Mechanical Properties of a Bone-like Bioceramic–Epoxy-Based Composite Material with Nanocellulose Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocellulose
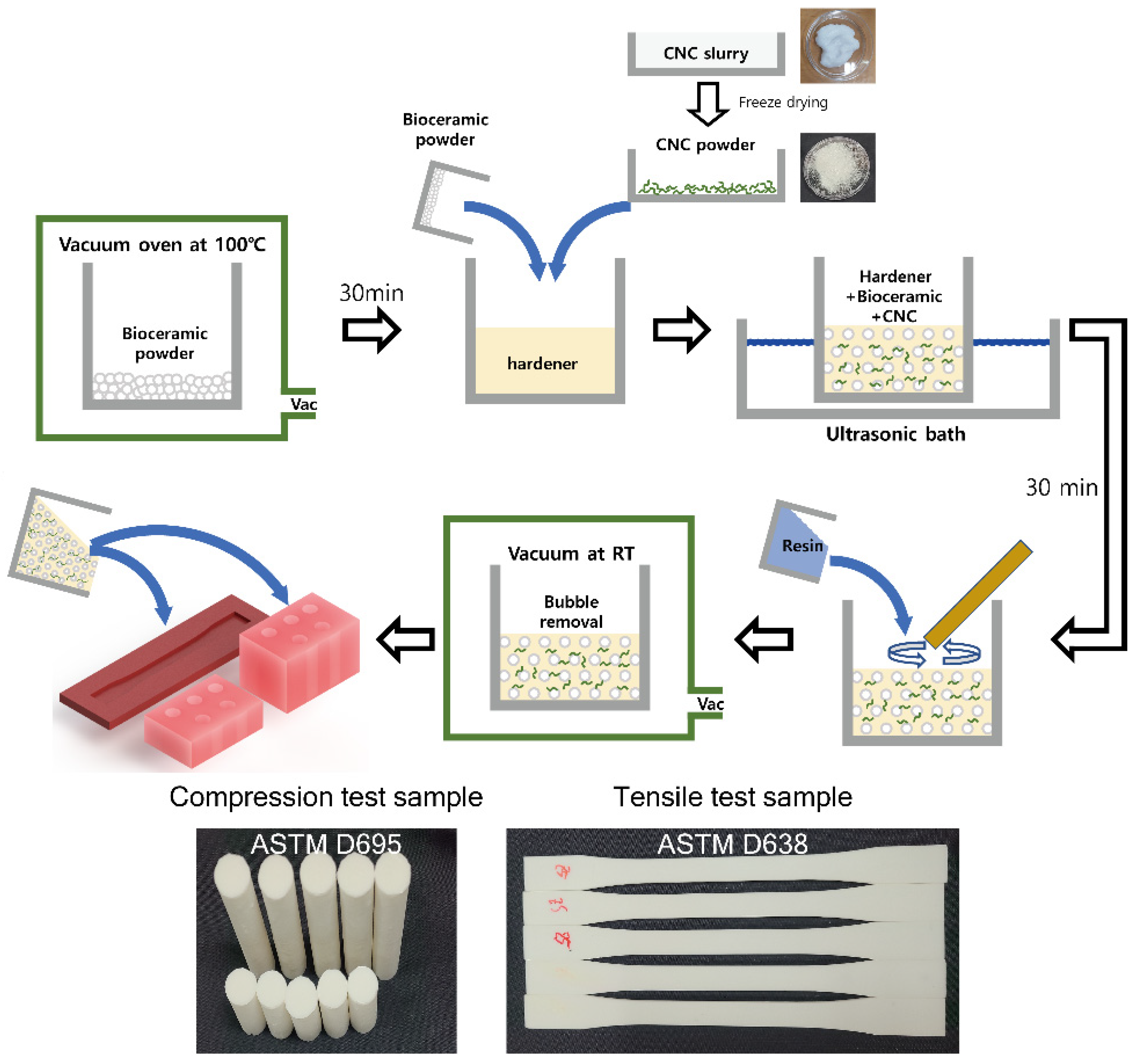
2.3. Preparation of Composite Materials
2.4. Material Characterization
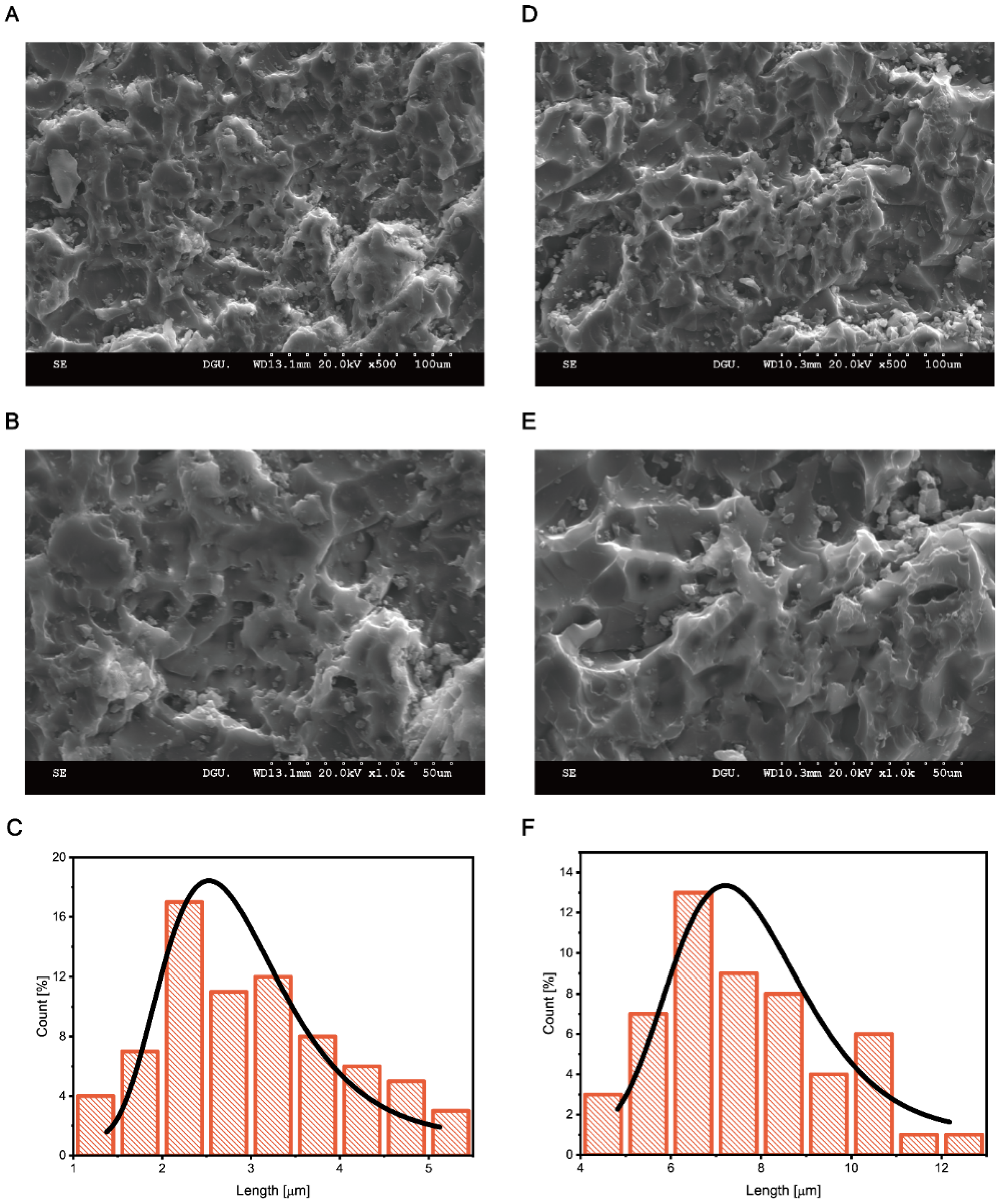
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, Z.; Axinte, D.; Gao, D. On modelling of cutting force and temperature in bone milling. J. Mater. Process. Technol. 2019, 266, 627–638. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, M.; Zhao, X.; Zhu, G.; McClean, C.; Zhao, Y.; Fan, Y. Experimental investigations and finite element simulation of cutting heat in vibrational and conventional drilling of cortical bone. Med. Eng. Phys. 2014, 36, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Micucci, S.; Chang, G.; Smith, E.; Cassidy, C.; Sagar, A.; James, T.P. Effect of oscillation speed and thrust force on cortical bone temperature during sagittal sawing. ASME International Mechanical Engineering Congress and Exposition. Am. Soc. Mech. Eng. 2013, 56215, V03AT03A023. [Google Scholar]
- Sabri, H.; Cowan, B.; Kapralos, B.; Porte, M.; Backstein, D.; Dubrowskie, A. Serious games for knee replacement surgery pro-cedure education and training. Procedia-Soc. Behav. Sci. 2010, 2, 3483–3488. [Google Scholar] [CrossRef]
- Cuneo, A.; Timossi, F.; Musenich, L.; Stagni, A.; Wilhelm, F.; Libonati, F. Design and Manufacturing of Bone-like Compo-sites. Procedia CIRP 2022, 110, 287–292. [Google Scholar] [CrossRef]
