Evaluation of Fillers for Magnesium Potassium Phosphate Cement (MKPC) for the Encapsulation of Low and Intermediate Level Metallic Radioactive Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of MKPC Mortars and Pastes
3. Results and Discussion
3.1. Mechanical Characterization
3.1.1. Fresh Paste Properties: Workability and Setting Time
3.1.2. Compressive Strength
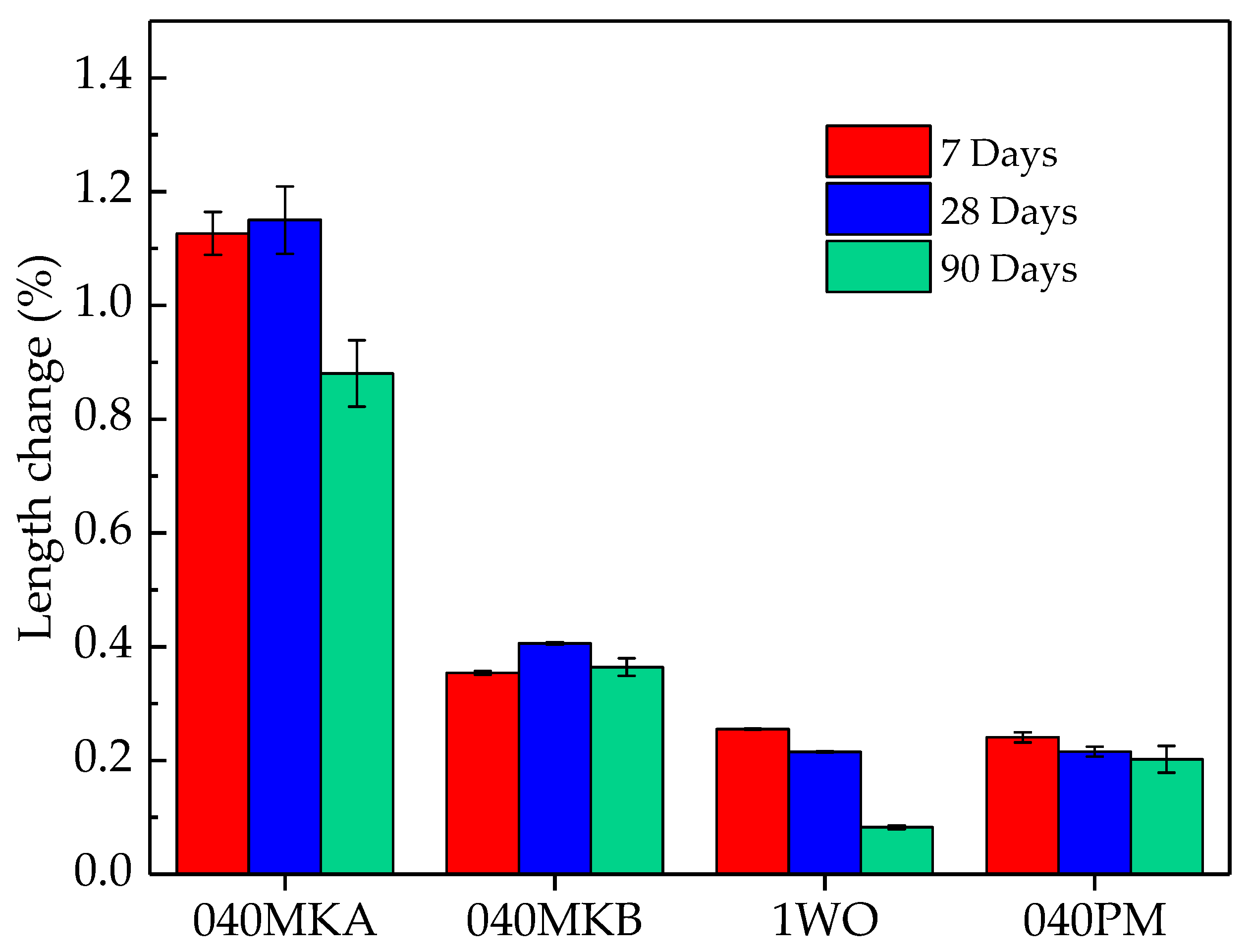
3.1.3. Dimensional Stability
3.2. Chemical Characterization
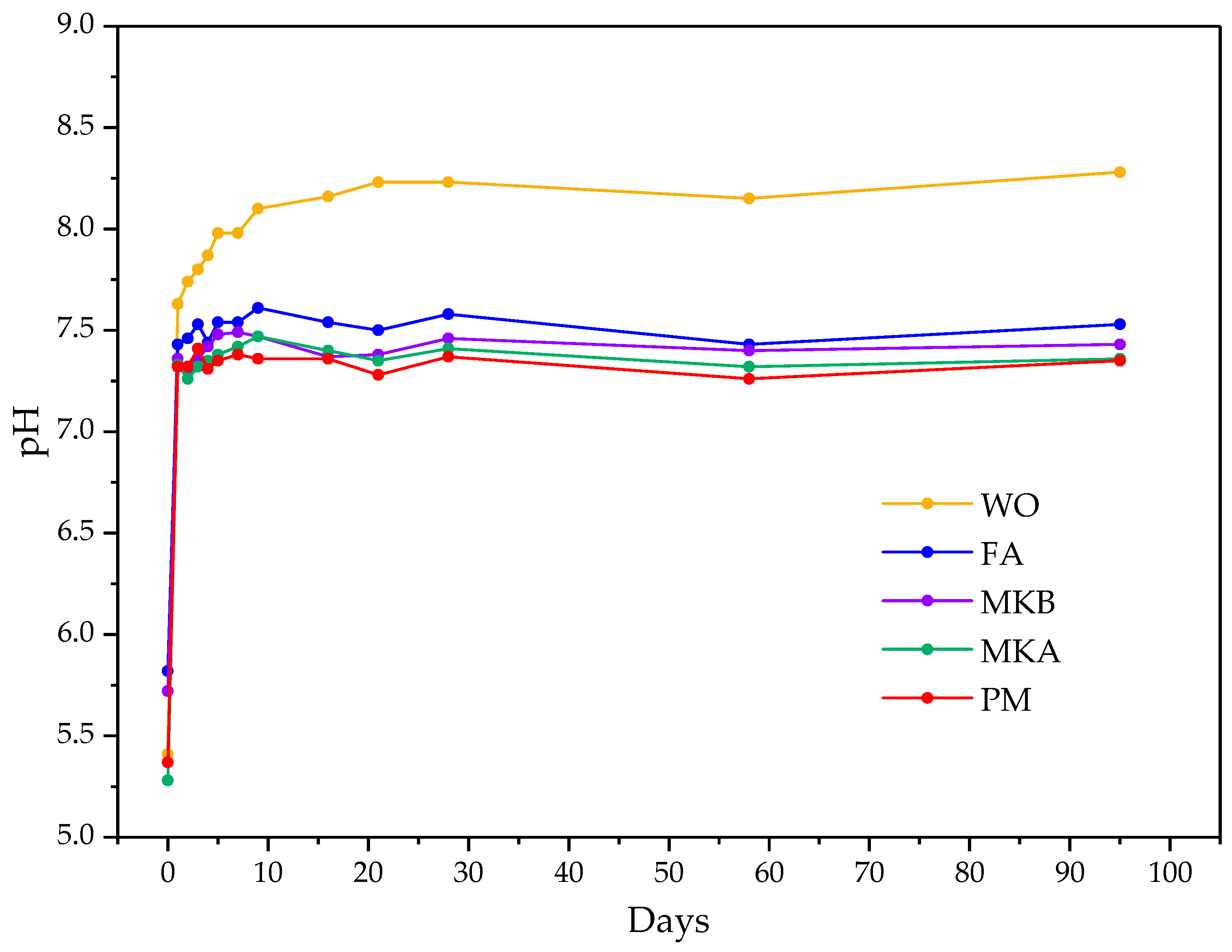
3.2.1. pH Measurement
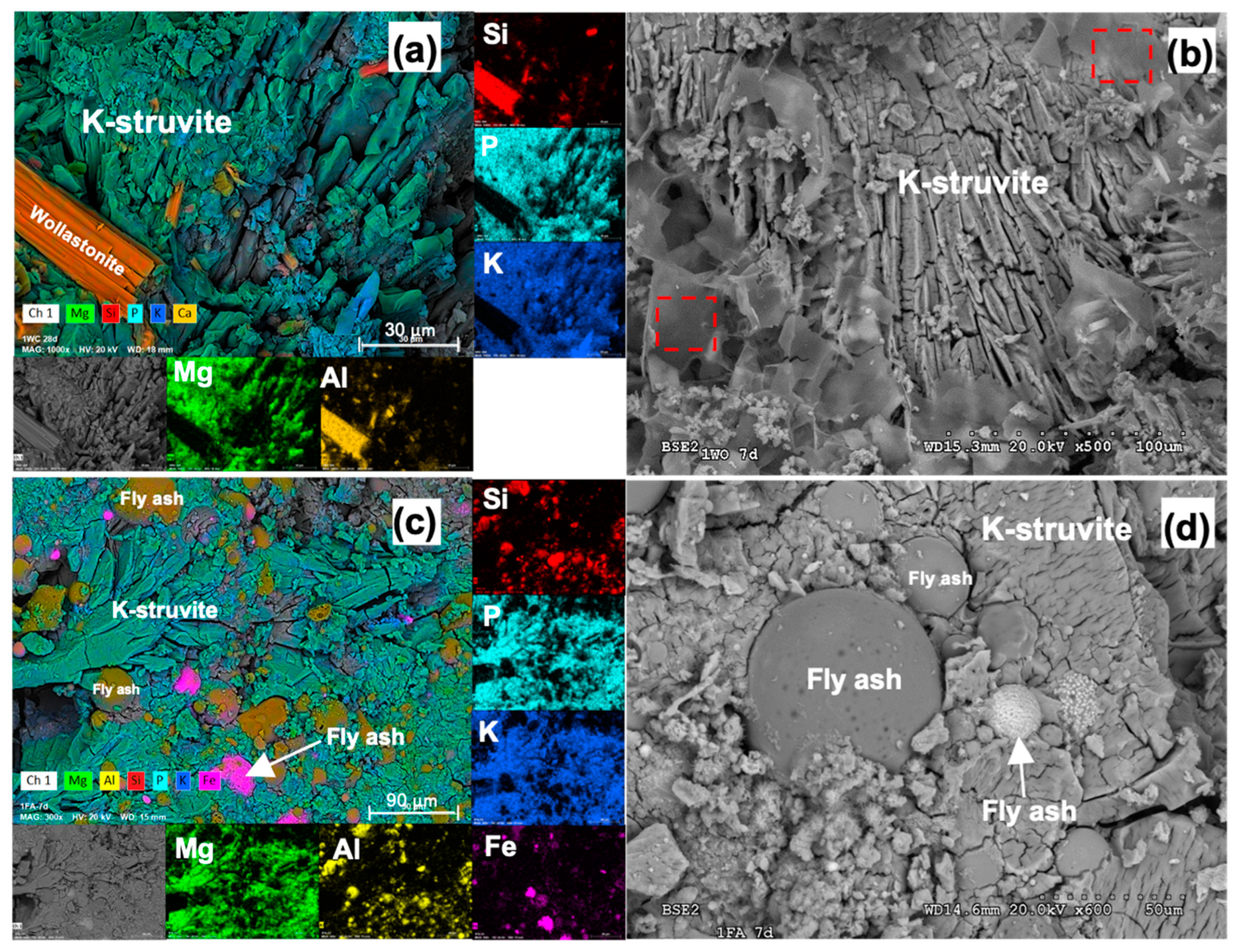
3.2.2. Mineralogical and Microstructural Analysis (XRD and SEM-EDX)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone Regeneration Capacity of Magnesium Phosphate Cements in a Large Animal Model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, B.; Ren, B.; Liu, Y.; Zhang, N.; Wang, Z.; Liu, J.; Mao, K. Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement. Life 2022, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Cao, Y. Laboratory Evaluation of Magnesium Phosphate Cement Paste and Mortar for Rapid Repair of Cement Concrete Pavement. Constr. Build. Mater. 2014, 58, 122–128. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Property Evaluation of Magnesium Phosphate Cement Mortar as Patch Repair Material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; Wang, R. Assessment of Magnesium Potassium Phosphate Cement for Waste Sludge Solidification: Macro- and Micro-Analysis. J. Clean. Prod. 2021, 294, 126365. [Google Scholar] [CrossRef]
- Buj, I.; Torras, J.; Casellas, D.; Rovira, M.; de Pablo, J. Effect of Heavy Metals and Water Content on the Strength of Magnesium Phosphate Cements. J. Hazard. Mater. 2009, 170, 345–350. [Google Scholar] [CrossRef]
- Iyengar, S.R.; Al-Tabbaa, A. Developmental Study of a Low-PH Magnesium Phosphate Cement for Environmental Applications. Environ. Technol. 2007, 28, 1387–1401. [Google Scholar] [CrossRef]
