Structural Diversity, XAS and Magnetism of Copper(II)-Nickel(II) Heterometallic Complexes Based on the [Ni(NCS)6]4− Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of [{Cu(pn)2}2Ni(NCS)6]n·2nH2O (1)
2.2. Synthesis of [{CuII(trien)}2Ni(NCS)6CuI(NCS)]n (2)
2.3. Synthesis of [Cu(tren)(NCS)]4[Ni(NCS)6] (3)
2.4. Methods
2.5. Single Crystal X-ray Diffraction Measurement
3. Results and Discussion
3.1. Infrared Spectra
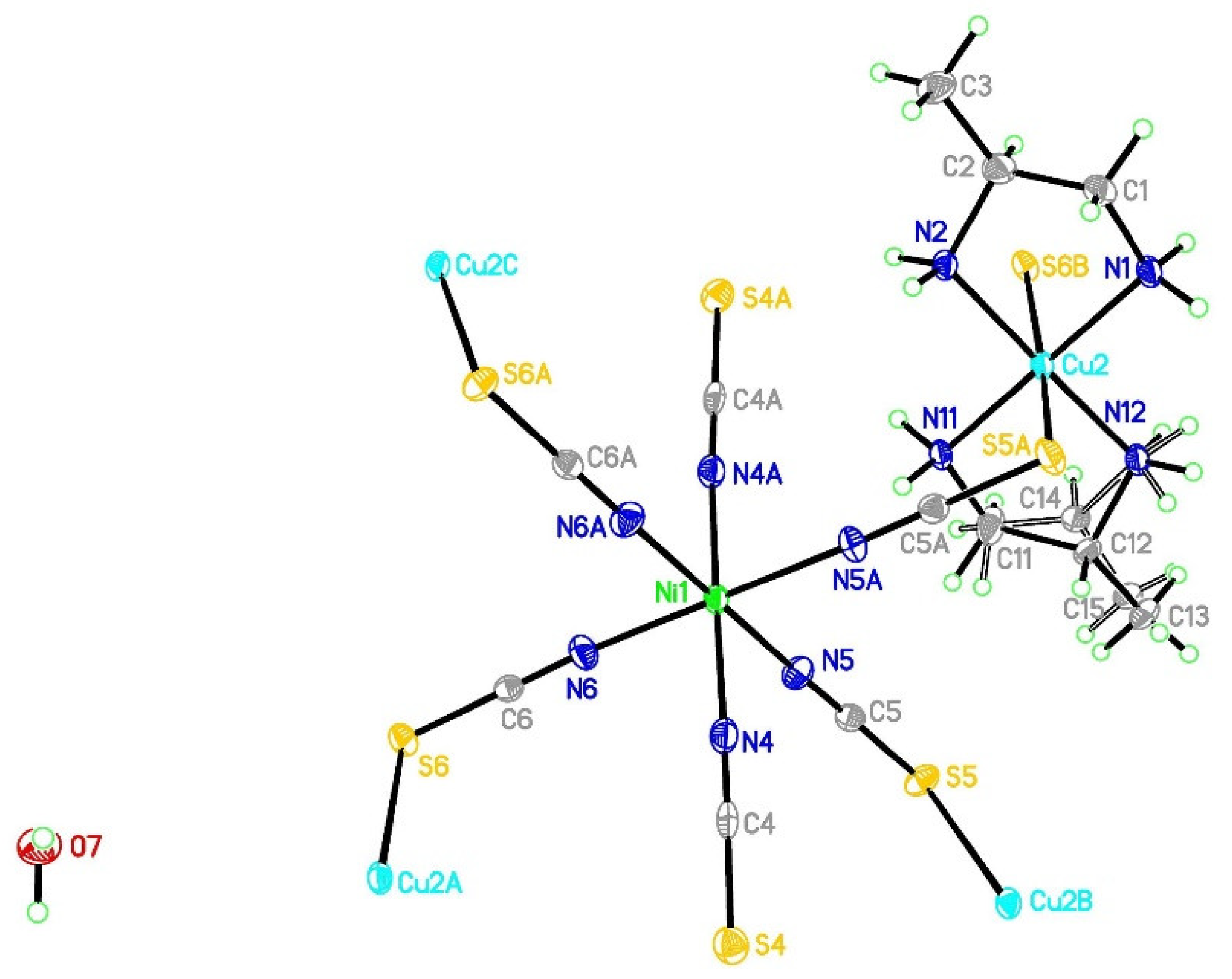
3.2. Structure of [{Cu(pn)2}2Ni(NCS)6]n·2nH2O (1)
3.3. Structure of [{CuII(trien)}2Ni(NCS)6CuI(NCS)]n (2)
3.4. Structure of [Cu(tren)(NCS)]4[Ni(NCS)6] (3)
3.5. X-ray Absorption Spectroscopy
3.6. Magnetism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Laan, G.; Figueroa, A.I. X-ray magnetic circular dichroism—A versatile tool to study magnetism. Coord. Chem. Rev. 2014, 277, 95–129. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Mingabudinova, L.R.; Vinogradov, V.V.; Milichko, V.A.; Hey-Hawkin, E.; Vinogradov, A.V. Metal–organic frameworks as competitive materials for non-linear optics. Chem. Soc. Rev. 2015, 45, 5408–5431. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Zayat, M.; Levy, D. Photochromic organic–inorganic hybrid materials. Chem. Soc. Rev. 2010, 40, 672–687. [Google Scholar] [CrossRef] [Green Version]
- Sodhi, R.K.; Paul, S. Metal Complexes in Medicine: An Overview and Update from Drug Design Perspective. Cancer Ther. Oncol. Int. J. 2019, 14, 555883. [Google Scholar] [CrossRef]
- Saini, P.; Sonika; Singh, G.; Kaur, G.; Singh, J.; Singh, H. Robust and Versatile Cu(I) metal frameworks as potential catalysts for azide-alkyne cycloaddition reactions: Review. Mol. Catal. 2021, 504, 111432. [Google Scholar] [CrossRef]
- Kalra, P.; Kaur, R.; Singh, G.; Singh, H.; Singh, G.; Pawan; Kaur, G.; Singh, J. Metals as “Click” catalysts for alkyne-azide cycloaddition reactions: An overview. J. Organomet. Chem. 2021, 944, 121846. [Google Scholar] [CrossRef]
- Nasibipour, M.; Safaei, E.; Wrzeszcz, G.; Wojtczak, A. Tuning of the redox potential and catalytic activity of a new Cu(II) complex by o-iminobenzosemiquinone as an electron-reservoir ligand†. New J. Chem. 2020, 44, 4426–4439. [Google Scholar] [CrossRef]
- Gispert, J.R. (Ed.) Coordination Chemistry; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Kinzel, N.W.; Demirbas, D.; Bill, E.; Weyhermüller, T.; Werlé, C.; Kaeffer, N.; Leitner, W. Systematic Variation of 3d Metal Centers in a Redox-Innocent Ligand Environment: Structures, Electrochemical Properties, and Carbon Dioxide Activation. Inorg. Chem. 2021, 60, 19062–19078. [Google Scholar] [CrossRef] [PubMed]
- Muzioł, T.M.; Tereba, N.; Podgajny, R.; Kędziera, D.; Wrzeszcz, G. Solvent-assisted structural conversion involving bimetallic complexes based on the tris(oxalato)ferrate(III) unit with the green → blue → red crystal color sequence. Dalton Trans. 2019, 48, 11536–11546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.-L.; Zhang, Q.; Yang, S.-Y.; Wei, X.-Q.; Tian, Z.; Shao, D. Supramolecular porous frameworks of two Ni(II) coordination polymers with varying structures, porosities, and magnetic properties. Polyhedron 2022, 225, 116078. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Zhu, J.-Y.; Shi, L.; You, W.-J.; Wei, X.-Q.; Tian, Z.; Shao, D. Synthesis, structure, magnetism and proton conductivity of a cyanide-bridged NiIICoIII framework. Polyhedron 2022, 228, 116181. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Mirica, K.A. Conductive two-dimensional metal–organic frameworks as multifunctional materials. Chem. Commun. 2018, 54, 7873–7891. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Zhang, K.; Teo, B.K. Molecular and crystal engineering of a new class of inorganic cadmium-thiocyanate polymers with host–guest complexes as organic spacers, controllers, and templates. Coord. Chem. Rev. 1990, 183, 157–195. [Google Scholar] [CrossRef]