- Huang, Q.-W.; Wang, L.-P.; Wang, J.-Y. Mechanical properties of artificial materials for bone repair. J. Shanghai Jiaotong Univ. Sci. 2014, 19, 675–680. [Google Scholar] [CrossRef]
- Elfar, J.; Stanbury, S. Composite bone models in orthopaedic surgery research and education. J. Am. Acad. Orthop. Surg. 2014, 22, 111. [Google Scholar]
- Kivanc, A.; Jay, M.; Laith, J.; Kenneth, E. Surgical simulation in orthopaedic skills training. J. Am. Acad. Orthop. Surg. 2012, 20, 410–422. [Google Scholar]
- Saceleanu, V.; Paz, R.; García, J.; Rivero, Y.; Cîndea, C.N.; Cacciotti, I.; Monzón, M. Production of synthetic models for neuro-oncology training by additive manufacturing. Appl. Sci. 2021, 11, 1182. [Google Scholar] [CrossRef]
- Goh, G.S.; Lohre, R.; Parvizi, J.; Goel, D.P. Virtual and augmented reality for surgical training and simulation in knee arthro-plasty. Arch. Orthop. Trauma Surg. 2021, 141, 2303–2312. [Google Scholar] [CrossRef]
- Pluim, J.M.; Jimenez-Bou, L.; Gerretsen, R.R.; Loeve, A.J. Aerosol production during autopsies: The risk of sawing in bone. Forensic Sci. Int. 2018, 289, 260–267. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Q.; Li, H.; Zhang, Q. Experimental investigation on cutting mechanisms in fixed diamond wire sawing of bone. Precis. Eng. 2021, 68, 319–325. [Google Scholar] [CrossRef]
- James, T.P.; Pearlman, J.J.; Saigal, A. Rounded Cutting Edge Model for the Prediction of Bone Sawing Forces. J. Biomech. Eng. 2012, 134, 071001. [Google Scholar] [CrossRef] [PubMed]
