Atherosclerotic-Derived Endothelial Cell Response Conducted by Titanium Oxide Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Nanotubes
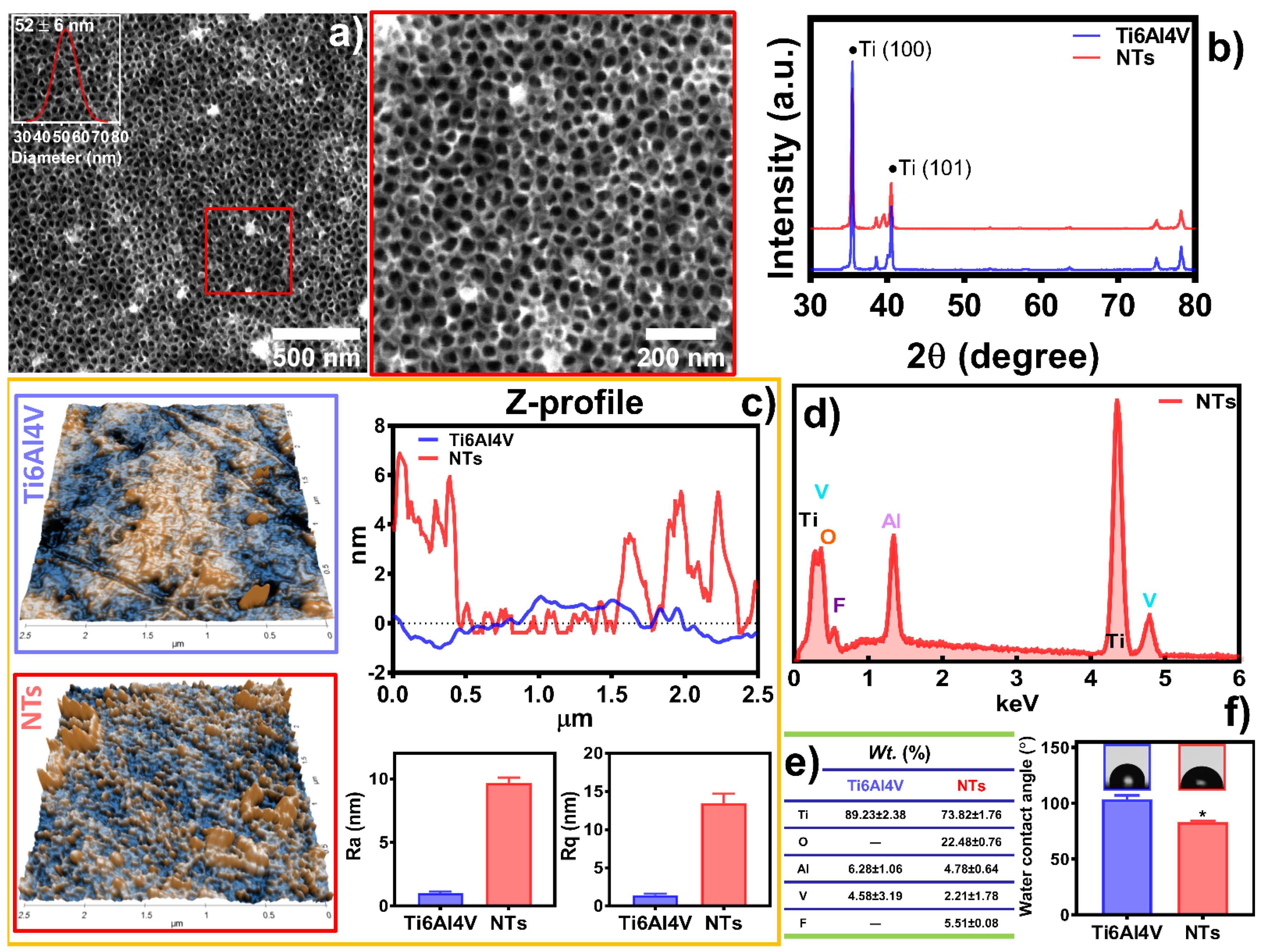
2.2. Surface Characterization
2.3. Isolation of Endothelial Cells from a Human Atherosclerotic Vessel
2.4. Atherosclerotic Endothelial Cell Characterization
2.5. Cell Culture
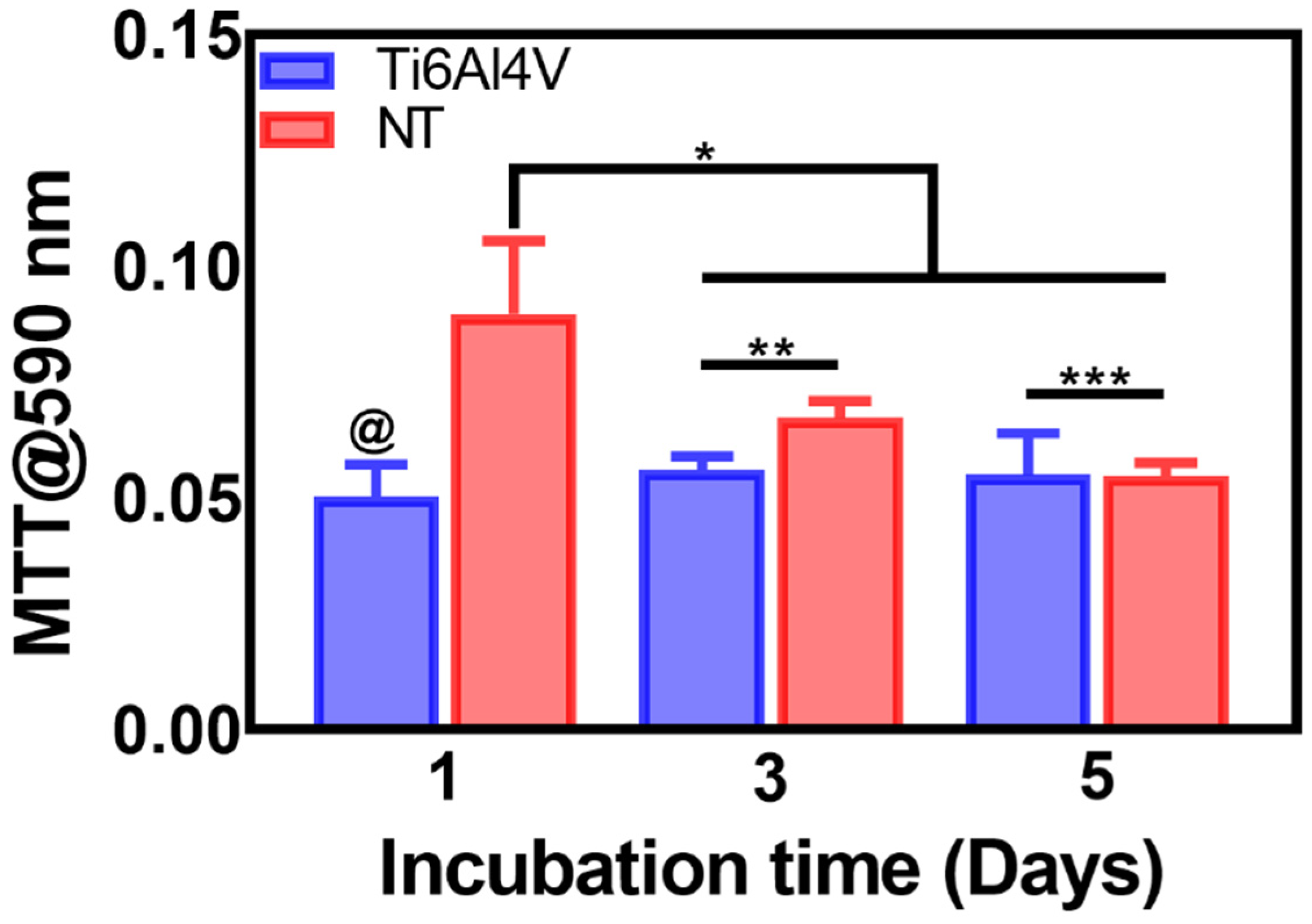
2.6. Cell Viability by MTT
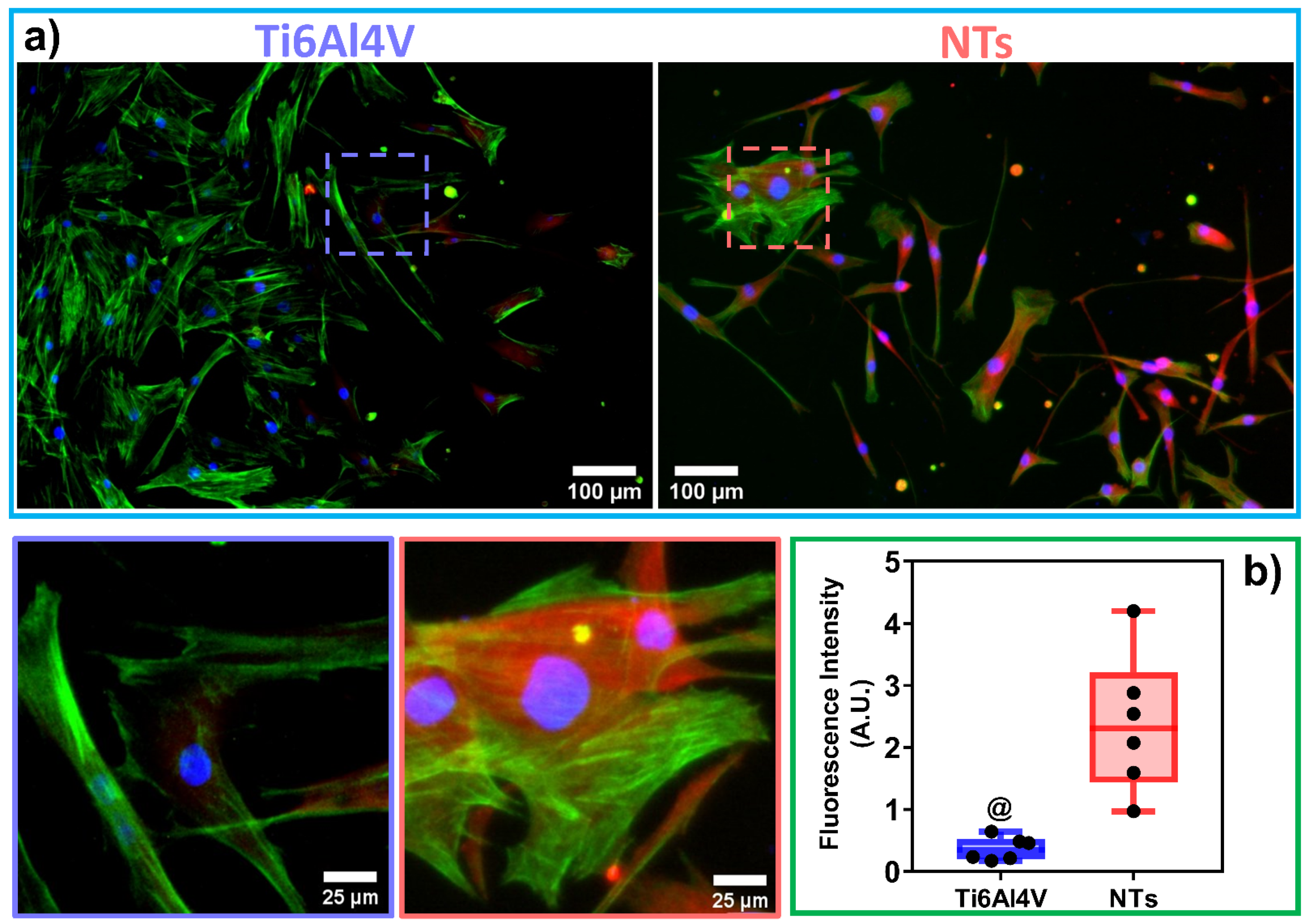
2.7. Immunofluorescence Staining
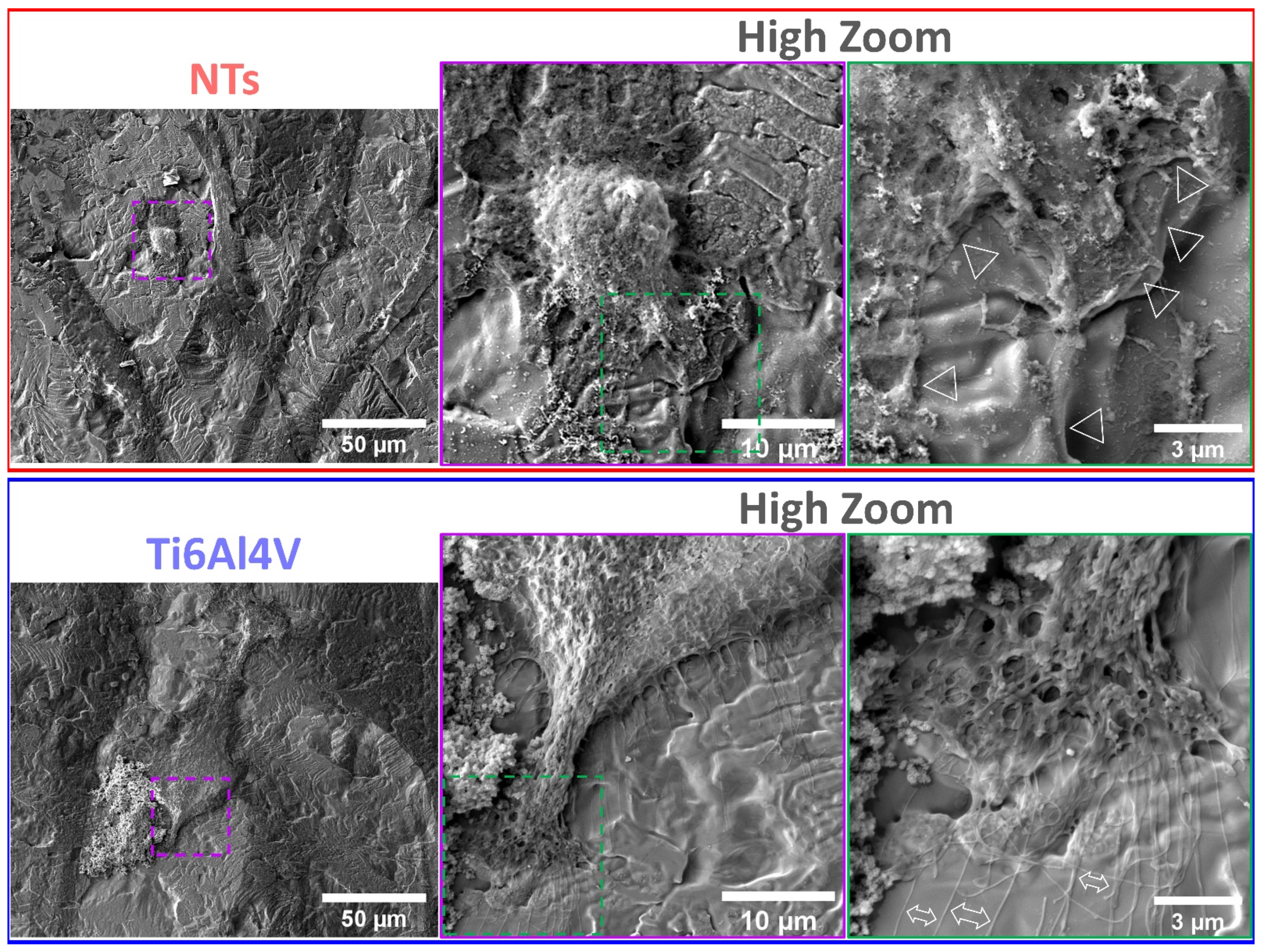
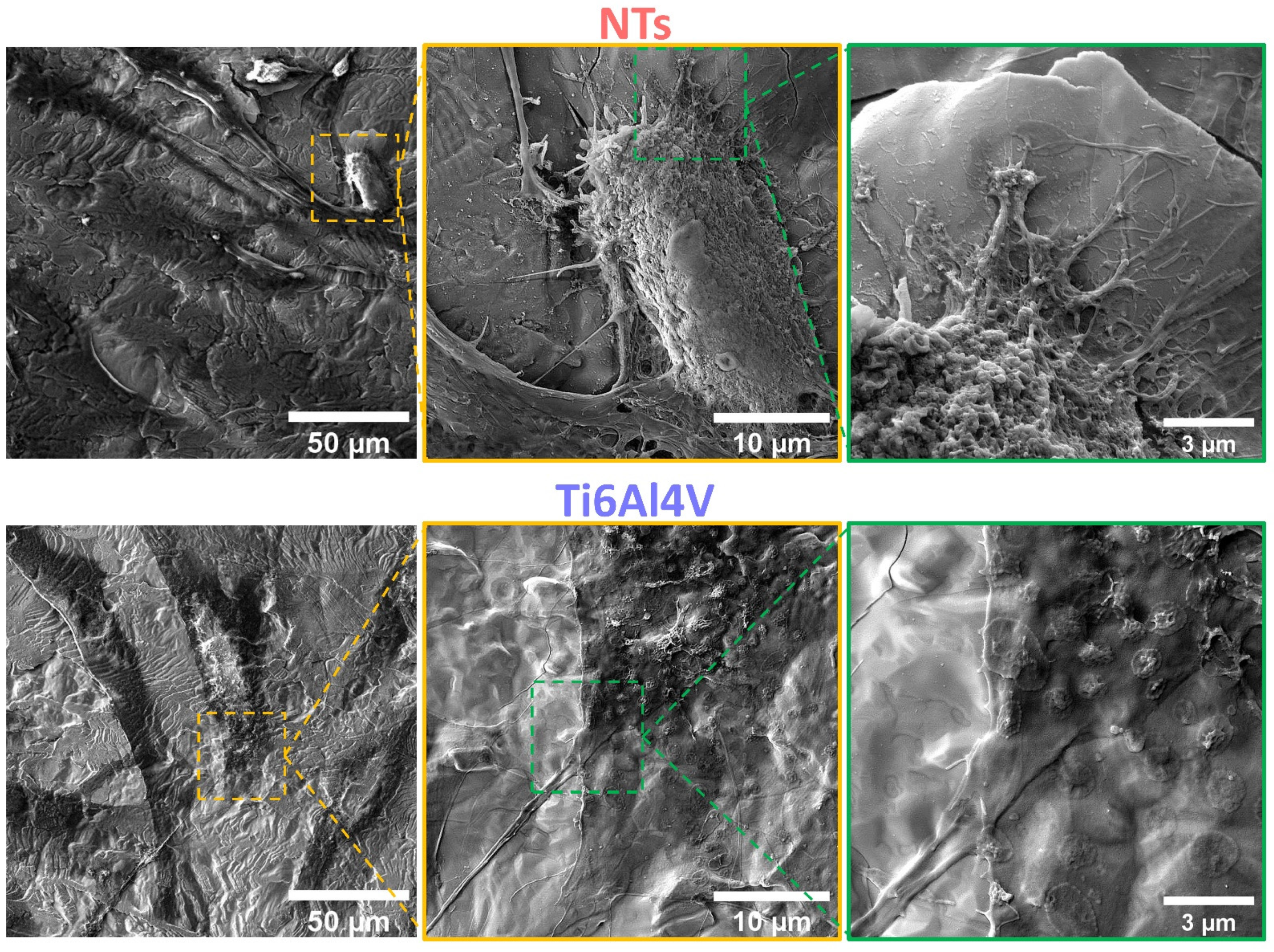
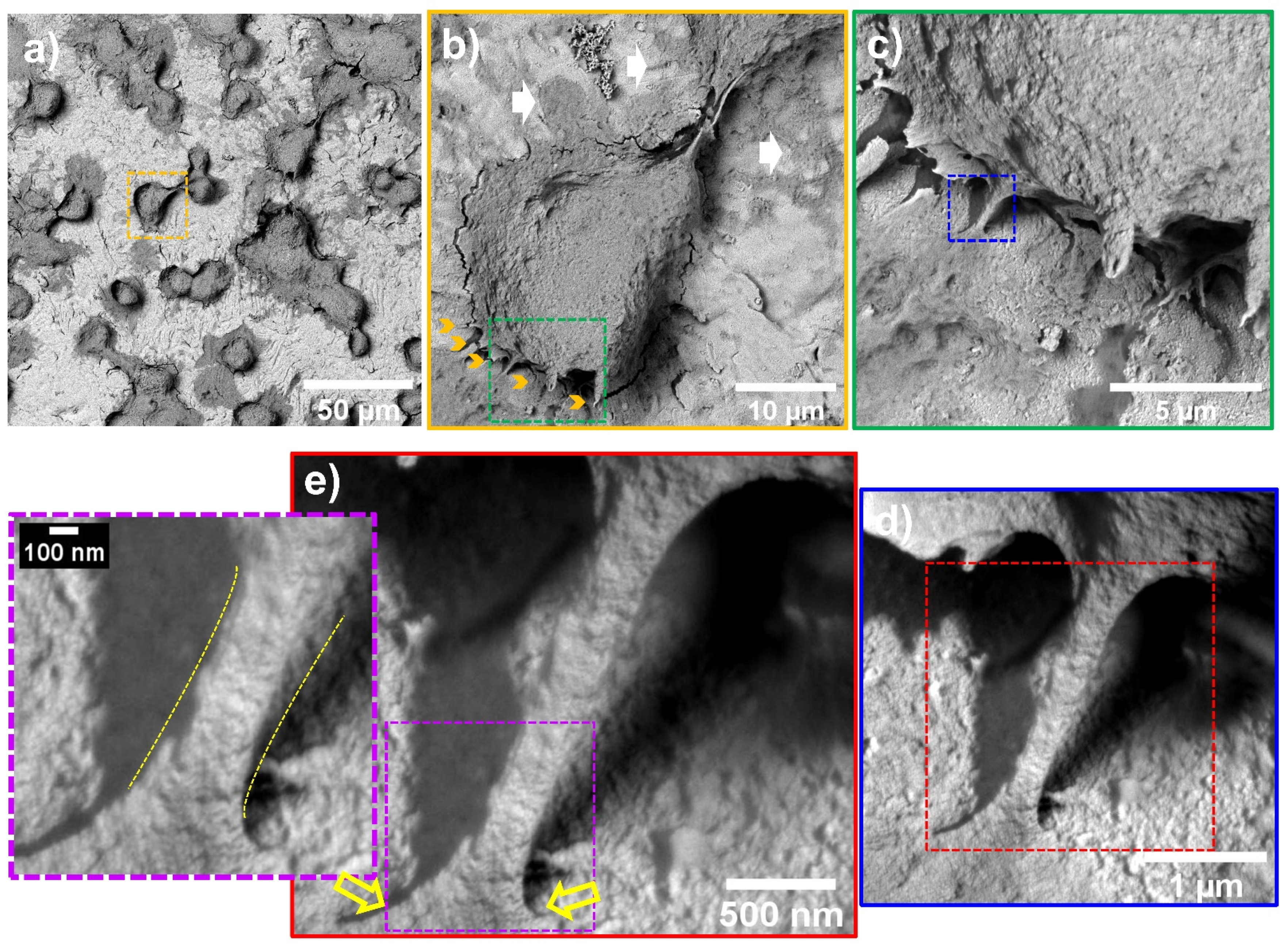
2.8. Atherosclerotic Endothelial Cells Characterization by FE-SEM
2.9. Endothelial Topography Analysis by AFM
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Hussain, R.; Iqbal, S.; Shah, M.; Rehman, W.; Khan, S.; Rasheed, L.; Rahim, F.; Dera, A.A.; Kehili, S.; Elkaeed, E.B.; et al. Synthesis of Novel Benzimidazole-Based Thiazole Derivatives as Multipotent Inhibitors of α-Amylase and α-Glucosidase: In Vitro Evaluation along with Molecular Docking Study. Molecules 2022, 27, 6457. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Shah, M.; Iqbal, S.; Rehman, W.; Khan, S.; Rasheed, L.; Naz, H.; Al-ghulikah, H.A.; Elkaeed, E.B.; Pashameah, R.A.; et al. Molecular iodine-promoted oxidative cyclization for the synthesis of 1,3,4-thiadiazole-fused-[1,2,4]-thiadiazole incorporating 1,4-benzodioxine moiety as potent inhibitors of α-amylase and α-glucosidase: In vitro and in silico study. Front. Chem. 2022, 10, 1023316. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komiyama, H.; Takano, M.; Hata, N.; Seino, Y.; Shimizu, W.; Mizuno, K. Neoatherosclerosis: Coronary stents seal atherosclerotic lesions but result in making a new problem of atherosclerosis. World J. Cardiol. 2015, 7, 776–783. [Google Scholar] [CrossRef]
- Ma, W.-R.; Chandrasekharan, K.H.; Nai, C.-S.; Zhu, Y.-X.; Iqbal, J.; Chang, S.; Cheng, Y.-W.; Wang, X.-Y.; Bourantas, C.V.; Zhang, Y.-J. Clinical outcomes of percutaneous coronary intervention for de novo lesions in small coronary arteries: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1017833. [Google Scholar] [CrossRef]
