Abstract
Carbon fiber (CF) composites performance enhancement is a research hotspot at present. In this work, first, a sandwich structure composite, CF@(carbon nanotube/Fe3O4)/epoxy (CF@(CNT/Fe3O4)/EP), is prepared by the free arc dispersion-CFs surface spraying-rolling process method, herein, CFs in the middle layer and (CNT/Fe3O4)/EP as top and substrate layer. Then, CF@(CNT/Fe3O4)/EP (on both sides) and CFs (in the middle) are overlapped by structure design, forming a multilayer CF@(CNT/Fe3O4)/EP-CFs composite with a CFs core sheath. A small amount of CNT/Fe3O4 is consumed, (CNT/Fe3O4)/EP and CFs core sheath realize thermal and electrical anisotropy and directional enhancement, and multilayer sandwich structure makes the electromagnetic interference (EMI) shielding performance better strengthened by multiple absorption–reflection/penetration–reabsorption. From CF-0 to CF-8, CNT/Fe3O4 content only increases by 0.045 wt%, axial thermal conductivity (λ‖) increases from 0.59 W/(m·K) to 1.1 W/(m·K), growth rate is 86%, radial thermal conductivity (λ⊥) only increases by 0.05 W/(m·K), the maximum λ‖/λ⊥ is 2.9, axial electrical conductivity (σ‖) increases from 6.2 S/cm to 7.7 S/cm, growth rate is 24%, radial electrical conductivity (σ⊥) only increases by 0.7 × 10−4 S/cm, the total EMI shielding effectiveness (EMI SET) increases by 196%, from 10.3 dB to 30.5 dB. This provides a new idea for enhancing CFs composite properties.
1. Introduction
With the rapid development of aerospace, transportation, energy, medical and health fields, there is an urgent need for materials with excellent thermal/electrical conductivity properties and electromagnetic interference shielding effectiveness (EMI SE) to adapt to the work in complex environments. Carbon fiber (CF) composites are widely used because of its high strength, high modulus, light weight and easy molding [1,2,3,4,5]. However, the poor magnetic property for CFs limits the further improvement of EMI SE [6]. In addition, the epoxy resin (EP), which is often used as the matrix of CF composites, has advantages of light weight, designability and easy processing [7], but its low intrinsic thermal conductivity (0.1~0.4 W/(M·K)) [8,9] and EMI SE (about 2 dB) [10] limit the performance of CF composites. Therefore, the preparation of CF composites with excellent thermal/electrical conductivity properties and EMI SE has become a research hotspot.
Adding nanofillers is one of the effective methods to prepare high performance composites [11,12,13]. Carbon nanotubes (CNTs) have excellent thermal and electrical properties [14,15] and are often used as an ideal material to enhance thermal/electrical conductivity properties of CF composites [16,17]. Moreover, because of their good dielectric loss characteristic, CNTs are a good choice for EMI shielding materials [18,19,20], and CFs have similar characteristic [21,22,23]. However, if CNTs and CFs are simply combined, although CF-CNT composites have good thermal and electrical properties, lacking magnetic property and impedance mismatch [6] will make CF-CNT composites have weak electromagnetic wave absorption and high electromagnetic reflectance, which result in secondary electromagnetic pollution [24,25]. The matching of electrical and magnetic properties is the key to obtain good shielding effect in the wide frequency range [26]. Therefore, in order to supplement the magnetic property lacking for CFs and CNTs and enhance magnetic loss, Fe3O4, Fe2O3, Fe, Ni, Co and other magnetic particles are usually introduced to cooperate with CNTs [27,28,29,30]. Main methods include in situ growth [31,32] and mechanical blending [33,34]. Among of them, Fe3O4 has a low toxicity and good biocompatibility as a more efficient shielding material. Furthermore, because of the large saturation magnetization of Fe3O4, they can provide a high value of complex permeability. Fe3O4 can exhibit the skin effect, their high resistivity allowing the electromagnetic waves to enter effectively. Therefore, CNT/Fe3O4 as a material with dual magnetic and dielectric properties could be important to achieve excellent thermal, electrical and EMI shielding effectiveness. On this basis, Li et al. [35,36] propose a free arc dispersion method, which can rapidly disperse nanomaterials and produce nanomaterials dispersion fog with good dispersion degree in the air. In addition, this method can disperse variety of nanomaterials at the same time, and the dispersed nanomaterials have a good mixing effect. This seems to well meet the need of collaborative use for the Fe3O4 and CNTs.
Another method to prepare high-performance composites is structure design to obtain composites with specific functions [37,38,39,40,41,42,43]. On the one hand, in order to meet the requirements of directional heat dissipation or electrical conductivity for composites, composites are required to have anisotropy [37,38]. On the other hand, the single-layer shielding structure is not easy to achieve high absorption loss, so sandwich structure, multilayer structure and porous structure begin to appear [39,40,41,42]. Based on the difference between the radial and axial thermoelectric properties of CFs and CNTs, if CNTs are extended along the CFs axial direction to form a CNTs network that is attached to the CFs surface, meanwhile, the Fe3O4 is mixed in the CNTs network by the free arc dispersion method, and CF composites with both thermal and electrical anisotropy and the EMI shielding property can be obtained. Furthermore, multilayer CF composites constructed by the above material can not only achieve the directional enhancement of thermal and electrical anisotropy for CF composites but also realize EMI shielding performance enhancement by multiple absorption–reflection/penetration–reabsorption when electromagnetic waves pass through each layer of CF composites.
Here, as step one, CF@(CNT/Fe3O4)/EP with sandwich structure is prepared by the free arc dispersion-CFs surface spraying-rolling process method, CFs in the middle layer, and (CNT/Fe3O4)/EP as top and substrate layer. Step two, CF@(CNT/Fe3O4)/EP (on both sides) and CFs (in the middle) are overlapped by structure design, forming multilayer CF@(CNT/Fe3O4)/EP-CFs composite with CFs core sheath. The structural morphology of CNT/Fe3O4 and CF@(CNT/Fe3O4)/EP are characterized by scanning electron microscopy (SEM), Raman spectroscopy and X-ray diffraction (XRD). The influence of multilayer sandwich structure on thermal and electrical anisotropy and EMI SE of multilayer CF@(CNT/Fe3O4)/EP-CFs composite is studied.
2. Materials and Experiments
2.1. Materials
CNTs (GT-300, length 15–30 μm, diameter 5–15 nm) were provided by Shandong Dazhan Nano Materials Co., Ltd., Binzhou, China. Fe3O4 (diameter 20 nm) was purchased from Nanjing Emperor Nano Materials Co., Ltd., Nanjing, China. CF (T700SC, diameter 7 μm) was supplied from Lianyungang Zhongfu Shenying Carbon Fiber Co., Ltd., Lianyungang, China. Epoxy resin (MF-4101H) and curing agent (ZH-520), Curing temperature T1 = 150 °C, T2 = 180 °C, curing time T1 = 2 h, 2 = 2 h, was obtained from Hubei Zhen Zhengfeng Advanced Materials Co., Ltd., Huanggang, China. Deionized water (DI water) was used as a dispersive working medium.
2.2. Experiments
Step1, free arc dispersion-CFs surface spraying-rolling process method
CNTs, Fe3O4 and DI water were mixed at a mass ratio of 1:3:10 and thoroughly stirred for 10 min, putting in the mold and applying 10 kg pressure to extrude into a cylindrical block (diameter 30 mm and height 10 mm). According to the free arc dispersion method of Li et al. [35,36], the cylindrical block was placed between the high-voltage pulse electrodes for dispersion, the voltage was 12 KV, the frequency was 10 Hz, the positive electrode used titanium grid, the negative electrode used titanium plate and CNT/Fe3O4 dispersion fog was obtained. At the same time, CNT/Fe3O4 dispersion fog passed through the spraying channel and was sprayed on continuously moving CFs surface by negative pressure airflow traction, and CFs movement speed was 0.01 m/s. The sprayed CFs moved into the heating box for heating at 100 °C to obtain CF@(CNT/Fe3O4). Finally, EP was poured on CF@(CNT/Fe3O4), after rolling, CNT/Fe3O4 was laid on the CFs surface to construct CNT/Fe3O4 network and CF@(CNT/Fe3O4)/EP was prepared. The above processes were simultaneous and continuous.
Step2, structure design
Pure CFs was placed in the middle as the core sheath, and CF@(CNT/Fe3O4)/EP was overlapped on the upper and lower sides of pure CFs, putting into the mold, and transferring to the heating box for curing, multilayer CF@(CNT/Fe3O4)/EP-CFs composite was obtained. Curing temperature T1 = 150 °C, T2 = 180 °C, curing time T1 = 2 h, T2 = 2 h. The sample size was 2 mm × 12 mm × 20 mm.
After calculation, the CFs volume fraction was 60% and the volume fraction of EP was 40% in the sample. Considering the sample performance gradient and consistency of composite size, the total number of layers for CF@(CNT/Fe3O4)/EP and pure CFs was fixed to 8. The schematic diagram of CFs overlapping method and the description of treatment for each experimental group were shown in Table 1.

