Effect of Ga2O3 Dopant on High Speed Sintered 5 mol% Y2O3 Stabilized Dental Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Piece

| Sample Name | Raw Material Powder | Chemical Composition of Zpex Smile (wt%) * | Ga Solution ** |
|---|---|---|---|
| Ga0 | Zpex Smile | ZrO2: 88.84 Y2O3: 9.29 Al2O3: 0.049 HfO2: 1.82 | - |
| Ga1.5 | GS1.5 | ||
| Ga2.5 | GS2.5 | ||
| Ga5.0 | GS5.0 |
| Measurement | Pre-Sintered Sample Size | Measured Sample Size |
|---|---|---|
| Density | φ18 mm × 18 mm | φ15 mm × 15 mm |
| XRD | φ18 mm × 1.9 mm | φ15 mm × 1.0 mm |
| SEM | φ18 mm × 1.9 and 18 mm | φ15 mm× 1.0 mm |
| Translucency | φ18 mm × 1.9, 4.0, 8.0, 12 and 18 mm | φ15 mm × 1.0 mm |
| Three-point flexural test | 20 mm (l) × 5.4 mm (w) × 2.3 mm (t) | 16 mm (l) × 4.0 mm (w) × 1.2 mm (t) |
| Sintering behavior | φ10.0 mm × 5.0 mm (tested on pre-sintered specimens) | |
| Ga Solution | Composition (wt%) | Ga Content (wt%) | ||
|---|---|---|---|---|
| Distilled Water * | Ga (NO3)3 · nH2O * | Urea * | ||
| GS1.5 | 65.7 | 8.5 | 25.8 | 1.5 |