- Fu, Y.C.; Cao, X.P.; Li, Z.J. Printability of Magnesium Potassium Phosphate Cement with Different Mixing Proportion for Repairing Concrete Structures in Severe Environment. Key Eng. Mater. 2016, 711, 989–995. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, M.; Xu, J.; Li, L.; Huang, Y.; Yang, L.; Zhao, P.; Lu, L. Mix Design and Rheological Properties of Magnesium Potassium Phosphate Cement Composites Based on the 3D Printing Extrusion System. Constr. Build. Mater. 2021, 284, 122797. [Google Scholar] [CrossRef]
- Weng, Y.; Ruan, S.; Li, M.; Mo, L.; Unluer, C.; Tan, M.J.; Qian, S. Feasibility Study on Sustainable Magnesium Potassium Phosphate Cement Paste for 3D Printing. Constr. Build. Mater. 2019, 221, 595–603. [Google Scholar] [CrossRef]
- Covill, A.; Hyatt, N.C.; Hill, J.; Collier, N.C. Development of Magnesium Phosphate Cements for Encapsulation of Radioactive Waste. Adv. Appl. Ceram. 2011, 110, 151–156. [Google Scholar] [CrossRef]
- Gardner, L.J.; Corkhill, C.L.; Walling, S.A.; Vigor, J.E.; Murray, C.A.; Tang, C.C.; Provis, J.L.; Hyatt, N.C. Early Age Hydration and Application of Blended Magnesium Potassium Phosphate Cements for Reduced Corrosion of Reactive Metals. Cem. Concr. Res. 2021, 143, 106375. [Google Scholar] [CrossRef]
- Pyo, J.-Y.; Um, W.; Heo, J. Magnesium Potassium Phosphate Cements to Immobilize Radioactive Concrete Wastes Generated by Decommissioning of Nuclear Power Plants. Nucl. Eng. Technol. 2021, 53, 2261–2267. [Google Scholar] [CrossRef]
- IAEA. Management of Problematic Waste and Material Generated during the Decommissioning of Nuclear Facilities; International Atomic Energy Agency: Vienna, Austria, 2006; p. 71. [Google Scholar]
- Vargel, C. Chapter B.1—The Corrosion of Aluminium. In Corrosion of Aluminium, 2nd ed.; Vargel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–61. ISBN 978-0-08-099925-8. [Google Scholar]
- Haque, M.A.; Chen, B. Research Progresses on Magnesium Phosphate Cement: A Review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Zheng, D.-D.; Ji, T.; Wang, C.-Q.; Sun, C.-J.; Lin, X.-J.; Hossain, K.M.A. Effect of the Combination of Fly Ash and Silica Fume on Water Resistance of Magnesium–Potassium Phosphate Cement. Constr. Build. Mater. 2016, 106, 415–421. [Google Scholar] [CrossRef]