Table 1.
Description of each experimental group treatment.
2.3. Characterizations
Field emission scanning electron microscope SEM (SU-8010, Hitachi, Tokyo, Japan) was applied to observe the surface distribution and morphology of CFs and composites. Raman spectrometer (InVia Reflex, Renishaw, London, UK) was used to analyze the material structure of CNT and Fe3O4, and the laser wavelength was 532 nm. X-ray diffractometer XRD (MiniFlex 600, Rigaku, Tokyo, Japan) was used to characterize the atomic structure of CNT and Fe3O4, and the scanning speed was 10°/min, the range was 20–80°. Thermal constant analyzer (TPS2500S, Hot Disk, Uppsala, Sweden) was used to test the thermal conductivity of multilayer CF@(CNT/Fe3O4)/EP-CFs composite according to the standard of ISO22007-2-2015. Electrical conductivity of multilayer CF@(CNT/Fe3O4)/EP-CFs composite was measured by four probes resistance tester (RTS-8, Guangzhou Four Probes Technology, Guangzhou, China), and micro-current tester (ST2643, Suzhou Jingge, Suzhou, China) was used to test interlaminar resistivity of multilayer CF@(CNT/Fe3O4)/EP-CFs composite. Vibrating sample magnetometer VSM (7404, LakeShore, OH, USA) was employed to test the magnetization hysteresis loops of CFs, CNT/Fe3O4 and CF@(CNT/Fe3O4)/EP at room temperature. Vector network analyzer (ZNB20, Rohde & Schwarz, Munich, Germany) was employed to measure the S11, S22, S12 and S21 parameters of multilayer CF@(CNT/Fe3O4)/EP-CFs composite according to the standard of ASTM D5568-08, frequency was X-band (8.2–12.4 GHz). the total EMI SE (SET), reflection EMI SE (SER) and the absorption EMI SE (SEA) of multilayer CF@(CNT/Fe3O4)/EP-CFs composite were calculated according to the following formula [44]:
3. Results and Discussion
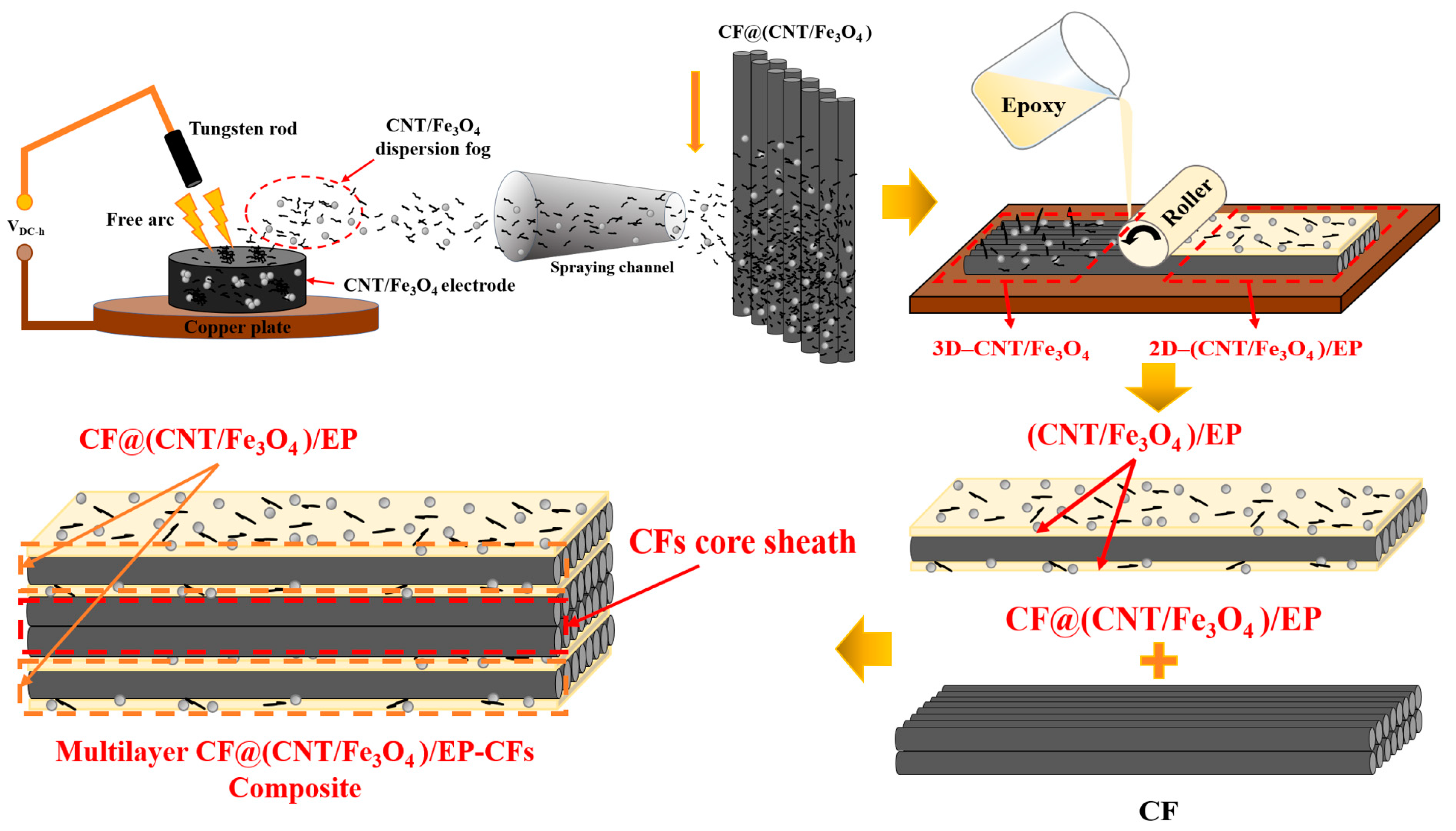
Figure 1 is the schematic diagram of multilayer CF@(CNT/Fe3O4)/EP-CFs composite preparation process. “Free arc dispersion” can disperse CNTs and Fe3O4 at the same time and obtain the CNT/Fe3O4 dispersion fog with well mix and dispersion degree. “CFs surface spraying” can spay CNT/Fe3O4 dispersion fog onto the CFs surface rapidly. “Rolling process” can flatten the 3D-CNT/Fe3O4 and form the 2D-(CNT/Fe3O4)/EP layer, while making CNTs has direction, which is beneficial to enhance the interlayer insulation performance, achieving thermal and electrical anisotropy of CF composites. Based on this, a sandwich structure composite (CF@(CNT/Fe3O4)/EP) with CFs in the middle layer and (CNT/Fe3O4)/EP as top and substrate layer is prepared. Furthermore, through the structure design, the CFs is placed in the middle as core sheath and CF@(CNT/Fe3O4)/EP is placed on both sides to prepare a multilayer sandwich structure CF composite (multilayer CF@(CNT/Fe3O4)/EP-CFs composite).
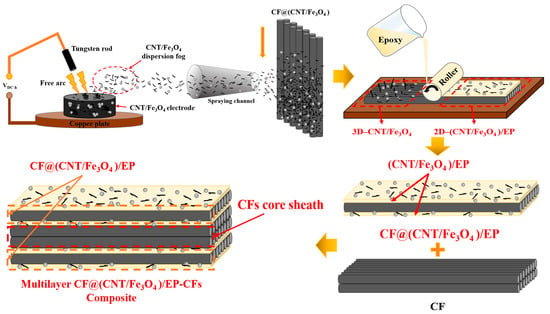
Figure 1.
Schematic diagram of CF@(CNT/Fe3O4)/EP composite preparation process.