| GS2.5 | 42.7 | 14.2 | 43.1 | 2.5 |
| GS5.0 | 37.2 | 28.3 | 34.5 | 5.0 |
2.1.1. Preparation of Pre-Sintered Test Piece
2.1.2. Ga2O3 Doping
2.1.3. Sintering of Test Piece
2.1.4. Processing of Sintered Body
2.2. Ga2O3 Content Evaluation
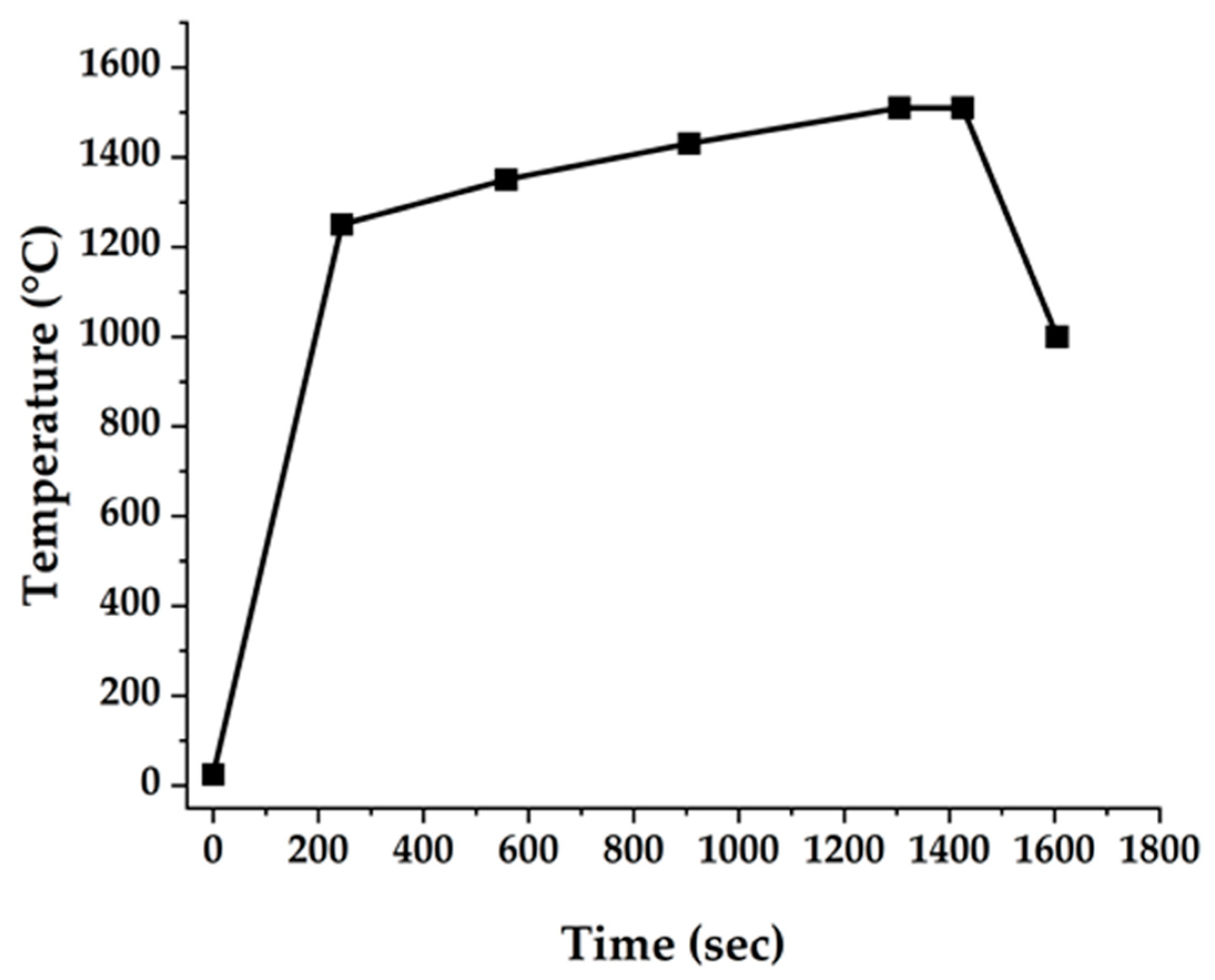
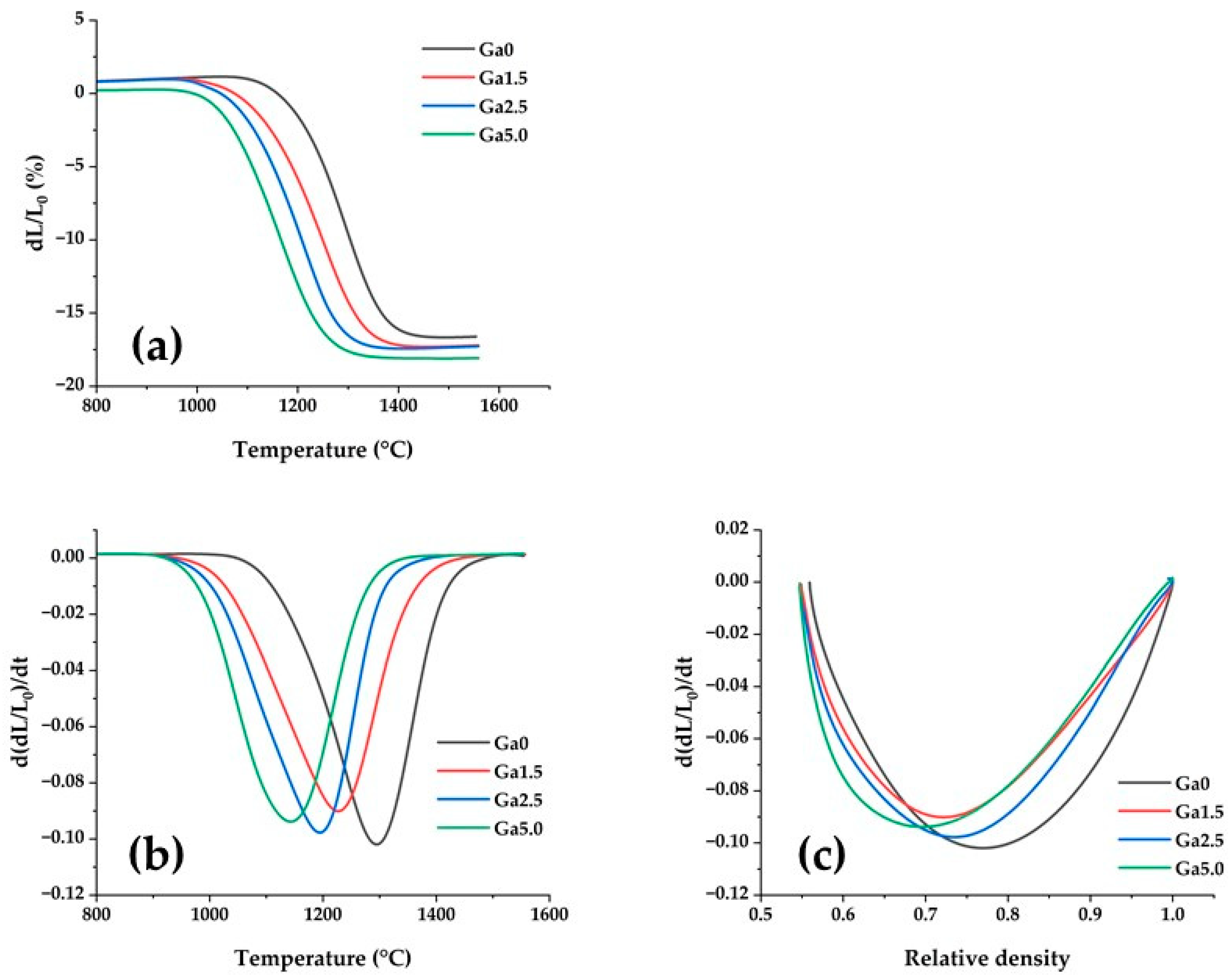
2.3. Sintering Behavior
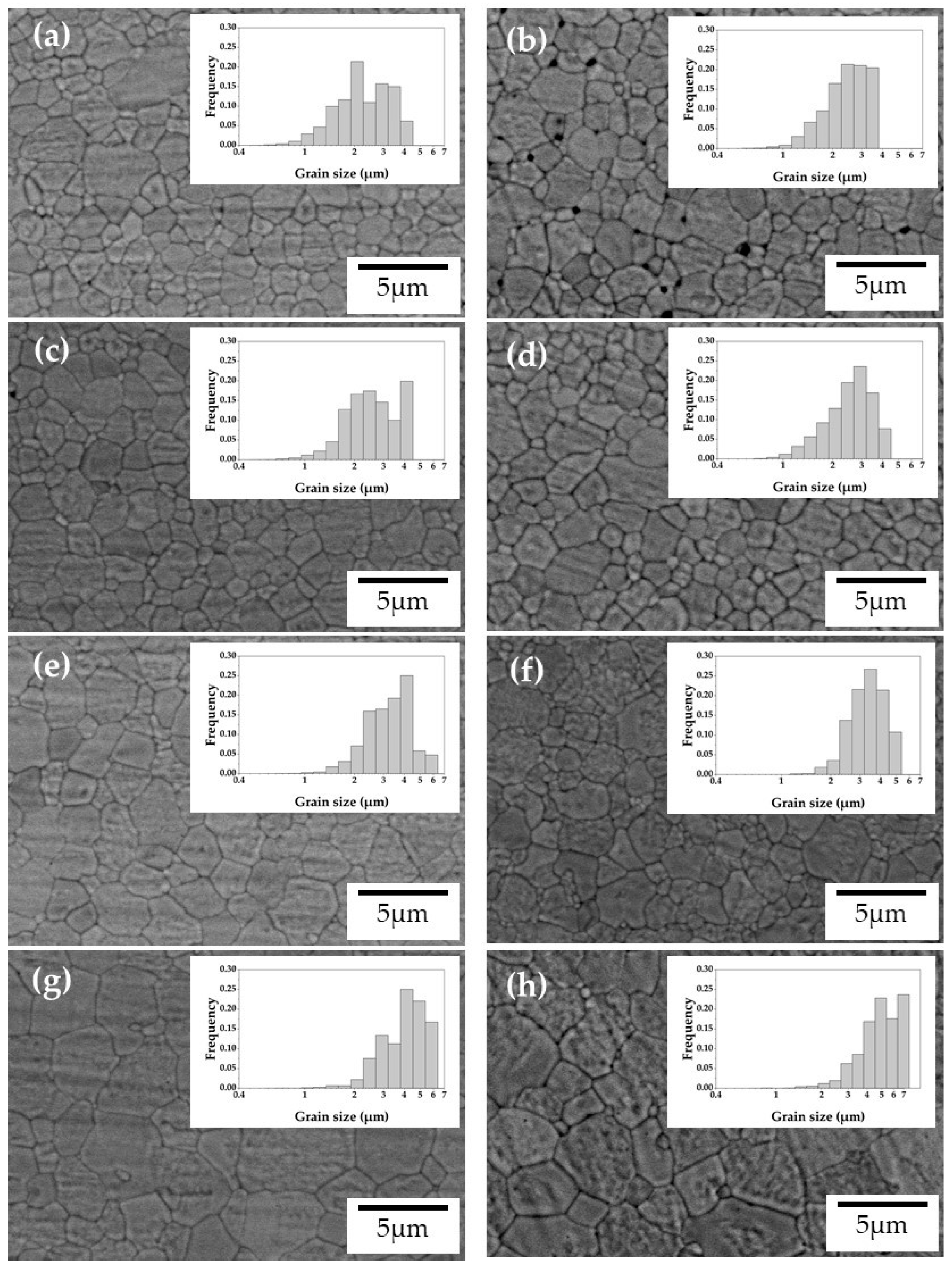
2.4. Microstructural Characterization
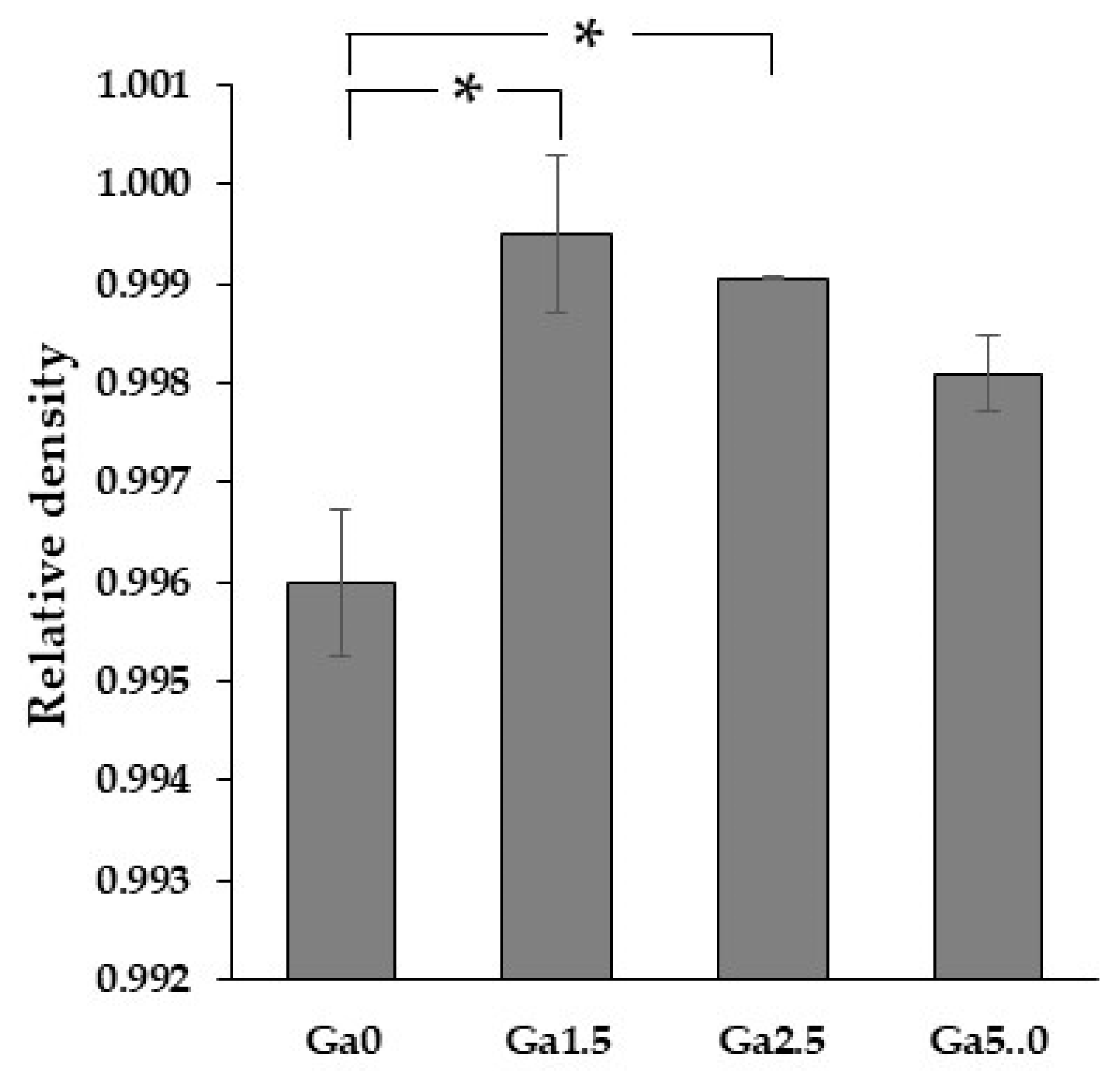
2.5. Density Measurement
- A: content of alumina (wt%)
- G: content of Ga2O3 (wt%)
- ρ: measured density (g/cm3)
- ρ0: theoretical density (g/cm3)
- ρA: theoretical density of alumina
- ρG: theoretical density of Ga2O3
- ρx: theoretical density of zirconia containing 5.33 mol% of yttria (6.028 g/cm3 [21]).
2.6. Crystallographic Characterization
2.7. Translucency
- Subscript w: measured on the white background
- Subscript b: measured on the black background.
2.8. Flexural Strength
- P: breaking load (N)
- l: test span (mm)
- w: width of the specimen (mm)
- b: thickness of the specimen (mm).