- Lu, X.; Chen, B. Experimental Study of Magnesium Phosphate Cements Modified by Metakaolin. Constr. Build. Mater. 2016, 123, 719–726. [Google Scholar] [CrossRef]
- Lahalle, H.; Cau Dit Coumes, C.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c Ratio on the Hydration Process of a Magnesium Phosphate Cement and on Its Retardation by Boric Acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Ma, C.; Wang, F.; Zhou, H.; Jiang, Z.; Ren, W.; Du, Y. Effect of Early-Hydration Behavior on Rheological Properties of Borax-Admixed Magnesium Phosphate Cement. Constr. Build. Mater. 2021, 283, 122701. [Google Scholar] [CrossRef]
- Liu, R.; Fang, B.; Zhang, G.; Guo, J.; Yang, Y. Investigation of Sodium Alginate as a Candidate Retarder of Magnesium Phosphate Cement: Hydration Properties and Its Retarding Mechanism. Ceram. Int. 2022, 48, 30846–30852. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Chen, B. Experimental Study of Magnesia and M/P Ratio Influencing Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Winnefeld, F. Influence of Wollastonite on Hydration and Properties of Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2020, 131, 106012. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the Influence of Mg/P Ratio on the Properties and Durability of Magnesium Potassium Phosphate Cement Pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Ma, C.; Zheng, Z.; Long, G.; Chen, B. Effects of Metakaolin on Properties and Microstructure of Magnesium Phosphate Cement. Constr. Build. Mater. 2020, 234, 117353. [Google Scholar] [CrossRef]
- Mo, L.; Lv, L.; Deng, M.; Qian, J. Influence of Fly Ash and Metakaolin on the Microstructure and Compressive Strength of Magnesium Potassium Phosphate Cement Paste. Cem. Concr. Res. 2018, 111, 116–129. [Google Scholar] [CrossRef]
- Alqarni, A.S. A Comprehensive Review on Properties of Sustainable Concrete Using Volcanic Pumice Powder Ash as a Supplementary Cementitious Material. Constr. Build. Mater. 2022, 323, 126533. [Google Scholar] [CrossRef]
