Anti-Oxidation Agents to Prevent Dye Degradation in Organic-Based Host–Guest Systems Suitable for Luminescent Solar Concentrators
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optical Characterization
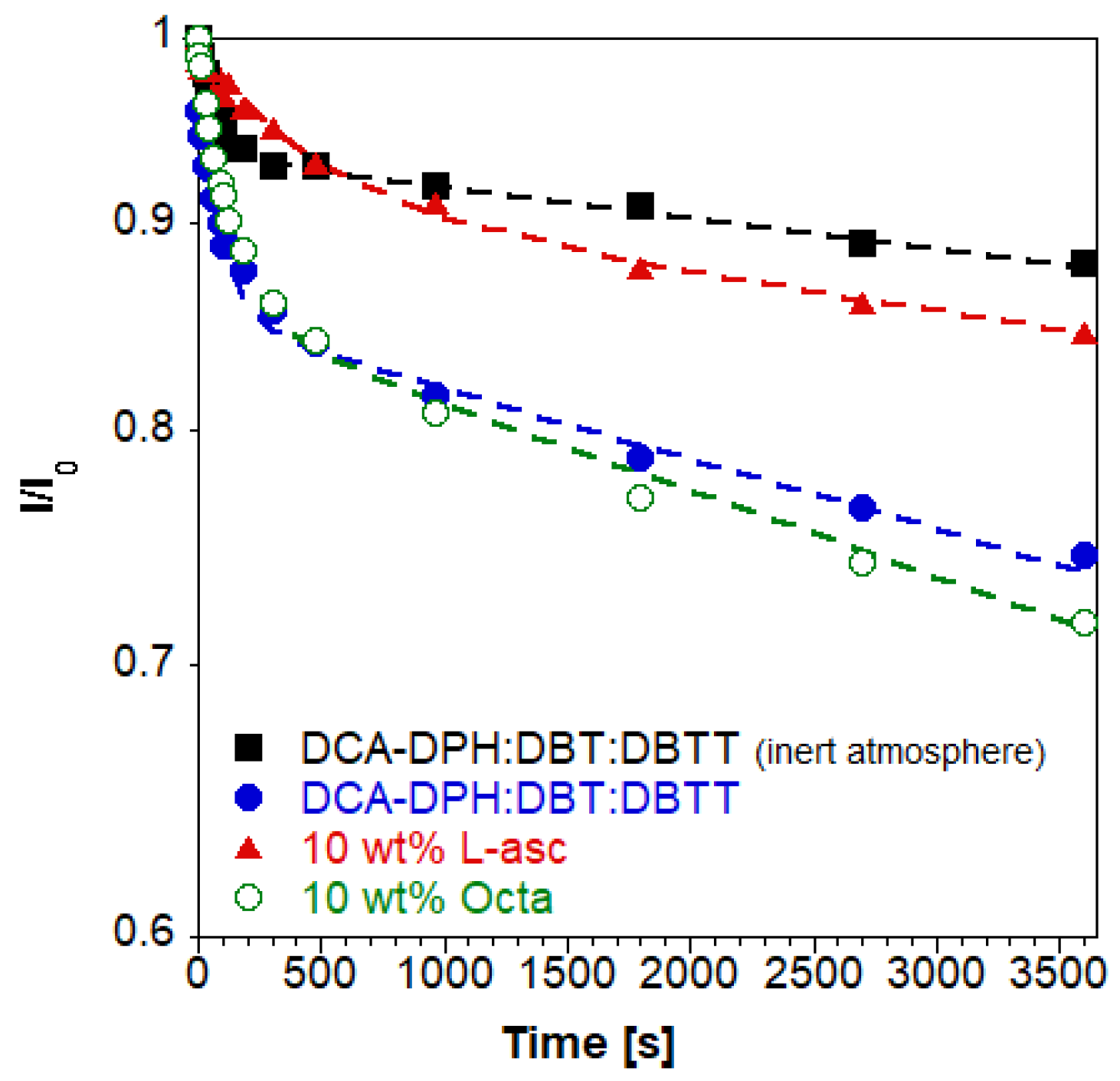
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gürtürk, M. Economic Feasibility of Solar Power Plants Based on PV Module with Levelized Cost Analysis. Energy 2019, 171, 866–878. [Google Scholar] [CrossRef]
- Alaaeddin, M.H.; Sapuan, S.M.; Zuhri, M.Y.M.; Zainudin, E.S.; Al-Oqla, F.M. Photovoltaic Applications: Status and Manufacturing Prospects. Renew. Sustain. Energy Rev. 2019, 102, 318–332. [Google Scholar] [CrossRef]
- Almosni, S.; Delamarre, A.; Jehl, Z.; Suchet, D.; Cojocaru, L.; Giteau, M.; Behaghel, B.; Julian, A.; Ibrahim, C.; Tatry, L.; et al. Material Challenges for Solar Cells in the Twenty-First Century: Directions in Emerging Technologies. Sci. Technol. Adv. Mater. 2018, 19, 336–369. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lieber, C.M. Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Y. Effects of Solar Photovoltaic Technology on the Environment in China. Environ. Sci. Pollut. Res. 2017, 24, 22133–22142. [Google Scholar] [CrossRef]
- Debije, M.G.; Verbunt, P.P.C. Thirty Years of Luminescent Solar Concentrator Research: Solar Energy for the Built Environment. Adv. Energy Mater. 2012, 2, 12–35. [Google Scholar] [CrossRef]
- Papakonstantinou, I.; Portnoi, M.; Debije, M.G. The Hidden Potential of Luminescent Solar Concentrators. Adv. Energy Mater. 2021, 11, 2002883. [Google Scholar] [CrossRef]
- Mazzaro, R.; Vomiero, A. The Renaissance of Luminescent Solar Concentrators: The Role of Inorganic Nanomaterials. Adv. Energy Mater. 2018, 8, 1801903. [Google Scholar] [CrossRef]
- Castillo, M.S.; Liu, X.; Abd-AlHamid, F.; Connelly, K.; Wu, Y. Intelligent Windows for Electricity Generation: A Technologies Review. Build. Simul. 2022, 15, 1747–1773. [Google Scholar] [CrossRef]
- Meinardi, F.; Bruni, F.; Brovelli, S. Luminescent Solar Concentrators for Building-Integrated Photovoltaics. Nat. Rev. Mater. 2017, 2, 17072. [Google Scholar] [CrossRef]