- Yanping, L.; Dedong, Y.; Xiaojun, C.; Xudong, W.; Guofang, S.; Chengtao, W. Simulation and Evaluation of a Bone Sawing Procedure for Orthognathic Surgery Based on an Experimental Force Model. J. Biomech. Eng. 2014, 136, 034501. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Satake, U.; Enomoto, T. Surgical oscillating saw blade to suppress forces in bone cutting. CIRP Ann. 2022, 71, 73–76. [Google Scholar] [CrossRef]
- Ramulu, M.; Kramlich, J. Machining of fiber reinforced composites: Review of environmental and health effects. Int. J. Environ. Conscious Des. Manuf. 2004, 11. [Google Scholar]
- Zustra, M.A. Evaluation of the Potential Health Hazards Associated with the Machining of Carbon Fiber Composites. Master’s Thesis, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 1987. [Google Scholar]
- Parsons, A.; Ahmed, I.; Han, N.; Felfel, R.; Rudd, C. Mimicking Bone Structure and Function with Structural Composite Materials. J. Bionic Eng. 2010, 7, S1–S10. [Google Scholar] [CrossRef]
- Minamoto, K.; Nagano, M.; Inaoka, T.; Kitano, T.; Ushijima, K.; Fukuda, Y.; Futatsuka, M. Skin Problems among Fiber-Glass Reinforced Plastics Factory Workers in Japan. Ind. Health 2002, 40, 42–50. [Google Scholar] [CrossRef]
- Hsieh, M.Y.; Guo, Y.L.; Shiao, J.S.C.; Sheu, H.M. Morphology of glass fibers in electronics workers with fiberglass dermatitis—A scanning electron microscopy study. Int. J. Dermatol. 2001, 40, 258–261. [Google Scholar] [CrossRef]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the effects of tensile and compressive loadings on human femur bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef]