- Cornelissen, A.; Vogt, F.J. The effects of stenting on coronary endothelium from a molecular biological view: Time for improvement? J. Cell. Mol. Med. 2019, 23, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, R.C.; Baughman, K.L.; Fallon, J.T.; Block, P.C. Scanning electron microscopy after coronary transluminal angioplasty of normal canine coronary arteries. Am. J. Cardiol. 1980, 45, 591–598. [Google Scholar] [CrossRef]
- Boos, C.J.; Balakrishnan, B.; Jessani, S.; Blann, A.D.; Lip, G.Y.H. Effects of Percutaneous Coronary Intervention on Peripheral Venous Blood Circulating Endothelial Cells and Plasma Indices of Endothelial Damage/Dysfunction. Chest 2007, 132, 1920–1926. [Google Scholar] [CrossRef]
- Blann, A.; Midgley, H.; Burrows, G.; Maxwell, S.; Utting, S.; Davies, M.; Waitet, M.; McCollum, C. Free radicals, antioxidants, and endothelial cell damage after percutaneous transluminal coronary angioplasty. Coron. Artery Dis. 1993, 4, 905–910. [Google Scholar] [CrossRef]
- Mungchan, P.; Glab-ampai, K.; Chruewkamlow, N.; Trakarnsanga, K.; Srisawat, C.; Nguyen, K.T.; Chaicumpa, W.; Punnakitikashem, P. Targeted Nanoparticles for the Binding of Injured Vascular Endothelium after Percutaneous Coronary Intervention. Molecules 2022, 27, 8144. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-C.; Xu, H.; Tran, R.T.; Tsai, Y.-T.; Tang, L.; Banerjee, S.; Yang, J.; Nguyen, K.T. In Situ Re-endothelialization via Multifunctional Nanoscaffolds. ACS Nano 2014, 8, 10826–10836. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Ge, S.; Luo, D.; Du, R.; Wang, Y.; Liu, W.; Wang, G.; Yin, T. The endothelium permeability after bioresorbable scaffolds implantation caused by the heterogeneous expression of tight junction proteins. Mater. Today Bio 2022, 16, 100410. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, X.; Yuan, K.; Yin, T.; Du, R.; Shen, L.; Zhu, Z.; Yu, S.; Zhang, H.; Wang, G. Structural and temporal dynamics analysis on drug-eluting stents: History, research hotspots and emerging trends. Bioact. Mater. 