Supplementation of Polymeric Reservoirs with Redox-Responsive Metallic Nanoparticles as a New Concept for the Smart Delivery of Insulin in Diabetes
Abstract
:1. Introduction
2. Materials and Methods
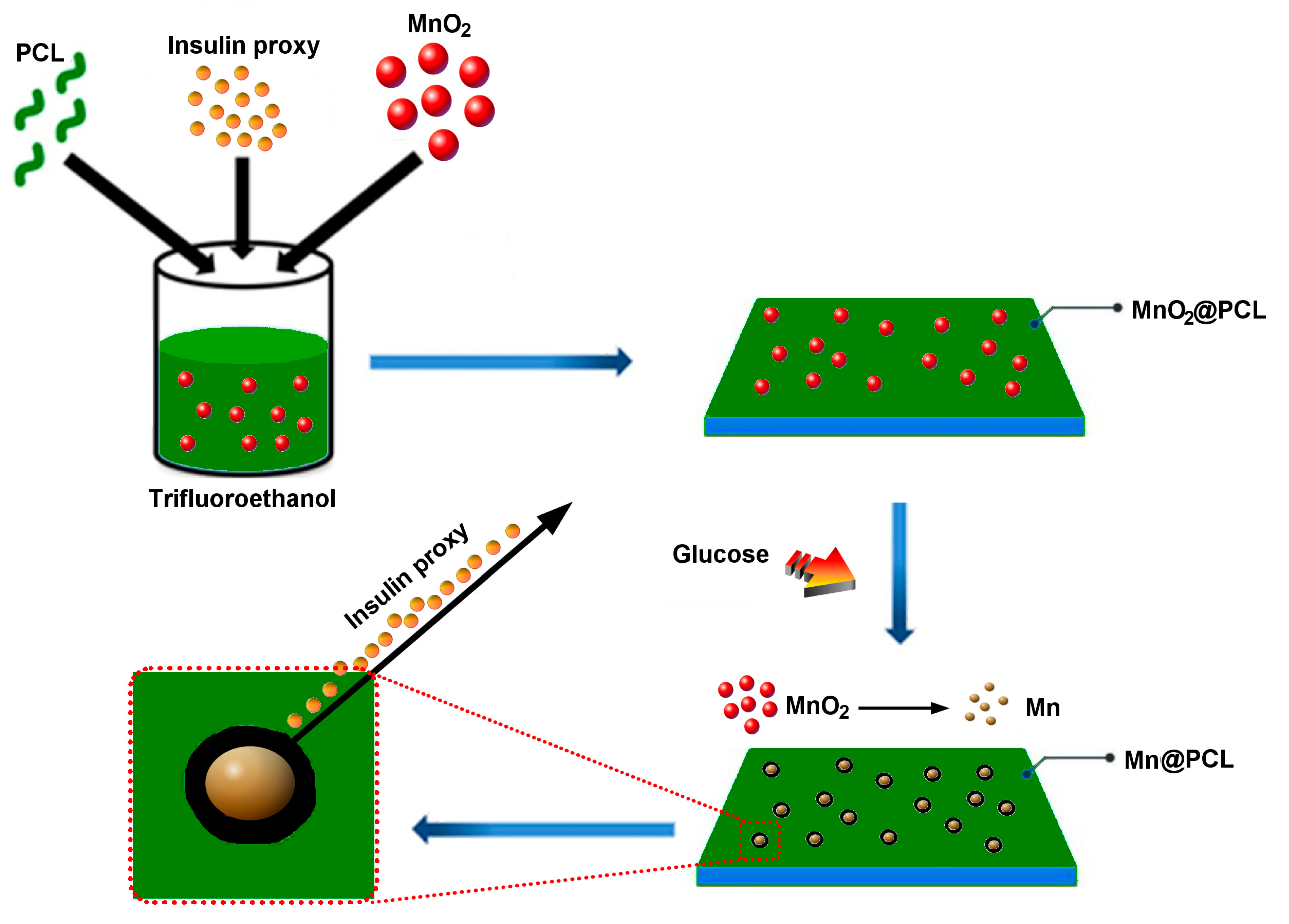
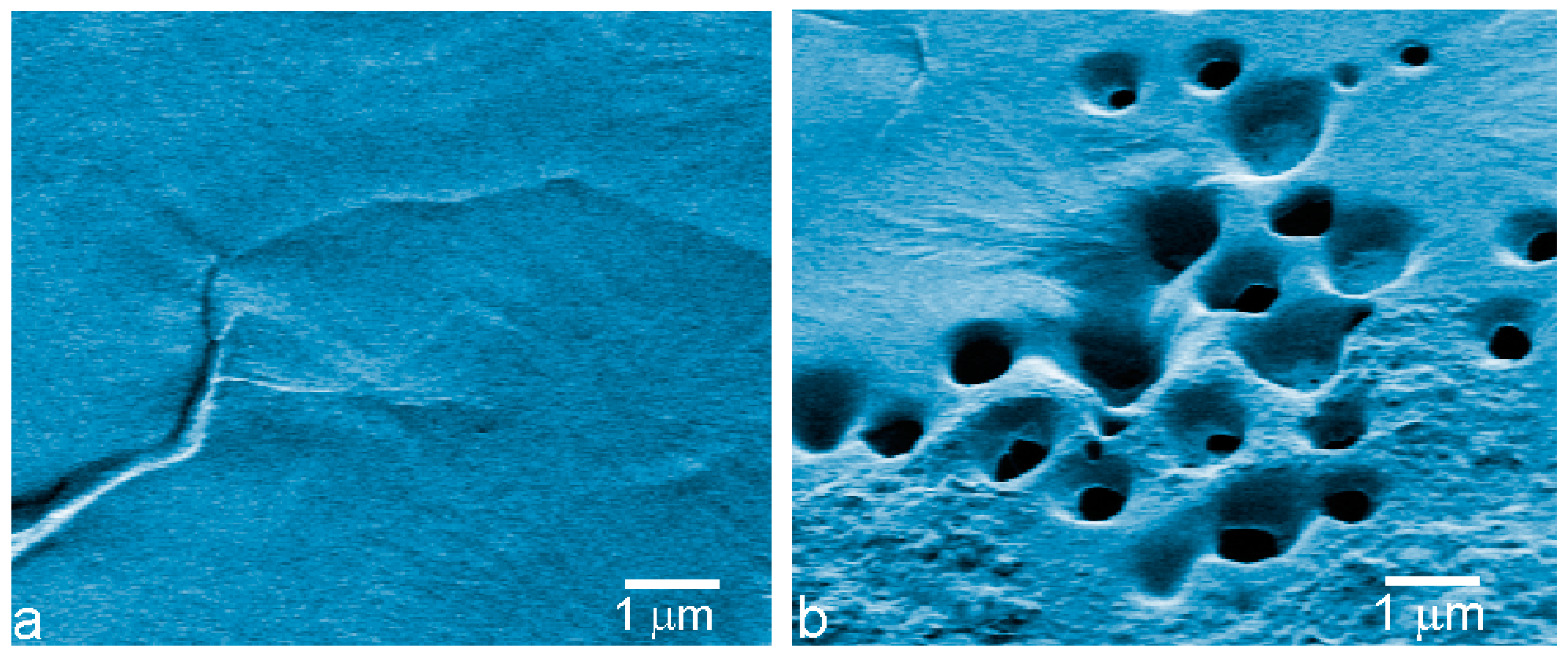
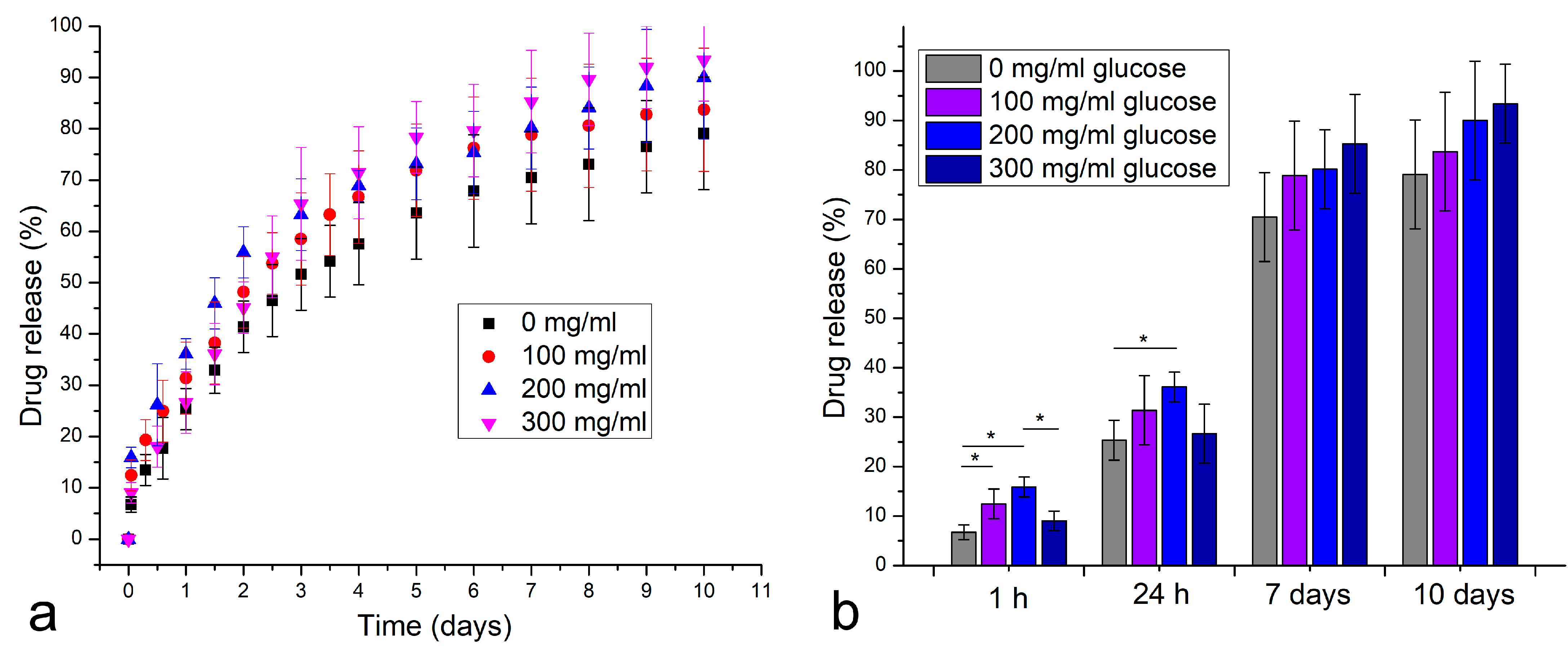
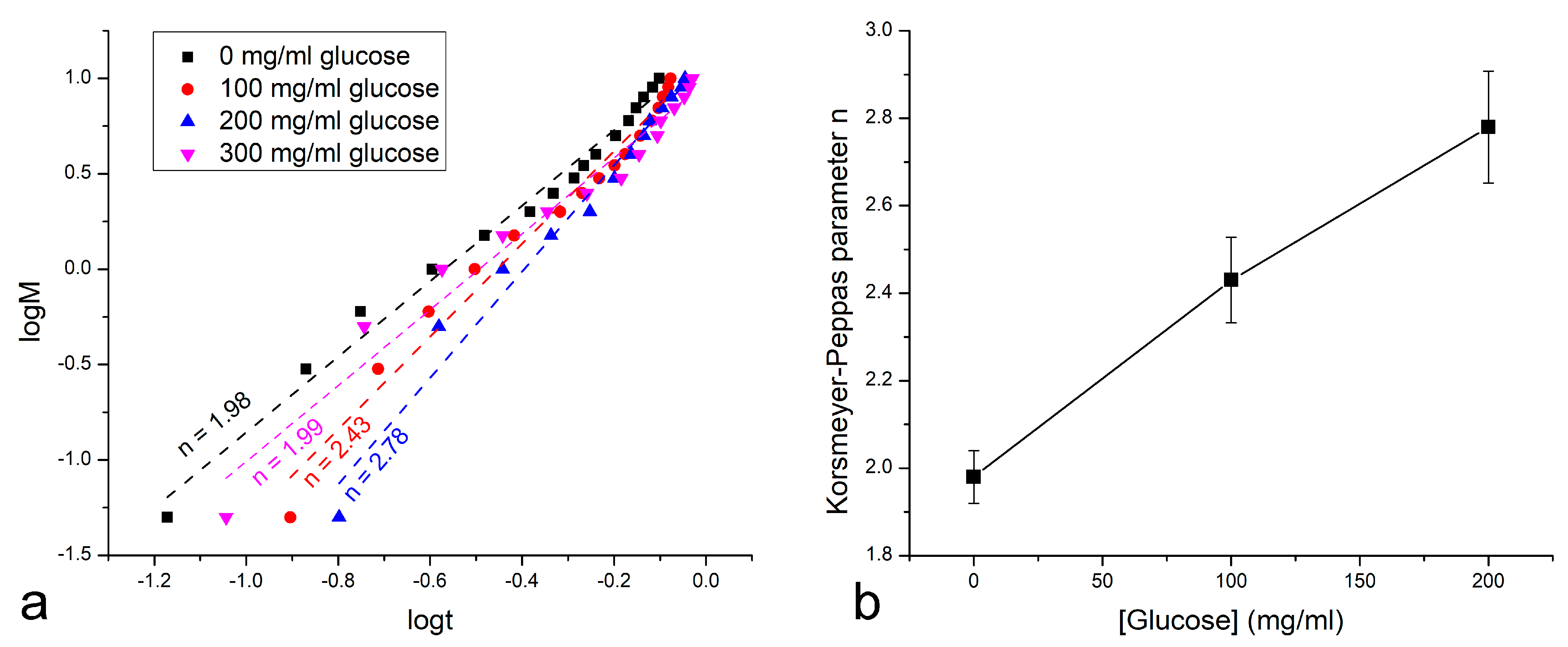
3. Results
4. Discussion
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Liu, S.; Kang, Y.; Wang, M. Glutathione- and pH-responsive nonporous silica prodrug nanoparticles for controlled release and cancer therapy. Nanoscale 2015, 7, 5859–5868. [Google Scholar] [CrossRef]
- Niu, B.; Zhou, Y.; Liao, K.; Wen, T.; Lao, S.; Quan, G.; Pan, X.; Wu, C. “Pincer movement”: Reversing cisplatin resistance based on simultaneous glutathione depletion and glutathione S-transferases inhibition by redox-responsive degradable organosilica hybrid nanoparticles. Acta Pharm. Sin. B 2022, 12, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Milcovich, G.; Contessotto, P.; Marsico, G.; Pandit, A. Synthetic/ECM-inspired hybrid platform for hollow microcarriers with ROS-triggered nanoporation hallmarks. Sci. Rep. 2017, 7, 13138. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, M.; Basu, S.M.; Yadava, S.K.; Sarviya, N.; Giri, J. A facile strategy for the preparation of polypropylene sulfide nanoparticles for hydrophobic and base-sensitive cargo. J. Appl. Polym. Sci. 2022, 139, 51767. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Guo, M.; Xu, W.; Wang, J.; Wang, Y.; Suo, A. Hydrazide-manganese coordinated multifunctional nanoplatform for potentiating immunotherapy in hepatocellular carcinoma. J. Colloid Interface Sci. 2022, 628, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Jia, X.L.; Qi, J.L.; Yang, L.P.; Sun, W.H.; Yan, X.; Yang, S.K.; Cao, D.Y.; Du, Q.; Qi, X.R. Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int. J. Nanomed. 2017, 12, 2385–2405. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zhuang, Y.; Liu, Z.; Mao, J.; Qian, S.; Zhao, Q.; Lu, B.; Mao, X.; Zhang, L.; Zhang, Y.; et al. Regulated extravascular microenvironment via reversible thermosensitive hydrogel for inhibiting calcium influx and vasospasm. Bioact. Mater. 2022, 21, 422–435. [Google Scholar] [CrossRef]