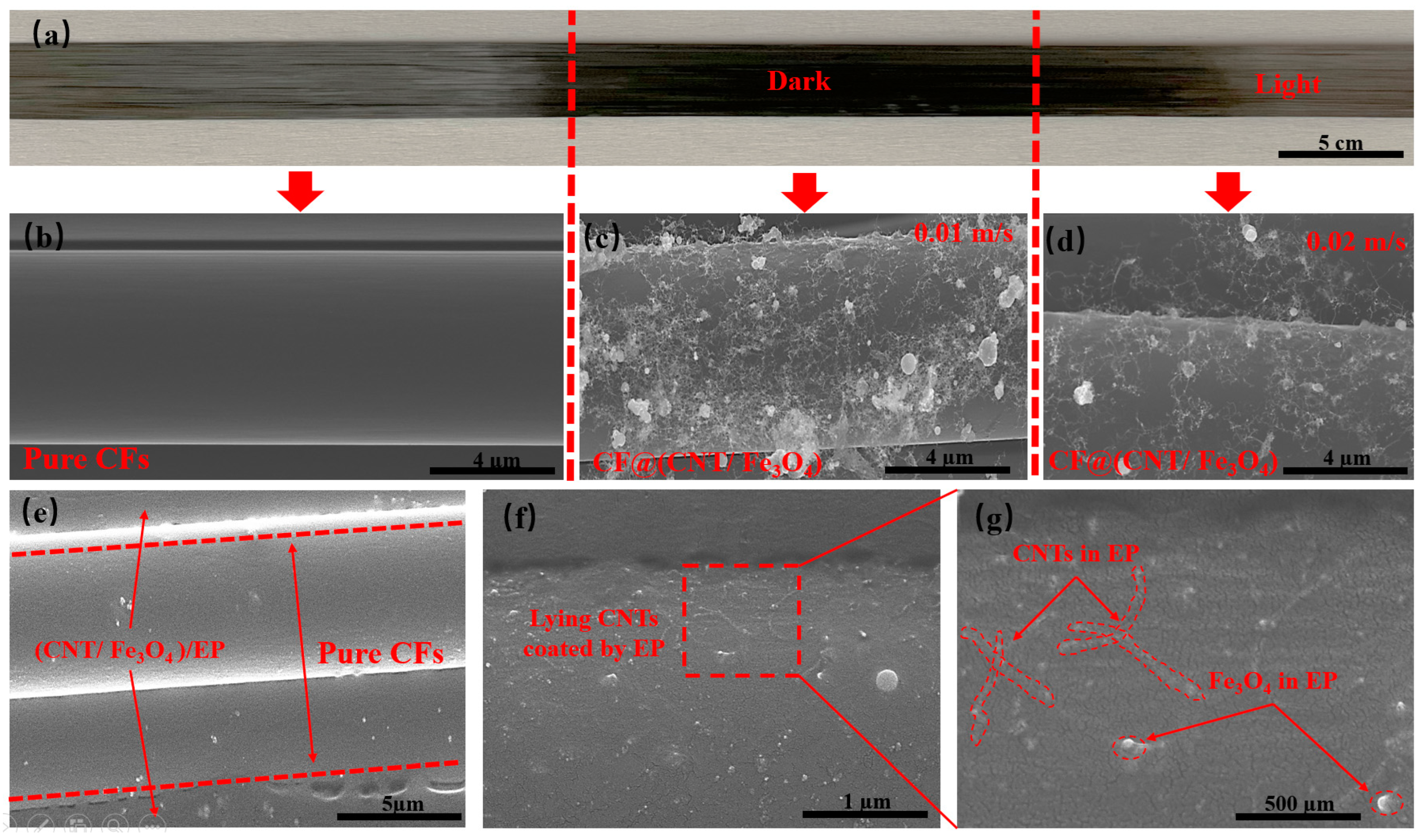
As is shown in Figure 2a, the left side is pure CFs, and the middle and right side are the CFs sprayed with CNT/Fe3O4 dispersion fog. It can be clearly seen that pure CFs has a light color and luster, but the CFs sprayed with CNT/Fe3O4 dispersion fog appears darker color. This is because the adsorption of CNTs and Fe3O4 on the CFs surface and changes the diffuse reflection of CFs surface. Figure 2b shows that pure CFs has a smooth surface without any substance. Figure 2c,d show the attachment of CNT/Fe3O4 when CFs moving speed is 0.01 m/s and 0.02 m/s, respectively. The faster CFs moving speed, the less CNT/Fe3O4 is deposited, and the lighter color is appeared on the macroscopic (Figure 2a, right). At the same time, CNTs and Fe3O4 have high dispersion degree and without obvious agglomeration, CNTs is connected to each other and extend to the radial and axial directions of CFs, presenting a 3D distribution, Fe3O4 is interspersed in the CNTs network, and adsorbed on the CFs surface. On the one hand, CNTs and Fe3O4 are coated by the size agent on the CFs surface, which establishes the physical association between CFs and CNT/Fe3O4 [45]. On the other hand, this may be related to the high specific surface area of CFs [46]. Figure 2e shows the sandwich structure CF@(CNT/Fe3O4)/EP composite obtained after rolling, with CFs in the middle and the thickness of (CNT/Fe3O4)/EP distributed on both sides is about 2 µm, which is uniformly attached to the CFs surface. It can be clearly seen in Figure 2f,g that CNTs and Fe3O4 are coated in EP, in which CNTs is attached to the CFs surface and distribute in the axial direction of CFs only, and Fe3O4 is interspersed in CNTs network with uniform distribution. This morphology is obviously different from Figure 2c,d; this indicates that the effect of rolling makes CNT/Fe3O4 change from 3D to 2D planar structure, which is conducive to maintaining the insulation between CFs layers.
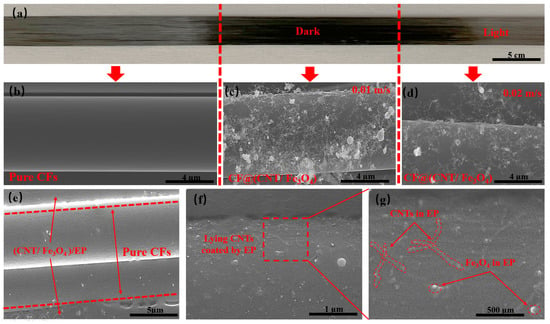
Figure 2.
(a) Pure CFs at the left, CF@(CNT/Fe3O4) with high content CNT/Fe3O4 at middle, and low content CNT/Fe3O4 at right. (b) SEM of pure CFs. (c) CF@(CNT/Fe3O4) with no rolling and high content CNT/Fe3O4, and (d) with no rolling and low content CNT/Fe3O4. (e) CF@(CNT/Fe3O4)/EP with rolled treatment, CFs in the middle and CF@(CNT/Fe3O4) on both sides. (f,g) CF@(CNT/Fe3O4)/EP locally enlarged image; lying CNTs are coated by EP.
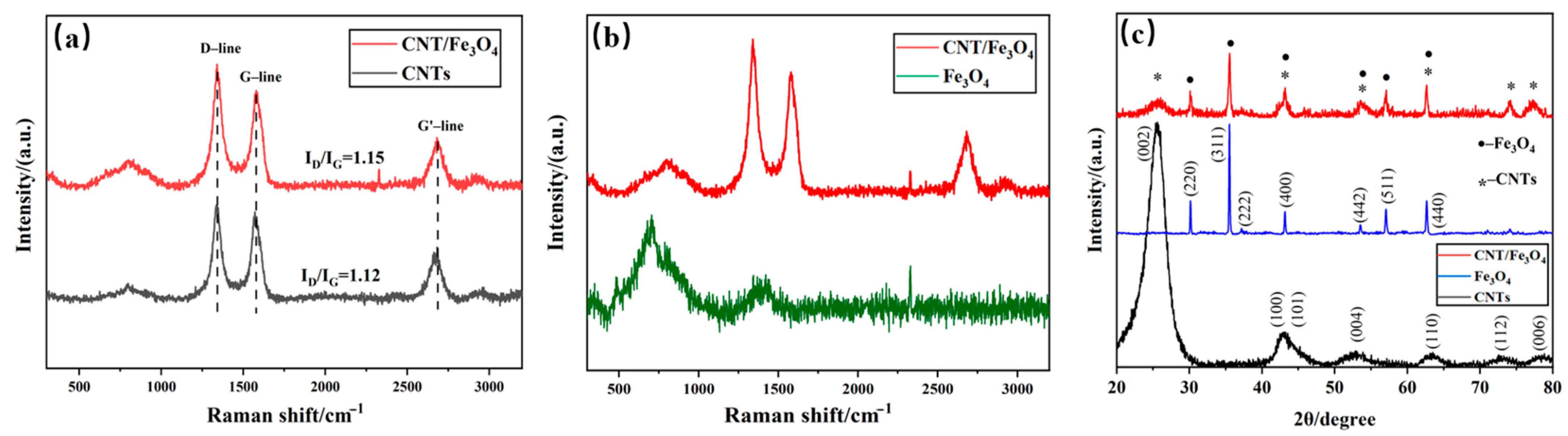
Considering that CNTs and Fe3O4 may change their properties under the action of free arc, Fe3O4 may be converted into Fe2O3 at high temperature [47]. The Raman of CNT/Fe3O4 dispersion fog obtained using the free arc dispersion method is compared with pure CNTs (Figure 3a). CNT/Fe3O4 dispersion fog has characteristic peaks at 1341 cm−1 (D-line) and 1578 cm−1 (G-line), which are the characteristic peaks of carbonaceous compounds [48,49]; this parameter complies with the CNT standard spectrum. In addition, the ID/IG values of CNTs/Fe3O4 and CNTs are 1.15 and 1.12, respectively; this indicates that the graphitization degree of CNTs is not affected by the free arc. Figure 3b shows the comparison of CNT/Fe3O4 dispersion fog Raman image and Fe3O4 standard spectrum, and the result is also consistent [50,51]. It demonstrated that the structure of CNTs and Fe3O4 do not change, and CNT/Fe3O4 dispersion fog has a higher purity, only containing CNTs and Fe3O4; there are no other substances. XRD image (Figure 3c) shows that the diffraction peaks of CNTs and Fe3O4 are in good agreement with CNT/Fe3O4 dispersion fog, respectively; there are corresponding diffraction peaks at particular diffraction angles [52,53]. The above characterizations indicate that CNTs and Fe3O4 maintain good material structure during free arc dispersion and spraying.