2.9. Statistical Analysis
3. Results and Discussion
3.1. Ga2O3 Content
3.2. Sintering Behavior
3.3. Microstructural Characterization
3.4. Density
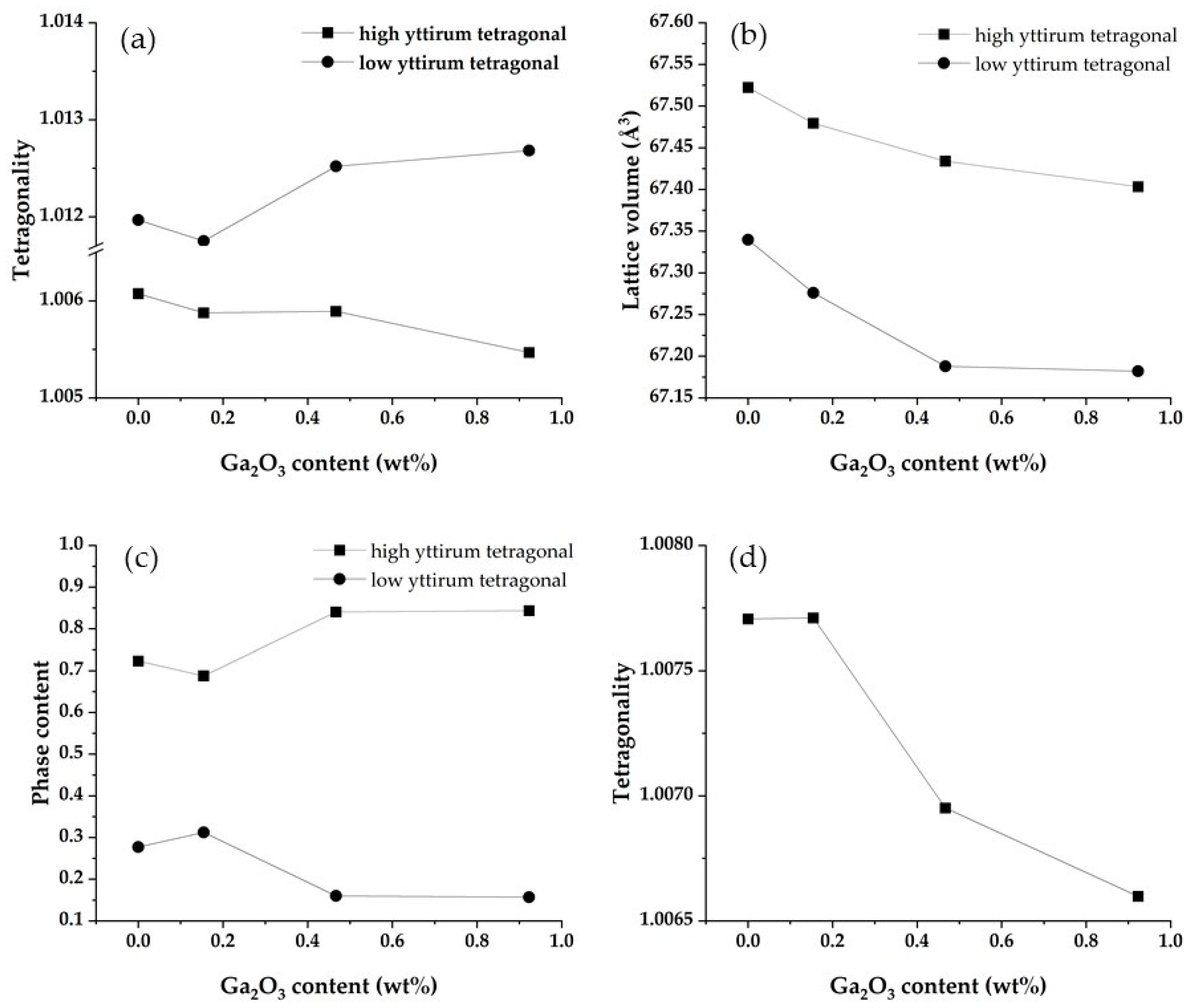
3.5. Crystallographic Characterization
- t: tetragonality
- c: phase content
- Subscript h: high yttrium tetragonal phase
- Subscript l: low yttrium tetragonal phase
- Ga2O3 dissolves in 5Y zirconia and reduces the lattice volume;
- Dissolved Ga2O3 functions as a stabilizer;
- Ga2O3 promotes the phase separation into a high yttrium tetragonal and low yttrium tetragonal phase.
