The Influence of Long-Time Storage on the Structure and Properties of Multi-Block Thermoplastic Polyurethanes Based on Poly(butylene adipate) Diol and Polycaprolactone Diol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
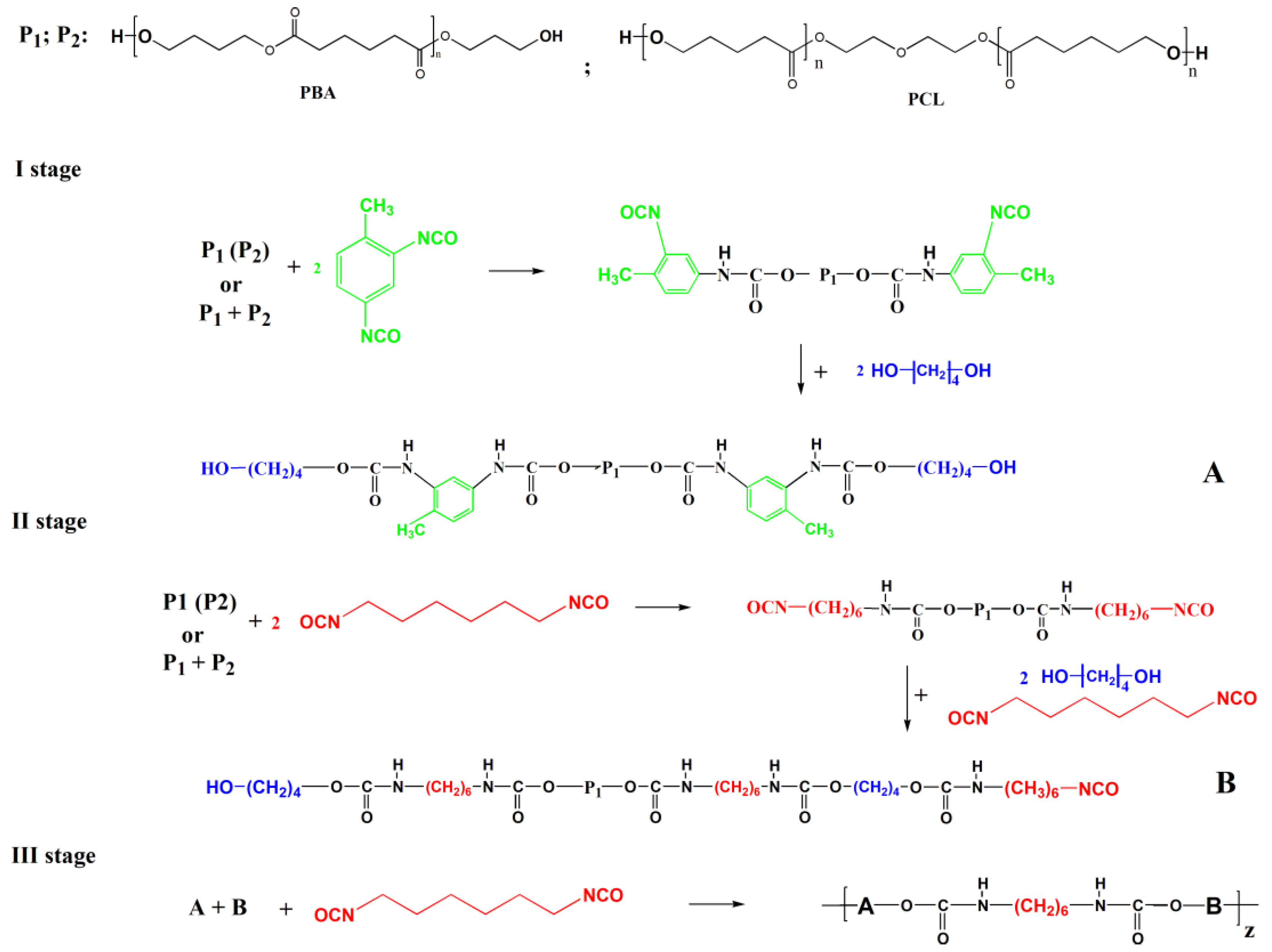