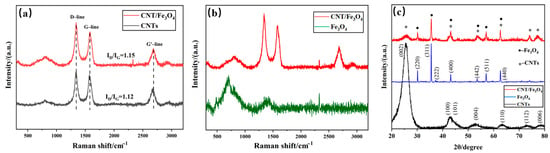
Figure 3.
Raman spectral comparison of CNT/Fe3O4 and CNTs (a), and CNT/Fe3O4 and Fe3O4 (b), XRD of Fe3O4, CNTs and CNT/Fe3O4 (c).
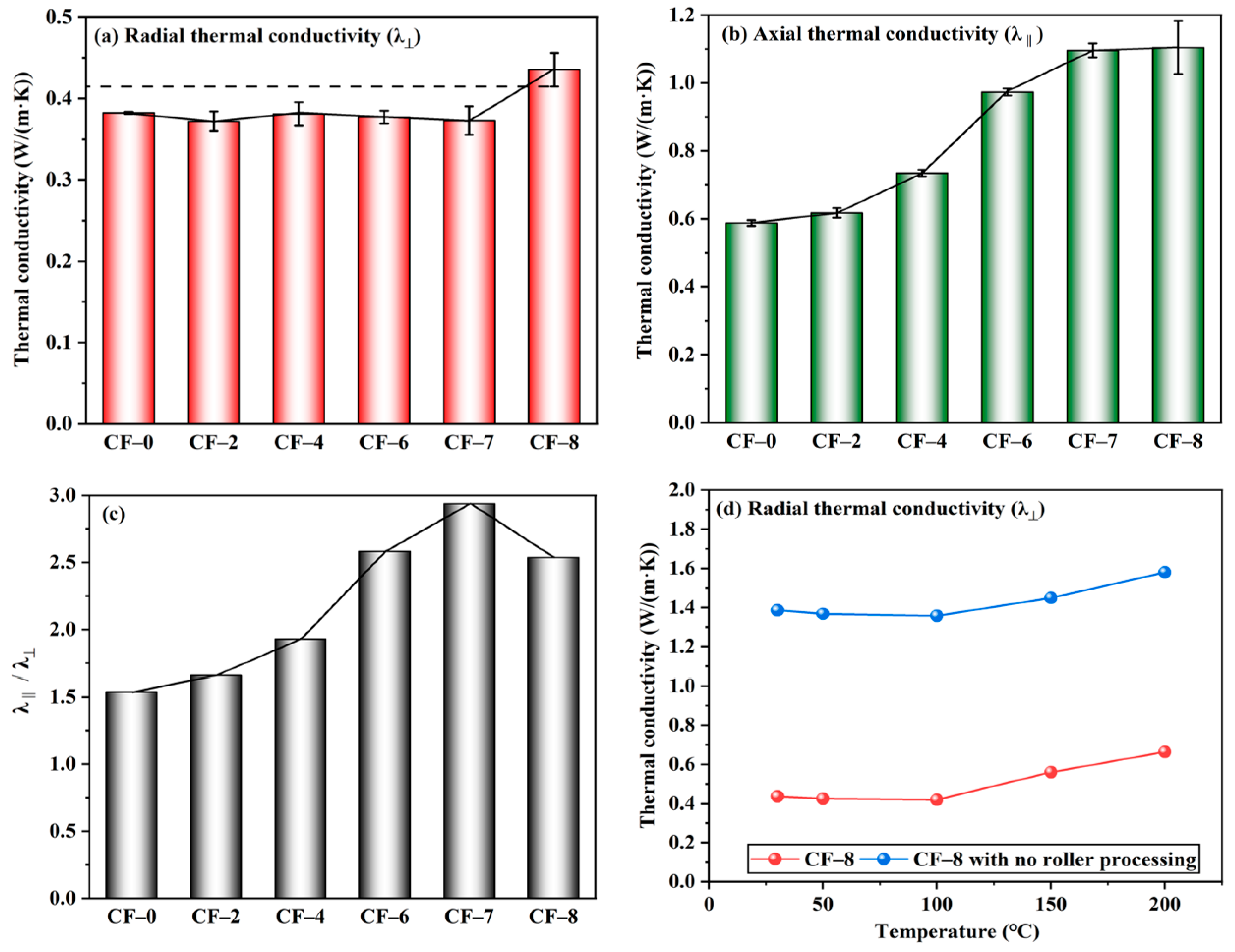
Radial thermal conductivity (λ⊥) of the multilayer CF@(CNT/Fe3O4)/EP-CFs composite is shown in Figure 4a. The λ⊥ of CF-0 is 0.38 W/(m·K), and the λ⊥ of CF-2 to CF-7 remains stable at about 0.38 W/(m·K) with the increase of CNT/Fe3O4. This is because CNT/Fe3O4 changes from 3D to 2D plane due to the rolling treatment. CNTs with excellent thermal conductivity (about 3000 W/(m·K)) [54] are attached to the CF surface and covered by EP [8] with high insulation, forming (CNT/Fe3O4)/EP. It makes cross-plane heat conduction in the multilayer CF@(CNT/Fe3O4)/EP-CFs composite not easy. In addition, due to the presence of contact thermal resistance between CFs [55], the CFs core sheath in CFs 2 to CFs 7 forms radial thermal insulation layer. Both (CNT/Fe3O4)/EP and CFs core sheath form the multilayer thermal insulation system. λ⊥ of CF-8 increases slightly. From CF-7 to CF-8, λ⊥ increases from 0.38 W/(m·K) to 0.44 W/(m·K). The main reason is that CF-8 does not contain a CFs core sheath; therefore, the radial thermal insulation layer is lost, but because of the (CNT/Fe3O4)/EP, the increase of λ⊥ is not significant.
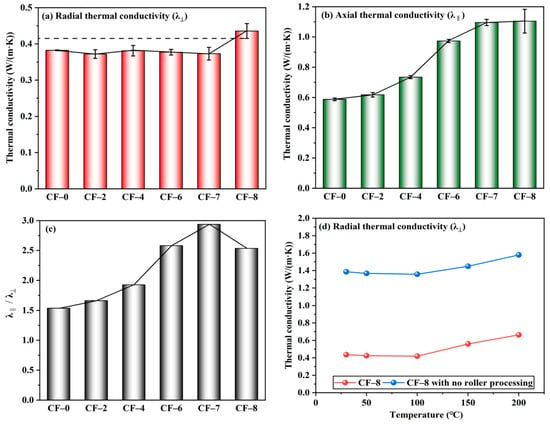
Figure 4.
Radial thermal conductivity λ⊥(a), axial thermal conductivity λ‖ (b), and λ‖/λ⊥(c) of multilayer CF@(CNT/Fe3O4)/EP-CFs composite, λ⊥ comparison of roll treatment (d).
Axial thermal conductivity (λ‖) of the multilayer CF@(CNT/Fe3O4)/EP-CFs composite is shown in Figure 4b. Different from λ⊥, with the increase of CNT/Fe3O4 content, λ‖ increases. When the amount of CNT/Fe3O4 is from 0 to 0.56 mg/cm3 (CF-0 to CF-7), λ‖ increases from 0.59 W/(m·K) to 1.1 W/(m·K), and the growth rate is 86%. The reason is that CFs has excellent thermal conductivity [56], and the axial heat conduction is not affected by EP and CFs core sheath. In addition, CNTs in the (CNT/Fe3O4)/EP forms a good thermal conductivity network [57]; the higher CNTs content, the more abundant CNTs network, and the higher heat conduction efficiency. When the amount of CNT/Fe3O4 is changed from 0.56 to 0.64 mg/cm3 (CF-7 to CF-8), λ‖ hardly changes, but the instability (standard deviation) increases. The main reason is that CF-8 does not have CFs core sheath, the barrier of radial heat conduction is greatly reduced, so the heat conduction has a component in radial. Thus, the increase in axial thermal conductivity is limited and the heat transfer randomness is increased.
The difference between λ‖ and λ⊥ indicates that the CFs core sheath and (CNT/Fe3O4)/EP have influence on the thermal anisotropy of multilayer CF@(CNT/Fe3O4)/EP-CFs composite. Figure 4c directly represents the difference between λ‖ and λ⊥of multilayer CF@(CNT/Fe3O4)/EP-CFs composite, the larger value of λ‖/λ⊥, the more significant thermal anisotropy. From CF-0 to CF-7, λ‖/λ⊥ gradually increases, while CF-7 to CF-8 starts to decrease. Obviously, the λ‖/λ⊥ of CF-7 is higher than CF-8; this is attributed to the CFs core sheath in CF-7. On the one hand, the CFs core sheath stabilizes λ⊥; on the other hand, the CFs core sheath eliminates the radial component of heat conduction to ensure λ‖ promotion. For CF-8, the large λ⊥ and the similar λ‖ make its thermal anisotropy insignificant compared to CF-7. Figure 4d shows that within the 30–200 °C, the λ⊥ of CF-8 with rolling treatment (about 0.4 W/(m·K)) is lower than no rolling treatment (about 1.4 W/(m·K)). This shows from the performance point of view that the (CNT/Fe3O4)/EP formed by rolling can effectively reduce the heat transfer between layers. Combined with the difference between λ‖ and λ⊥, the main reason is that CNTs have high axial thermal conductivity [58], and rolling makes CNTs attach to the CFs surface and extend along the axial direction of CFs. At this time, heat can be transferred along the CFs axial direction; however, due to the coverage of EP and the direction of CNTs, radial heat transfer is difficult.
