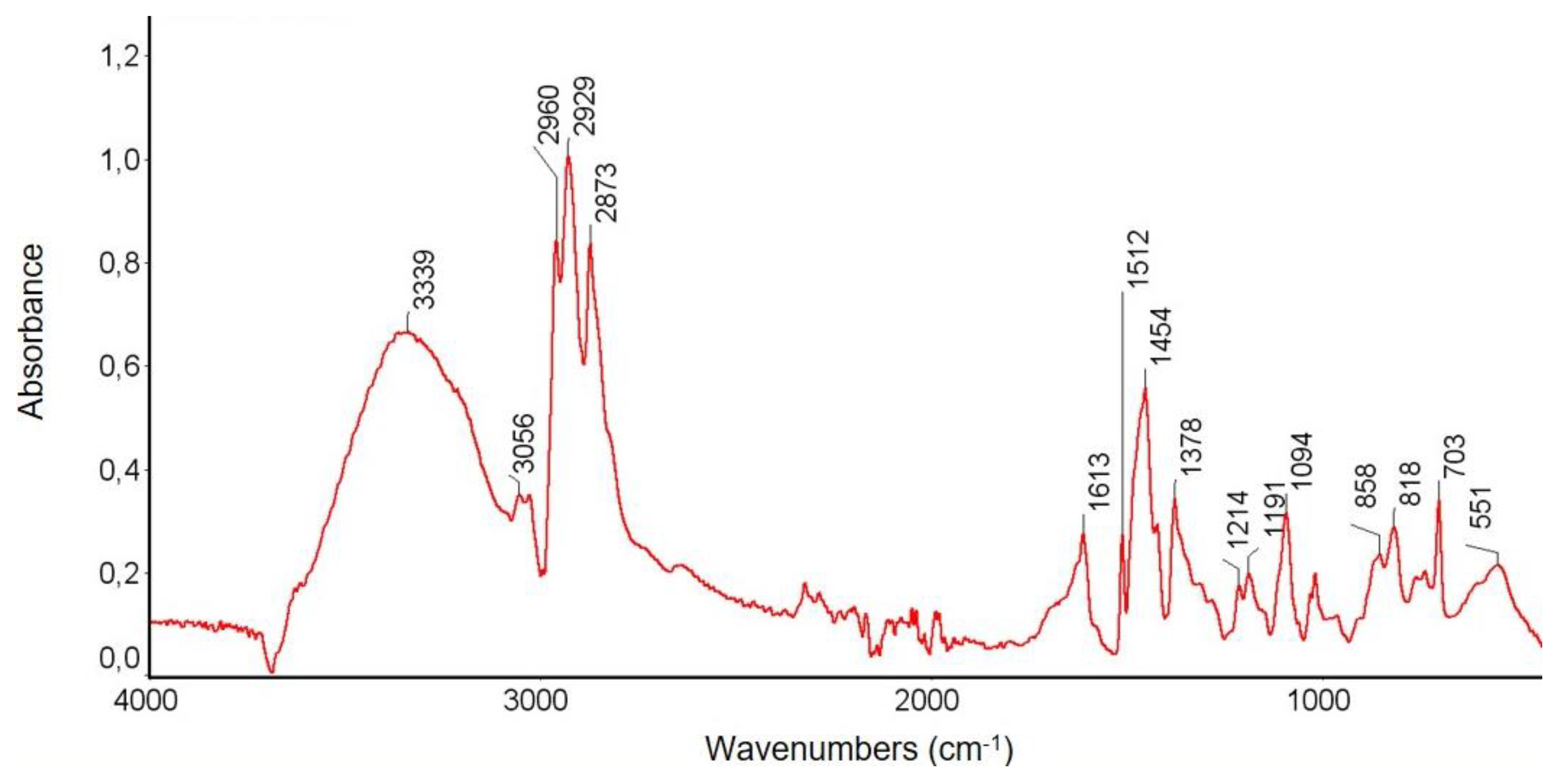
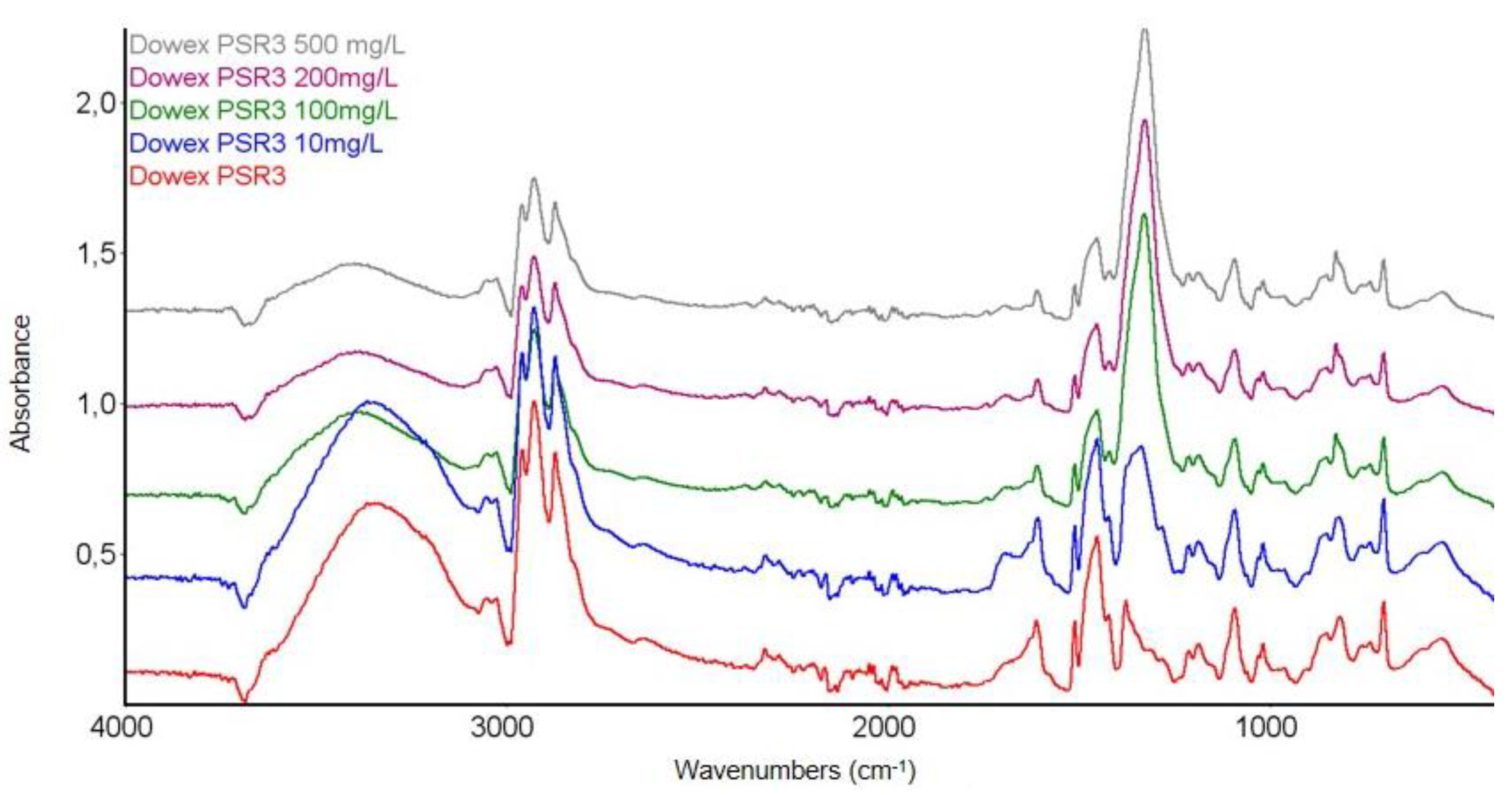
3.3.1. FTIR Spectroscopy
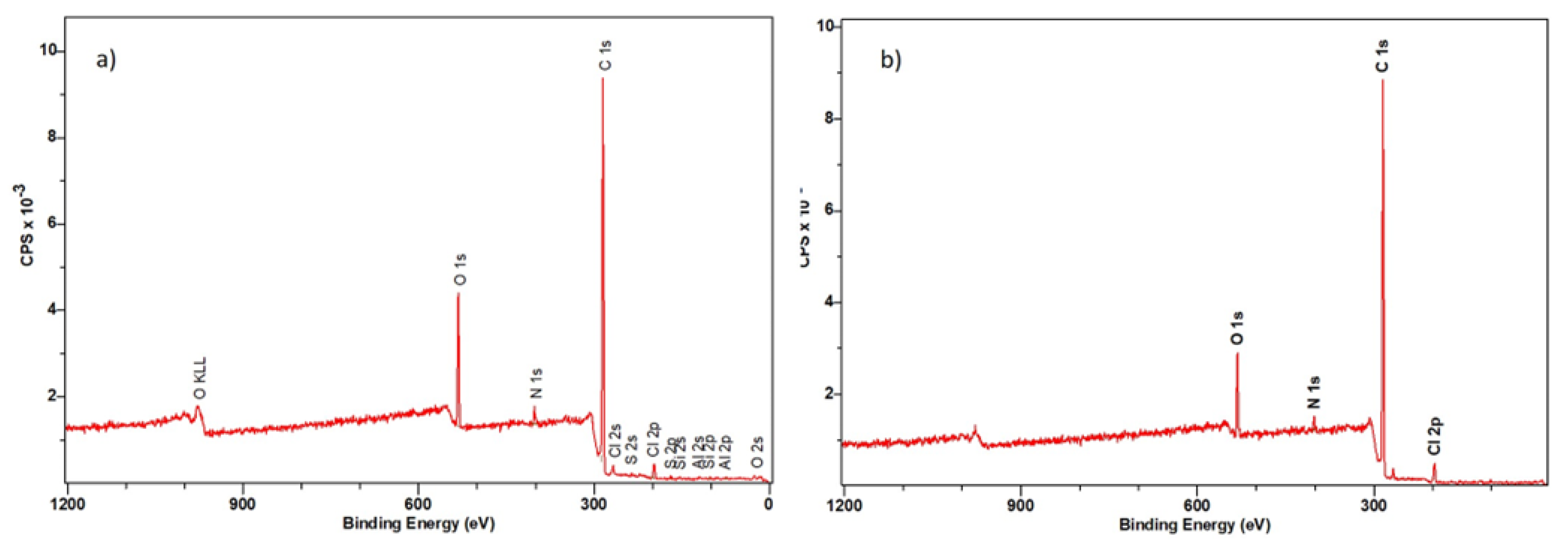
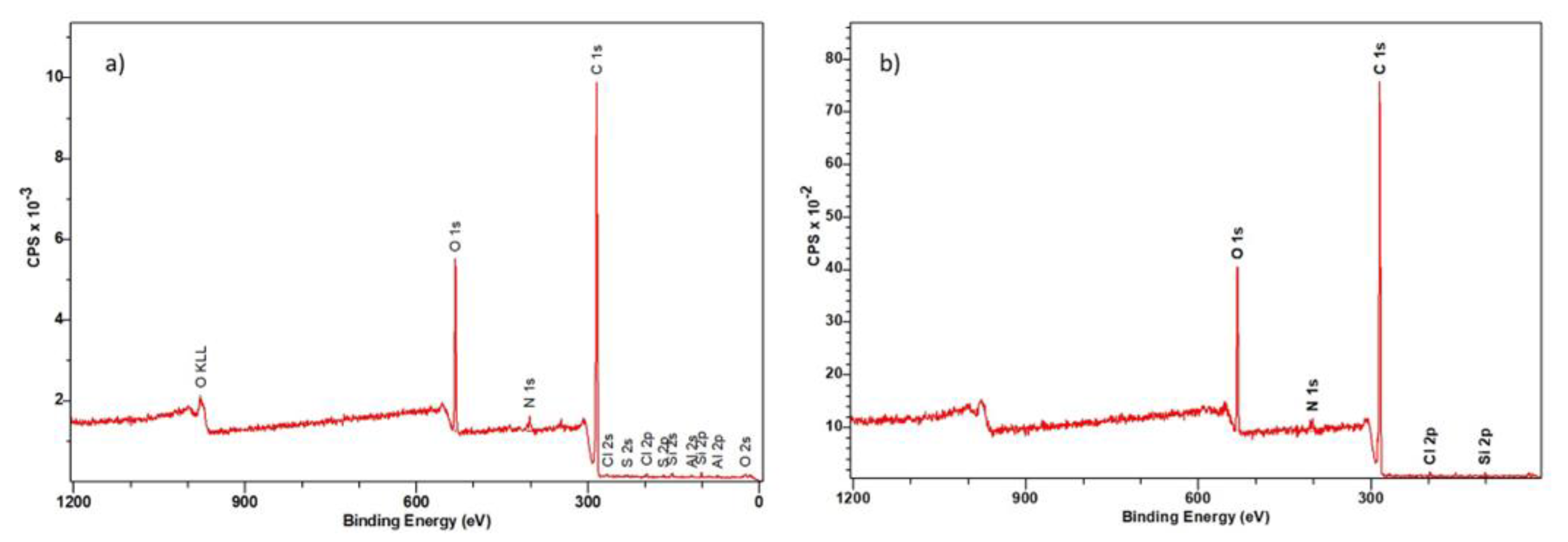
3.3.2. XPS Spectroscopy
3.3.3. Elemental Analysis of CHN
3.4. Sorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anthony, F.; Godfrey, K. Simultaneous Adsorption of Ni(II) and Mn(II) Ions from Aqueous Solution unto a Nigerian Kaolinite Clay. J. Mater. Res. Technol. 2014, 3, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Flieger, J.; Kawka, J.; Płazinski, W.; Panek, R.; Madej, J. Sorption of Heavy Metal Ions of Chromium, Manganese, Selenium, Nickel, Cobalt, Iron from Aqueous Acidic Solutions in Batch and Dynamic Conditions on Natural and Synthetic Aluminosilicate Sorbents. Materials 2020, 13, 5271. [Google Scholar] [CrossRef] [PubMed]
- Šuránek, M.; Melichová, Z.; Kureková, V.; Kljajevic, L.; Nenadovic, S. Removal of Nickel from Aqueous Solutions by Natural Bentonites from Slovakia. Materials 2021, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Charazinska, S.; Burszta-Adamiak, E.; Lochynski, P. Recent Trends in Ni(II) Sorption from Aqueous Solutions Using Natural Materials. Rev. Environ. Sci. Biotechnol. 2022, 5, 105–138. [Google Scholar] [CrossRef]
- Ebisike, K.; Okoronkwo, A.E.; Alaneme, K.K. Nickel Sorption onto Chitosan-Silica Hybrid Aerogel from Aqueous Solution. Walailak J. Sci. Technol. 2021, 18, 9454. [Google Scholar] [CrossRef]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. The Influence of Salt Anions on Heavy Metal Ion Adsorption on the Example of Nickel. Materials 2018, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Henckens, M.L.C.M.; Worrell, E. Reviewing the Availability of Copper and Nickel for Future Generations. The Balance between Production Growth, Sustainability and Recycling Rates. J. Clean. Prod. 2020, 264, 121460. [Google Scholar] [CrossRef]
- Farooq, A.; Ahmad, S.; Hamad, K.; Deen, K.M. Effect of Ni Concentration on the Surface Morphology and Corrosion Behavior of Zn-Ni Alloy Coatings. Metals 2022, 12, 96. [Google Scholar] [CrossRef]
- Aramini, M.; Magnani, G.; Pontiroli, D.; Milanese, C.; Girella, A.; Bertoni, G.; Gaboardi, M.; Zacchini, S.; Marini, A.; Riccò, M. Nickel Addition to Optimize the Hydrogen Storage Performance of Lithium Intercalated Fullerides. Mater. Res. Bull. 2020, 126, 110848. [Google Scholar] [CrossRef]
- Blumbergs, E.; Serga, V.; Platacis, E.; Maiorov, M.; Shishkin, A. Cadmium Recovery from Spent Ni-Cd Batteries: A Brief Review. Metals 2021, 11, 1714. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saradesh, K.M.; Vinodkumar, G.S. Metallurgical Processes for Hardening of 22Karat Gold for Light Weight and High Strength Jewelry Manufacturing. J. Mater. Res. Technol. 2020, 9, 2009–2020. [Google Scholar] [CrossRef]