2023, 23, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Råmunddal, T.; Holck, E.N.; Karim, S.; Eftekhari, A.; Escaned, J.; Ioanes, D.; Walsh, S.; Spratt, J.; Veien, K.; Jensen, L.O.; et al. International randomized trial on the effect of revascularization or optimal medical therapy of chronic total coronary occlusions with myocardial ischemia—ISCHEMIA-CTO trial—Rationale and design. Am. Heart J. 2023, 257, 41–50. [Google Scholar] [CrossRef]
- Dong, J.; Pacella, M.; Liu, Y.; Zhao, L. Surface engineering and the application of laser-based processes to stents—A review of the latest development. Bioact. Mater. 2022, 10, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.M.; Joseph, J.; Nair, M.B.; Nair, S.V.; Vijayakumar, M.; Menon, D. Coupled benefits of nanotopography and titania surface chemistry in fostering endothelialization and reducing in-stent restenosis in coronary stents. Biomater. Adv. 2022, 142, 213149. [Google Scholar] [CrossRef]
- Oliver, A.A.; Sikora-Jasinska, M.; Demir, A.G.; Guillory, R.J. Recent advances and directions in the development of bioresorbable metallic cardiovascular stents: Insights from recent human and in vivo studies. Acta Biomater. 2021, 127, 1–23. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.-S.; Brott, B.C.; Jun, H.-W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef]
- Hu, X.; Neoh, K.G.; Zhang, J.; Kang, E.-T.; Wang, W. Immobilization strategy for optimizing VEGF’s concurrent bioactivity towards endothelial cells and osteoblasts on implant surfaces. Biomaterials 2012, 33, 8082–8093. [Google Scholar] [CrossRef]
- Bruni, S.; Martinesi, M.; Stio, M.; Treves, C.; Bacci, T.; Borgioli, F. Effects of surface treatment of Ti–6Al–4V titanium alloy on biocompatibility in cultured human umbilical vein endothelial cells. Acta Biomater. 2005, 1, 223–234. [Google Scholar] [CrossRef]
- Treves, C.; Martinesi, M.; Stio, M.; Gutiérrez, A.; Jiménez, J.A.; López, M.F. In vitro biocompatibility evaluation of surface-modified titanium alloys. J. Biomed. Mater. Res. Part A 2010, 92, 1623–1634. [Google Scholar]