- Yang, C.; Liu, D.; Renny, A.; Kuttipillai, P.S.; Lunt, R.R. Integration of Near-Infrared Harvesting Transparent Luminescent Solar Concentrators onto Arbitrary Surfaces. J. Lumin. 2019, 210, 239–246. [Google Scholar] [CrossRef]
- Van Sark, W.G.J.H.M.; Barnham, K.W.J.; Slooff, L.H.; Chatten, A.J.; Büchtemann, A.; Meyer, A.; McCormack, S.J.; Koole, R.; Farrell, D.J.; Bose, R.; et al. Luminescent Solar Concentrators—A Review of Recent Results. Opt. Express 2008, 16, 21773. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, Y.; Dong, R.; Luscombe, C.K. Review on the Role of Polymers in Luminescent Solar Concentrators. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 201–215. [Google Scholar] [CrossRef]
- Rondão, R.; Frias, A.R.; Correia, S.F.H.; Fu, L.; de Zea Bermudez, V.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. High-Performance Near-Infrared Luminescent Solar Concentrators. ACS Appl. Mater. Interfaces 2017, 9, 12540–12546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, P.; Wilson, L.J.; Subbiah, J.; Yang, H.; Mulvaney, P.; Jones, D.J.; Ghiggino, K.P.; Wong, W.W.H. High-Performance Large-Area Luminescence Solar Concentrator Incorporating a Donor–Emitter Fluorophore System. ACS Energy Lett. 2019, 4, 1839–1844. [Google Scholar] [CrossRef]
- Corsini, F.; Tatsi, E.; Colombo, A.; Dragonetti, C.; Botta, C.; Turri, S.; Griffini, G. Highly Emissive Fluorescent Silica-Based Core/Shell Nanoparticles for Efficient and Stable Luminescent Solar Concentrators. Nano Energy 2021, 80, 105551. [Google Scholar] [CrossRef]
- Lyu, G.; Kendall, J.; Meazzini, I.; Preis, E.; Bayseç, S.; Scherf, U.; Clément, S.; Evans, R.C. Luminescent Solar Concentrators Based on Energy Transfer from an Aggregation-Induced Emitter Conjugated Polymer. ACS Appl. Polym. Mater. 2019, 1, 3039–3047. [Google Scholar] [CrossRef]
- Correia, S.F.H.; De Zea Bermudez, V.; Ribeiro, S.J.L.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. Luminescent Solar Concentrators: Challenges for Lanthanide-Based Organic-Inorganic Hybrid Materials. J. Mater. Chem. A 2014, 2, 5580–5596. [Google Scholar] [CrossRef]