- Veneu, D.M.; Yokoyama, L.; Cunha, O.G.C.; Schneider, C.L.; Monte, M.B.D.M. Nickel Sorption Using Bioclastic Granules as a Sorbent Material: Equilibrium, Kinetic and Characterization Studies. J. Mater. Res. Technol. 2019, 8, 840–852. [Google Scholar] [CrossRef]
- Abou-Mesalam, M.M. Sorption Kinetics of Copper, Zinc, Cadmium and Nickel Ions on Synthesized Silico-Antimonate Ion Exchanger. Colloids Surf. A Physicochem. Eng. Asp. 2003, 225, 85–94. [Google Scholar] [CrossRef]
- Jumadilov, T.; Yskak, L.; Imangazy, A.; Suberlyak, O. Ion Exchange Dynamics in Cerium Nitrate Solution Regulated by Remotely Activated Industrial Ion Exchangers. Materials 2021, 14, 3491. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Selective Sorption of Nickel and Cobalt from Sulphate Solutions Using Chelating Resins. Int. J. Miner. Process. 2004, 74, 359–371. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Wang, J.; Wei, W.; Hao, B.; Yang, L.; Tang, J.; Zhang, C. Removal of Nickel Ions from Automobile Industry Wastewater Using Ion Exchange Resin: Characterization and Parameter Optimization. IOP Conf. Ser. Earth Environ. Sci. 2020, 467, 012182. [Google Scholar] [CrossRef]
- Kołodyńska, D. Cu(II), Zn(II), Ni(II), and Cd(II) Complexes with HEDP Removal from Industrial Effluents on Different Ion Exchangers. Ind. Eng. Chem. Res. 2010, 49, 2388–2400. [Google Scholar] [CrossRef]
- Stefan, D.S.; Meghea, I. Mechanism of Simultaneous Removal of Ca2+, Ni2+, Pb2+ and Al3+ Ions from Aqueous Solutions Using Purolite® S930 Ion Exchange Resin. Comptes Rendus Chim. 2014, 17, 496–502. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B.; Barlas, H. Sorption of Ni(II) Ions from Aqueous Solution by Lewatit Cation-Exchange Resin. J. Hazard. Mater. 2009, 167, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Bąk, J.; Thomas, P.; Kołodyńska, D. Chitosan-Modified Biochars to Advance Research on Heavy Metal Ion Removal: Roles, Mechanism and Perspectives. Materials 2022, 15, 6108. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wołowicz, A. Zinc(II) Removal from Model Chloride and Chloride-Nitrate(V) Solutions Using Various Sorbents. Physicochem. Probl. Miner. Process. 2019, 55, 1517–1534. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Sorption Behavior of Dowex PSR-2 and Dowex PSR-3 Resins of Different Structures for Metal(II) Removal. Solvent Extr. Ion Exch. 2016, 34, 375–397. [Google Scholar] [CrossRef]