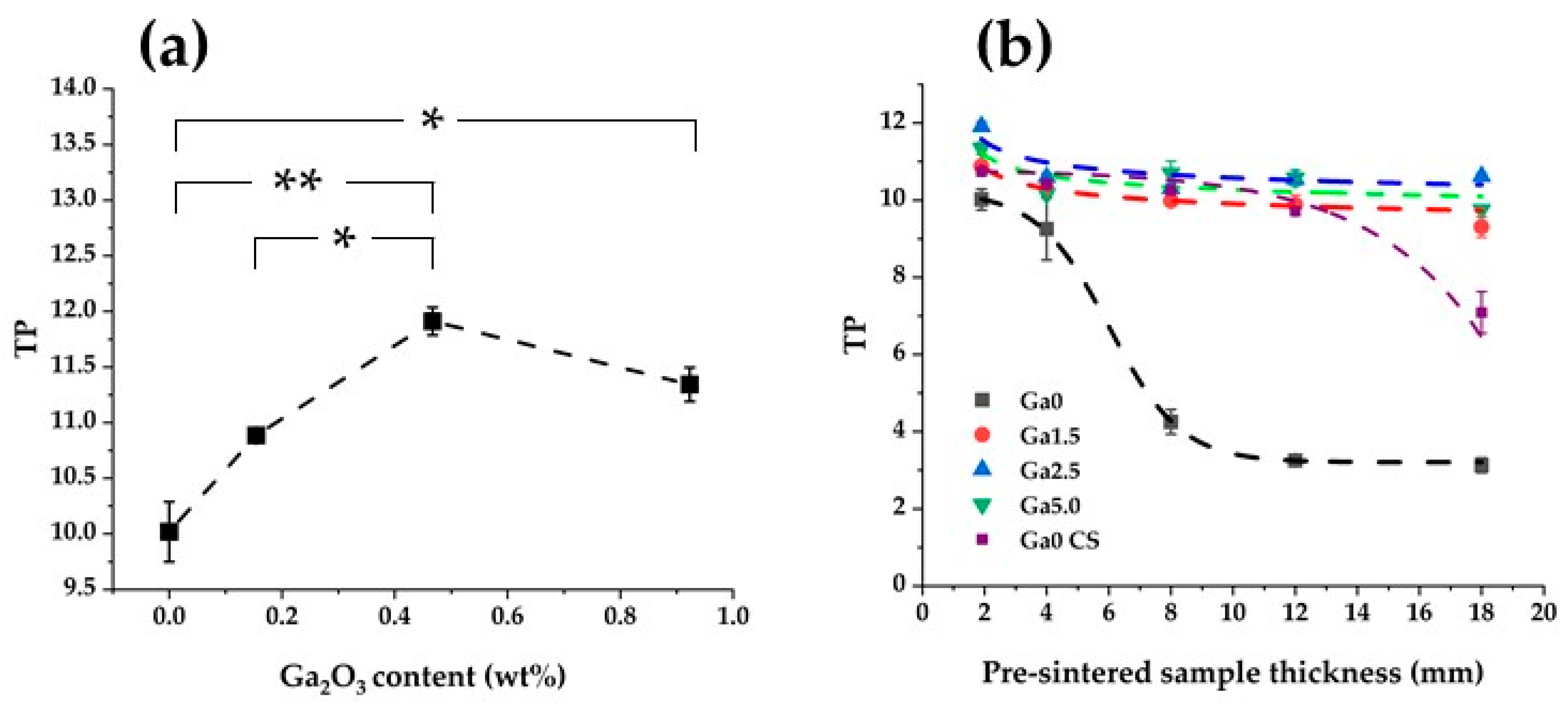
3.6. Translucency
3.7. Flexural Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [Green Version]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Too, T.D.C.; Inokoshi, M.; Nozaki, K.; Shimizubata, M.; Nakai, H.; Liu, H. Influence of sintering conditions on translucency, biaxial flexural strength, microstructure, and low-temperature degradation of highly translucent dental zirconia. Dent. Mater. J. 2021, 40, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Ban, S. Classification and Properties of Dental Zirconia as Implant Fixtures and Superstructures. Materials 2021, 14, 4879. [Google Scholar] [CrossRef]
- Bogna, S.; Christine, K.; Marlis, E.; David, F.; Daniel, E.; Nina, L. Three generations of zirconia: From veneered to monolithic. Part I. Quintessence Int. 2017, 48, 369–380. [Google Scholar]
- Carrabba, M.; Keeling, A.J.; Aziz, A.; Vichi, A.; Fonzar, R.F.; Wood, D.; Ferrari, M. Translucent zirconia in the ceramic scenario for monolithic restorations: A flexural strength and translucency comparison test. J. Dent. 2017, 60, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Omar, G.; Majid, K.; Suhail, B.; Hasan, T.; Wael, E.; Muhammad, Z. Revolution of Current Dental Zirconia: A Comprehensive Review. Molecules 2022, 27, 1699. [Google Scholar] [CrossRef]
- Skramstad, M.; Fasbinder, D.J. Full-Contour Zirconia Fixed Partial Dentures as Chairside Applications: A Case Report. Compendium 2016, 37, 648–654. [Google Scholar]
- Jassim, Z.M.; Majeed, M.A. Comparative Evaluation of the Fracture Strength of Monolithic Crowns Fabricated from Different all-ceramic CAD/CAM Materials (an In Vitro study). Biomed. Pharmacol. J. 2018, 11, 1689–1697. [Google Scholar] [CrossRef]
- Jansen, J.U.; Lümkemann, N.; Letz, I.; Pfefferle, R.; Sener, B.; Stawarczyk, B. Impact of high-speed sintering on translucency, phase content, grain sizes, and flexural strength of 3Y-TZP and 4Y-TZP zirconia materials. J. Prosthet. Dent. 2019, 122, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Cokic, S.M.; Vleugels, J.; Meerbeek, B.V.; Camargo, B.; Willems, E.; Li, M.; Zhang, F. Mechanical properties, aging stability and translucency of speed sintered zirconia for chairside restrations. Dent. Mater. 2020, 36, 959–972. [Google Scholar] [CrossRef]
- Lawson, N.C.; Maharishi, A. Strength and translucency of zirconia after high-speed sintering. J. Esthet. Restor. Dent. 2020, 32, 219–225. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Qiu, D.Y.; Wenjie, S. Rapid-sintered dental zirconia for chair-side one-visit application. Int. J. Appl. Ceram. Technol. 2019, 16, 1830–1835. [Google Scholar] [CrossRef]
- Nonaka, K.; Teramae, M.; Pezzotti, G. Evaluation of the Effect of High-Speed Sintering and Specimen Thickness on the Properties of 5 mol% Yttria-Stabilized Dental Zirconia Sintered Bodies. Materials 2022, 15, 5685. [Google Scholar] [CrossRef]
- Nonaka, K.; Takeuchi, N.; Morita, T.; Pezzotti, G. Evaluation of the effect of high-speed sintering on the mechanical and crystallographic properties of dental zirconia sintered bodies. J. Eur. Ceram. Soc. 2023, 43, 510–520. [Google Scholar] [CrossRef]