- González, R.; Acosta, A.; Chiozzone, R.; Kremer, C.; Armentano, D.; De Munno, G.; Julve, M.; Lloret, F.; Faus, J. New Family of Thiocyanate-Bridged Re(IV)-SCN-M(II) (M = Ni, Co, Fe, and Mn) Heterobimetallic Compounds: Synthesis, Crystal Structure, and Magnetic Properties. Inorg. Chem. 2012, 51, 5737–5747. [Google Scholar] [CrossRef]
- Bieńko, A.; Kłak, J.; Mroziński, J.; Boča, R.; Brüdgam, I.; Hartl, H. Trinuclear thiocyanate-bridged compounds of the type [ML]2[Mn(NCS)4](ClO4)2 (where M = Cu(II), Ni(II); L = N-dl-5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene). Dalton Trans. 2007, 2681–2688. [Google Scholar] [CrossRef]
- Quan, Y.-P.; Yin, P.; Han, N.-N.; Yang, A.-H.; Gao, H.-L.; Cui, J.-Z.; Shi, W.; Cheng, P. Novel hetero-polynuclear metal complexes (CuL)3[Mn(NCS)5]2 and (NiL)3[Mn(NCS)5]2 containing trigonal bipyramidal geometric [Mn(NCS)5]3– as bridging ligand. Inorg. Chem. Comm. 2009, 12, 469–472. [Google Scholar] [CrossRef]
- Bose, D.; Mostafa, G.; Bailey Walsh, R.D.; Zaworotko, M.J.; Ghosh, B.K. Bimetallic complex of the type [Cu(tren)(NCS)]4[Mn(NCS)6]: A hydrogen bonded network structure. Polyhedron 2006, 25, 663–670. [Google Scholar] [CrossRef]
- Kobayashi, M.; Savard, D.; Geisheimer, A.R.; Sakai, K.; Leznoff, D.B. Heterobimetallic Coordination Polymers Based on the [Pt(SCN)4]2− and [Pt(SeCN)4]2− Building Blocks. Inorg. Chem. 2013, 52, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Khandar, A.A.; Klein, A.; Bakhtiari, A.; Mahjoub, A.R.; Pohl, R.W.H. One-dimensional ladder like and two-dimensional polymorphs of heterometallic thiocyanate bridged copper(II) and mercury(II) coordination polymer: Syntheses, structural, vibration, luminescence and EPR studies. Inorg. Chim. Acta 2011, 366, 184–190. [Google Scholar] [CrossRef]
- Pryma, O.V.; Petrusenko, S.R.; Kokozay, V.N.; Skelton, B.W.; Shishkin, O.V.; Teplytska, T.S. A Facile Direct Synthesis of Bimetallic CuIIZnII Complexes with Ethylenediamine Revealing Different Types of Chain Crystal Structures. Eur. J. Inorg. Chem. 2003, 2003, 1426–1432. [Google Scholar] [CrossRef]
- Wrzeszcz, G.; Muzioł, T.M.; Tereba, N. Synthesis, characterization and crystal structure of a 1D thiocyanato bridged [Cu(en)2Zn(NCS)4]∙H2O. Comparison of the three structures with the same [Cu(en)2Zn(NCS)4] unit—different in structural terms. J. Mol. Struct. 2015, 1083, 374–380. [Google Scholar] [CrossRef]
- Tereba, N.; Muzioł, T.M.; Podgajny, R.; Wrzeszcz, G. Influence of the Substituted Ethylenediamine Ligand on the Structure and Properties of [Cu(diamine)2Zn(NCS)4]⋅Solv. Compounds. Crystals 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, M.; Duhayon, C.; Bretosh, K.; Bereau, V.; Sutter, J.-P. Molybdenum(III) Thiocyanate- and Selenocyanate-Based One-Dimensional Heteronuclear Polymers: Coordination Affinity-Controlled Assemblage of Mixed Spin and Mixed Valence Derivatives with Ni(II) and Co(II/III). Inorg. Chem. 2020, 59, 7603–7612. [Google Scholar] [CrossRef] [PubMed]