- Nikodem, A. Correlations between structural and mechanical properties of human trabecular femur bone. Acta Bioeng. Biomech. 2012, 14, 37–46. [Google Scholar] [PubMed]
- Frankel, V.H.; Nordin, M. Basic Biomechanics of the Skeletal System; Lea & Febiger: Philadelphia, PA, USA, 1980. [Google Scholar]
- Lüders, C. Nonlinear-Elastic Orthotropic Material Modeling of an Epoxy-Based Polymer for Predicting the Material Behavior of Transversely Loaded Fiber-Reinforced Composites. J. Compos. Sci. 2020, 4, 46. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Kang, J.-H. Mechanical Properties of Compact Bone Defined by the Stress-Strain Curve Measured Using Uniaxial Tensile Test: A Concise Review and Practical Guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; LeGeros, J.P. Dense Hydroxyapatite. An Introduction to Bioceramics; Hench, L.L., Wilson, J., Eds.; Word Scientific: Hackensack, NJ, USA, 1993. [Google Scholar]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Yao, Y.; Qin, W.; Xing, B.; Sha, N.; Jiao, T.; Zhao, Z. High performance hydroxyapatite ceramics and a triply periodic minimum surface structure fabricated by digital light processing 3D printing. J. Adv. Ceram. 2021, 10, 39–48. [Google Scholar] [CrossRef]
- Opuroğlu, M.; Şen, M. Synthesis and characterization of a Zr-containing silicate-based epoxy-functional polymer nanocom-posite system. Polym. Eng. Sci. 2015, 55, 792–798. [Google Scholar] [CrossRef]
- Masaki, T. Mechanical properties of toughened ZrO2-Y2O3 Ceramics. J. Am. Ceram. Soc. 1986, 69, 638–640. [Google Scholar] [CrossRef]