- Flegler, A.; Burye, T.; Yang, Q.; Nicholas, J. Cubic yttria stabilized zirconia sintering additive impacts: A comparative study. Ceram. Int. 2014, 40, 16323–16335. [Google Scholar] [CrossRef]
- Matsui, K.; Yamakawa, M.; Uehara, M.; Enomoto, N.; Hojo, J. Mechanism of Alumina-Enhanced Sintering of Fine Zirconia Powder: Influence of Alumina Concentration on the Initial Stage Sintering. J. Am. Ceram. Soc. 2008, 91, 1888–1897. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagayama, H.; Sakuma, T. Small dopant effect on static grain growth and flow stress in superplastic TZP. Mater. Trans. 2003, 44, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Cochis, A.; Azzimonti, B.; Chiesa, R.; Rimondini, L.; Gasik, M. Metallurgical gallium additions to titanium alloys demonstrate a strong time-increasing antibacterial activity without any cellular toxicity. ACS Biomater. 2019, 5, 2815–2820. [Google Scholar] [CrossRef] [Green Version]
- Ingel, R.P.; Lewis, D., III. Lattice and Density for Y2O3-Stabilized ZrO2. J. Am. Ceram. Soc. 1986, 69, 325–332. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 30, 15–20. [Google Scholar] [CrossRef]
- Belli, R.; Hurle, K.; Schürrlein, J.; Petschelt, A.; Werbach, K.; Peterlik, H.; Rabe, T.; Mieller, B.; Lohbauer, U. A Revised Relationship Between Fracture Toughness and Y2O3 Content in Modern Dental Zirconias. ChemRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Yamashita, I.; Tsukuma, K.; Tojo, T.; Kawaji, H.; Atake, T. Synchrotron X-ray Study of the Crystal Structure and Hydrothermal Degradation of Yttria-Stabilized Tetragonal Zirconia Polycrystal. J. Am. Ceram. Soc. 2008, 91, 1634–1639. [Google Scholar] [CrossRef]
- Lamas, D.G.; Walsöe De Reca, N.E. X-ray diffraction study of compositionally homogeneous, nanocrystalline yttria-doped zirconia powders. J. Mater. Sci. 2000, 35, 5563–5567. [Google Scholar] [CrossRef]
- Matsui, K.; Tanaka, K.; Yamakawa, M.; Uehara, M.; Enomoto, N.; Hojo, J. Sintering Kinetics at Isothermal Shrinkage: II, Effect of Y2O3 Concentration on the Initial Sintering Stage of Fine Zirconia Powder. J. Am. Ceram. Soc. 2007, 90, 443–447. [Google Scholar] [CrossRef]
- Matsui, K.; Ohmichi, N.; Ohgai, M.; Enomoto, N.; Hojo, J. Sintering kinetics at constant rates of heating: Effect of Al2O3 on the initial sintering stage of fine zirconia powder. J. Am. Ceram. Soc. 2005, 88, 3346–3352. [Google Scholar] [CrossRef]
- Matsui, K.; Ohomichi, N.; Ohgai, M.; Yoshida, H.; Ikuhara, Y. Grain Boundary Segregation-Induced Phase Transformation in Yttria-Stabilized Tetragonal Zirconia Polycrystal. J. Ceram. Soc. Jpn. 2006, 114, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Yoshida, H.; Ikuhara, Y. Isothermal Sintering Effects on Phase Separation and Grain Growth in Yttria-Stabilized Tetragonal Zirconia Polycrystal. J. Am. Ceram. Soc. 2009, 92, 467–475. [Google Scholar] [CrossRef]