- Burla, M.C.; Chiari, B.; Cinti, A.; Piovesana, O. Structure and Magnetism of a New 2-D Bimetallic Compound of Mn(II) and Cu(II). Mol. Cryst. Liq. Cryst. 1995, 273, 211–217. [Google Scholar] [CrossRef]
- Shen, L.; Xu, Y.-Z. Structure and magnetic properties of a novel two-dimensional thiocyanato-bridged heterometallic polymer {Cu(en)2[Ni(en)(SCN)3]2}n. J. Chem. Soc. Dalton Trans. 2001, 3413–3414. [Google Scholar] [CrossRef]
- Shi, J.-M.; Xu, W.; Zhao, B.; Cheng, P.; Liao, D.-Z.; Chen, X.-Y. A 2D Thiocyanato-Bridged Copper(II)-Manganese(II) Bimetallic Coordination Polymer with Ferromagnetic Interactions. Eur. J. Inorg. Chem. 2005, 2005, 55–58. [Google Scholar] [CrossRef]
- Mousavi, M.; Béreau, V.; Duhayon, C.; Guionneauc, P.; Sutter, J.-P. First magnets based on thiocyanato-bridges. Chem. Commun. 2012, 48, 10028–10030. [Google Scholar] [CrossRef]
- Xie, K.-P.; Xu, W.-J.; He, C.-T.; Huang, B.; Du, Z.-Y.; Su, Y.-J.; Zhang, W.-X.; Chena, X.-M. Order–disorder phase transition in the first thiocyanate-bridged double perovskite-type coordination polymer: [NH4]2[NiCd(SCN)6]. CrystEngComm 2016, 18, 4495–4498. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Keyzer, E.N.; Bond, A.D.; Astle, M.A.; Greya, C.P. The structures of ordered defects in thiocyanate analogues of Prussian Blue. Chem. Sci. 2020, 11, 4430–4438. [Google Scholar] [CrossRef] [Green Version]
- Maity, D.; Chattopadhyay, S.; Ghosh, A.; Drew, M.G.B.; Mukhopadhyay, G. Syntheses, characterization and X-ray crystal structures of a mono- and a penta-nuclear nickel(II) complex with oximato Schiff base ligands. Inorg. Chim. Acta 2011, 365, 25–31. [Google Scholar] [CrossRef]
- Shurdha, E.; Moore, C.E.; Rheingold, A.L.; Lapidus, S.H.; Stephens, P.W.; Arif, A.M.; Miller, J.S. First Row Transition Metal(II) Thiocyanate Complexes, and Formation of 1-, 2-, and 3-Dimensional Extended Network Structures of M(NCS)2(Solvent)2 (M = Cr, Mn, Co) Composition. Inorg. Chem. 2013, 52, 10583–10594. [Google Scholar] [CrossRef]
- Hofffman, D.W.; Wood, J.S. Tetramethylammonium hexaisothiocyanatonickelate(II) [(CH3)4]4Ni(NCS)6. Cryst. Struct. Commun. 1982, 11, 691–694. [Google Scholar]
- Kruger, P.E.; McKee, V. Tetrakis(triethylammonium) Hexakis(isothiocyanato-N)nickel(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1996, 52, 617–619. [Google Scholar] [CrossRef]
- Vijayakanth, T.; Ram, F.; Praveenkumar, B.; Shanmuganathan, K.; Boomishankar, R. Piezoelectric Energy Harvesting from a Ferroelectric Hybrid Salt [Ph3MeP]4[Ni(NCS)6] Embedded in a Polymer Matrix. Angew. Chem. Int. Ed. 2020, 59, 10368–10373. [Google Scholar] [CrossRef]
- Bi, J.-H.; Bi, W.-T.; Huang, Z.-X.; Hu, N.-L. Synthesis and Crystal Structure of [(phen)3Co]2⋅Ni(SCN)6. Asian J. Chem. 2009, 21, 6622–6624. [Google Scholar]
- Song, M.-P.; Li, L.-K.; Wu, B.-L.; Niu, Y.-Y. Bis(5,8-diazoniadispiro[4.2.4.2]tetradecane) hexakis(thiocyanato-κN)-nickelate(II) dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m2217. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zhang, S.-Y.; Zeng, Y.; Shu, X.; Du, Z.-Y.; He, C.-T.; Zhang, W.-X.; Chen, X.-M. Molecular Dynamics, Phase Transition and Frequency-Tuned Dielectric Switch of an Ionic Co-Crystal. Angew. Chem. Int. Ed. 2018, 57, 8032–8036. [Google Scholar] [CrossRef]
- Kushch, N.D.; Bardin, A.A.; Buravov, L.I.; Glushakova, N.M.; Shilov, G.V.; Dmitriev, A.I.; Morgunov, R.B.; Kulikov, A.V. Synthesis particularities, structure and properties of the radical cationsalts ω-(BEDT-TTF)5M(SCN)6·C2H5OH, M = Mn, Ni. Synth. Met. 2014, 195, 75–82. [Google Scholar] [CrossRef]