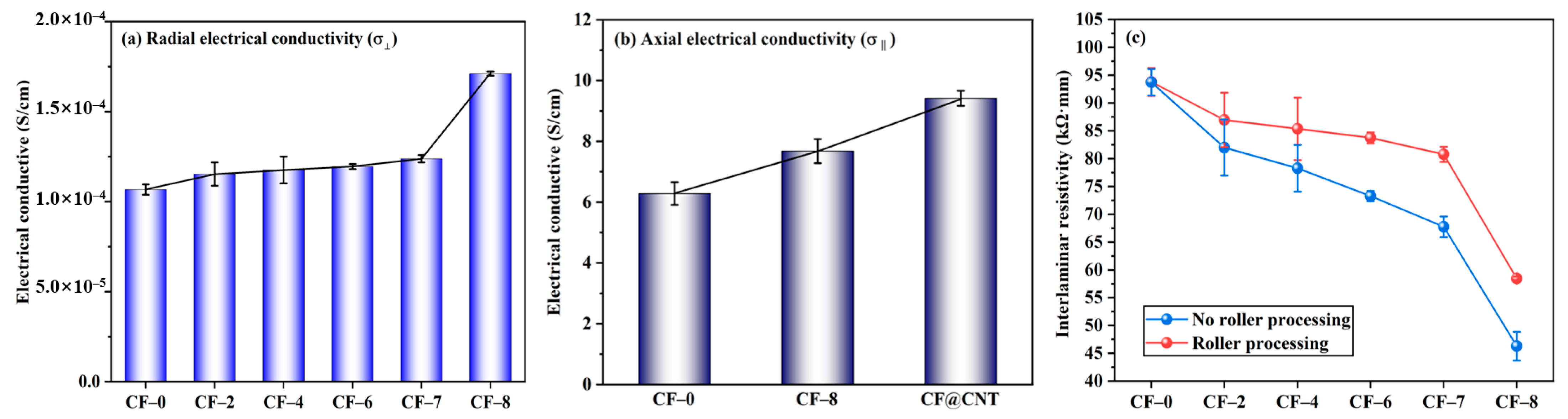
Radial electrical conductivity (σ⊥) of multilayer CF@(CNT/Fe3O4)/EP-CFs composite is shown in Figure 5a. The σ⊥ of CF-0 to CF-7 is generally stable, maintaining at 1.1 × 10−4 (S/cm), while the σ⊥ of CF-8 is increased to 1.7 × 10−4 (S/cm), showing a slight improvement. The main reason is that CNTs has low resistance/high electrical conductivity (105–107 S/m) [59,60], and adding CNTs to the composite can improve electrical conductivity. Similar to the thermal conductivity, due to the insulation effect of (CNT/Fe3O4)/EP and CFs core sheath, the cross-plane electrical conduction in multilayer CF@(CNT/Fe3O4)/EP-CFs composite is difficult to carry out. Because CF-8 does not have CFs core sheath and CNTs enhance the electrical conductivity of EP [61], so the σ⊥ of CF-8 obtains some improvement.
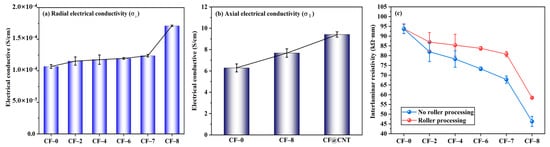
Figure 5.
Radial electrical conductivity σ⊥ of multilayer CF@(CNT/Fe3O4)/EP-CFs composite (a), axial electrical conductivity σ‖comparison of CF, CF@ (CNT/Fe3O4) and CF@CNT composite (b), interlaminar resistivity comparison of roll treatment (c).
As shown in Figure 5b, axial electrical conductivity (σ‖) is higher than σ⊥, the σ‖ of CF-0, CF-8 and CF@CNTs (the content of CNTs is 0.64 mg/cm3) are 6.2 S/m, 7.7 S/m and 9.4 S/m, respectively, showing increase trend. The main reason is that σ‖ is not restricted by (CNT/Fe3O4)/EP, CFs core sheath and interlamination contact resistance, and CFs have high axial electrical conductivity (about 670 S/cm) [62]. The σ‖ of CF-8 is higher than CF-0 because CNTs is contained in the filler, and the CNTs direction is along the CFs axial direction, which helps to improve the axial electrical conductivity of EP and composite. In addition, although the same mass of CNTs (0.64 mg/cm3) is added in CF@CNT composite, the σ‖ of CF@CNT is higher than CF-8. This is attributed to the fact that Fe3O4 has poor electrical conductivity [63], CF-8 contains 0.48 mg/cm3 Fe3O4 and CNTs content is much lower than CF@CNT, which makes low electrical conductivity for CF-8.
Figure 5c shows that the interlaminar resistivity of multilayer CF@(CNT/Fe3O4)/EP-CFs composite with rolling is generally higher than no rolling. The main reason is that CNTs no rolling may penetrate EP, thus connecting adjacent CFs, forming CFs-CNTs-CFs interlayer electric conduction pathway, which reduces the macroscopic resistivity and influences the interlamination insulation performance of the composite.
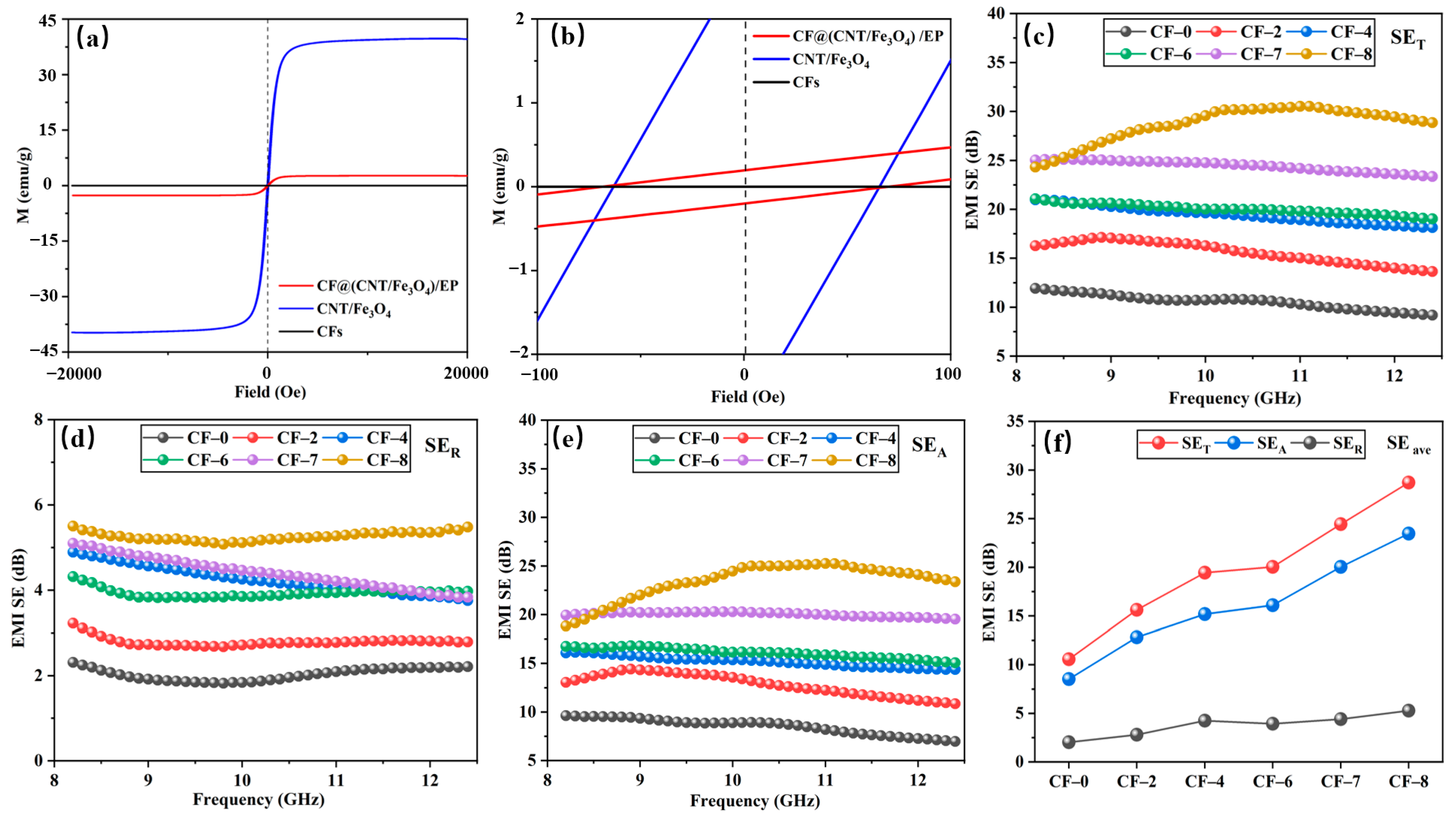
The magnetic property of CFs, CNT/Fe3O4 powder and CF@(CNT/Fe3O4)/EP are tested, and the results are shown in Figure 6a. CFs has no magnetic, and the saturation magnetization (Ms) of CNT/Fe3O4 powder is 40 emu/g, when combined with CFs and EP, the Ms decreases to 2.6 emu/g, which is mainly attributed to CNT/Fe3O4 is coated [64]. It can be seen from local magnification (Figure 6b) that the coercivity (Hc) of CNT/Fe3O4 powder and CF@(CNT/Fe3O4)/EP are 66.6Oe and 64.6Oe, respectively. The similar Hc values indicate that free arc has no effect on the antidemagnetization ability of CNT/Fe3O4.