- Nolasco, M.M.; Vaz, P.M.; Freitas, V.T.; Lima, P.P.; André, P.S.; Ferreira, R.A.S.; Vaz, P.D.; Ribeiro-Claro, P.; Carlos, L.D. Engineering Highly Efficient Eu(III)-Based Tri-Ureasil Hybrids toward Luminescent Solar Concentrators. J. Mater. Chem. A 2013, 1, 7339–7350. [Google Scholar] [CrossRef]
- Wu, K.; Li, H.; Klimov, V.I. Tandem Luminescent Solar Concentrators Based on Engineered Quantum Dots. Nat. Photonics 2018, 12, 105–110. [Google Scholar] [CrossRef]
- Sadeghi, S.; Bahmani Jalali, H.; Melikov, R.; Ganesh Kumar, B.; Mohammadi Aria, M.; Ow-Yang, C.W.; Nizamoglu, S. Stokes-Shift-Engineered Indium Phosphide Quantum Dots for Efficient Luminescent Solar Concentrators. ACS Appl. Mater. Interfaces 2018, 10, 12975–12982. [Google Scholar] [CrossRef] [PubMed]
- Bergren, M.R.; Makarov, N.S.; Ramasamy, K.; Jackson, A.; Guglielmetti, R.; McDaniel, H. High-Performance CuInS2 Quantum Dot Laminated Glass Luminescent Solar Concentrators for Windows. ACS Energy Lett. 2018, 3, 520–525. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, H.; Cao, M.; Rao, Z.; Zhao, X.; Vomiero, A. Eu-Doped ZnO Quantum Dots with Solid-State Fluorescence and Dual Emission for High-Performance Luminescent Solar Concentrators. Mater. Chem. Front. 2021, 5, 4746–4755. [Google Scholar] [CrossRef]
- Selopal, G.S.; Mohammadnezhad, M.; Besteiro, L.V.; Cavuslar, O.; Liu, J.; Zhang, H.; Navarro-Pardo, F.; Liu, G.; Wang, M.; Durmusoglu, E.G.; et al. Synergistic Effect of Plasmonic Gold Nanoparticles Decorated Carbon Nanotubes in Quantum Dots/TiO2 for Optoelectronic Devices. Adv. Sci. 2020, 7, 2001864. [Google Scholar] [CrossRef]
- Cheng, Y.; Ding, L. Perovskite/Si Tandem Solar Cells: Fundamentals, Advances, Challenges, and Novel Applications. SusMat 2021, 1, 324–344. [Google Scholar] [CrossRef]
- Wei, M.; de Arquer, F.P.G.; Walters, G.; Yang, Z.; Quan, L.N.; Kim, Y.; Sabatini, R.; Quintero-Bermudez, R.; Gao, L.; Fan, J.Z.; et al. Ultrafast Narrowband Exciton Routing within Layered Perovskite Nanoplatelets Enables Low-Loss Luminescent Solar Concentrators. Nat. Energy 2019, 4, 197–205. [Google Scholar] [CrossRef]