- Kołodyńska, D. Polyacrylate Anion Exchangers in Sorption of Heavy Metal Ions with the Biodegradable Complexing Agent. Chem. Eng. J. 2009, 150, 280–288. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Polyacrylate Ion Exchangers in Sorption of Noble and Base Metal Ions from Single and Tertiary Component Solutions. Solvent Extr. Ion Exch. 2014, 32, 189–205. [Google Scholar] [CrossRef]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. The Use of the Chelating Resin of a New Generation Lewatit MonoPlus TP-220 with the Bis-Picolylamine Functional Groups in the Removal of Selected Metal Ions from Acidic Solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Carbon-Based Adsorber Resin Lewatit AF 5 Applicability in Metal Ion Recovery. Microporous Mesoporous Mater. 2016, 224, 400–414. [Google Scholar] [CrossRef]
- Fila, D.; Hubicki, Z.; Kołodyńska, D. Recovery of Metals from Waste Nickel-Metal Hydride Batteries Using Multifunctional Diphonix Resin. Adsorption 2019, 25, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Keränen, A.; Leiviskä, T.; Salakka, A.; Tanskanen, J. Removal of Nickel and Vanadium from Ammoniacal Industrial Wastewater by Ion Exchange and Adsorption on Activated Carbon. Desalin. Water Treat. 2015, 53, 2645–2654. [Google Scholar] [CrossRef]
- Kiefer, R.; Höll, W.H. Sorption of Heavy Metals onto Selective Ion-Exchange Resins with Aminophosphonate Functional Groups. Ind. Eng. Chem. Res. 2001, 40, 4570–4576. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Shen, X.; Qiao, X.; Jia, L.; Xiang, J.; Jin, Y. Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents. Materials 2020, 13, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunanithi, R.; Ok, Y.S.; Dharmarajan, R.; Ahmad, M.; Seshadri, B.; Bolan, N.; Naidu, R. Sorption, Kinetics and Thermodynamics of Phosphate Sorption onto Soybean Stover Derived Biochar. Environ. Technol. Innov. 2017, 8, 113–125. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle Diffusion Processes during Acid Dye Adsorption onto Chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Aniagor, C.O.; Afifi, M.A.; Hashem, A. Modelling of Basic Blue-9 Dye Sorption onto Hydrolyzed Polyacrylonitrile Grafted Starch Composite. Carbohydr. Polym. Technol. Appl. 2021, 2, 100141. [Google Scholar] [CrossRef]
- Osemeahon, S.A.; Dimas, B.J. Removal of Crude Oil from Aqueous Medium by Sorption on Sterculis Setigera. Asian J. Appl. Chem. Res. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Al-Degs, Y.; Barral, M.T.; Paradelo, R. Optimization of Direct Blue 71 Sorption by Organic Rich-Compost Following Multilevel Multifactor Experimental Design. Arab. J. Chem. 2022, 15, 103468. [Google Scholar] [CrossRef]