- Khan, M.A.; Wu, V.M.; Ghosh, S.; Uskoković, V. Gene Delivery Using Calcium Phosphate Nanoparticles: Optimization of the Transfection Process and the Effects of Citrate and Poly(L-Lysine) as Additives. J. Colloid Interface Sci. 2016, 471, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Uskoković, V. An Odyssey at the Interface–A Study in the Stream of Consciousness. Biointerface Res. Appl. Chem. 2022, 12, 5150–5160. [Google Scholar]
- Ghiasi, B.; Sefidbakht, Y.; Mozaffari-Jovin, S.; Gharachloo, B.; Mehraria, M.; Khodadadi, A.; Rezaei, M.; Ranaei-Siadat, S.O.; Uskoković, V. Hydroxyapatite as a Biomaterial—A Gift that Keeps on Giving. Drug Devel. Indust. Pharm. 2020, 46, 1035–1062. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.S.; Gonçalves, F.A.M.M.; Mendonça, P.V.; Gil, M.H.; Coelho, J.F.J. Temperature and pH responsive polymers based on chitosan: Applications and new graft copolymerization strategies based on living radical polymerization. Carbohydr. Polym. 2010, 80, 618–630. [Google Scholar] [CrossRef]
- Yang, H.; Sun, A.; Yang, J.; Cheng, H.; Yang, X.; Chen, H.; Ding, H.; Falahati, M. Development of doxorubicin-loaded chitosan–heparin nanoparticles with selective anticancer efficacy against gastric cancer cells in vitro through regulation of intrinsic apoptosis pathway. Arabian J. Chem. 2021, 14, 103266. [Google Scholar] [CrossRef]
- Wu, G.; Panahi-Sarmad, M.; Vlierberghe, S.V.; Xu, R.; Hou, X.; Cui, Z.; Xiao, X. Multi-stimuli responsive shape memory behavior of dual-switch TPU/CB/CNC hybrid nanocomposites as triggered by heat, water, ethanol, and pH. Chem. Eng. J. 2022, 450, 138253. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.-K. Principles for Controlling the Shape Recovery and Degradation Behavior of Biodegradable Shape-Memory Polymers in Biomedical Applications. Micromachines 2021, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Miao, L.F.; Qian, L.L.; Wang, N.; Qi, M.M.; Zhang, Y.M.; Dang, S.P.; Wu, Y.; Wang, R.X. Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Front. Endocrinol. 2019, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Uskoković, V.; Lee, K.; Lee, P.P.; Fischer, K.E.; Desai, T.A. Shape Effect in the Design of Nanowire-Coated Microparticles as Epithelial Drug Delivery Devices. ACS Nano 2012, 6, 7832–7841. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin delivery methods: Past, present and future. Int. J. Pharm. Investig. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; He, D.; Cai, L.; He, X.; Wang, K.; Yang, X.; Li, L.; Li, S.; Su, X. Alizarin Complexone Functionalized Mesoporous Silica Nanoparticles: A Smart System Integrating Glucose-Responsive Double-Drugs Release and Real-Time Monitoring Capabilities. ACS Appl. Mater. Interfaces 2016, 8, 8358–8366. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Li, M.; Ge, S.; Yang, J.; Sun, Q.; Xiong, L. Glucose-responsive biopolymer nanoparticles prepared by co-assembly of concanavalin A and amylopectin for insulin delivery. Indust. Crops Prod. 2018, 112, 98–104. [Google Scholar] [CrossRef]
- Chen, S.; Miyazaki, T.; Itoh, M.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Temperature-Stable Boronate Gel-Based Microneedle Technology for Self-Regulated Insulin Delivery. ACS Appl. Polymer Mater. 2020, 2, 2781–2790. [Google Scholar] [CrossRef]
- Lee, J.; Saha, B.; Vlachos, D.G. Pt catalysts for efficient aerobic oxidation of glucose to glucaric acid in water. Green Chem. 2016, 18, 3815–3822. [Google Scholar] [CrossRef]
- Granata, G.; Onoguchi, A.; Tokoro, C. Preparation of copper nanoparticles for metal-metal bonding by aqueous reduction with d-glucose and PVP. Chem. Eng. Sci. 2019, 209, 115210. [Google Scholar] [CrossRef]
- Suvarna, S.; Das, U.; Kc, S.; Mishra, S.; Sudarshan, M.; Saha, K.D.; Dey, S.; Chakraborty, A.; Narayana, Y. Synthesis of a novel glucose capped gold nanoparticle as a better theranostic candidate. PLoS ONE 2017, 12, e0178202. [Google Scholar] [CrossRef] [Green Version]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Engelbrekt, C.; Sørensen, K.H.; Lübcke, T.; Zhang, J.; Li, Q.; Pan, C.; Bjerrum, N.J.; Ulstrup, J. 1.7 nm platinum nanoparticles: Synthesis with glucose starch, characterization and catalysis. ChemPhysChem 2010, 11, 2844–2853. [Google Scholar] [CrossRef]