- López Lago, E.; Seijas, J.A.; de Pedro, I.; Rodríguez Fernández, J.; Vázquez-Tato, M.P.; González, J.A.; Rilo, E.; Segade, L.; Cabeza, O.; Rodríguez Fernández, C.D.; et al. Structural and physical properties of a new reversible and continuous thermochromic ionic liquid in a wide temperature interval: [BMIM]4[Ni(NCS)6]. New J. Chem. 2018, 42, 15561–15571. [Google Scholar] [CrossRef]
- Brinzari, T.V.; Tian, C.; Halder, G.J.; Musfeldt, J.L.; Whangbo, M.-H.; Schlueter, J.A. Properties and Structural Phase Transition in Penta- And Hexacoordinate Isothiocyanato Ni(II) Compounds. Inorg. Chem. 2009, 48, 7650–7658. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Escuer, A.; Solanas, X.; Font-Bardía, M. Aqueous syntheses and crystal structures of the hexa- and pentacoordinated nickel(II) isothiocyanato derivatives of bulky triamines: (H2Et5dien)2[Ni(NCS)6], [Ni(Me5dien)(NCS)2] and [(Me4Etdien)(NCS)2]. Inorg. Chim. Acta 1996, 248, 59–65. [Google Scholar] [CrossRef]
- Jia, Z.-H.; Liu, J.-Y.; Liu, D.-X.; Zhang, S.-Y.; Du, Z.-Y.; He, C.-T.; Zhang, W.-X.; Chenc, X.-M. Four-step thermosensitive dielectric response arising from motionable low-symmetry ammonium confined in deformable supramolecular cages. J. Mater. Chem. C 2021, 9, 8076–8082. [Google Scholar] [CrossRef]
- Tomkiewicz, A.; Kłak, J.; Mroziński, J. Bimetallic complexes with macrocyclic ligands. Variation of magnetic exchange interactions in some heteronuclear thiocyanato-bridged compounds. Mat. Sci. Pol. 2004, 22, 253–263. [Google Scholar]
- Rad, A.R.S.; Khoshgouei, M.B.; Rezvani, A.R. Water gas shift reaction over Zn–Ni/SiO2 catalyst prepared from [Zn(H2O)6]2[Ni(NCS)6]·H2O/SiO2 precursor. J. Mol. Catal. A Chem. 2011, 344, 11–17. [Google Scholar] [CrossRef]
- Laure, B.; Tran, L.-T.; Luneau, D.; Reber, C. Crystal structures, magnetic properties, and absorption spectra of nickel(II) thiocyanato complexes: A comparison of different coordination geometries. Can. J. Chem. 2003, 81, 1168–1179. [Google Scholar] [CrossRef]
- Cabeza, O.; Varela, L.M.; Rilo, E.; Segade, L.; Domínguez-Pérez, M.; Ausín, D.; de Pedro, I.; Rodríguez Fernández, J.; González, J.; Vazquez-Tato, M.P.; et al. Synthesis, microstructure and volumetry of novel metal thiocyanate ionic liquids with [BMIM] cation. J. Mol. Liq. 2019, 238, 638–651. [Google Scholar] [CrossRef]
- House, J.E., Jr.; Marquardt, L.A. Synthesis and thermal decomposition of piperidinium hexathiocyanatonickelate(II). Thermochim. Acta 1989, 153, 231–236. [Google Scholar] [CrossRef]
- Wang, C.-F.; Zhu, Z.-Y.; Zhou, X.-G.; Weng, L.-H.; Shen, Q.-S.; Yan, Y.-G. Polymorphism and reactivity of [Ni(pyridine)4(NCS)2]: Two new supramolecular isomers and one macro-ionic derivative [(N-Methylpyridinium)n]22n+·[Ni(μ1,3–SCN)2(NCS)2]n2n−. Inorg. Chem. Commun. 2006, 9, 1326–1330. [Google Scholar] [CrossRef]
- Ju, Z.-F.; Yao, Q.-X.; Wu, W.; Zhang, J. Strong electron-accepting methylviologen dication confined in magnetic hosts: Synthesis, structural characterization, charge-transfer and magnetic properties of {(MV)2[Ni(SCN)5]·Cl·2H2O}n and {(MV)[M(N3)2(SCN)2]}n (M = Mn, Co). Dalton Trans. 2008, 355–362. [Google Scholar] [CrossRef]
- Fleck, M. Thiocyanates of nickel and caesium: Cs2NiAg2(SCN)6·2H2O and CsNi(SCN)3. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, i63–i65. [Google Scholar] [CrossRef] [Green Version]