- Roszowska-Jarosz, M.; Masiewicz, J.; Kostrzewa, M.; Kucharczyk, W.; Żurowski, W.; Kucińska-Lipka, J.; Przybylek, P. Me-chanical properties of bio-composites based on epoxy resin and nanocellulose fibres. Materials 2021, 14, 3576. [Google Scholar] [CrossRef]
- Goodsell, J.E.; Moon, R.J.; Huizar, A.; Pipes, R.B. A strategy for prediction of the elastic properties of epoxy-cellulose nano-crystal-reinforced fiber networks. Nord. Pulp Pap. Res. J. 2014, 29, 85–94. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites–fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar]
- Luo, Y.; Ren, Y.; Shu, Y.; Mao, C.; Zhou, Z.; Bi, Z.M. Cutting Behavior of Cortical Bone in Different Bone Osteon Cutting Angles and Depths of Cut. Chin. J. Mech. Eng. 2022, 35, 1–12. [Google Scholar] [CrossRef]
- Margareta, N.; Frankel, V.H. Basic Biomechanics of the Musculoskeletal System; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Dickenson, R.P.; Hutton, W.C.; Stott, J.R. The mechanical properties of bone in osteoporosis. J. Bone Jt. Surg. 1981, 63, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, W.H.; Eastwood, D.M.; Allgrove, J.; Hvid, I.; Weinans, H.H.; Bank, R.A.; Sakkers, R.J. Current concepts in osteogenesis imperfecta: Bone structure, biomechanics and medical management. J. Child. Orthop. 2019, 13, 1–11. [Google Scholar] [CrossRef]
- Beloshenko, V.A.; Borzenko, A.P.; Glazunova, V.A.; Konstantinova, T.E.; Yakovleva, R.A.; Popov, Y.V. Properties of Polymer Composites Filled with Zirconium Dioxide Nanopowders. Int. Polym. Sci. Technol. 2009, 36, 27–30. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