- Sakr, A.K.; Cheira, M.F.; Hassanin, M.A.; Mira, H.I.; Mohamed, S.A.; Khandaker, M.U.; Osman, H.; Eed, E.M.; Sayyed, M.I.; Hanfi, M.Y. Adsorption of Yttrium Ions on 3-Amino-5-Hydroxypyrazole Impregnated Bleaching Clay, a Novel Sorbent Material. Appl. Sci. 2021, 11, 10320. [Google Scholar] [CrossRef]
- Menkiti, M.C.; Aniagor, C.O.; Agu, C.M.; Ugonabo, V.I. Effective Adsorption of Crystal Violet Dye from an Aqueous Solution Using Lignin-Rich Isolate from Elephant Grass. Water Conserv. Sci. Eng. 2018, 3, 33–46. [Google Scholar] [CrossRef]
- Kamaraj, R.; Pandiarajan, A.; Jayakiruba, S.; Naushad, M.; Vasudevan, S. Kinetics, Thermodynamics and Isotherm Modeling for Removal of Nitrate from Liquids by Facile One-Pot Electrosynthesized Nano Zinc Hydroxide. J. Mol. Liq. 2016, 215, 204–211. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of a Cationic Dye from Aqueous Solutions by Adsorption onto Bentonite Clay. Chemosphere 2006, 63, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Diclofenac Sorption from Synthetic Water: Kinetic and Thermodynamic Analysis. J. Environ. Chem. Eng. 2020, 8, 104105. [Google Scholar] [CrossRef]
- Song, Y.; Wang, K.; Zhao, F.; Du, Z.; Zhong, B.; An, G. Preparation of Powdered Activated Carbon Composite Material and Its Adsorption Performance and Mechanisms for Removing RhB. Water 2022, 14, 3048. [Google Scholar] [CrossRef]
- Shi, J.; Yi, S.; He, H.; Long, C.; Li, A. Preparation of Nanoscale Zero-Valent Iron Supported on Chelating Resin with Nitrogen Donor Atoms for Simultaneous Reduction of Pb2+ and NO3-. Chem. Eng. J. 2013, 230, 166–171. [Google Scholar] [CrossRef]
- Silverstein, F.X.M.; Webster, D.J.K. Spektroskopowe Metody Identyfikacji Związków Organicznych; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2007. [Google Scholar]
- Zieliński, W. Metody Spektroskopowe i Ich Zastosowanie Do Identyfikacji Związków Organicznych; WNT: Warszawa, Poland, 1996. [Google Scholar]
- Gabrienko, A.A.; Ewing, A.V.; Chibiryaev, A.M.; Agafontsev, A.M.; Dubkov, K.A.; Kazarian, S.G. New Insights into the Mechanism of Interaction between CO2 and Polymers from Thermodynamic Parameters Obtained by in Situ ATR-FTIR Spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 6465–6475. [Google Scholar] [CrossRef]
- Zong, L.; Liu, F.; Chen, D.; Zhang, X.; Ling, C.; Li, A. A Novel Pyridine Based Polymer for Highly Efficient Separation of Nickel from High-Acidity and High-Concentration Cobalt Solutions. Chem. Eng. J. 2018, 334, 995–1005. [Google Scholar] [CrossRef]