- Chekhlov, A.N. (18-crown-6)potassium tris(thiocyanato)nickelate(II): Synthesis and crystal structure. Russ. J. Coord. Chem. 2008, 34, 434–437. [Google Scholar] [CrossRef]
- Dobrzańska, L.; Wrzeszcz, G.; Grodzicki, A.; Rozpłoch, F. Synthesis and Characterization of Thiocyanato-Bridged Heteropolynuclear Chromium(III)–Copper(II) Complexes. Pol. J. Chem. 2000, 74, 199–206. [Google Scholar]
- Dobrzańska, L.; Wrzeszcz, G.; Grodzicki, A.; Rozpłoch, F. Synthesis and Properties of Thiocyanato-Bridged Chromium(III)-Copper(II) Hydroxo Complexes. Pol. J. Chem. 2000, 74, 1017–1021. [Google Scholar]
- Dobrzańska, L.; Wrzeszcz, G.; Grodzicki, A.; Rozpłoch, F. Synthesis and Properties of New Bimetallic Complexes of General Formula: [Ni(diamine2]3[Cr(NCS)6]2·nH2O. Pol. J. Chem. 2001, 75, 909–914. [Google Scholar]
- Wrzeszcz, G.; Dobrzańska, L.; Grodzicki, A.; Wojtczak, A. Magnetostructural characterisation of the first bimetallic assemblies derived from the anionic building block [Cr(NCS)6]3−, [M(en)3]n[{M(en)2-µ-SCN-Cr(NCS)4-µ-NCS}2n] with M = Ni(II), Zn(II). J. Chem. Soc. Dalton Trans. 2002, 2862–2867. [Google Scholar] [CrossRef]
- Wrzeszcz, G.; Dobrzańska, L.; Grodzicki, A.; Rozpłoch, F. Synthesis and Characterization of New Thiocyanato Bridged Complexes with the General Formula [MLn]3[Cr(NCS)6]2∙mH2O, where M = Cu(II), Ni(II), Co(II); L = Various Substituted Imidazoles. Pol. J. Chem. 2003, 77, 147–156. [Google Scholar]
- Dobrzańska, L.; Wrzeszcz, G.; Grodzicki, A.; Rozpłoch, F. Synthesis, Spectroscopy and Magnetism of New µ-Thiocyanato Polynuclear Copper(II)–Chromium(III) Complexes. Pol. J. Chem. 2001, 75, 1689–1694. [Google Scholar]
- Wrzeszcz, G.; Dobrzańska, L. Magnetic and Thermal Properties of New Thiocyanato Bridged Complexes of the Type [M(diamine)2]3[Cr(NCS)6]2∙nH2O, where M = Cu(II), Ni(II). Pol. J. Chem. 2003, 77, 1245–1254. [Google Scholar]
- Wrzeszcz, G. Synthesis and Characterization of New Thiocyanato Bridged Heterobimetallic Complexes with the General Formula: [Cu(diamine)2]3[Cr(NCS)6]2∙nH2O. Pol. J. Chem. 2003, 77, 845–854. [Google Scholar]
- Wrzeszcz, G.; Grzebielucha, T. Synthesis and Properties of New Bimetallic Complexes of General Formula: [Cu(diamine)2][Cr(NCS)4(NH3)2]2. Pol. J. Chem. 2009, 83, 1575–1582. [Google Scholar]
- Baker, M.L.; Mara, M.W.; Yan, J.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. K- and L-edge X-ray absorption spectroscopy (XAS) and resonant inelastic X-ray scattering (RIXS) determination of differential orbital covalency (DOC) of transition metal sites. Coord. Chem. Rev. 2017, 345, 182–208. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Figgis, N.; Nyholm, R.S. A Convinient Solid for Calibration of the Gouy Magnetic Susceptibility Apparatus. J. Chem. Soc. 1958, 4190–4191. [Google Scholar] [CrossRef]
- CrysAlis RED and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, Oxfordshire, UK, 2000.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, K. DIAMOND, Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: Un update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, pt. B, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Buckley, R.C.; Wardeska, J.G. Linkage isomerism of bridging thiocyanate in binuclear complexes. Inorg. Chem. 1972, 11, 1723–1726. [Google Scholar] [CrossRef]
- Baer, C.; Pike, J. Infrared Spectroscopic Analysis of Linkage Isomerism in Metal−Thiocyanate Complexes. J. Chem. Educ. 2010, 87, 724–726. [Google Scholar] [CrossRef]