| Composite Materials | Resin | Hardener | Bioceramic | CNC |
|---|---|---|---|---|
| Bioceramic 1 wt% | 74.25 | 24.75 | 1 | - |
| Bioceramic 2 wt% | 73.5 | 24.5 | 2 | - |
| Bioceramic 3 wt% | 72.75 | 24.25 | 3 | - |
| Bioceramic 4 wt% | 72 | 24 | 4 | - |
| Bioceramic 5 wt% | 71.25 | 23.75 | 5 | - |
| Bioceramic with CNC 1 wt% | 70.5 | 23.5 | 5 | 1 |
| Bioceramic with CNC 2 wt% | 69 | 23 | 5 | 3 |
| Bioceramic with CNC 3 wt% | 67.5 | 22.5 | 5 | 5 |
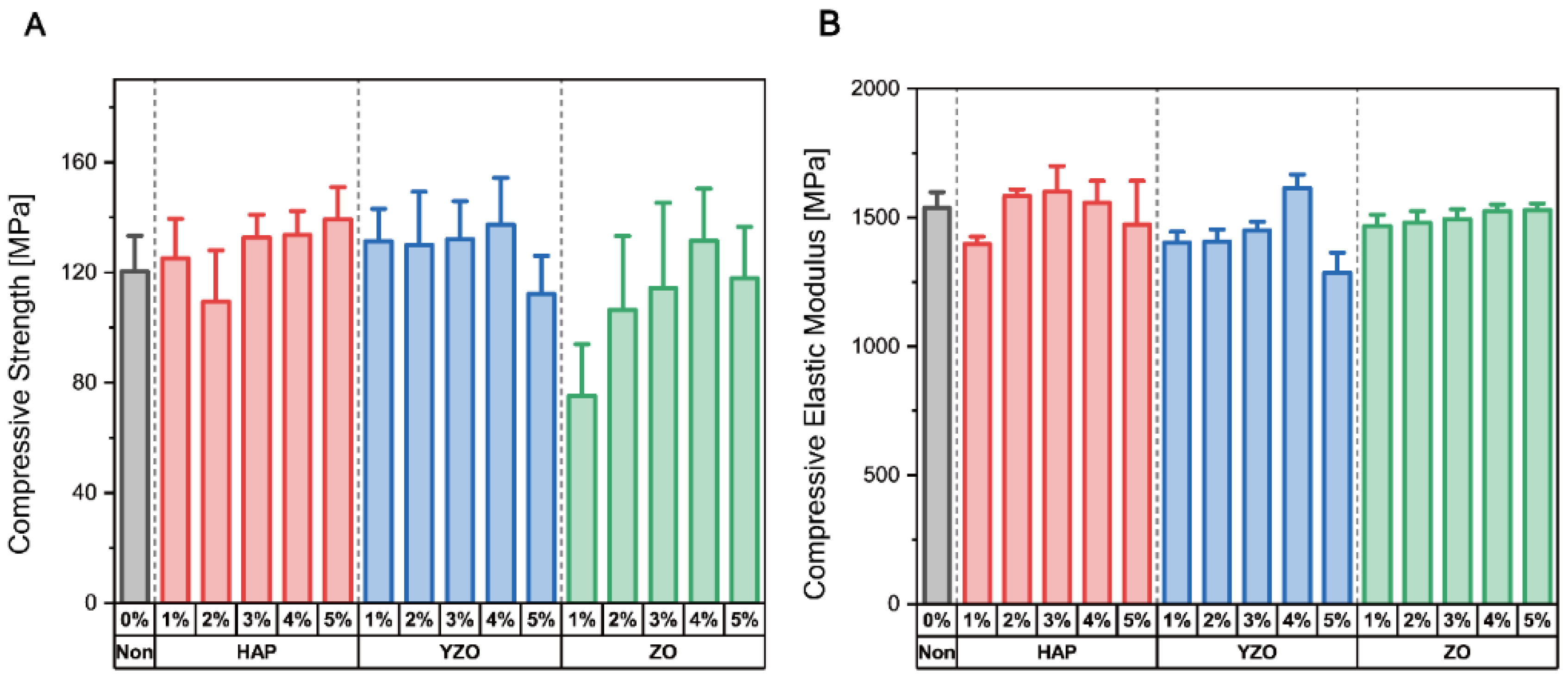
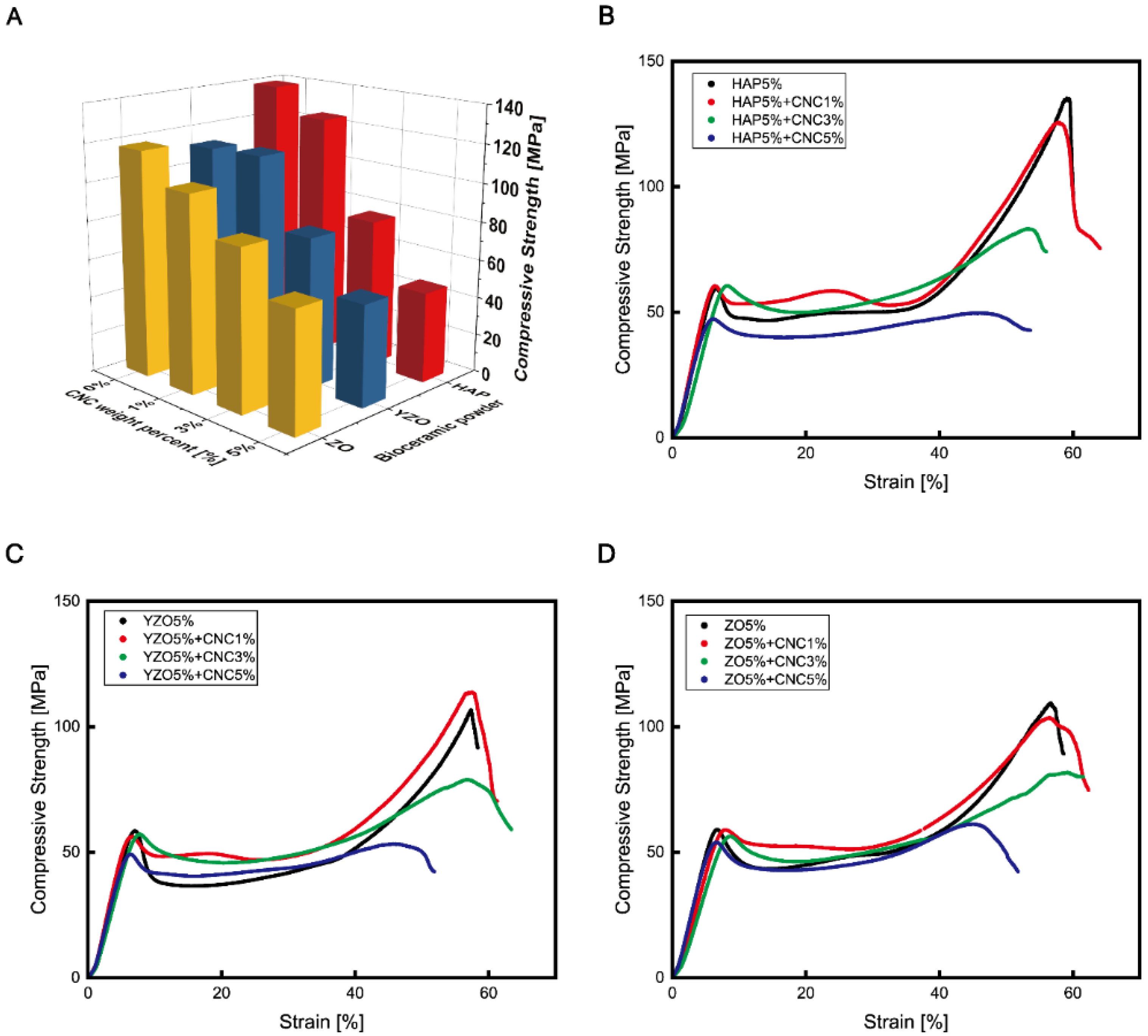
| Powder | Weight Percent | Compressive Strength [MPa] | Compressive Elastic Modulus [MPa] |
|---|---|---|---|
| Epoxy | - | 120.41 ± 19.25 | 1536.76 ± 91.43 |
| HAP | 1% | 125.02 ± 21.57 | 1396.91 ± 42.92 |
| 2% | 109.39 ± 27.85 | 1583.09 ± 34.96 | |
| 3% | 132.63 ± 12.46 | 1599.72 ± 150.37 | |
| 4% | 133.56 ± 12.96 | 1556.37 ± 128.70 | |
| 5% | 139.23 ± 15.61 | 1473.01 ± 195.34 | |
| YZO | 1% | 131.39 ± 17.39 | 1402.60 ± 61.59 |
| 2% | 130.04 ± 28.64 | 1406.72 ± 71.12 | |
| 3% | 132.10 ± 18.46 | 1450.46 ± 44.84 | |
| 4% | 137.43 ± 22.69 | 1613.84 ± 79.85 | |
| 5% | 112.15 ± 18.46 | 1285.45 ± 103.97 | |
| ZO | 1% | 75.149 ± 28 | 1466.73 ± 65.58 |
| 2% | 106.43 ± 35.66 | 1479.09 ± 68.44 | |
| 3% | 114.33 ± 46.12 | 1493.41 ± 58.03 | |
| 4% | 131.50 ± 28.23 | 1524.79 ± 37.90 | |
| 5% | 117.83 ± 27.82 | 1529.30 ± 37.19 |
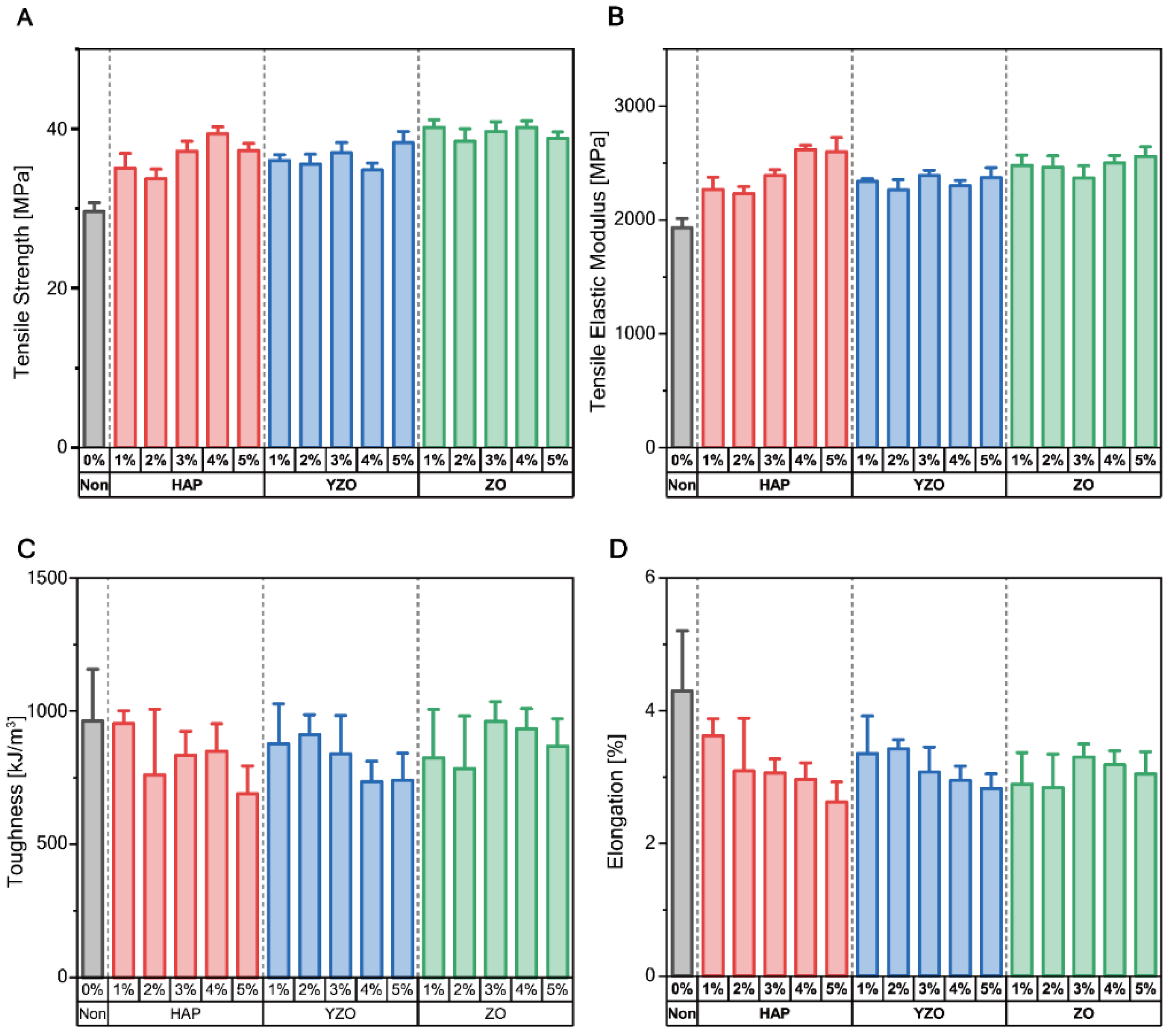
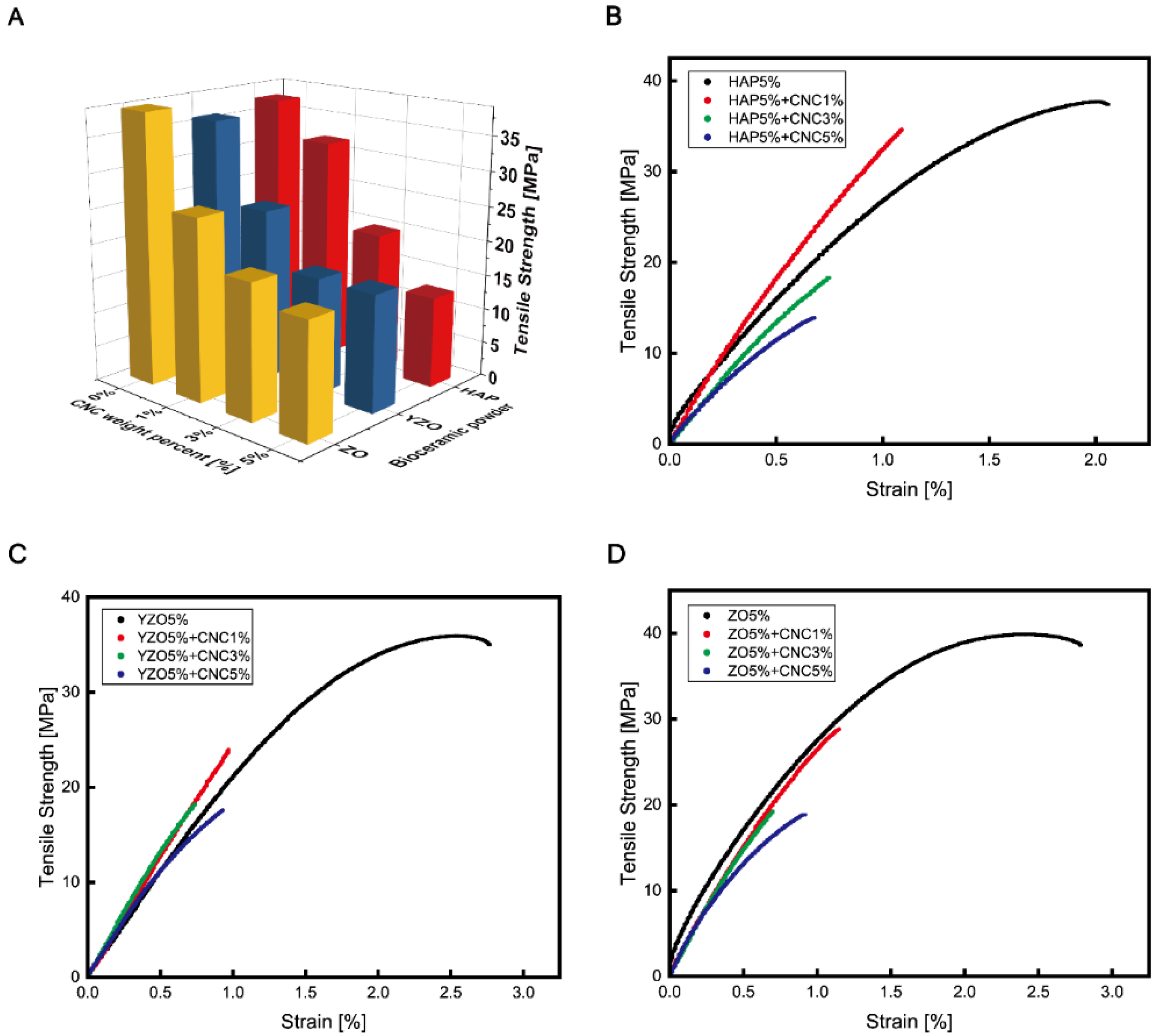