- Zagorodni, A.A.; Kotova, D.L.; Selemenev, V.F. Infrared Spectroscopy of Ion Exchange Resins: Chemical Deterioration of the Resins. React. Funct. Polym. 2002, 53, 157–171. [Google Scholar] [CrossRef]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. X-Ray Photoelectron Spectroscopy Studies of Reactions on Chromium Metal and Chromium Oxide Surfaces. J. Electron Spectros. Relat. Phenom. 2011, 184, 29–37. [Google Scholar] [CrossRef]
- Gobbo, P.; Novoa, S.; Biesinger, M.C.; Workentin, M.S. Interfacial Strain-Promoted Alkyne-Azide Cycloaddition (I-SPAAC) for the Synthesis of Nanomaterial Hybrids. Chem. Commun. 2013, 49, 3982–3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raicopol, M.; Andronescu, C.; Atasiei, R.; Hanganu, A.; Pilan, L. Post-Polymerization Electrochemical Functionalization of a Conducting Polymer: Diazonium Salt Electroreduction at Polypyrrole Electrodes. J. Electrochem. Soc. 2014, 161, G103–G113. [Google Scholar] [CrossRef]
- Wanger, W.C.; Riggs, L.; Davis, J.; Moulder, G.M. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics Division: Eden Prairie, MN, USA, 1979. [Google Scholar]




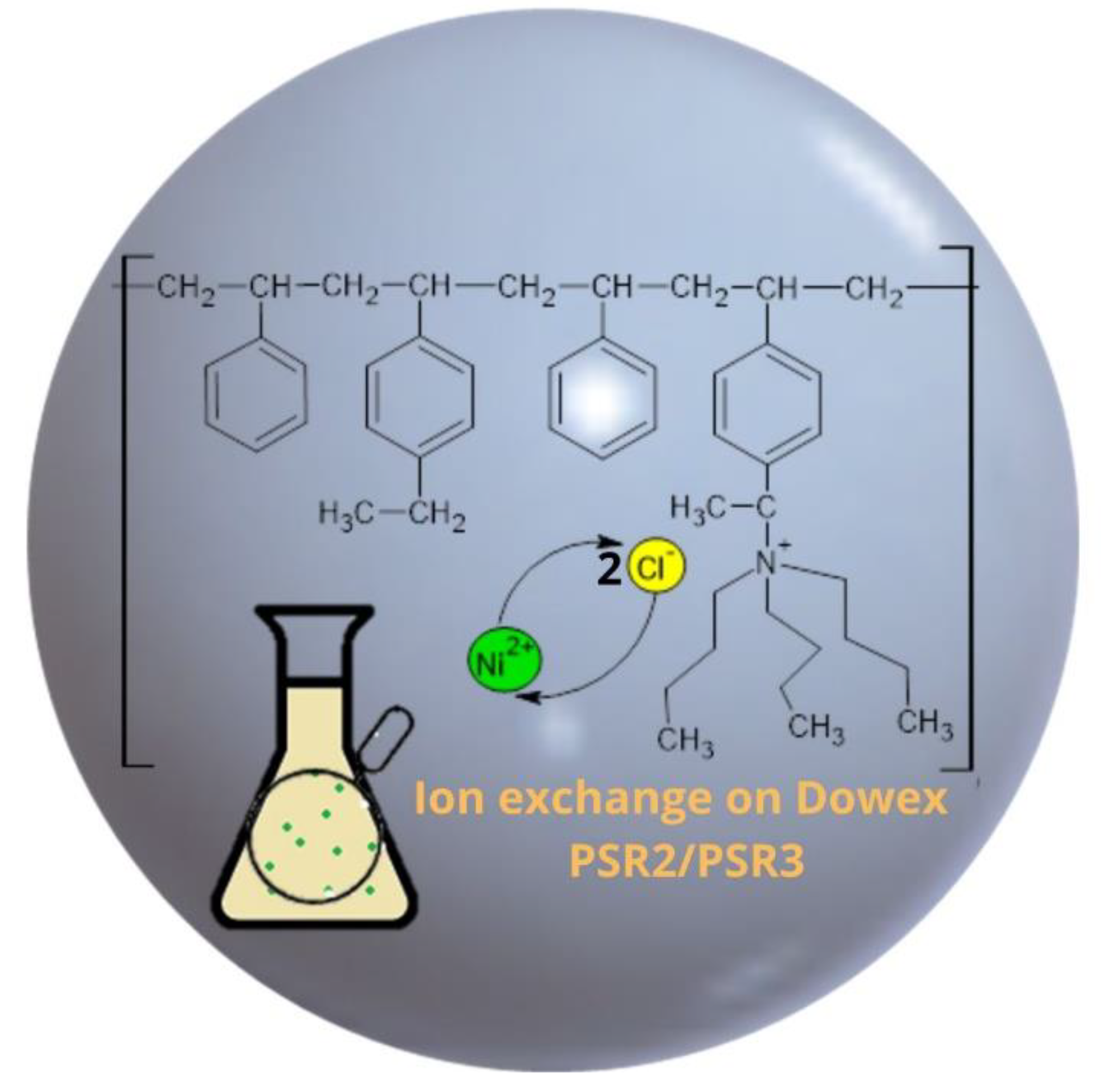
| Features | Ion Exchangers | |
|---|---|---|
| Dowex PSR2 | Dowex PSR3 | |
| Resin type | strongly basic anion exchangers | strongly basic anion exchangers |
| Physical form | beige beads | beige beads |
| Skeleton | microporous, cross-linked polystyrene DVB | macroporous, cross-linked polystyrene DVB |
| Functional groups Matrix | tri-n-butyl ammonium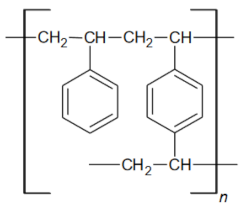 | tri-n-butyl ammonium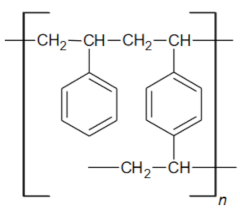 |
| Ion exchange capacity (val/L) | 0.65 | 0.60 |
| Water retention (%) | 40–48 | 50–65 |
| Mean bead size (mm) | 0.3–1.2 | 0.3–1.2 |
| Working temperature (K) | <373 | <373 |
| Producer | DOW Chemical Company | DOW Chemical Company |
| Model | Equation | Parameters |
|---|---|---|
| Kinetic | ||
| PFO | k1—the rate constant of PFO equation (1/min) | |
| PSO | k2—the rate constant of PSO equation (g/mg∙min) | |
| EKE | α—the initial adsorption rate (mg/g∙min) β—the constant of desorption connected with the activaton energy chemisorption and the range of surface coverage (g/mg) | |
| IPD | ki—the rate constant of intraparticle diffusion equation (mg/g/min0.5) C—the intercept which reflects the boundary layer impact | |
| Isotherm | ||
| Langmuir | q0—the Langmuir monolayer sorption capacity (mg/g) KL—the characteristics of Langmuir equation (L/mg) | |
| Freundlich | KF—the adsorption capacity of the Freundlich equation (mg/g) n—the Freundlich constant associated with the surface heterogenity | |