- Guo, G.; Xu, Y.; Cao, J.; Hu, C. The {V4Nb6O30} Cluster: A New Type of Vanadoniobate Anion Structure. Chem. Eur. J. 2012, 18, 3493–3497. [Google Scholar] [CrossRef] [PubMed]
- Triščíková, L.; Chomič, J.; Abboud, K.A.; Park, J.-H.; Meisel, M.W.; Černák, J. Trinuclear Cu(pn)2Ag2(CN)4: Preparation, crystal structure and properties (pn=1,2-diaminopropane). Inorg. Chim. Acta 2004, 357, 2763–2768. [Google Scholar] [CrossRef]
- Mistry, S.; Natarajan, S. Synthesis, structures and magnetic studies of new copper-azides. Inorg. Chim. Acta 2018, 483, 26–38. [Google Scholar] [CrossRef]
- Mroziński, J.; Kłak, J.; Kruszyński, R. Crystal structure and magnetic properties of the 1D bimetallic thiocyanate bridged compound: {(CuL1)[Co(NCS)4]}(L1 = N-rac-5,12-Me2-[14]-4,11-dieneN4). Polyhedron 2008, 27, 1401–1407. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the ProgramPackage ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Bieńko, A.; Kłak, J.; Mroziński, J.; Domagała, S.; Korybut-Daszkiewicz, B.; Woźniak, K. Magnetism and crystal structures of CuIIMnII and CuIINiII ordered bimetallic chains. Polyhedron 2007, 26, 5030–5038. [Google Scholar] [CrossRef]
- Ribas, J.; Diaz, C.; Costa, R.; Tercero, J.; Solans, X.; Font-Bardía, M.; Stoeckli-Evans, H. Synthesis and Magnetic Properties of Four New (Cu−Ni)2 Tetranuclear Complexes of General Formula [Cu(oxpn)Ni(μ-NCS)(H2O)(aa)]2(X)2 (oxpn = N,N‘-Bis(3-aminopropyl)oxamide; aa = Bidentate Amine; X = ClO4-or PF6-). Ferro- and Antiferromagnetic Alternation. Inorg. Chem. 1998, 37, 233–239. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Addison, A.W.; Nageswara Rao, T.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Marongiu, G.; Lingafelter, E.C.; Paoletti, P. Crystal structure of thiocyanatotriethylenetetraminecopper(II) thiocyanate. Inorg. Chem. 1969, 8, 2763–2767. [Google Scholar] [CrossRef]
- Marongiu, G.; Cannas, M. Crystal structures of thiocyanate polyamine copper(II) complexes. Part 7. (3,6-Diazaoctane-1,8-diamine)isothiocyanatocopper(II) perchlorate: A disordered structure. J. Chem. Soc. Dalton Trans. 1979, 41–44. [Google Scholar] [CrossRef]
- Sharma, M.; Ganeshpandian, M.; Majumder, M.; Tamilarasan, A.; Sharma, M.; Mukhopadhyay, R.; Islam, N.S.; Palaniandavar, M. Octahedral copper(ii)-diimine complexes of triethylenetetramine: Effect of stereochemical fluxionality and ligand hydrophobicity on CuII/CuI redox, DNA binding and cleavage, cytotoxicity and apoptosis-inducing ability. Dalton Trans. 2020, 49, 8282–8297. [Google Scholar] [CrossRef]
- Tian, C.-B.; Li, Z.-H.; Lin, J.-D.; Wu, S.-T.; Du, S.-W.; Lin, P. Cluster-Based CuII–Azide Polymers: Synthesis, Structure, Magnetic Properties, and Effect of Polyamines on Crystal Structures. Eur. J. Inorg. Chem. 2010, 2010, 427–437. [Google Scholar] [CrossRef]
- Kitajgorodskij, A.I. Molecular Crystals and Molecules; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Woollard-Shore, J.G.; Holland, J.P.; Jones, M.W.; Dilworth, J.R. Nitrite reduction by copper complexes. Dalton Trans. 2010, 39, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Toro, I.; Domínguez-Martín, A.; Choquesillo-Lazarte, D.; García-Rubiño, M.E.; González-Pérez, J.M.; Castiñeiras, A.; Bauzá, A.; Frontera, A.; Niclós-Gutiérrez, J. Copper(II) polyamine chelates as efficient receptors for acyclovir: Syntheses, crystal structures and dft study. Polyhedron 2018, 145, 218–226. [Google Scholar] [CrossRef]