- Miller, K.; Hsu, J.E.; Soslowsky, L.J. 6.618—Materials in Tendon and Ligament Repair. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 257–279. [Google Scholar]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer- polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Heimowska, A.; Morawska, M.; Bocho-Janiszewska, M. Biodegradation of poly(ε-caprolactone) in natural water environments. Pol. J. Chem. Tech. 2017, 19, 120–126. [Google Scholar] [CrossRef]
- Cercone, M.; Chevalier, J.; Kennedy, J.G.; Miller, A.D.; Fortier, L.A. Early Failure of a Polyvinyl Alcohol Hydrogel Implant with Osteolysis and Foreign Body Reactions in an Ovine Model of Cartilage Repair. Am. J. Sports Med. 2021, 49, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.D., Jr.; Mullarky, R.H.; Ryan, D.E. Tissue biocompatibility of kevlar aramid fibers and polymethylmethacrylate, composites in rabbits. J. Biomed. Mater. Res. 1987, 21, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Bliley, J.M.; Marra, K.G. Polymeric Biomaterials as Tissue Scaffolds. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Zayed, M.A.; El-dek, S.I.; Ahmed, M.K.; Hady, M.A.; El Sherbiny, D.H.; Uskoković, V. Nanofibrous ε-Polycaprolactone Scaffolds Containing Ag-Doped Magnetite Nanoparticles: Physicochemical Characterization and Biological Testing for Wound Dressing Applications in vitro and in vivo. Bioact. Mater. 2021, 6, 2070–2088. [Google Scholar]
- Ahmed, M.K.; Ramadan, R.; El-dek, S.I.; Uskoković, V. Complex Relationship between Alumina and Selenium-Doped Carbonated Hydroxyapatite as the Ceramic Additives to Electrospun Polycaprolactone Scaffolds for Bone Tissue Engineering. J. Alloys Compd. 2019, 801, 70–81. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ε-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opt. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Uskoković, V.; Drofenik, M. Gold-Embellished Mixed-Valence Manganite as a Smart, Self-Regulating Magnetoplasmonic Nanomaterial. Mat. Chem. Phys. 2021, 271, 124870. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, S.; Chen, J.; Feng, J.; Hou, Y.; Ruan, Y.; Weng, X.; Milcovich, G. Gold nanoparticles/electrochemically expanded graphite composite: A bifunctional platform toward glucose sensing and SERS applications. J. Electroanal. Chem. 2019, 851, 113471. [Google Scholar] [CrossRef]
- Valera, P.; Zavattari, P.; Sanna, A.; Pretti, S.; Marcello, A.; Mannu, C.; Targhetta, C.; Bruno, G.; Songini, M. Zinc and Other Metals Deficiencies and Risk of Type 1 Diabetes: An Ecological Study in the High Risk Sardinia Island. PLoS ONE 2015, 10, e0141262. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jouihan, H.A.; Cooksey, R.C.; Jones, D.; Kim, H.J.; Winge, D.R.; McClain, D.A. Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology 2013, 154, 1029–1038. [Google Scholar] [CrossRef]
- Balan, L.; Ghimbeu, C.M.; Vidal, L.; Guterl, C.V. Synthesis of manganese oxide nanostructures using visible light at room temperature. Green Chem. 2013, 15, 2191–2199. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Pauline, S. Synthesis, Structural and Morphological Characterization of CTAB-Mn3O4 by CO Precipitation Method. ChemTech Res. 2014, 6, 3813–3815. [Google Scholar]
- Baykal, A.; Koseoglu, Y.; Senel, M. Low temperature synthesis and characterization of Mn3O4 nano particles. Cent. Eur. J. Chem. 2007, 5, 169–176. [Google Scholar]
- Cherian, E.; Rajan, A.; Baskar, G. Synthesis of manganese dioxide nanoparticles using co-precipitation method and its antimicrobial activity. Int. J. Mod. Sci. Tech. 2016, 1, 17–22. [Google Scholar]
- Andal, V.; Buvaneswari, G. Effect of reducing agents in the conversion of Cu2O nanocolloid to Cu nanocolloid. Eng. Sci. Tech. Int. J. 2017, 20, 340–344. [Google Scholar]
- Dong, C.; Zhang, X.; Cai, H.; Cao, C. Sodium Alginate Mediated Route for the Synthesis of Monodisperse Silver Nanoparticles Using Glucose as Reducing Agents. Rare Metal Mater. Eng. 2016, 45, 261–266. [Google Scholar]