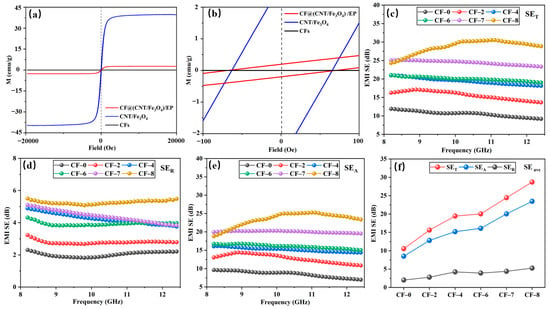
Figure 6.
Magnetic hysteresis loops at room temperature (a,b), EMI SET (c), EMI SER (d), EMI SEA (e) and EMI SEave (f) at 8.2 to 12.4 GHz.
The SET, SER and SEA of CF-0 to CF-8 are shown in Figure 6c–e. The higher CNT/Fe3O4 content, the higher SET, SER and SEA value, and the SEA value is greater than SER. In CF@(CNT/Fe3O4)/EP, electromagnetic wave interacts with Fe3O4 first when passing through (CNT/Fe3O4)/EP, part of the electromagnetic wave is absorbed due to hysteresis loss and natural resonance, and the rest will reach the CFs surface. Here, a part of electromagnetic wave is reflected back to (CNT/Fe3O4)/EP due to impedance mismatch, and the remaining part will pass through CFs to (CNT/Fe3O4)/EP on the other side [55]. CF@(CNT/Fe3O4)/EP with sandwich structure attenuates electromagnetic wave by multiple absorption, reflection and scattering processes and improves its EMI shielding performance [65,66,67]. Due to multilayer CF@(CNT/Fe3O4)/EP-CFs composite having more than one layer of CF@(CNT/Fe3O4)/EP, it provides more opportunities for electromagnetic wave propagation, so the above attenuation process of absorption–reflection/penetration–reabsorption for electromagnetic wave will be repeated many times and strengthens EMI shielding performance. In this process, since absorption is the main attenuation mode of electromagnetic wave, so the value of SEA is greater than SER. In addition, as the electromagnetic wave is absorbed, the heat (converted by the electromagnetic wave) generated by the electrical loss and magnetic loss accumulates inside the composite, which will cause the composite temperature increase.
Figure 6f shows the average values (SEave) of SET, SER and SEA in X-band (8.2–12.4 GHz). When the amount of CNT/Fe3O4 is 0 (CF-0), the SEave of SET, SER and SEA are 10.56 dB, 2.03 dB and 8.53 dB, respectively. When the addition of CNT/Fe3O4 is increased to 0.64 mg/cm3 (CF-8), compared with CF-0, the SEave of SET, SER and SEA are increased by 172%, 159% and 175% respectively. The reason is that CF-8 contains Fe3O4 while CF-0 does not, so the magnetic loss and dielectric loss for Fe3O4 are missing, which greatly reduces the electromagnetic wave absorption effect and leads to relatively poor EMI shielding performance [68,69].
Table 2 summarizes the λ, σ and EMI SE for some related polymer composites; it is observed that multilayer CF@(CNT/Fe3O4)/EP-CFs composite prepared by this work has good performances.

Table 2.
Comparison of the λ, σ and EMI SE for some related composites.
4. Conclusions
In this work, the multilayer CF@(CNT/Fe3O4)/EP-CFs composite is obtained by free arc dispersion-CFs surface spraying-rolling process method and structural design. Under circumstance of the content for CNT/Fe3O4 is very small, the (CNT/Fe3O4)/EP and CFs core sheath achieve thermal and electrical anisotropy and directional enhancement for multilayer CF@(CNT/Fe3O4)/EP-CFs composite, multilayer sandwich structure makes the EMI shielding performance better strengthened by multiple absorption–reflection/penetration–reabsorption of electromagnetic wave. From CF-0 to CF-8, the content of CNT/Fe3O4 only increases by 0.045 wt%, λ‖ increases from 0.59 W/(m·K) to 1.1 W/(m·K), the growth rate is 86%, λ⊥ only increases by 0.05 W/(m·K), and the maximum λ‖/λ⊥ is 2.9, σ‖ increases from 6.2 S/cm to 7.7 S/cm, growth rate is 24%, σ⊥ only increases by 0.7 × 10−4 S/cm and EMI SET increases by 196%, from 10.3 dB to 30.5 dB. This provides a new idea for enhancing CFs composite properties.
Author Contributions
Conceptualization, C.Z. and L.B.; methodology, C.Z.; software, S.S. and L.B.; validation, H.W. and D.Z.; formal analysis, S.S.; investigation, S.S.; data curation, L.B.; writing—original draft preparation, C.Z.; writing—review and editing, C.Z.; visualization, C.Z.; project administration, Y.H. and W.L.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 52176076 and 51676103, Taishan Scholar Project of Shandong Province, grant number NO. ts20190937.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors acknowledge the support from Qingdao University of Science and Technology. This research made use of the Ministry of Science and Technology of polymer processing technology and the related thermal physics international cooperation base.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qiu, L.; Zheng, X.H.; Zhu, J.; Su, G.P.; Tang, D.W. The effect of grain size on the lattice thermal conductivity of an individual polyacrylonitrile-based carbon fiber. Carbon 2013, 51, 265–273. [Google Scholar] [CrossRef]
- Wang, Q.F.; Ma, Y.; Liang, X.; Zhang, D.H.; Miao, M.H. Flexible supercapacitors based on carbon nanotube-MnO2 nanocomposite film electrode. Chem. Eng. J. 2019, 371, 145–153. [Google Scholar] [CrossRef]
- Cui, Y.; Qin, Z.H.; Wu, H.; Li, M.; Hu, Y.J. Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management. Nat. Commun. 2021, 12, 1284. [Google Scholar] [CrossRef]
- Saylor, R.A.; Hersey, M.; West, A.; Buchanan, A.M.; Berger, S.N.; Nijhout, H.F.; Reed, M.C.; Best, J.; Hashemi, P. In vivo Hippocampal Serotonin Dynamics in Male and Female Mice: Determining Effects of Acute Escitalopram Using Fast Scan Cyclic Voltammetry. Front. Neurosci. 2019, 13, 103389. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, H.A.; Hassan, M.M.; Safar, S.S. Parametric study of shear strength of CFRP strengthened end-web panels. Steel Compos. Struct. 2019, 31, 159–172. [Google Scholar]
- Kim, J.T.; Park, C.W.; Kim, B.J. A study on synergetic EMI shielding behaviors of Ni-Co alloy-coated carbon fibers-reinforced composites. Synthetic. Met. 2017, 223, 212–217. [Google Scholar] [CrossRef]
- Xiao, S.H.; Zhou, X.; Deng, H.; Fu, Q. Preparation of elastic conductor with high stretchability and stable conductivity under strain via pre-stretching and spraying approach. Compos. Commun. 2021, 24, 100641. [Google Scholar] [CrossRef]
- Ruan, K.P.; Zhong, X.; Shi, X.T.; Dang, J.J.; Gu, J.W. Liquid crystal epoxy resins with high intrinsic thermal conductivities and their composites: A mini-review. Mater. Today Phys. 2021, 20, 100456. [Google Scholar] [CrossRef]
- Ruan, K.P.; Gu, J.W. Ordered Alignment of Liquid Crystalline Graphene Fluoride for Significantly Enhancing Thermal Conductivities of Liquid Crystalline Polyimide Composite Films. Macromolecules 2022, 55, 4134–4145. [Google Scholar] [CrossRef]
- Song, P.; Liu, B.; Qiu, H.; Shi, X.T.; Cao, D.P.; Gu, J.W. MXenes for polymer matrix electromagnetic interference shielding composites: A review. Compos. Commun. 2021, 24, 100653. [Google Scholar] [CrossRef]
- Qian, G.; Wu, B.; Qin, Z.; Li, X.; Zheng, Z.; Xia, R.; Qian, J. Enhanced Thermal Conductivity via In Situ Constructed CNT Aerogel Structure in Composites. Adv. Mater. Interfaces 2022, 9, 2102098. [Google Scholar] [CrossRef]
- Liu, Z.F.; Chen, Z.H.; Yu, F. Enhanced thermal conductivity of microencapsulated phase change materials based on graphene oxide and carbon nanotube hybrid filler. Sol. Energy Mater. Sol. Cells 2019, 192, 72–80. [Google Scholar] [CrossRef]