- Herrera, J.M.; Marvaud, V.; Verdaguer, M.; Marrot, J.; Kalisz, M.; Mathonière, C. Reversible Photoinduced Magnetic Properties in the Heptanuclear Complex [MoIV(CN)2(CN-CuL)6]8+: A Photomagnetic High-Spin Molecule. Angew. Chem. Int. Ed. 2004, 43, 5468–5471. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Na, J.-J.; Wang, B.-X.; Xiao, H.-P.; Li, Z. Novel copper-azido magnetic molecular tapes: Syntheses, structures, and magnetic properties. CrystEngComm 2011, 13, 6415–6421. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- de Groot, F.M.F. Differences between L3 and L2 X-ray absorption spectra. Phys. B Condens. Matter 1995, 208–209, 15–18. [Google Scholar] [CrossRef]
- Hocking, R.K.; DeBeer George, S.; Raymond, K.N.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Fe L-Edge X-ray Absorption Spectroscopy Determination of Differential Orbital Covalency of Siderophore Model Compounds: Electronic Structure Contributions to High Stability Constants. J. Am. Chem. Soc. 2010, 132, 4006–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocking, R.K.; Wasinger, E.C.; de Groot, F.M.F.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Fe L-Edge XAS Studies of K4[Fe(CN)6] and K3[Fe(CN)6]: A Direct Probe of Back-Bonding. J. Am. Chem. Soc. 2006, 128, 10442–10451. [Google Scholar] [CrossRef] [Green Version]
- van Elp, J.; Peng, G.; Zhou, Z.H.; Adams, M.W.W.; Baidya, N.; Mascharak, P.K.; Cramer, S.P. Nickel L-Edge X-ray Absorption Spectroscopy of Pyrococcus furiosus Hydrogenase. Inorg. Chem. 1995, 34, 2501–2504. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Boča, R.; Svoboda, I.; Trávníček, Z.; Dlháň, L.; Matelková, K.; Fuess, H. Heterobimetallic assemblies of Ni(II) complexes with a tetradentate amine ligand and diamagnetic cyanidometallates. Inorg. Chim. Acta 2011, 366, 366–372. [Google Scholar] [CrossRef]
- Baran, P.; Boča, M.; Boča, R.; Krutošíková, A.; Miklovič, J.; Pelikán, J.; Titiš, J. Structural characterization, spectral and magnetic properties of isothiocyanate nickel(II) complexes with furopyridine derivatives. Polyhedron 2005, 24, 1510–1516. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-Block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]















| Identification Code | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C18 H44 Cu2 N14 Ni O2 S6 | C19 H36 Cu3 N15 Ni S7 | C34 H72 Cu4 N26 Ni S10 |
| Formula weight | 866.82 | 948.38 | 1478.64 |
| Temperature [K] | 293(2) | 293(2) | 293(2) |
| Wavelength [Å] | 0.71073 | 0.71073 | 0.71073 |
| Crystal system, space group | monoclinic, C2/c (no 15) | orthorhombic, Pnma (62) | monoclinic, P21/c (14) |
| Unit cell dimensions [Å] and [°] | a = 15.2714(7) b = 16.7046(6) β = 98.419(4) c = 14.2730(7) | a = 16.6621(10) b = 12.5136(7) c = 17.6717(12) | a = 11.0628(9) b = 14.5681(11) β = 92.158(7) c = 19.3533(14) |
| Volume [Å3] | 3601.9(3) | 3684.6(4) | 3116.8(4) |
| Z, Calculated density [Mg·m−3] | 4, 1.598 | 4, 1.710 | 2, 1.576 |
| Absorption coefficient [mm−1] | 2.076 | 2.645 | 2.025 |
| F(000) | 1792 | 1928 | 1524 |
| Crystal size [mm] | 0.360 × 0.310 × 0.160 | 0.570 × 0.320 × 0.110 | 0.430 × 0.330 × 0.130 |
| Theta range for data collection [°] | 2.194 to 26.367 | 2.305 to 26.372 | 2.512 to 26.370 |
| Limiting indices | −19 ≤ h ≤ 18 −20 ≤ k ≤ 20 −17 ≤ l ≤ 17 | −20 ≤ h ≤ 20 −15 ≤ k ≤ 15 −22 ≤ l ≤ 20 | −13 ≤ h ≤ 13 −18 ≤ k ≤ 17 −24 ≤ l ≤ 23 |