- Zhao, Y.; Yin, G.; Zeng, X.; Zhao, J.; Yao, G. Potential application of carbohydrate biomass in hydrometallurgy: One-pot reduction of metal oxides/salts under mild hydrothermal conditions. RSC Adv. 2022, 12, 20747–20754. [Google Scholar] [CrossRef] [PubMed]
- Witzemann, E.J. Disodium phosphate as a catalyst for the quantitative oxidation of glucose to carbon dioxide with hydrogen peroxide. J. Biol. Chem. 1920, 45, 1–22. [Google Scholar] [CrossRef]
- Swensson, B.; Ek, M.; Gray, D.G. In Situ Preparation of Silver Nanoparticles in Paper by Reduction with Alkaline Glucose Solutions. ACS Omega 2018, 3, 9449–9452. [Google Scholar] [CrossRef]
- Eddy, C.W. A Study of the Oxidation of d-Glucose with Air in a Saturated Solution of Barium Hydroxide. Master’s Thesis, University of Nebraska–Lincoln, Lincoln, NE, USA, 1929. Available online: https://digitalcommons.unl.edu/opentheses/62/ (accessed on 25 December 2022).
- Witzemann, E.J. A new method of preparation and some interesting transformations of colloidal manganese dioxide. J. Am. Chem. Soc. 1915, 37, 1079–1091. [Google Scholar] [CrossRef] [Green Version]
- Cross, C.F.; Bevan, E.J.; Smith, C. XLIX.—Reactions of the carbohydrates with hydrogen peroxide. J. Chem. Soc. Trans. 1898, 73, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Spoehr, H.A. The oxidation of carbohydrates with air. J. Am. Chem. Soc. 1924, 46, 1494–1502. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Calzada-Funes, J.; Hueso, J.L. Manganese oxide nano-platforms in cancer therapy: Recent advances on the development of synergistic strategies targeting the tumor microenvironment. Appl. Mater. Today 2022, 29, 101628. [Google Scholar] [CrossRef]
- Henkel, J.V.; Schulz-Vogt, H.N.; Dellwig, O.; Pollehne, F.; Schott, T.; Meeske, C.; Beier, S.; Jurgens, K. Biological manganese-dependent sulfide oxidation impacts elemental gradients in redox-stratified systems: Indications from the Black Sea water column. ISME J. 2022, 16, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Q.; Liu, H.; Shan, X.; Pang, Z.; Song, P.; Niu, F.; Hu, L. Glucose-Responsive Microspheres as a Smart Drug Delivery System for Controlled Release of Insulin. Eur. J. Drug Metabol. Pharmacokin. 