- Wu, Y.; Zhan, Y.; Xin, W.; Cao, W.; Li, J.; Chen, M.; Jiang, X.; Wang, J.; Sun, Z. Highly Emissive Carbon Dots/Organosilicon Composites for Efficient and Stable Luminescent Solar Concentrators. ACS Appl. Energy Mater. 2022, 5, 1781–1792. [Google Scholar] [CrossRef]
- Gong, X.; Zheng, S.; Zhao, X.; Vomiero, A. Engineering High-Emissive Silicon-Doped Carbon Nanodots towards Efficient Large-Area Luminescent Solar Concentrators. Nano Energy 2022, 101, 107617. [Google Scholar] [CrossRef]
- Griffini, G.; Levi, M.; Turri, S. Novel High-Durability Luminescent Solar Concentrators Based on Fluoropolymer Coatings. Prog. Org. Coatings 2014, 77, 528–536. [Google Scholar] [CrossRef]
- Griffini, G.; Brambilla, L.; Levi, M.; Del Zoppo, M.; Turri, S. Photo-Degradation of a Perylene-Based Organic Luminescent Solar Concentrator: Molecular Aspects and Device Implications. Sol. Energy Mater. Sol. Cells 2013, 111, 41–48. [Google Scholar] [CrossRef]
- Tanaka, N.; Barashkov, N.; Heath, J.; Sisk, W.N. Photodegradation of Polymer-Dispersed Perylene Di-Imide Dyes. Appl. Opt. 2006, 45, 3846. [Google Scholar] [CrossRef] [PubMed]
- Earp, A.A.; Rawling, T.; Franklin, J.B.; Smith, G.B. Perylene Dye Photodegradation Due to Ketones and Singlet Oxygen. Dye. Pigment. 2010, 84, 59–61. [Google Scholar] [CrossRef]
- Krumer, Z.; Pera, S.J.; van Dijk-Moes, R.J.A.; Zhao, Y.; de Brouwer, A.F.P.; Groeneveld, E.; van Sark, W.G.J.H.M.; Schropp, R.E.I.; de Mello Donegá, C. Tackling Self-Absorption in Luminescent Solar Concentrators with Type-II Colloidal Quantum Dots. Sol. Energy Mater. Sol. Cells 2013, 111, 57–65. [Google Scholar] [CrossRef]
- Mateen, F.; Lee, S.Y.; Hong, S.-K. Luminescent Solar Concentrators Based on Thermally Activated Delayed Fluorescence Dyes. J. Mater. Chem. A 2020, 8, 3708–3716. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Y.; Bronstein, N.D.; Li, L.; Alivisatos, A.P.; Nuzzo, R.G. Enhanced Photon Collection in Luminescent Solar Concentrators with Distributed Bragg Reflectors. ACS Photonics 2016, 3, 278–285. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Haraldsson, T.; Clemments, A.; Fujii, M.; Sugimoto, H.; Xu, B.; Sychugov, I. Triplex Glass Laminates with Silicon Quantum Dots for Luminescent Solar Concentrators. Sol. RRL 2020, 4, 2000195. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Peng, W.-T.; Sheng, W.; Liu, D.; Kuttipillai, P.S.; Young, M.; Donahue, M.R.; Levine, B.G.; Borhan, B.; et al. Impact of Stokes Shift on the Performance of Near-Infrared Harvesting Transparent Luminescent Solar Concentrators. Sci. Rep. 2018, 8, 16359. [Google Scholar] [CrossRef]
- Corsini, F.; Nitti, A.; Tatsi, E.; Mattioli, G.; Botta, C.; Pasini, D.; Griffini, G. Large-Area Semi-Transparent Luminescent Solar Concentrators Based on Large Stokes Shift Aggregation-Induced Fluorinated Emitters Obtained Through a Sustainable Synthetic Approach. Adv. Opt. Mater. 2021, 9, 2100182. [Google Scholar] [CrossRef]
- Banal, J.L.; White, J.M.; Ghiggino, K.P.; Wong, W.W.H. Concentrating Aggregation-Induced Fluorescence in Planar Waveguides: A Proof-of-Principle. Sci. Rep. 2015, 4, 4635. [Google Scholar] [CrossRef]
- Delgado-Sanchez, J.-M. Luminescent Solar Concentrators: Photo-Stability Analysis and Long-Term Perspectives. Sol. Energy Mater. Sol. Cells 2019, 202, 110134. [Google Scholar] [CrossRef]
- Mateen, F.; Hwang, T.G.; Boesel, L.F.; Choi, W.J.; Kim, J.P.; Gong, X.; Park, J.M.; Hong, S. Luminescent Solar Concentrator Utilizing Energy Transfer Paired Aggregation-induced Emissive Fluorophores. Int. J. Energy Res. 2021, 45, 17971–17981. [Google Scholar] [CrossRef]
- Botta, C.; Betti, P.; Pasini, M. Organic Nanostructured Host–Guest Materials for Luminescent Solar Concentrators. J. Mater. Chem. A 2013, 1, 510–514. [Google Scholar] [CrossRef]
- Cappelli, A.; Villafiorita-Monteleone, F.; Grisci, G.; Paolino, M.; Razzano, V.; Fabio, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; et al. Highly Emissive Supramolecular Assemblies Based on π-Stacked Polybenzofulvene Hosts and a Benzothiadiazole Guest. J. Mater. Chem. C 2014, 2, 7897–7905. [Google Scholar] [CrossRef]