- Wang, J.-L.; Li, B.-C.; Li, Z.-J.; Ren, K.-F.; Jin, L.-J.; Zhang, S.-M.; Chang, H.; Sun, Y.-X.; Ji, J. Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials 2014, 35, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.W.; Liau, L.L.; Chua, K.H.; Ahmad, R.; Akbar, S.A.; Pingguan-Murphy, B. Enhanced in vitro angiogenic behaviour of human umbilical vein endothelial cells on thermally oxidized TiO2 nanofibrous surfaces. Sci. Rep. 2016, 6, 21828. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Haberstroh, K.M.; Webster, T.J. Enhanced Functions of Vascular Cells on Nanostructured Ti for Improved Stent Applications. Tissue Eng. 2007, 13, 1421–1430. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Gallagher, J.O.; Jin, S. Enhanced Cellular Mobility Guided by TiO2 Nanotube Surfaces. Nano Lett. 2008, 8, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Partida, E.; Valdéz-Salas, B.; Moreno-Ulloa, A.; Escamilla, A.; Curiel, M.A.; Rosales-Ibáñez, R.; Villarreal, F.; Bastidas, D.M.; Bastidas, J.M. Improved in vitro angiogenic behavior on anodized titanium dioxide nanotubes. J. Nanobiotechnol. 2017, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.; Jung, F.; Pietzsch, J. Human Endothelial Cell Models in Biomaterial Research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Valdez-Salas, B.; Escamilla, A.; Moreno-Ulloa, A.; Burtseva, L.; Valdez-Salas, E.; Curiel Alvarez, M.; Nedev, N. The Promotion of Antibacterial Effects of Ti6Al4V Alloy Modified with TiO2 Nanotubes Using a Superoxidized Solution. J. Nanomater. 2015, 2015, 818565. [Google Scholar] [CrossRef] [Green Version]
- Valdez-Salas, B.; Beltrán-Partida, E.; Curiel-Álvarez, M.; Guerra-Balcázar, M.; Arjona, N. Crystallographic Pattern Mediates Fungal Nanoadhesion Bond Formation on Titanium Nanotubes. ACS Omega 2021, 6, 15625–15636. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltrán-Partida, E.; Nedev, N.; Ibarra-Wiley, R.; Salinas, R.; Curiel-Álvarez, M.; Valenzuela-Ontiveros, Y.; Pérez, G. Controlled antifungal behavior on Ti6Al4V nanostructured by chemical nanopatterning. Mater. Sci. Eng. C 2019, 96, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Salas, B.; Castillo-Uribe, S.; Beltran-Partida, E.; Curiel-Alvarez, M.; Perez-Landeros, O.; Guerra-Balcazar, M.; Cheng, N.; Gonzalez-Mendoza, D.; Flores-Peñaloza, O. Recovering Osteoblast Functionality on TiO(2) Nanotube Surfaces Under Diabetic Conditions. Int. J. Nanomed. 