| Temkin | bT—the Temkin constant related to the sorption heat (kJ/mol) A—the equilibrium Temkin binding constant (L/g) | |
| Dubinin-Raduszkiewicz | qm—the Dubinin–Raduszkiewicz constant associated with the adsorption capacity (mg/g) β—the Dubinin–Raduszkiewicz constant related to the adsorption mean free energy (mol2/kJ2) Ea—the activation energy (kJ/mol) | |
| Sips | qm, K—model’s constants (mg/g) and (L/mg) n—the heterogeneity index whose magnitude increases with heterogeneity | |
| Ion Exchangers | Sorption Conditions | Equilibrium Capacity (mg/g) | References |
|---|---|---|---|
| Amberlite IRA458 | C0 58.7 mg/L, t 240 min, T 293 K, in aqueous solution (Ni(II)-IDS 1-1) | 5.89 | [26] |
| Amberlite IRA958 | 5.84 | ||
| Amberlite IRA67 | 5.73 | ||
| Purolite S984 | C0 100 mg/L, t 240 min, T 298 K, in chloride solution (0.1 mol/L HCl) | 4.95 | [27] |
| Purolite A830 | 4.60 | [28] | |
| Lewatit MonoPlus SR7 | 4.56 | ||
| Purolite A400TL | 4.72 | ||
| Dowex PSR2 | 3.70 | [25] | |
| Dowex PSR3 | 4.73 | ||
| Lewatit MonoPlus TP220 | 6.24 | [29] | |
| Lewatit AF5 | 4.89 | [30] | |
| Diphonix | C0 100 mg/L, t 120 min, T 293 K, in nitrate solution (0.2 mol/L HNO3) | 5.03 | [31] |
| AmberjetTM 1200H | C0 2.44 mg/L, t 24 h, in real ammoniacal industrial wastewater | 4.36 | [32] |
| Purolite S940 | C0 2935 mg/L, t 7 days, 298 K, in chloride solution | 4.22 | [33] |
| Purolite S950 | 3.31 | ||
| Dowex PSR2 | C0 100 mg/L, t 240 min, T 293 K, in aqueous solution | 10.30 | this research |
| Model | Parameters | Units | Ion Exchangers | ||
|---|---|---|---|---|---|
| PSR2 | PSR3 | ||||
| PFO | qe | mg/g | 10.30 | 19.10 | |
| k1 | 1/min | 0.028 | 0.024 | ||
| q1 | mg/g | 7.57 | 4.97 | ||
| R2 | - | 0.976 | 0.931 | ||
| PSO | k2 | (g/mg∙min) | 0.008 | 0.023 | |
| q2 | (mg/g) | 10.79 | 19.20 | ||
| R2 | - | 0.998 | 1.000 | ||
| EKE | α | (mg/g∙min) | 3.227 | 11,471.622 | |
| β | (g/mg) | 0.562 | 0.735 | ||
| R2 | - | 0.960 | 0.950 | ||
| IPD | first step | ki1 | mg/g/min0.5 | 1.534 | 1.988 |
| C1 | - | 0.168 | 9.916 | ||
| R21 | - | 0.749 | 0.873 | ||
| second step | ki2 | mg/g/min0.5 | 0.966 | 0.410 | |
| C2 | - | 1.627 | 14.703 | ||
| R22 | - | 0.864 | 0.821 | ||
| third step | ki3 | mg/g/min0.5 | 0.060 | 0.061 | |
| C3 | - | 9.374 | 18.152 | ||
| R23 | - | 1.000 | 1.000 | ||
| Model | Parameters | Units | Ion Exchangers | |
|---|---|---|---|---|
| PSR2 | PSR3 | |||
| Langmuir | qm | mg/g | 19.16 | 31.72 |
| KL | L/mg | 0.012 | 0.021 | |
| R2 | - | 0.972 | 0.995 | |
| Freundlich | KF | mg/g | 1.15 | 2.00 |
| n | - | 2.257 | 2.172 | |
| R2 | - | 0.964 | 0.937 | |
| Temkin | A | L/g | 0.257 | 0.376 |
| bT | kJ/mol | 0.762 | 0.442 | |
| R2 | - | 0.915 | 0.968 | |