- Hossain, K.M.A. Blended Cement Using Volcanic Ash and Pumice. Cem. Concr. Res. 2003, 33, 1601–1605. [Google Scholar] [CrossRef]
- Anwar Hossain, K.M. Properties of Volcanic Pumice Based Cement and Lightweight Concrete. Cem. Concr. Res. 2004, 34, 283–291. [Google Scholar] [CrossRef]
- Pınarcı, İ.; Kocak, Y. Hydration Mechanisms and Mechanical Properties of Pumice Substituted Cementitious Binder. Constr. Build. Mater. 2022, 335, 127528. [Google Scholar] [CrossRef]
- Nuclear Decommissioning Authority. Geological Disposal. Guidance on the Application of the Waste Package Specifications for Unshielded Waste Packages; Radioactive Waste Management Limited: Didcot, UK, 2014; p. 84.
- Nirex. WPS/903: Guidance on the Immobilisation of Radionuclides in Wasteforms; United Kingdom Nirex Limited: Harwell, UK, 2007; p. 39. [Google Scholar]
- EN 196-1; Methods of Testing Cement. Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible Mini-Slump Test Procedure for Measuring the Yield Stress of Cementitious Pastes. Mater. Struct. 2017, 50, 235. [Google Scholar] [CrossRef]
- ASTM C191-08; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2008.
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore Suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.C.; Garcia, J.; Walker, C.; Naito, M.; Pettersson, S.; Puigdomenech, I.; Cuñado, M.A.; Vuorio, M.; Weber, H.; Ueda, H.; et al. Development of an Accurate PH Measurement Methodology for the Pore Fluids of Low PH Cementitious Materials; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2012. [Google Scholar]
- Alonso, M.C.; Calvo, J.L.G.; Pettersson, S.; Puigdomenech, I.; Cuñado, M.A.; Vuorio, M.; Weber, H.; Ueda, H.; Naito, M.; Walker, C.; et al. Round Robin Test for Defining an Accurate Protocol to Measure the Pore Fluid PH of Low-PH Cementitious Materials. In Cement-Based Materials for Nuclear Waste Storage; Bart, F., Cau-di-Coumes, C., Frizon, F., Lorente, S., Eds.; Springer: New York, NY, USA, 2013; pp. 251–259. ISBN 978-1-4614-3445-0. [Google Scholar]