2022, 17, 5469–5488. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.; Robert-Ebadi, H.; Ricco, J.B.; Setacci, C.; Cao, P.; de Donato, G.; Eckstein, H.H.; De Rango, P.; Diehm, N.; Schmidli, J.; et al. Chapter I: Definitions, Epidemiology, Clinical Presentation and Prognosis. Eur. J. Vasc. Endovasc. Surg. 2011, 42, S4–S12. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Ulloa, A.; Nájera-García, N.; Hernández, M.; Ramírez-Sánchez, I.; Taub, P.R.; Su, Y.; Beltrán-Partida, E.; Ceballos, G.; Dugar, S.; Schreiner, G.; et al. A pilot study on clinical pharmacokinetics and preclinical pharmacodynamics of (+)-epicatechin on cardiometabolic endpoints. Food Funct. 2018, 9, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Partida, E.; Valdez-Salas, B.; Curiel-Álvarez, M.; Castillo-Uribe, S.; Escamilla, A.; Nedev, N. Enhanced antifungal activity by disinfected titanium dioxide nanotubes via reduced nano-adhesion bonds. Mater. Sci. Eng. C 2017, 76, 59–65. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Valdez-Salas, B.; Escamilla, A.; Curiel, M.; Valdez-Salas, E.; Nedev, N.; Bastidas, J.M. Disinfection of titanium dioxide nanotubes using super-oxidized water decrease bacterial viability without disrupting osteoblast behavior. Mater. Sci. Eng. C 2016, 60, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Feng, Y.; Rijnaarts, H.H.M.; Yntema, D.; Gong, Z.; Dionysiou, D.D.; Cao, Z.; Miao, S.; Chen, Y.; Ye, Y.; Wang, Y. Applications of anodized TiO2 nanotube arrays on the removal of aqueous contaminants of emerging concern: A review. Water Res. 2020, 186, 116327. [Google Scholar] [CrossRef]