2020, 45, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. Mechanism of Formation Governs the Mechanism of Release of Antibiotics from Calcium Phosphate Powders and Cements in a Drug-Dependent Manner. J. Mater. Chem. B 2019, 7, 3982–3992. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Pitt, C.G.; Gu, Z.W. Modification of the rates of chain cleavage of poly(ε-caprolactone) and related polyesters in the solid state. J. Control Release 1987, 4, 283–292. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Charaabi, S.; Carbonnier, B. Glucose-sensitive capsules based on hydrogen-bonded (polyvinylpyrrolidone/phenylboronic–modified alginate) system. Colloids Surf. B 2019, 177, 416–424. [Google Scholar] [CrossRef]
- Terada, H.; Inagi, T. Partition of methyl orange between H2O and n-octanol. Membrane 1977, 2, 63–68. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Kawashima, Y.; Xia, D.; Cui, F. Hydrophobic ion pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int. J. Nanomed. 2011, 6, 3049–3056. [Google Scholar]
- Kurpiers, M.; Wolf, J.D.; Spleis, H.; Steinbring, C.; Jörgensen, A.M.; Matuszczak, B.; Bernkop-Schnürch, A. Lysine-Based Biodegradable Surfactants: Increasing the Lipophilicity of Insulin by Hydrophobic Ion Paring. J. Pharm. Sci. 2021, 110, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. The alkaline diet: Is there evidence that an alkaline pH diet benefits health? J. Environ. Public Health 2012, 2012, 727630. [Google Scholar] [CrossRef] [PubMed]
- Irwin, N.J.; Bryant, M.G.; McCoy, C.P.; Trotter, J.L.; Turner, J. Multifunctional, Low Friction, Antimicrobial Approach for Biomaterial Surface Enhancement. ACS Appl. Bio Mater. 2020, 3, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.A.; Silva, J.B.A.; Giulietti, M. Solubility of d-Glucose in Water and Ethanol/Water Mixtures. J. Chem. Eng. Data 2007, 52, 2166–2170. [Google Scholar] [CrossRef]