- Park, J.M.; Kwon, D.J.; Wang, Z.J.; Roh, J.U.; Lee, W.I.; Park, J.K.; DeVries, K.L. Effects of carbon nanotubes and carbon fiber reinforcements on thermal conductivity and ablation properties of carbon/phenolic composites. Compos. Part. B-Eng. 2014, 67, 22–29. [Google Scholar] [CrossRef]
- Han, Z.D.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Jouni, M.; Djurado, D.; Massardier, V.; Boiteux, G. A representative and comprehensive review of the electrical and thermal properties of polymer composites with carbon nanotube and other nanoparticle fillers. Polym. Int. 2017, 66, 1237–1251. [Google Scholar] [CrossRef]
- Deng, S.L.; Lin, Z.D.; Xu, B.F.; Lin, H.B.; Du, C.M. Effects of Carbon Fillers on Crystallization Properties and Thermal Conductivity of Poly(phenylene sulfide). Polym.-Plast. Technol. 2015, 54, 1017–1024. [Google Scholar] [CrossRef]
- Shin, Y.C.; Novin, E.; Kim, H. Electrical and Thermal Conductivities of Carbon Fiber Composites with High Concentrations of Carbon Nanotubes. Int. J. Precis. Eng. Man. 2015, 16, 465–470. [Google Scholar] [CrossRef]
- Kornev, K.G.; Halverson, D.; Korneva, G.; Gogotsi, Y.; Friedman, G. Magnetostatic interactions between carbon nanotubes filled with magnetic nanoparticles. Appl. Phys. Lett. 2008, 92, 233117. [Google Scholar] [CrossRef]
- Korneva, G.; Ye, H.; Gogotsi, Y.; Halverson, D.; Friedman, G.; Bradley, J.C.; Kornev, K.G. Carbon Nanotubes Loaded with Magnetic Particles. Nano Lett. 2005, 5, 879–884. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.W.; Yuan, X.Y.; Zhang, Y.J.; Liu, X.M.; Cheng, L.F.; Zhang, L.T. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly(dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Chu, Z.Y.; Cheng, H.F.; Xie, W.; Sun, L.K. Effects of diameter and hollow structure on the microwave absorption properties of short carbon fibers. Ceram. Int. 2012, 38, 4867–4873. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.Q.; Xiao, S.T.; Qiang, C.W.; Tian, L.L.; Xu, J.C. Preparation and properties of cobalt oxides coated carbon fibers as microwave-absorbing materials. Appl. Surf. Sci. 2011, 257, 7678–7683. [Google Scholar] [CrossRef]
- Qiang, C.W.; Xu, J.C.; Zhang, Z.Q.; Tian, L.L.; Xiao, S.T.; Liu, Y.; Xu, P. Magnetic properties and microwave absorption properties of carbon fibers coated by Fe3O4 nanoparticles. J. Alloys Compd. 2010, 506, 93–97. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tu, T.X.; Dai, Y.; Tang, P.P.; Zhang, Y.; Deng, Z.M.; Li, L.L.; Zhang, H.B.; Yu, Z.Z. Direct Ink Writing of Highly Conductive MXene Frames for Tunable Electromagnetic Interference Shielding and Electromagnetic Wave-Induced Thermochromism. Nano-Micro. Lett. 2021, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.M.; Tang, P.P.; Wu, X.Y.; Zhang, H.B.; Yu, Z.Z. Superelastic, Ultralight, and Conductive Ti3C2Tx MXene/Acidified Carbon Nanotube Anisotropic Aerogels for Electromagnetic Interference Shielding. ACS. Appl. Mater. Interfaces 2021, 13, 20539–20547. [Google Scholar] [CrossRef]
- Gao, J.S.; Wang, H.H.; Zhou, Y.; Liu, Z.M.; He, Y. Self-template and in-situ synthesis strategy to construct MnO2/Mn3O4@Ni-Co/GC nanocubes for efficient microwave absorption properties. J. Alloy Compd. 2022, 892, 162151. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Li, Y.G.; Wang, H.L.; Zhou, J.G.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Ohlan, A.; Singh, K.; Chandra, A.; Dhawan, S.K. Microwave absorption properties of conducting polymer composite with barium ferrite nanoparticles in 12.4–18 GHz. Appl. Phys. Lett. 2008, 93, 053114. [Google Scholar] [CrossRef]
- Xu, H.F.; Zhang, H.J.; Lv, T.; Wei, H.W.; Song, F. Study on Fe3O4/polyaniline electromagnetic composite hollow spheres prepared against sulfonated polystyrene colloid template. Colloid. Polym. Sci. 2013, 291, 1713–1720. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.P.; Kumari, S.; Srivastava, A.K.; Bathula, S.; Dhawan, S.K.; Duttab, P.K.; Dhar, A. EM shielding effectiveness of Pd-CNT-Cu nanocomposite buckypaper. J. Mater. Chem. A 2015, 3, 13986–13993. [Google Scholar] [CrossRef]
- Li, D.X.; Zhou, X.W.; Guo, X.J.; Yuan, B.; Liu, Y.J.; Ortega, C.M.; Sun, L.; Liu, Z. A novel Fe3O4/buckypaper composite as free-standing anode for lithium-ion battery. J. Alloy Compd. 2016, 657, 109–114. [Google Scholar] [CrossRef]
- Chaudhary, A.; Kumar, R.; Teotia, S.; Dhawan, S.K.; Dhakate, S.R.; Kumari, S. Integration of MCMBs/MWCNTs with Fe3O4 in a flexible and light weight composite paper for promising EMI shielding applications. J. Mater. Chem. C 2017, 5, 322–332. [Google Scholar] [CrossRef]
- Rao, B.V.B.; Chengappa, M.; Kale, S.N. Lightweight, flexible and thin Fe3O4-loaded, functionalized multi walled carbon nanotube buckypapers for enhanced X-band electromagnetic interference shielding. Mater. Res. Express. 2017, 4, 045012. [Google Scholar]
- Li, S.L.; He, Y.; Jing, C.W.; Gong, X.B.; Cui, L.L.; Cheng, Z.Y.; Zhang, C.Q.; Nan, F. A novel preparation and formation mechanism of carbon nanotubes aerogel. Carbon Lett. 2018, 28, 16–23. [Google Scholar]
- Li, S.L.; Zhang, C.Q.; He, Y.; Feng, M.; Ma, C.; Cui, Y. Multi-interpolation mixing effects under the action of micro-scale free arc. J. Mater. Process. Technol. 2019, 271, 645–650. [Google Scholar] [CrossRef]
- Uetani, K.; Takahashi, K.; Watanabe, R.; Tsuneyasu, S.; Satoh, T. Thermal Diffusion Films with In-Plane Anisotropy by Aligning Carbon Fibers in a Cellulose Nanofiber Matrix. ACS. Appl. Mater. Inter. 2022, 14, 33903–33911. [Google Scholar] [CrossRef]
- Nagai, H.; Fujita, K.; Urabe, K.; Iwashita, N. FEM analysis of flexural modulus of carbon fiber monofilament considering anisotropy. Adv. Compos. Mater. 2022, 31, 137–150. [Google Scholar] [CrossRef]
- Danlee, Y.; Bailly, C.; Huynen, I. Thin and flexible multilayer polymer composite structures for effective control of microwave electromagnetic absorption. Compos. Sci. Technol. 2014, 100, 182–188. [Google Scholar] [CrossRef]
- Yang, L.; Fan, H.L.; Liu, J.; Ma, Y.; Zheng, Q. Hybrid lattice-core sandwich composites designed for microwave absorption. Mater. Des. 2013, 50, 863–871. [Google Scholar] [CrossRef]
- Agarwal, P.R.; Kumar, R.; Kumari, S.; Dhakate, S.R. Three-dimensional and highly ordered porous carbon-MnO2 composite foam for excellent electromagnetic interference shielding efficiency. RSC Adv. 2016, 6, 100713–100722. [Google Scholar] [CrossRef]
- Kumar, R.; Dhakate, S.R.; Saini, P.; Mathur, R.B. Improved electromagnetic interference shielding effectiveness of light weight carbon foam by ferrocene accumulation. RSC Adv. 2013, 3, 4145–4151. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ruan, K.P.; Gu, J.W. Flexible Sandwich-Structured Electromagnetic Interference Shielding Nanocomposite Films with Excellent Thermal Conductivities. Small 2021, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, M.H.; Saadeh, W.H.; Sundararaj, U. EMI shielding effectiveness of carbon based nanostructured polymeric materials: A comparative study. Carbon 2013, 60, 146–156. [Google Scholar] [CrossRef]