- Wang, L.; Gong, T.; Brown, Z.; Gu, Y.; Teng, K.; Ye, W.; Ming, W. Preparation of Ascidian-Inspired Hydrogel Thin Films to Selectively Induce Vascular Endothelial Cell and Smooth Muscle Cell Growth. ACS Appl. Bio Mater. 2020, 3, 2068–2077. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Zanetti, A.; Corada, M.; Takahashi, T.; Balconi, G.; Breviario, F.; Orsenigo, F.; Cattelino, A.; Kemler, R.; Daniel, T.O.; et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, β-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 2003, 161, 793–804. [Google Scholar] [CrossRef]
- Hood, J.D.; Meininger, C.J.; Ziche, M.; Granger, H.J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 1998, 274, H1054–H1058. [Google Scholar] [CrossRef]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell–scaffold interactions in tissue engineering for oral and craniofacial reconstruction. Bioact. Mater. 2023, 23, 16–44. [Google Scholar] [CrossRef]
- Pesen, D.; Hoh, J.H. Micromechanical Architecture of the Endothelial Cell Cortex. Biophys. J. 2005, 88, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Lappalainen, P.; Kotila, T.; Jégou, A.; Romet-Lemonne, G. Biochemical and mechanical regulation of actin dynamics. Nat. Rev. Mol. Cell Biol. 2022, 23, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Yadunandanan Nair, N.; Samuel, V.; Ramesh, L.; Marib, A.; David, D.T.; Sundararaman, A. Actin cytoskeleton in angiogenesis. Biol. Open 2022, 11, bio058899. [Google Scholar] [CrossRef] [PubMed]
- Cutiongco, M.F.A.; Chua, B.M.X.; Neo, D.J.H.; Rizwan, M.; Yim, E.K.F. Functional differences between healthy and diabetic endothelial cells on topographical cues. Biomaterials 2018, 153, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Alushin, G.M. Cellular force-sensing through actin filaments. FEBS J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.C.; Sreerekha, P.R.; Divyarani, V.V.; Nair, S.; Chennazhi, K.; Menon, D. Influence of titania nanotopography on human vascular cell functionality and its proliferation in vitro. J. Mater. Chem. 2012, 22, 1326–1340. [Google Scholar] [CrossRef]
- Wu, B.; Tang, Y.; Wang, K.; Zhou, X.; Xiang, L. Nanostructured Titanium Implant Surface Facilitating Osseointegration from Protein Adsorption to Osteogenesis: The Example of TiO(2) NTAs. Int. J. Nanomed. 2022, 17, 1865–1879. [Google Scholar] [CrossRef]