- Shenoy, U.S.; Shetty, A.N. Simple glucose reduction route for one-step synthesis of copper nanofluids. Appl. Nanosci. 2014, 4, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Rauf, S.; Ali, N.; Tayyab, Z.; Shah, M.Y.; Yang, C.P.; Hu, J.F.; Kong, W.; Huang, Q.A.; Hayat, A.; Muhammad, M. Ionic liquid coated zerovalent manganese nanoparticles with stabilized and enhanced peroxidase-like catalytic activity for colorimetric detection of hydrogen peroxide. Mater. Res. Express 2020, 7, 035018. [Google Scholar] [CrossRef]
- Shi, L.; Jiao, R.; Liu, W.; Li, B.; Jin, Y. Visual detection of glucose by hydrogen peroxide test strips. New J. Chem. 2022, 46, 4162–4166. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Liu, F.; Fu, Q.; Chen, X.; Xu, J.; Zhang, Z.; Huang, Y.; Tang, Y.; Guo, T.; et al. Hydrogen peroxide and glucose concentration measurement using optical fiber grating sensors with corrodible plasmonic nanocoatings. Biomed. Opt. Express 2018, 9, 1735–1744. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Franc, A.; Muselík, J.; Sabadková, D.; Neumann, D. Preparation of pellets with controlled release of glucose as prevention of hypoglycaemia in paediatric patients. Eur. J. Pharm. Sci. 2015, 75, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Sun, W.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Insulin-Responsive Glucagon Delivery for Prevention of Hypoglycemia. Small 2017, 13, 1603028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uskoković, V. When 1 + 1 > 2: Nanostructured Composite Materials for Hard Tissue Engineering Applications. Mat. Sci. Eng. C 2015, 57, 434–451. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uskoković, V. Supplementation of Polymeric Reservoirs with Redox-Responsive Metallic Nanoparticles as a New Concept for the Smart Delivery of Insulin in Diabetes. Materials 2023, 16, 786. https://doi.org/10.3390/ma16020786
Uskoković V. Supplementation of Polymeric Reservoirs with Redox-Responsive Metallic Nanoparticles as a New Concept for the Smart Delivery of Insulin in Diabetes. Materials. 2023; 16(2):786. https://doi.org/10.3390/ma16020786
Chicago/Turabian StyleUskoković, Vuk. 2023. "Supplementation of Polymeric Reservoirs with Redox-Responsive Metallic Nanoparticles as a New Concept for the Smart Delivery of Insulin in Diabetes" Materials 16, no. 2: 786. https://doi.org/10.3390/ma16020786