- Huber, S.; Ruiz, A.Z.; Li, H.; Patrinoiu, G.; Botta, C.; Calzaferri, G. Optical Spectroscopy of Inorganic–Organic Host–Guest Nanocrystals Organized as Oriented Monolayers. Inorg. Chim. Acta 2007, 360, 869–875. [Google Scholar] [CrossRef]
- Viani, L.; Tolbod, L.P.; Jazdzyk, M.; Patrinoiu, G.; Cordella, F.; Mura, A.; Bongiovanni, G.; Botta, C.; Beljonne, D.; Cornil, J.; et al. Spatial Control of 3D Energy Transfer in Supramolecular Nanostructured Host−Guest Architectures. J. Phys. Chem. B 2009, 113, 10566–10570. [Google Scholar] [CrossRef]
- Karnjkar, Y.S.; Dinde, R.M.; Dinde, N.M.; Bawankar, K.N.; Hinge, S.P.; Mohod, A.V.; Gogate, P.R. Degradation of Magenta Dye Using Different Approaches Based on Ultrasonic and Ultraviolet Irradiations: Comparison of Effectiveness and Effect of Additives for Intensification. Ultrason. Sonochem. 2015, 27, 117–124. [Google Scholar] [CrossRef]
- Moura, J.C.V.P.; Oliveira-Campos, A.M.F.; Griffiths, J. The Effect of Additives on the Photostability of Dyed Polymers. Dye. Pigment. 1997, 33, 173–196. [Google Scholar] [CrossRef]
- Moreau, J.; Giovanella, U.; Bombenger, J.P.; Porzio, W.; Vohra, V.; Spadacini, L.; Di Silvestro, G.; Barba, L.; Arrighetti, G.; Destri, S.; et al. Highly Emissive Nanostructured Thin Films of Organic Host-Guests for Energy Conversion. ChemPhysChem 2009, 10, 647–653. [Google Scholar] [CrossRef]
- Kitamura, C.; Tanaka, S.; Yamashita, Y. Design of Narrow-Bandgap Polymers. Syntheses and Properties of Monomers and Polymers Containing Aromatic-Donor and o-Quinoid-Acceptor Units. Chem. Mater. 1996, 8, 570–578. [Google Scholar] [CrossRef]
- Sonar, P.; Singh, S.P.; Leclère, P.; Surin, M.; Lazzaroni, R.; Lin, T.T.; Dodabalapur, A.; Sellinger, A. Synthesis, Characterization and Comparative Study of Thiophene-Benzothiadiazole Based Donor-Acceptor-Donor (D-A-D) Materials. J. Mater. Chem. 2009, 19, 3228–3237. [Google Scholar] [CrossRef]