| Reflections collected/unique | 12068/3677 [R(int) = 0.0426] | 25450/3949 [R(int) = 0.0255] | 20016/6372 [R(int) = 0.0481] |
| Completeness [%] to theta [°] | 25.242° 99.9% | 25.242° 99.9% | 25.242° 99.9% |
| Absorption correction | Numerical | Numerical | Numerical |
| Max. and min. transmission | 0.732 and 0.522 | 0.760 and 0.314 | 0.779 and 0.476 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3677/3/219 | 3949/6/253 | 6372/0/367 |
| Goodness-of-fit on F2 | 0.836 | 1.065 | 0.996 |
| Final R Indices [I>2sigma(I)] | R1 a = 0.0396, wR2 b = 0.0936 | R1 a = 0.0312, wR2 b = 0.0740 | R1 a = 0.0386, wR2 b = 0.0902 |
| R indices (all data) | R1 a = 0.0713, wR2 b = 0.1049 | R1 a = 0.0375, wR2 b = 0.0778 | R1 a = 0.0590, wR2 b = 0.1007 |
| Largest diff. peak and hole [eÅ−3] | 0.604 and −0.346 | 1.013 and −0.629 | 0.387 and −0.369 |
| Ni1-N5 i | 2.061(3) | Cu2-N2 | 2.003(3) |
| Ni1-N5 | 2.061(3) | Cu2-N12 | 2.010(3) |
| Ni1-N6i | 2.073(3) | Cu2-N11 | 2.013(3) |
| Ni1-N6 | 2.073(3) | Cu2-N1 | 2.028(3) |
| Ni1-N4 i | 2.118(4) | Cu2-S5 i | 2.9883(11) |
| Ni1-N4 | 2.118(4) | Cu2-S6 ii | 3.0224(10) |
| Ni1-N5 | 2.048(3) | Cu2-N14 | 1.999(2) |
| Ni1-N2 | 2.084(2) | Cu2-N17 | 2.014(2) |
| Ni1-N2#1 | 2.084(2) | Cu2-N20 | 2.017(2) |
| Ni1-N1 | 2.082(3) | Cu2-N11 | 2.029(2) |
| Ni1-N4 | 2.091(3) | Cu2-S4 | 2.6422(4) |
| Ni1-N3 | 2.103(3) | ||
| Cu3-N6 | 1.946(5) | Cu3-S2#3 | 2.3520(8) |
| Cu3-S2#2 | 2.3520(8) | Cu3-S3 | 2.3711(1) |
| Cu1-N5 | 1.950(3) | Cu3-N4 | 1.941(3) |
| Cu1-N27 | 2.041(3) | Cu3-N14 | 2.034(2) |
| Cu1-N24 | 2.042(2) | Cu3-N11 | 2.061(3) |
| Cu1-N21 | 2.042(3) | Cu3-N17 | 2.081(3) |
| Cu1-N30 | 2.128(3) | Cu3-N20 | 2.109(3) |
| Ni2-N2 | 2.075(3) | Ni2-N3 | 2.082(3) |
| Ni2-N2 i | 2.075(3) | Ni2-N1 i | 2.102(3) |
| Ni2-N3 i | 2.082(3) | Ni2-N1 | 2.102(3) |
| Complex | Integrated L3-Edge Intensity 925–935 | Integrated L2-Edge Intensity 945–955 | Energy of Maximum L3-Edge eV | Energy of Maximum L2-Edge eV |
|---|---|---|---|---|
| [{Cu(pn)2}2Ni(NCS)6]n∙2nH2O (1) | 10.5 | 1.8 | 931.6 | 951.4 |
| [{CuII(trien)}2Ni(NCS)6CuI(NCS)]n (2) | 9.2 | 2.0 | 931.4 | 951.2 |
| [Cu(tren)(NCS)]4[Ni(NCS)6] (3) | 7.1 | 3.2 | 931.2 | 951.2 |
| Complex | Integrated L3-Edge Intensity 850–860 | Integrated L2-Edge Intensity 865–875 | Energy of Maximum L3-Edge eV | Energy of Maximum L2-Edge eV |
|---|---|---|---|---|
| [{Cu(pn)2}2Ni(NCS)6]n∙2nH2O (1) | 11.9 | 4.2 | 853.4 | 870.6 |
| 855.4 | 871.8 | |||
| [{CuII(trien)}2Ni(NCS)6CuI(NCS)]n (2) | 10.2 | 3.9 | 853.4 | 870.4 |
| 855.4 | 871.6 | |||
| [Cu(tren)(NCS)]4[Ni(NCS)6] (3) | 10.1 | 4.5 | 853.4 | 870.6 |
| 855.4 | 871.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tereba, N.; Muzioł, T.M.; Wiśniewska, J.; Podgajny, R.; Bieńko, A.; Wrzeszcz, G. Structural Diversity, XAS and Magnetism of Copper(II)-Nickel(II) Heterometallic Complexes Based on the [Ni(NCS)6]4− Unit. Materials 2023, 16, 731. https://doi.org/10.3390/ma16020731
Tereba N, Muzioł TM, Wiśniewska J, Podgajny R, Bieńko A, Wrzeszcz G. Structural Diversity, XAS and Magnetism of Copper(II)-Nickel(II) Heterometallic Complexes Based on the [Ni(NCS)6]4− Unit. Materials. 2023; 16(2):731. https://doi.org/10.3390/ma16020731
Chicago/Turabian StyleTereba, Natalia, Tadeusz M. Muzioł, Joanna Wiśniewska, Robert Podgajny, Alina Bieńko, and Grzegorz Wrzeszcz. 2023. "Structural Diversity, XAS and Magnetism of Copper(II)-Nickel(II) Heterometallic Complexes Based on the [Ni(NCS)6]4− Unit" Materials 16, no. 2: 731. https://doi.org/10.3390/ma16020731