| Dubinin-Raduszkiewicz | qm | mg/g | 0.0005 | 0.0011 |
| β | mol2/kJ2 | 0.0049 | 0.0053 | |
| Ea | kJ/mol | 10.074 | 9.753 | |
| R2 | - | 0.945 | 0.969 | |
| qm | mg/g | 27.74 | 25.53 | |
| Sips | K | L/mg | 0.002 | 0.0002 |
| n | 0.634 | 0.469 | ||
| R2 | 0.982 | 0.989 | ||
| Parameters | Temperature (K) | Ion Exchangers | |
|---|---|---|---|
| PSR2 | PSR3 | ||
| Kd | 293 | 37.7 | 58.7 |
| 313 | 34.3 | 52.5 | |
| 323 | 31.1 | 47.1 | |
| 333 | 28.9 | 43.7 | |
| ΔH° (kJ/mol) | - | −5.37 | −6.01 |
| ΔS° (kJ/mol) | - | 11.96 | 13.47 |
| ΔG° (kJ/mol) | 293 | −8.87 | −9.96 |
| 313 | −9.11 | −10.23 | |
| 323 | −9.23 | −10.36 | |
| 333 | −9.35 | −10.50 | |
| Ion Exchanger | Name | Position (eV) | Raw Area | % Atom Concentration |
|---|---|---|---|---|
| Dowex PSR2 | C 1s | 284.7 | 23,366.6 | 83.3 |
| N 1s | 401.7 | 618.9 | 2.2 | |
| O 1s | 532.2 | 3235.6 | 11.5 | |
| Al 2p | 74.7 | 139.3 | 0.5 | |
| Si 2p | 101.7 | 134.5 | 0.5 | |
| S 2p | 167.7 | 106.8 | 0.4 | |
| Cl 2p | 197.0 | 459.8 | 1.6 | |
| Dowex PSR3 | C 1s | 284.7 | 20,357.8 | 87.7 |
| N 1s | 401.7 | 649.0 | 1.6 | |
| O 1s | 531.5 | 5529.7 | 8.1 | |
| Cl 2p | 197.0 | 1420.3 | 2.7 |
| Element | Position (eV) | Raw Area | % Atom Concentration | Phase |
|---|---|---|---|---|
| Dowex PSR2 | ||||
| C 1s | 284.7 | 3338.9 | 74.7 | C=C, C-H, C-C |
| 285.9 | 1086.3 | 24.3 | -CH-, CN | |
| 288.6 | 45.3 | 1 | C-H–Ar | |
| O 1s | 531.9 | 404.9 | 52.1 | C-O |
| 532.9 | 372.3 | 47.9 | nitrates | |
| Cl 2p | 196.8 | 68.3 | 50.7 | Cl- |
| 198.4 | 66.5 | 49.3 | – | |
| N 1s | 399.90 | 33.1 | 31.5 | quaternary amine |
| 402.13 | 65.0 | 61.9 | protonated amine | |
| 406.14 | 7.0 | 6.6 | nitro group | |
| Dowex PSR3 | ||||
| C 1s | 284.7 | 7633.66 | 86.5 | C=C, C-H, C-C |
| 286.2 | 1119.66 | 12.7 | -CH-, CN | |
| 288.7 | 74.7348 | 0.8 | C-H–Ar | |
| O 1s | 532.2 | 2210.1 | 84.4 | C-O |
| 533.8 | 408.3 | 15.6 | nitrates | |
| Cl 2p | 196.6 | 264.8 | 74.4 | Cl- |
| 197.9 | 132.4 | - | - | |
| 198.6 | 91.2 | 25.6 | chlorates | |
| 199.7 | 45.6 | - | - | |
| N 1s | 401.9 | 374.6 | 100 | quaternary amine |
| Ion Exchanger | Name | Position (eV) | Raw Area | % Atom Concentration |
|---|---|---|---|---|
| Dowex PSR2 | C 1s | 284.7 | 23,842.4 | 80.1 |
| N 1s | 401.7 | 889.7 | 3.0 | |
| O 1s | 532.2 | 4281.0 | 14.4 | |
| Al 2p | 73.2 | 277.1 | 0.9 | |
| Si 2p | 101.7 | 363.1 | 1.2 | |
| S 2p | 167.7 | 48.8 | 0.2 | |
| Cl 2p | 197.0 | 81.4 | 0.3 | |
| Dowex PSR3 | C 1s | 284.7 | 18,315.3 | 82.3 |
| N 1s | 401.7 | 1249.1 | 3.1 | |
| O 1s | 532.2 | 9236.3 | 14.2 | |
| Cl 2p | 197.7 | 235.3 | 0.5 | |
| Ni 2p | 856.2 | 482.5 | - |