- Soudée, E.; Péra, J. Influence of Magnesia Surface on the Setting Time of Magnesia–Phosphate Cement. Cem. Concr. Res. 2002, 32, 153–157. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Z. Effect of Aggregates and Water Contents on the Properties of Magnesium Phospho-Silicate Cement. Cem. Concr. Compos. 2005, 27, 11–18. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Kaufmann, J.; Lothenbach, B. Influence of Magnesium-to-Phosphate Ratio and Water-to-Cement Ratio on Hydration and Properties of Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2019, 123, 105781. [Google Scholar] [CrossRef]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterisation of Magnesium Potassium Phosphate Cements Blended with Fly Ash and Ground Granulated Blast Furnace Slag. Cem. Concr. Res. 2015, 74, 78–87. [Google Scholar] [CrossRef] [Green Version]
- De Campos, M.; Davy, C.A.; Djelal, N.; Rivenet, M.; Garcia, J. Development of a Stoichiometric Magnesium Potassium Phosphate Cement (MKPC) for the Immobilization of Powdered Minerals. Cem. Concr. Res. 2021, 142, 106346. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B. Factors That Affect the Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2013, 47, 977–983. [Google Scholar] [CrossRef]
- Deltombe, E.; Pourbaix, M. The Electrochemical Behavior of Aluminum—Potential PH Diagram of the System AI-H2O at 25 C. Corrosion 1958, 14, 16–20. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Setting and Strength Development of Magnesium Phosphate Cement Paste. Adv. Cem. Res. 2009, 21, 175–180. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction Mechanism of Magnesium Potassium Phosphate Cement with High Magnesium-to-Phosphate Ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]




| wt.% | Fly Ash | Wollastonite | Pumice | Metakaolin A | Metakaolin B |
|---|---|---|---|---|---|
| SiO2 | 52.9 | 41.21 | 55.4 | 57.31 | 52.94 |
| Al2O3 | 21.85 | 4.98 | 17.65 | 37.01 | 43.18 |
| CaO | 3.79 | 43.31 | 1.23 | 0.11 | 0.02 |
| Fe2O3 | 10.55 | 3.68 | 3.26 | 1.62 | 0.54 |
| MgO | 1.55 | 2.45 | - | 0.32 | - |
| K2O | 1.77 | 1.64 | 9.7 | 0.73 | 0.19 |