- Sonar, P.; Singh, S.P.; Sudhakar, S.; Dodabalapur, A.; Sellinger, A. High-Mobility Organic Thin Film Transistors Based on Benzothiadiazole- Sandwiched Dihexylquaterthiophenes. Chem. Mater. 2008, 20, 3184–3190. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Hintz, H.; Egelhaaf, H.J.; Peisert, H.; Chassé, T. Photo-Oxidation and Ozonization of Poly(3-Hexylthiophene) Thin Films as Studied by UV/VIS and Photoelectron Spectroscopy. Polym. Degrad. Stab. 2010, 95, 818–825. [Google Scholar] [CrossRef]
- Zhen, Y.; Wang, J.; Li, J.; Fu, M.; Fu, F.; Zhang, Y.; Feng, J. Enhanced Photocatalytic Degradation for Thiophene by Ag/α-MoO3 Heterojunction under Visible-Light Irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 3672–3681. [Google Scholar] [CrossRef]
- Abdou, M.S.A.; Holdcroft, S. Mechanisms of Photodegradation of Poly(3-Alkylthiophenes) in Solution. Macromolecules 1993, 26, 2954–2962. [Google Scholar] [CrossRef]
- Che, Y.; Niazi, M.R.; Izquierdo, R.; Perepichka, D.F. Mechanism of the Photodegradation of A-D-A Acceptors for Organic Photovoltaics. Angew. Chemie Int. Ed. 2021, 60, 24833–24837. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Vohra, V.; Devaux, A.; Dieu, L.-Q.; Scavia, G.; Catellani, M.; Calzaferri, G.; Botta, C. Energy Transfer in Fluorescent Nanofibers Embedding Dye-Loaded Zeolite L Crystals. Adv. Mater. 2009, 21, 1146–1150. [Google Scholar] [CrossRef]
- Calzaferri, G.; Huber, S.; Maas, H.; Minkowski, C. Host–Guest Antenna Materials. Angew. Chem. Int. Ed. 2003, 42, 3732–3758. [Google Scholar] [CrossRef]
- Xu, X.; Chueh, C.-C.; Yang, Z.; Rajagopal, A.; Xu, J.; Jo, S.B.; Jen, A.K.Y. Ascorbic Acid as an Effective Antioxidant Additive to Enhance the Efficiency and Stability of Pb/Sn-Based Binary Perovskite Solar Cells. Nano Energy 2017, 34, 392–398. [Google Scholar] [CrossRef]
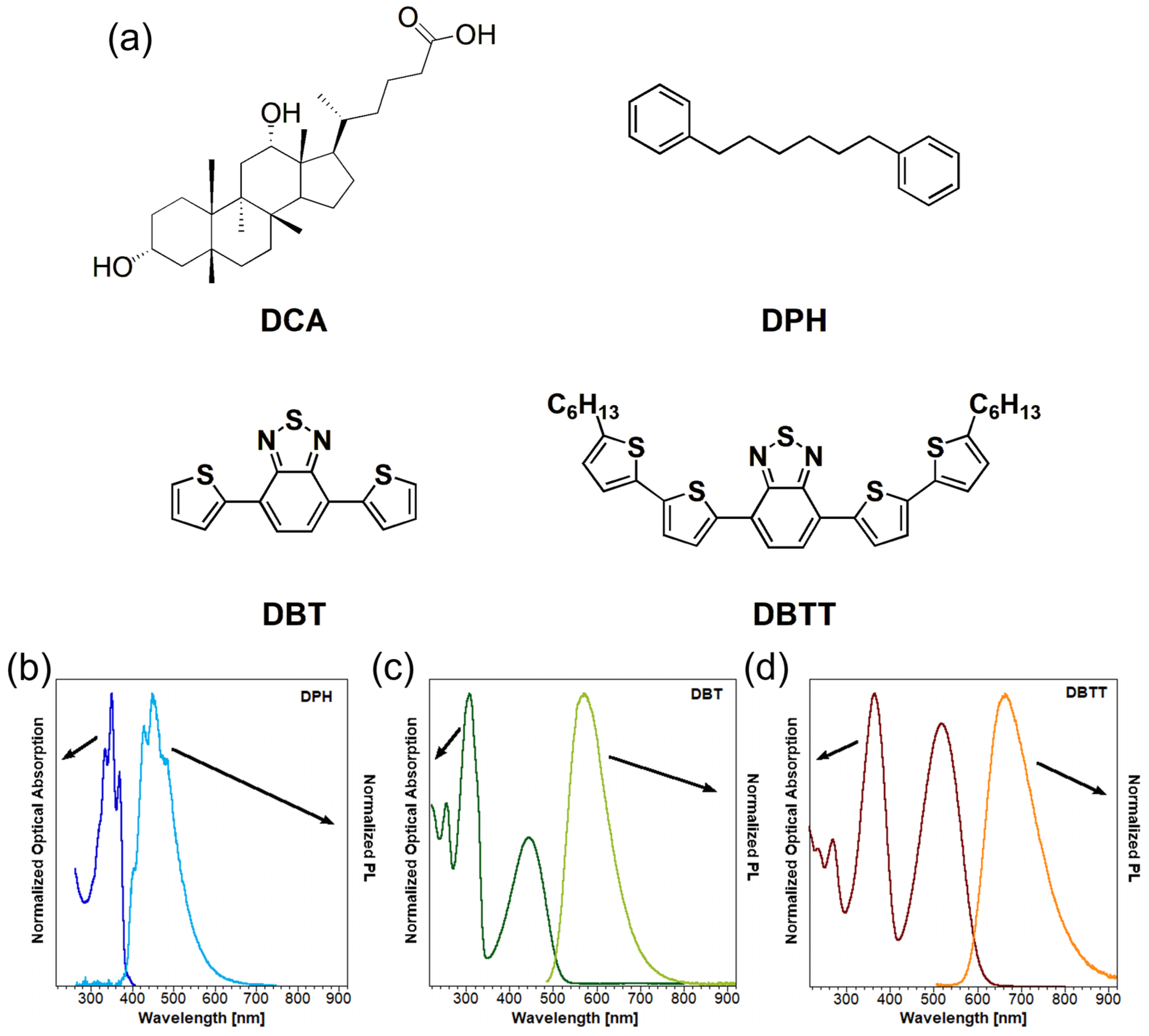
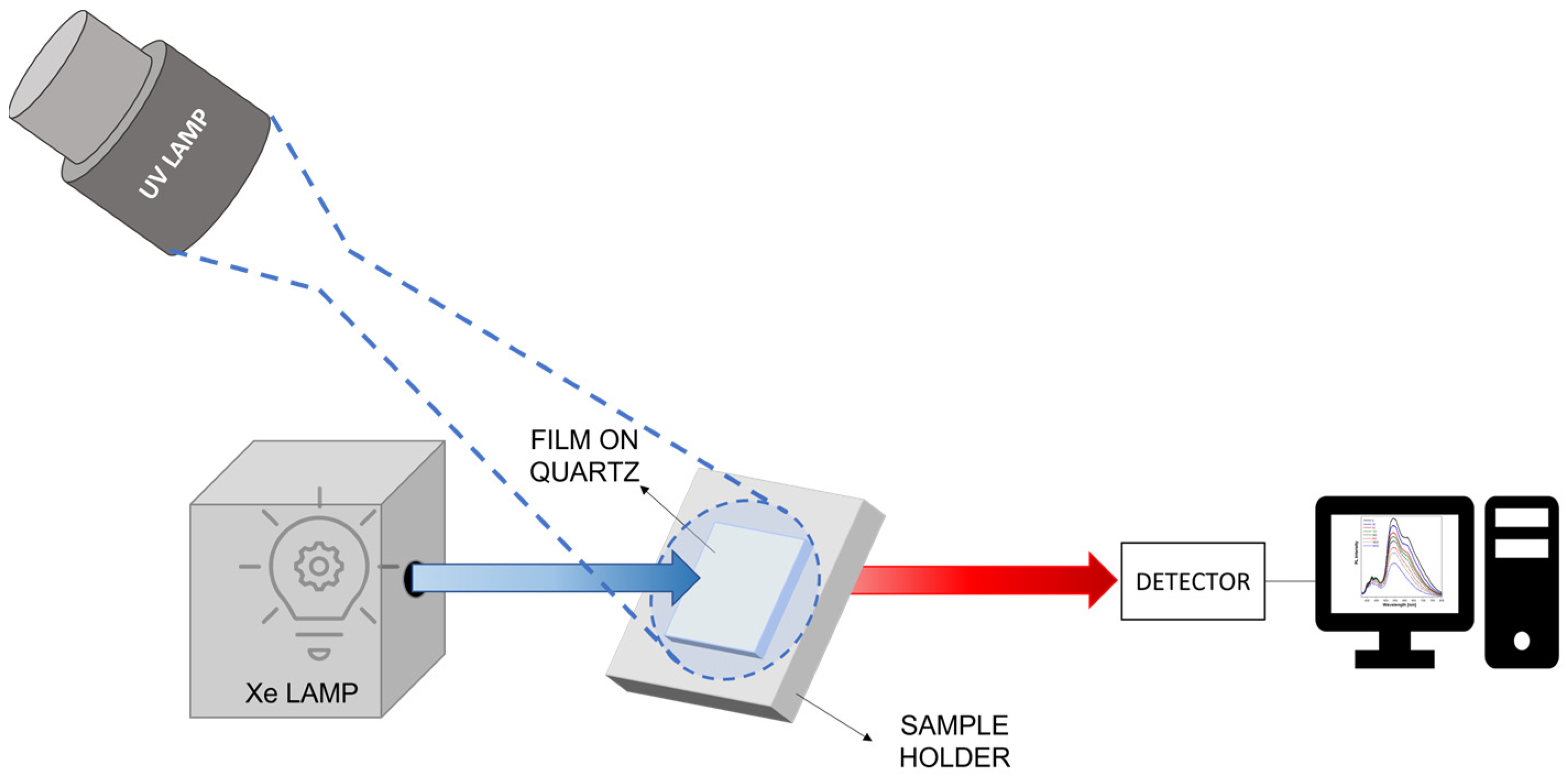
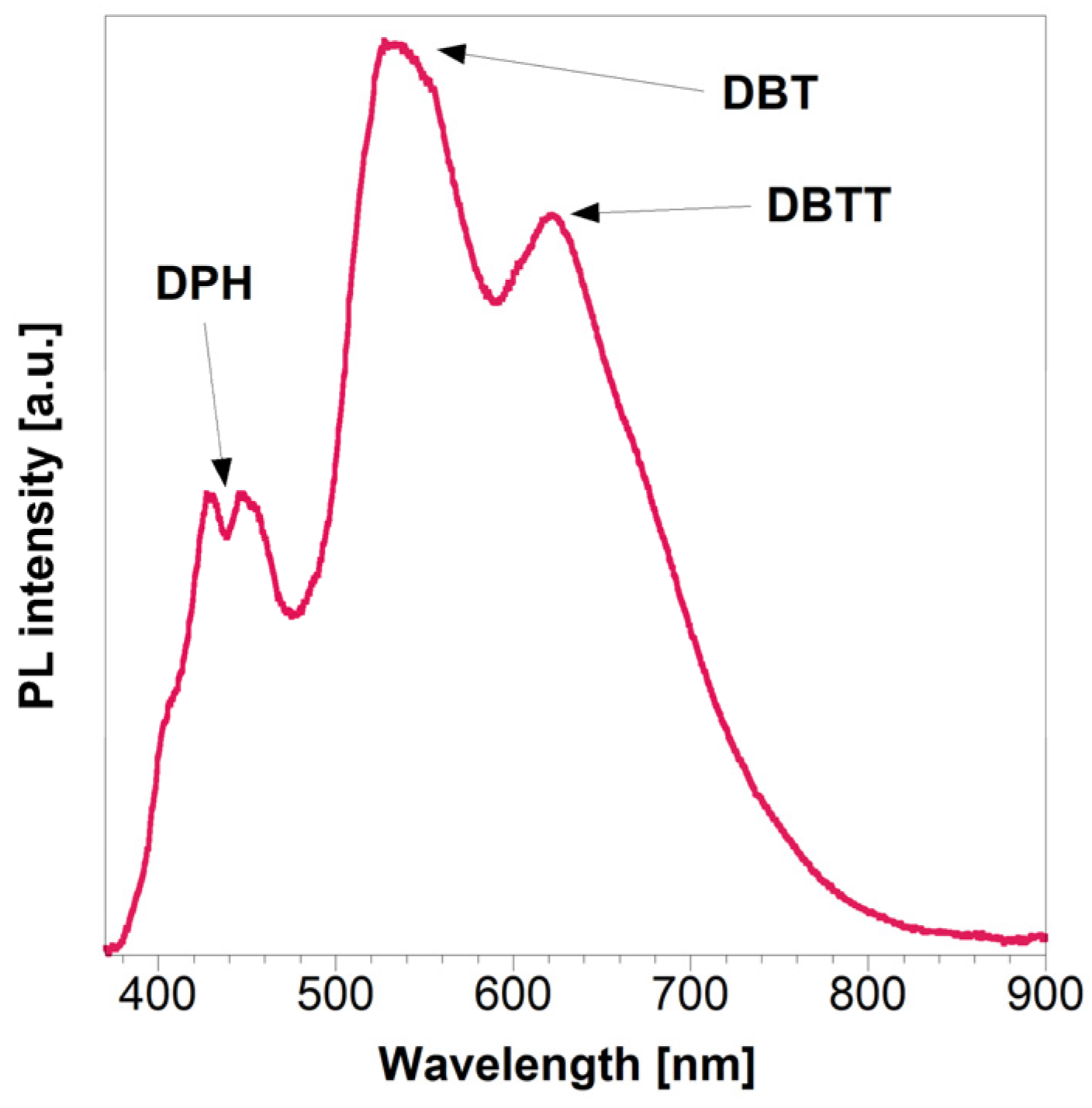
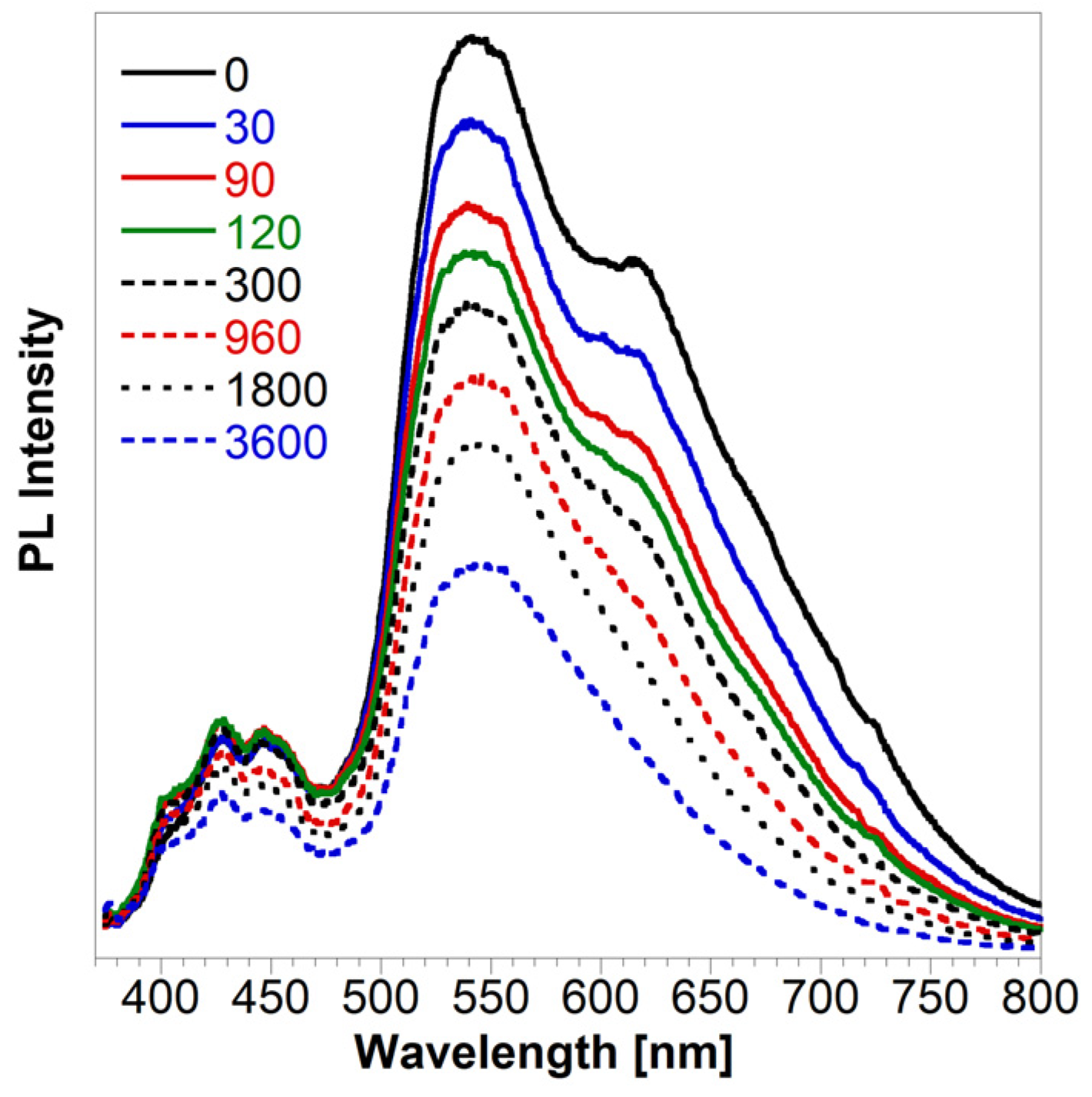
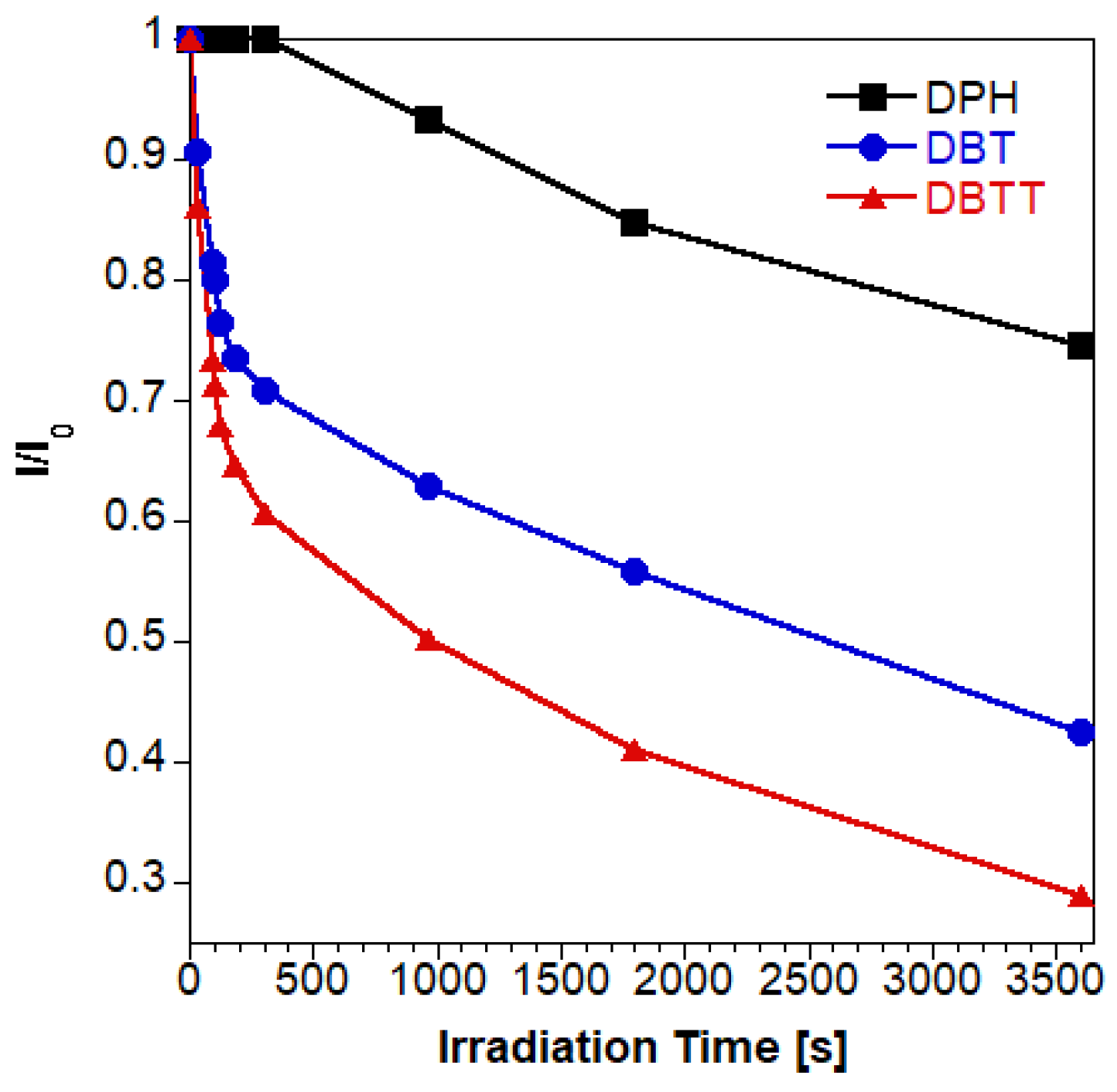
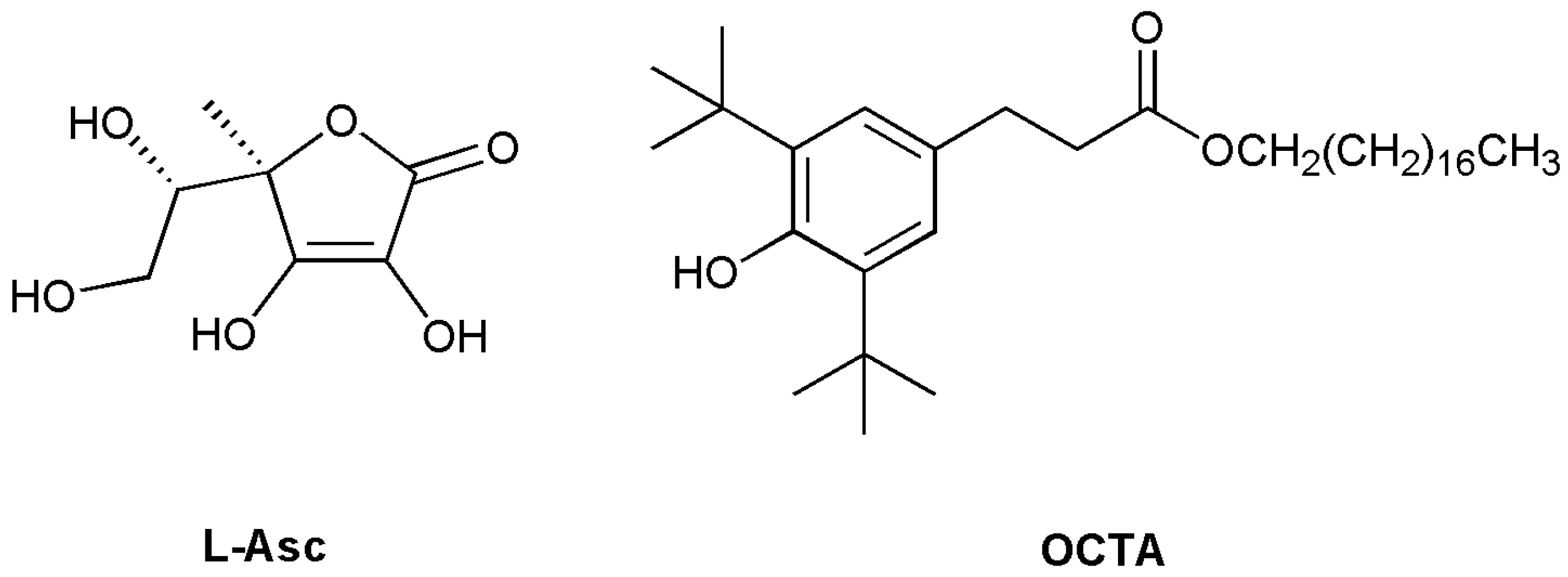
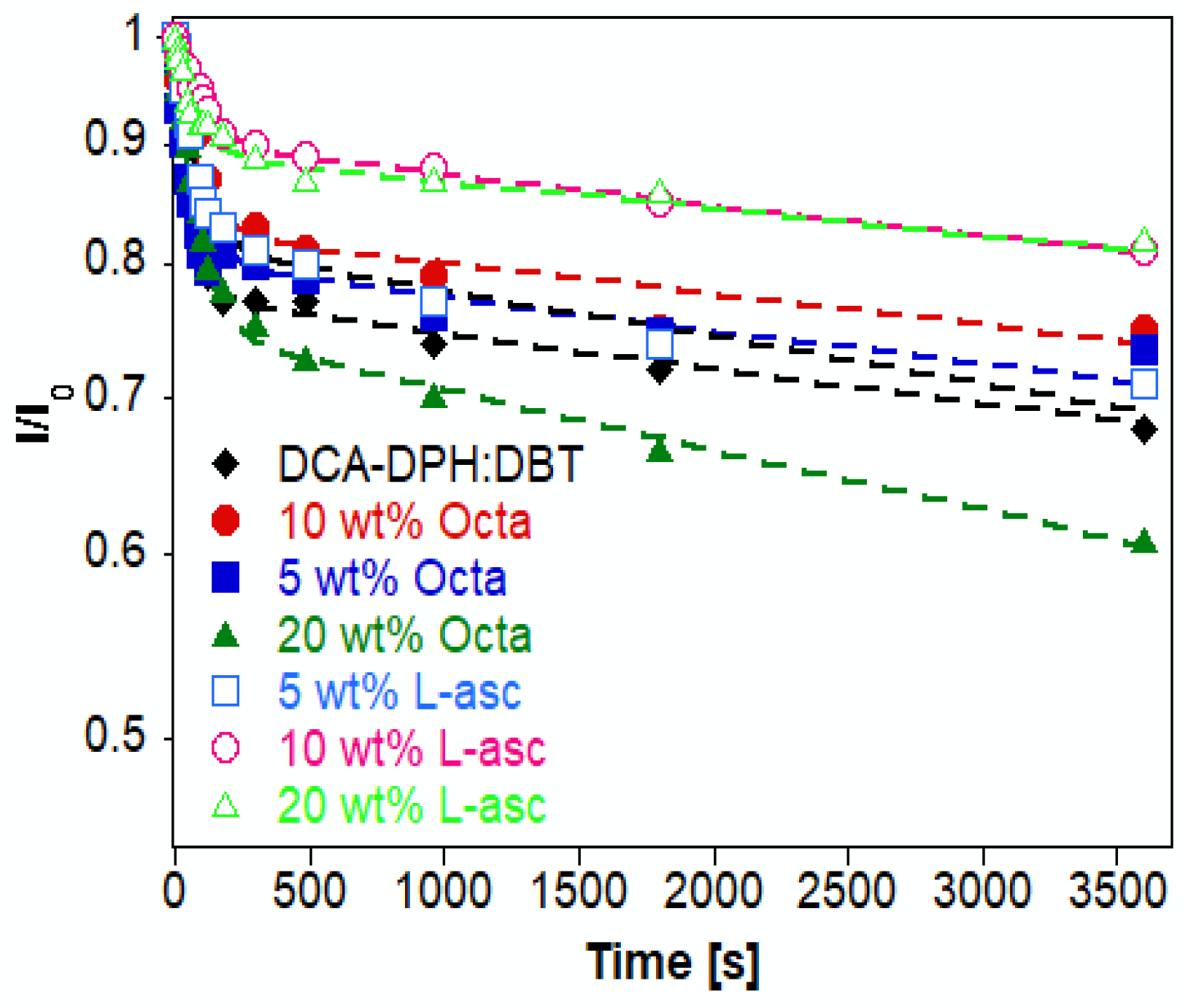
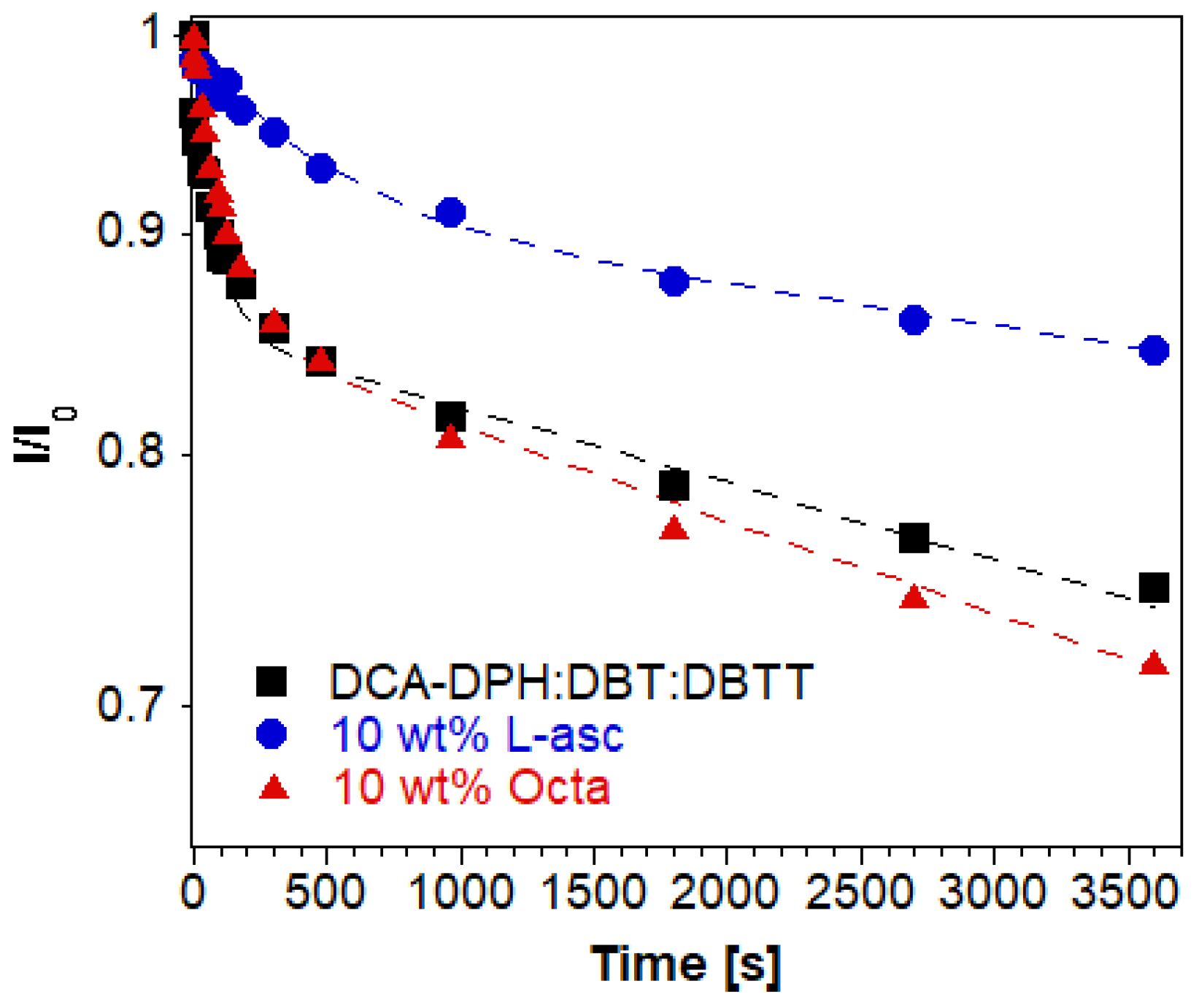
| t1 (min) | A1 | t2 (min) | A2 | Adj. R-Square | tav (h) | |
|---|---|---|---|---|---|---|