- Chen, I.W.; Xue, L.A. Development of Superplastic Structural Ceramics. J. Am. Ceram. Soc. 1990, 73, 2585–2609. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.L.; Chen, I.W. Size Control of Tetragonal Zirconia Polycrystals Using the Space Charge Concept. J. Am. Ceram. Soc. 1990, 73, 3269–3277. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Ohmichi, N.; Ohgai, M.; Yoshida, H.; Ikuhara, Y. Effect of alumina-doping on grain boundary segregation-induced phase transformation in yttria-stabilized tetragonal zirconia polycrystal. J. Mater. Res. 2006, 21, 2278–2289. [Google Scholar] [CrossRef]
- Bykov, Y.V.; Rybakov, K.I.; Semenov, V.E. High-temperature microwave processing of materials. J. Phys. D 2001, 34, R55–R75. [Google Scholar] [CrossRef]
- Charmond, S.; Carry, C.P.; Bouvard, D. Densification and microstructure evolution of Y-Tetragonal Zirconia Polycrystal powder during direct and hybrid microwave sintering in a single-mode cavity. J. Eur. Ceram. Soc. 2010, 30, 1211–1221. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.; Penner-Hahn, J. Effect of Dopants on Zirconia Stabi-lization-An X-ray Absorption Study: I, Trivalent Dopants. J. Am. Ceram. Soc. 1994, 77, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, I.; Tsukuma, K. Light scattering by residual pores in transparent zirconia ceramics. J. Ceram. Soc. Jpn. 2011, 119, 133–135. [Google Scholar] [CrossRef] [Green Version]
- Carniglia, S.C. Petch Relation in Single-Phase Oxide Ceramics. J. Am. Ceram. Soc. 1965, 48, 580–583. [Google Scholar] [CrossRef]



| Crystal Phase | Chemical Formula | Space Group | ICDD Number | Authors |
|---|---|---|---|---|
| 4Y tetragonal (low yttrium region) | (ZrO2)0.96(Y2O3)0.04 | P42/nmc | 00-060-0503 | Yamashita et al. [24] |
| 5Y tetragonal | (ZrO2)0.95(Y2O3)0.05 | P42/nmc | 01-070-4428 | Lamas and Walsöe De Reca. [25] |
| Sample Name | Ga2O3 Content (wt%) |
|---|---|
| Ga0 | 0.000 |
| Ga1.5 | 0.154 |
| Ga2.5 | 0.467 |
| Ga5.0 | 0.923 |
| Ga0 | Ga1.5 | Ga2.5 | Ga5.0 | ||
|---|---|---|---|---|---|
| tetragonality | high yttrium tetragonal | 1.0061 | 1.0059 | 1.0059 | 1.0055 |
| low yttrium tetragonal | 1.0120 | 1.0117 | 1.0125 | 1.0127 | |
| lattice volume (Å3) | high yttrium tetragonal | 67.52 | 67.48 | 67.43 | 67.40 |
| low yttrium tetragonal | 67.34 | 67.28 | 67.19 | 67.18 | |
| phase content (wt%) | high yttrium tetragonal | 0.72 | 0.69 | 0.84 | 0.84 |
| low yttrium tetragonal | 0.28 | 0.31 | 0.16 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonaka, K.; Teramae, M.; Pezzotti, G. Effect of Ga2O3 Dopant on High Speed Sintered 5 mol% Y2O3 Stabilized Dental Zirconia. Materials 2023, 16, 714. https://doi.org/10.3390/ma16020714
Nonaka K, Teramae M, Pezzotti G. Effect of Ga2O3 Dopant on High Speed Sintered 5 mol% Y2O3 Stabilized Dental Zirconia. Materials. 2023; 16(2):714. https://doi.org/10.3390/ma16020714
Chicago/Turabian StyleNonaka, Kazumichi, Mitsuji Teramae, and Giuseppe Pezzotti. 2023. "Effect of Ga2O3 Dopant on High Speed Sintered 5 mol% Y2O3 Stabilized Dental Zirconia" Materials 16, no. 2: 714. https://doi.org/10.3390/ma16020714