| Element | Position (eV) | Raw Area | % Atom Concentration | Phase |
|---|---|---|---|---|
| Dowex PSR2 | ||||
| C 1s | C 1s A | 284.7 | 77.5 | C=C, C-H, C-C |
| C 1s B | 286.0 | 21.4 | -CH-, CN | |
| C 1s C | 288.7 | 1.1 | C-H–Ar | |
| O 1s | O 1s A | 533.9 | 5.2 | C-O |
| O 1s B | 532.2 | 94.8 | nitrates | |
| Cl 2p | Cl 2p 3/2 | 11.4 | 50.7 | Cl- |
| Cl 2p 1/2 | 11.1 | 49.3 | – | |
| N 1s | N 1s A | 399.8 | 21.2 | quaternary amine |
| N 1s B | 402.2 | 50.7 | protonated amine | |
| N 1s C | 406.1 | 28.1 | nitro group | |
| Dowex PSR3 | ||||
| C 1s | 284.7 | 5194.3 | 63.6 | C=C, C-H, C-C |
| 284.9 | 1122.9 | 13.7 | -CH-, CN | |
| 286.1 | 1378.5 | 16.9 | C-H–Ar | |
| 288.7 | 457.8 | 5.6 | -COOH | |
| 291.2 | 18.9 | 0.2 | π→π * | |
| O 1s | 531.9 | 2817.6 | 63.5 | C-O |
| 533.4 | 1621.2 | 36.5 | nitrates | |
| Cl 2p | 196.8 | 114.2 | 100.0 | Cl- |
| 199.5 | 57.1 | - | - | |
| N 1s | 401.9 | 239.7 | 66.1 | quaternary amine |
| 405.9 | 123.0 | 33.9 | nitro group | |
| Ion Exchanger | Element | Measurement I [%] | Measurement II [%] | Mean (%) |
|---|---|---|---|---|
| Dowex PSR2 | C | 70.08 | 70.22 | 70.15 |
| H | 8.81 | 8.79 | 8.80 | |
| N | 2.80 | 2.77 | 2.78 | |
| Dowex PSR3 | C | 74.81 | 74.73 | 74.77 |
| H | 12.07 | 12.10 | 12.09 | |
| N | 2.41 | 2.41 | 2.41 |
| Ion Exchanger | Element | % Content Determined by the CHN Method | Theoretical% Content (1:1)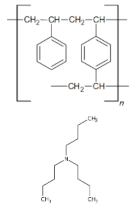 | Theoretical% Content (2:1) | Theoretical% Content (1:2) |
|---|---|---|---|---|---|
| Dowex PSR2 | C | 70.15 | 86.12 | 88.34 | 83.72 |
| H | 8.80 | 10.53 | 9.51 | 11.63 | |
| N | 2.78 | 3.35 | 2.15 | 4.65 | |
| Dowex PSR3 | C | 74.77 | 86.12 | 88.34 | 83.72 |
| H | 12.09 | 10.53 | 9.51 | 11.63 | |
| N | 2.41 | 3.35 | 2.15 | 4.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk, J.; Sofińska-Chmiel, W.; Gajewska, M.; Malinowska, P.; Kołodyńska, D. Determination of the Ni(II) Ions Sorption Mechanism on Dowex PSR2 and Dowex PSR3 Ion Exchangers Based on Spectroscopic Studies. Materials 2023, 16, 644. https://doi.org/10.3390/ma16020644
Bąk J, Sofińska-Chmiel W, Gajewska M, Malinowska P, Kołodyńska D. Determination of the Ni(II) Ions Sorption Mechanism on Dowex PSR2 and Dowex PSR3 Ion Exchangers Based on Spectroscopic Studies. Materials. 2023; 16(2):644. https://doi.org/10.3390/ma16020644
Chicago/Turabian StyleBąk, Justyna, Weronika Sofińska-Chmiel, Maria Gajewska, Paulina Malinowska, and Dorota Kołodyńska. 2023. "Determination of the Ni(II) Ions Sorption Mechanism on Dowex PSR2 and Dowex PSR3 Ion Exchangers Based on Spectroscopic Studies" Materials 16, no. 2: 644. https://doi.org/10.3390/ma16020644