- Li, S.L.; Zhang, C.Q.; Fu, J.F.; Zhou, Y.S.; Sun, J.Q.; He, Y.; Nan, F.; Yu, Z.Z. Interfacial modification of carbon fiber by carbon nanotube gas-phase dispersion. Compos. Sci. Technol. 2020, 195, 108196. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, L.; Zheng, J.; Liu, L.B.; Alsulami, H.; Kutbi, M.A.; Xu, J.L. Surface modification of carbon fibers with hydrophilic Fe3O4 nanoparticles for nickel-based multifunctional composites. Appl. Surf. Sci. 2020, 509, 145348. [Google Scholar] [CrossRef]
- Panupakorn, P.; Chaichana, E.; Praserthdam, P.; Jongsomjit, B. Polyethylene/Clay Nanocomposites Produced by In Situ Polymerization with Zirconocene/MAO Catalyst. J. Nanomater. 2013, 2013, 154874. [Google Scholar] [CrossRef]
- Mueller, F.; Bresser, D.; Paillard, E.; Winter, M.; Passerini, S. Influence of the carbonaceous conductive network on the electrochemical performance of ZnFe2O4 nanoparticles. J. Power Sources. 2013, 236, 87–94. [Google Scholar] [CrossRef]
- Pan, Y.; Zeng, W.J.; Li, L.; Zhang, Y.Z.; Dong, Y.N.; Ye, K.; Cheng, K.; Cao, D.X.; Wang, G.L.; Lucht, B.L. Surfactant assisted, one-step synthesis of Fe3O4 nanospheres and further modified Fe3O4/C with excellent lithium storage performance. J. Electroanal. Chem. 2018, 810, 248–254. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, P.H.; Zhao, W.J.; Lu, J.; Zhao, J.H. Raman study of ultrathin Fe3O4 films on GaAs(001) substrate: Stoichiometry, epitaxial orientation and strain. J. Raman Spectrosc. 2011, 42, 1388–1391. [Google Scholar] [CrossRef]
- Wang, L.P.; Huang, Y.B.; Lai, Y.H. Surface enhanced Raman scattering activity of dual-functional Fe3O4/Au composites. Appl. Surf. Sci. 2018, 435, 290–296. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mat. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Pan, L.; Tang, J.; Chen, Y.H. Synthesis of Fe3O4, Fe2O3, Ag/Fe3O4 and Ag/Fe2O3 nanoparticles and their electrocatalytic properties. Sci. China Chem. 2013, 56, 362–369. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Da, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Qiu, H.; Ruan, K.P.; Wang, S.S.; Zhang, Y.L.; Gu, J.W. Flexible and insulating silicone rubber composites with sandwich structure for thermal management and electromagnetic interference shielding. Compos. Sci. Technol. 2022, 219, 109253. [Google Scholar] [CrossRef]
- Bily, M.A.; Kwon, Y.W.; Pollak, R.D. Study of Composite Interface Fracture and Crack Growth Monitoring Using Carbon Nanotubes. Appl. Compos. Mater. 2010, 17, 347–362. [Google Scholar] [CrossRef]
- Zheng, Y.D.; Wang, R.; Dong, X.Y.; Wu, L.X.; Zhang, X. High Strength Conductive Polyamide 6 Nanocomposites Reinforced by Prebuilt Three-Dimensional Carbon Nanotube Networks. ACS Appl. Mater. Interfaces 2018, 10, 28103–28111. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; Mceuen, P.L. Thermal Transport Measurements of Individual Multiwalled NanoTubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, S.; Salman, S.M. Effectiveness of Polystyrene/Carbon Nanotube Composite in Electromagnetic Interference Shielding Materials: A Review. Polym.-Plast. Technol. 2017, 56, 1027–1042. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K. Prediction of electrical conductivity of carbon fiber-carbon nanotube-reinforced polymer hybrid composites. Compos. Part B-Eng. 2019, 167, 728–735. [Google Scholar] [CrossRef]
- Ji, X.Y.; Matsuo, S.; Sottos, N.R.; Cahill, D.G. Anisotropic thermal and electrical conductivities of individual polyacrylonitrile-based carbon fibers. Carbon 2022, 197, 1–9. [Google Scholar] [CrossRef]
- Bagheli, S.; Fadafan, H.K.; Orimi, R.L.; Ghaemi, M. Synthesis and experimental investigation of the electrical conductivity of water based magnetite nanofluids. Powder Technol. 2015, 274, 426–430. [Google Scholar] [CrossRef]
- Qiao, M.T.; Li, J.X.; Wei, D.; He, X.W.; Lei, X.F.; Wei, J.; Zhang, Q.Y. Chain-like Fe3O4@void@mSiO2@MnO2 composites with multiple porous shells toward highly effective microwave absorption application. Micropor. Mesopor. Mat. 2021, 314, 110867. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, X.; Wang, Y.Y.; Gong, Y.; Zheng, K.; Chen, L.; Tian, X.Y.; Zhang, X. Segregated double network enabled effective electromagnetic shielding composites with extraordinary electrical insulation and thermal conductivity. Compos. Part A-Appl. S. 2019, 117, 56–64. [Google Scholar] [CrossRef]
- Feng, C.P.; Wan, S.S.; Wu, W.C.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Chen, J.; Yang, W. Electrically insulating, layer structured SiR/GNPs/BN thermal management materials with enhanced thermal conductivity and breakdown voltage. Compos. Sci. Technol. 2018, 167, 456–462. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Li, Y.C.; Liang, C.B.; Zhang, J.L. Novel Ti3C2Tx MXene/epoxy intumescent fire-retardant coatings for ancient wooden architectures. J. Appl. Polym. Sci. 2021, 138, 50649. [Google Scholar] [CrossRef]
- Liu, Z.S.; Zhang, Y.; Zhang, H.B.; Dai, Y.; Liu, J.; Li, X.F.; Yu, Z.Z. Electrically conductive aluminum ion-reinforced MXene films for efficient electromagnetic interference shielding. J. Mater. Chem. C 2020, 8, 1673–1678. [Google Scholar] [CrossRef]
- Luo, J.Q.; Zhao, S.; Zhang, H.B.; Deng, Z.M.; Li, L.L.; Yu, Z.Z. Flexible, stretchable and electrically conductive MXene/natural rubber nanocomposite films for efficient electromagnetic interference shielding. Compos. Sci. Technol. 2019, 182, 107754. [Google Scholar] [CrossRef]
- Liu, Z.F.; Ge, H.Y.; Wu, J.M.; Chen, J. Enhanced electromagnetic interference shielding of carbon fiber/cement composites by adding ferroferric oxide nanoparticles. Constr. Build. Mater. 2017, 151, 575–581. [Google Scholar] [CrossRef]
- Gholampour, M.; Movassagh-Alanagh, F.; Salimkhani, H. Fabrication of nano-Fe3O4 3D structure on carbon fibers as a microwave absorber and EMI shielding composite by modified EPD method. Solid State Sci. 2017, 64, 51–61. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Bordbar-Khiabani, A.; Ahangari-Asl, A. Three-phase PANI@nano-Fe3O4@CFs heterostructure: Fabrication, characterization and investigation of microwave absorption and EMI shielding of PANI@nano-Fe3O4@CFs/epoxy hybrid composite. Compos. Sci. Technol. 2017, 150, 65–78. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, J.J.; Xia, L.C.; Wang, J.F.; Li, C.H.; Xu, F.; Zhang, X.L.; Wu, H.; Guo, S.Y. Achieving high-efficiency and robust 3D thermally conductive while electrically insulating hybrid filler network with high orientation and ordered distribution. Chem. Eng. J. 2018, 334, 247–256. [Google Scholar] [CrossRef]
- Wan, X.R.; Lu, H.; Kang, J.F.; Li, S.; Yue, Y.L. Preparation of graphene-glass fiber-resin composites and its electromagnetic shielding performance. Compos. Interface 2018, 25, 883–900. [Google Scholar] [CrossRef]
- Yim, Y.J.; Rhee, K.Y.; Park, S.J. Electromagnetic interference shielding effectiveness of nickel-plated MWCNTs/high-density polyethylene composites. Compos. Part B-Eng. 2016, 98, 120–125. [Google Scholar] [CrossRef]
- Feng, A.; Jia, Z.; Yu, Q.; Zhang, H.; Wu, G. Preparation and Characterization of Carbon Nanotubes/Carbon Fiber/Phenolic Composites on Mechanical and Thermal Conductivity Properties. Nano 2018, 13, 1850037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).