| Na2O | 1.24 | 1.26 | 10.24 | - | 0.31 |
| TiO2 | 0.97 | 0.54 | 0.32 | 2.37 | 2.47 |
| P2O5 | 0.15 | - | 0.04 | - | 0.11 |
| - | - | 0.53 | - | - | |
| - | - | 0.36 | - | - | |
| ∑ others | 0.81 | 0.94 | 1.29 | 0.54 | 0.25 |
| SSA* (m2/g) | 11.68 | 6.54 | 2.69 | 21.28 | 12.61 |
| Filler | MgO/KH2PO4(Molar) | H2O/Cement (Mass) | Filler/Cement (Mass) | Sand/Cement (Mass) | H3BO3/Cement (Mass) |
|---|---|---|---|---|---|
| Mortars | |||||
| 1FA | 1.00 | 0.51 | 1.00 | 1.00 | 0.02 |
| 1WO | 0.51 | 1.00 | |||
| 1PM | 0.75 | 1.00 | |||
| 040PM | 0.51 | 0.40 | |||
| 1MKA | 0.65 | 1.00 | |||
| 040MKA | 0.51 | 0.40 | |||
| 040MKB | 0.51 | 0.40 | |||
| Pastes | |||||
| FA | 1.00 | 0.4 | 1.00 | 0.00 | 0.02 |
| WO | 1.00 | ||||
| PM | 0.40 | ||||
| MKA | 0.40 | ||||
| MKB | 0.40 | ||||
| Formulation | Initial Setting Time (h) | Final Setting Time (h) | Flow Area (cm2) |
|---|---|---|---|
| 1FA | 2.0 | 4.0 | 34.3 ± 4.5 |
| 1WO | 3.2 | 6.2 | 40.9 ± 2.4 |
| 040PM | 2.2 | 2.6 | 39.0 ± 3.2 |
| 040MKA | 2.0 | 2.4 | 41.9 ± 1.6 |
| 040MKB | 1.7 | 2.1 | 36.9 ± 1.1 |
| No Filler | 5.2 | 5.8 | 81.3 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieguez, M.; Ruiz, A.I.; Cuevas, J.; Alonso, M.C.; García-Lodeiro, I.; Fernández, R. Evaluation of Fillers for Magnesium Potassium Phosphate Cement (MKPC) for the Encapsulation of Low and Intermediate Level Metallic Radioactive Wastes. Materials 2023, 16, 679. https://doi.org/10.3390/ma16020679
Dieguez M, Ruiz AI, Cuevas J, Alonso MC, García-Lodeiro I, Fernández R. Evaluation of Fillers for Magnesium Potassium Phosphate Cement (MKPC) for the Encapsulation of Low and Intermediate Level Metallic Radioactive Wastes. Materials. 2023; 16(2):679. https://doi.org/10.3390/ma16020679
Chicago/Turabian StyleDieguez, Mikel, Ana Isabel Ruiz, Jaime Cuevas, María Cruz Alonso, Inés García-Lodeiro, and Raúl Fernández. 2023. "Evaluation of Fillers for Magnesium Potassium Phosphate Cement (MKPC) for the Encapsulation of Low and Intermediate Level Metallic Radioactive Wastes" Materials 16, no. 2: 679. https://doi.org/10.3390/ma16020679