| Powder | Weight Percent | Tensile Strength [MPa] | Tensile Elastic Modulus [MPa] | Toughness [kJ/m3] | Yield Strength [MPa] | Elongation [%] |
|---|---|---|---|---|---|---|
| Epoxy | - | 29.63 ± 1.66 | 1931.98 ± 118.63 | 962.65 ± 289.36 | 25.66 ± 0.96 | 4.30 ± 1.35 |
| HAP | 1% | 35.10 ± 2.70 | 2266.89 ± 162.73 | 953.48 ± 70.29 | 29.78 ± 1.88 | 3.62 ± 0.38 |
| 2% | 33.75 ± 1.78 | 2229.62 ± 94.68 | 759.83 ± 368.83 | 29.47 ± 1.63 | 3.09 ± 1.18 | |
| 3% | 37.22 ± 1.86 | 2388.49 ± 77.33 | 833.69 ± 133.41 | 32.41 ± 1.90 | 3.06 ± 0.32 | |
| 4% | 39.39 ± 1.31 | 2617.47 ± 57.80 | 849.02 ± 154.76 | 34.16 ± 1.66 | 2.97 ± 0.37 | |
| 5% | 37.29 ± 1.33 | 2597.85 ± 187.93 | 689.28 ± 155.16 | 32.28 ± 1.18 | 2.62 ± 0.45 | |
| YZO | 1% | 36.06 ± 1.05 | 2337.50 ± 37.13 | 876.80 ± 223.92 | 30.77 ± 0.77 | 3.35 ± 0.84 |
| 2% | 35.56 ± 1.85 | 2263.95 ± 137.20 | 911.64 ± 111.33 | 30.21 ± 0.92 | 3.42 ± 0.21 | |
| 3% | 37.00 ± 1.91 | 2389.97 ± 68.35 | 839.06 ± 215.80 | 31.60 ± 1.67 | 3.08 ± 0.56 | |
| 4% | 34.84 ± 1.31 | 2302.19 ± 67.55 | 735.30 ± 113.88 | 29.27 ± 0.87 | 2.95 ± 0.33 | |
| 5% | 38.24 ± 1.91 | 2372.80 ± 130.17 | 740.02 ± 135.55 | 31.55 ± 1.36 | 2.83 ± 0.30 | |
| ZO | 1% | 40.20 ± 1.39 | 2480.57 ± 133.21 | 823.57 ± 273.50 | 36.88 ± 1.27 | 2.89 ± 0.71 |
| 2% | 38.46 ± 2.31 | 2464.00 ± 147.75 | 783.97 ± 293.82 | 34.62 ± 2.33 | 2.84 ± 0.75 | |
| 3% | 39.67 ± 1.85 | 2366.60 ± 161.64 | 961.80 ± 109.43 | 34.67 ± 1.76 | 3.30 ± 0.29 | |
| 4% | 40.22 ± 1.20 | 2504.22 ± 92.83 | 932.90 ± 114.47 | 34.56 ± 1.70 | 3.19 ± 0.31 | |
| 5% | 38.83 ± 1.16 | 2559.81 ± 123.21 | 868.60 ± 153.28 | 33.66 ± 0.63 | 3.05 ± 0.50 |