| DCA-DPH:DBT | 0.926 | 0.23 | 476.2 | 0.77 | 0.87195 | 6.121 |
| 5 wt.% Octa | 1.191 | 0.18 | 505.1 | 0.82 | 0.98173 | 6.868 |
| 10 wt.% Octa | 1.191 | 0.18 | 537.6 | 0.82 | 0.97987 | 7.368 |
| 20 wt.% Octa | 1.235 | 0.25 | 282.5 | 0.75 | 0.99558 | 3.533 |
| 5% L-Asc | 1.235 | 0.185 | 366.3 | 0.815 | 0.99370 | 4.979 |
| 10% L-Asc | 1.587 | 0.10 | 555.6 | 0.90 | 0.98965 | 8.345 |
| 20% L-Asc | 1.282 | 0.11 | 656.2 | 0.89 | 0.97563 | 9.734 |
| t1 (min) | A1 | t2 (min) | A2 | Adj. R-Square | tav (h) | |
|---|---|---|---|---|---|---|
| DCA-DPH:DBT:DBTT | 1.389 | 0.14 | 411.5 | 0.86 | 0.98145 | 5.926 |
| 10 wt.% Octa | 2.083 | 0.15 | 340.1 | 0.85 | 0.9907 | 4.826 |
| 10 wt.% L-Asc | 7.576 | 0.09 | 793.7 | 0.91 | 0.99124 | 12.10 |
| DCA-DPH:DBT:DBTT (inert atmosphere) | 1.235 | 0.07 | 957.9 | 0.94 | 0.99223 | 14.93 |
| Inert Atmosphere | 1 Week Old | Air | 1 Week Old | |
|---|---|---|---|---|
| DCA-DPH:DBT:DBTT | 1 | 0.83 | 0.97 | 0.81 |
| 10 wt.% L-Asc | - | - | 1 | 1 |
| 10 wt.% Octa | - | - | 0.83 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafiorita-Monteleone, F.; Pasini, M.; Botta, C. Anti-Oxidation Agents to Prevent Dye Degradation in Organic-Based Host–Guest Systems Suitable for Luminescent Solar Concentrators. Materials 2023, 16, 656. https://doi.org/10.3390/ma16020656
Villafiorita-Monteleone F, Pasini M, Botta C. Anti-Oxidation Agents to Prevent Dye Degradation in Organic-Based Host–Guest Systems Suitable for Luminescent Solar Concentrators. Materials. 2023; 16(2):656. https://doi.org/10.3390/ma16020656
Chicago/Turabian StyleVillafiorita-Monteleone, Francesca, Mariacecilia Pasini, and Chiara Botta. 2023. "Anti-Oxidation Agents to Prevent Dye Degradation in Organic-Based Host–Guest Systems Suitable for Luminescent Solar Concentrators" Materials 16, no. 2: 656. https://doi.org/10.3390/ma16020656
APA StyleVillafiorita-Monteleone, F., Pasini, M., & Botta, C. (2023). Anti-Oxidation Agents to Prevent Dye Degradation in Organic-Based Host–Guest Systems Suitable for Luminescent Solar Concentrators. Materials, 16(2), 656. https://doi.org/10.3390/ma16020656