- Li, S.; Huang, N.F.; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005, 96, 1110–1126. [Google Scholar] [CrossRef]
- Charbonier, F.W.; Zamani, M.; Huang, N.F. Endothelial Cell Mechanotransduction in the Dynamic Vascular Environment. Adv. Biosyst. 2019, 3, 1800252. [Google Scholar] [CrossRef]
- Modaresifar, K.; Ganjian, M.; Díaz-Payno, P.J.; Klimopoulou, M.; Koedam, M.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Mechanotransduction in high aspect ratio nanostructured meta-biomaterials: The role of cell adhesion, contractility, and transcriptional factors. Mater. Today Bio 2022, 16, 100448. [Google Scholar] [CrossRef]
- Morgan, J.T.; Wood, J.A.; Shah, N.M.; Hughbanks, M.L.; Russell, P.; Barakat, A.I.; Murphy, C.J. Integration of basal topographic cues and apical shear stress in vascular endothelial cells. Biomaterials 2012, 33, 4126–4135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, T.; Angom, R.S.; Das, P.; Bhattacharya, S.; Mukhopadhyay, D. Nanomechanical insights: Amyloid beta oligomer-induced senescent brain endothelial cells. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2019, 1861, 183061. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; He, H.; Chen, Y.; Zeng, F.; Huang, J.; Wu, L.; Chen, Y. Effects of long-term serial cell passaging on cell spreading, migration, and cell-surface ultrastructures of cultured vascular endothelial cells. Cytotechnology 2014, 66, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebda, N.; Dubrovskyi, O.; Birukov, K.G. Focal Adhesion Kinase Regulation of Mechanotransduction and its Impact on Endothelial Cell Functions. Microvasc. Res. 2012, 83, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Farhat, N.; Thorin-Trescases, N.; Voghel, G.; Villeneuve, L.; Mamarbachi, M.; Perrault, L.P.; Carrier, M.; Thorin, E. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can. J. Physiol. Pharmacol. 2008, 86, 761–769. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular Senescence in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2018, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Kim, E.