| Group | df | TS | CS | TM | CM | T | YS | E |
|---|---|---|---|---|---|---|---|---|
| HAP wt% | 4 | 0.00 | 0.18 | 0.00 | 0.09 | 0.34 | 0.00 | 0.20 |
| YZO wt% | 4 | 0.04 | 0.55 | 0.26 | 0.00 | 0.40 | 0.02 | 0.35 |
| ZO wt% | 4 | 0.34 | 0.14 | 0.27 | 0.31 | 0.63 | 0.06 | 0.64 |
| HAP with CNC | 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.00 |
| YZO with CNC | 3 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | - | 0.00 |
| ZO with CNC | 3 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | - | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Baek, J.W.; Jin, Z.; Jeon, H.C.; Han, M.-W.; Lim, J.Y. Mechanical Properties of a Bone-like Bioceramic–Epoxy-Based Composite Material with Nanocellulose Fibers. Materials 2023, 16, 739. https://doi.org/10.3390/ma16020739
Kim Y-S, Baek JW, Jin Z, Jeon HC, Han M-W, Lim JY. Mechanical Properties of a Bone-like Bioceramic–Epoxy-Based Composite Material with Nanocellulose Fibers. Materials. 2023; 16(2):739. https://doi.org/10.3390/ma16020739
Chicago/Turabian StyleKim, Young-Seong, Jin Woo Baek, Zhengyun Jin, Hee Chang Jeon, Min-Woo Han, and Joong Yeon Lim. 2023. "Mechanical Properties of a Bone-like Bioceramic–Epoxy-Based Composite Material with Nanocellulose Fibers" Materials 16, no. 2: 739. https://doi.org/10.3390/ma16020739
APA StyleKim, Y.-S., Baek, J. W., Jin, Z., Jeon, H. C., Han, M.-W., & Lim, J. Y. (2023). Mechanical Properties of a Bone-like Bioceramic–Epoxy-Based Composite Material with Nanocellulose Fibers. Materials, 16(2), 739. https://doi.org/10.3390/ma16020739