-C.; Son, Y.; Lee, D.-W.; Park, Y.S.; Choi, J.H.; Cho, K.-H.; Kwon, K.-S.; Kim, J.-R. CD9 induces cellular senescence and aggravates atherosclerotic plaque formation. Cell Death Differ. 2020, 27, 2681–2696. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial Cell Senescence in Human Atherosclerosis. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef]

| Age, Years | 62 |
| Gender | Male |
| Nicotine use (Smoker) | - |
| Comorbidity | |
| Type 2 Diabetes mellitus | 10 years of follow up |
| Hypoglycemic drugs | Metformin |
| Hypertension | - |
| Dyslipidemia | - |
| Cerebrovascular disease | 3 years of follow up |
| Blood analysis | |
| Glucose, mg/dL | 89.1 |
| Urea, mg/dL | 56.3 |
| Creatinine, mg/dL | 1.7 |
| Hemoglobin, g/dL | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Partida, E.; Valdez-Salas, B.; García-López Portillo, M.; Gutierrez-Perez, C.; Castillo-Uribe, S.; Salvador-Carlos, J.; Alcocer-Cañez, J.; Cheng, N. Atherosclerotic-Derived Endothelial Cell Response Conducted by Titanium Oxide Nanotubes. Materials 2023, 16, 794. https://doi.org/10.3390/ma16020794
Beltrán-Partida E, Valdez-Salas B, García-López Portillo M, Gutierrez-Perez C, Castillo-Uribe S, Salvador-Carlos J, Alcocer-Cañez J, Cheng N. Atherosclerotic-Derived Endothelial Cell Response Conducted by Titanium Oxide Nanotubes. Materials. 2023; 16(2):794. https://doi.org/10.3390/ma16020794
Chicago/Turabian StyleBeltrán-Partida, Ernesto, Benjamín Valdez-Salas, Martha García-López Portillo, Claudia Gutierrez-Perez, Sandra Castillo-Uribe, Jorge Salvador-Carlos, José Alcocer-Cañez, and Nelson Cheng. 2023. "Atherosclerotic-Derived Endothelial Cell Response Conducted by Titanium Oxide Nanotubes" Materials 16, no. 2: 794. https://doi.org/10.3390/ma16020794
APA StyleBeltrán-Partida, E., Valdez-Salas, B., García-López Portillo, M., Gutierrez-Perez, C., Castillo-Uribe, S., Salvador-Carlos, J., Alcocer-Cañez, J., & Cheng, N. (2023). Atherosclerotic-Derived Endothelial Cell Response Conducted by Titanium Oxide Nanotubes. Materials, 16(2), 794. https://doi.org/10.3390/ma16020794