2.2. Synthesis of Multi-Block Thermoplastic Polyurethane (TPU)
2.3. Characterization
3. Results
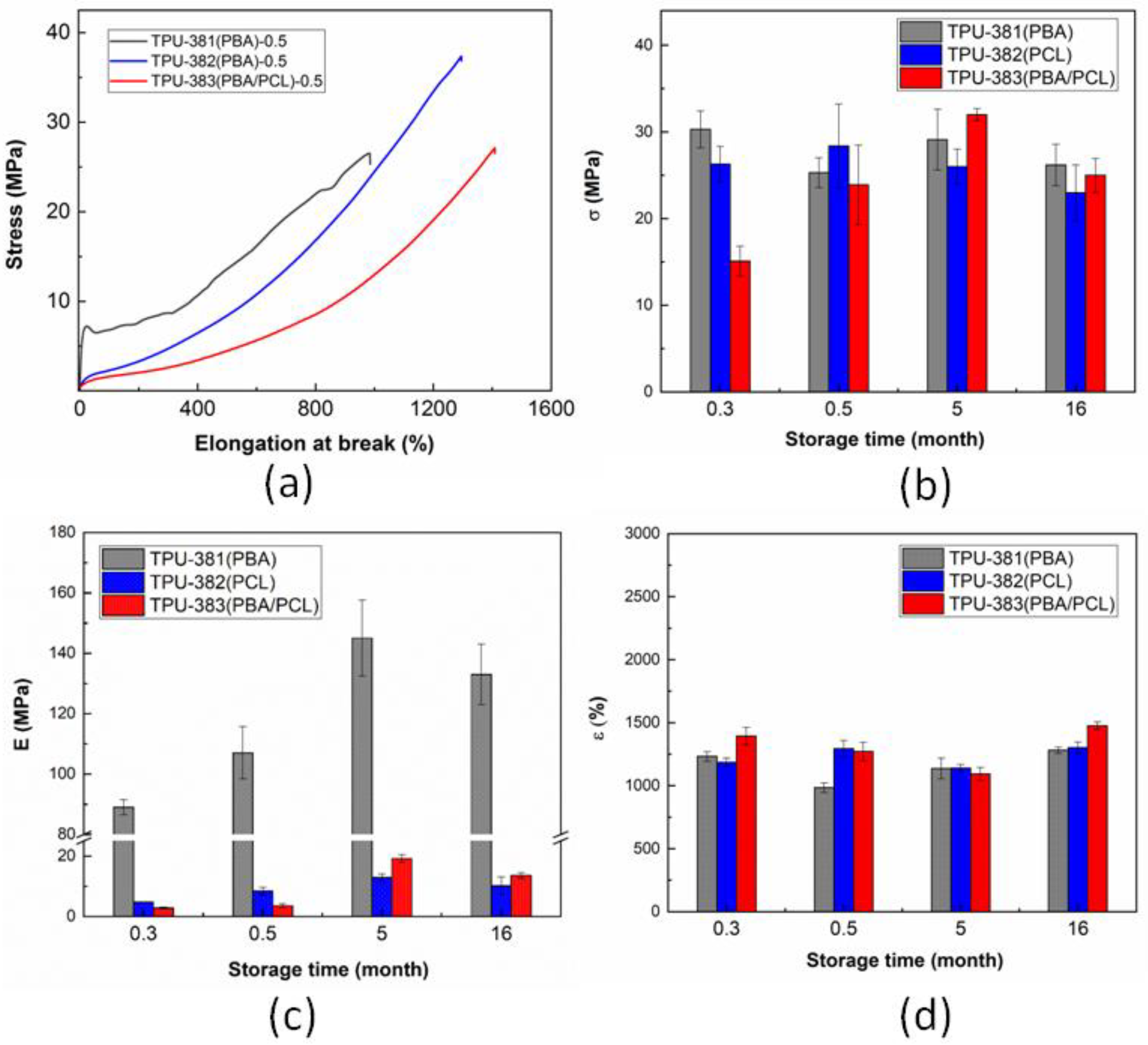
3.1. Tensile Behavior of TPUs at Long-Time Storage
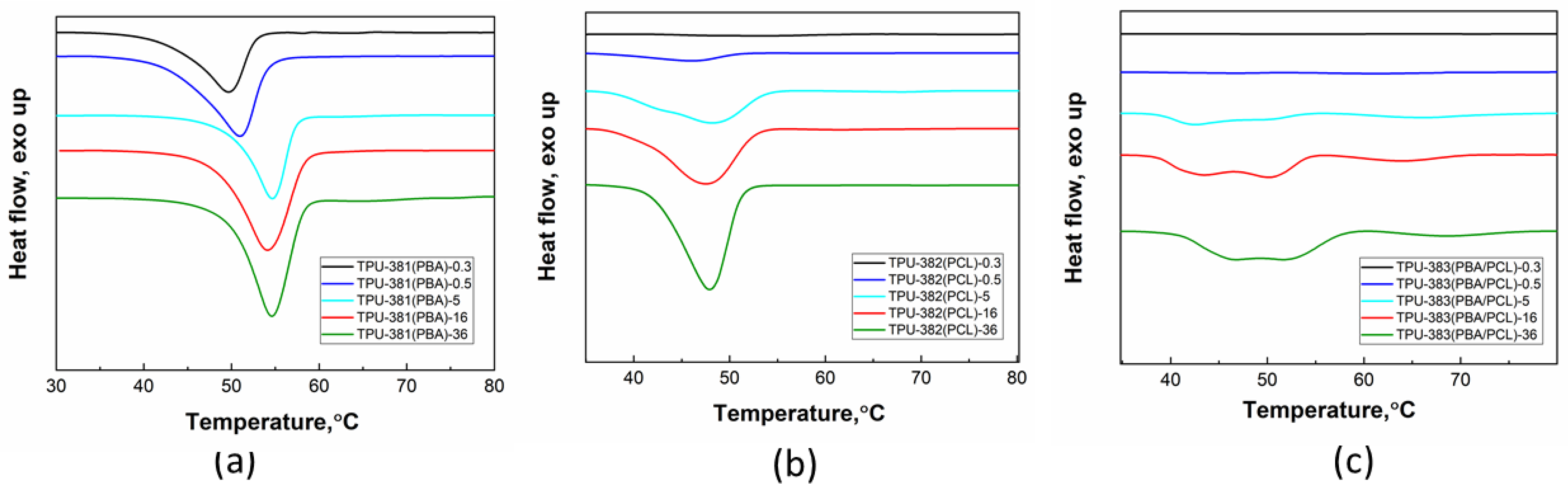
3.2. Thermal Properties of TPUs at Long-Time Storage
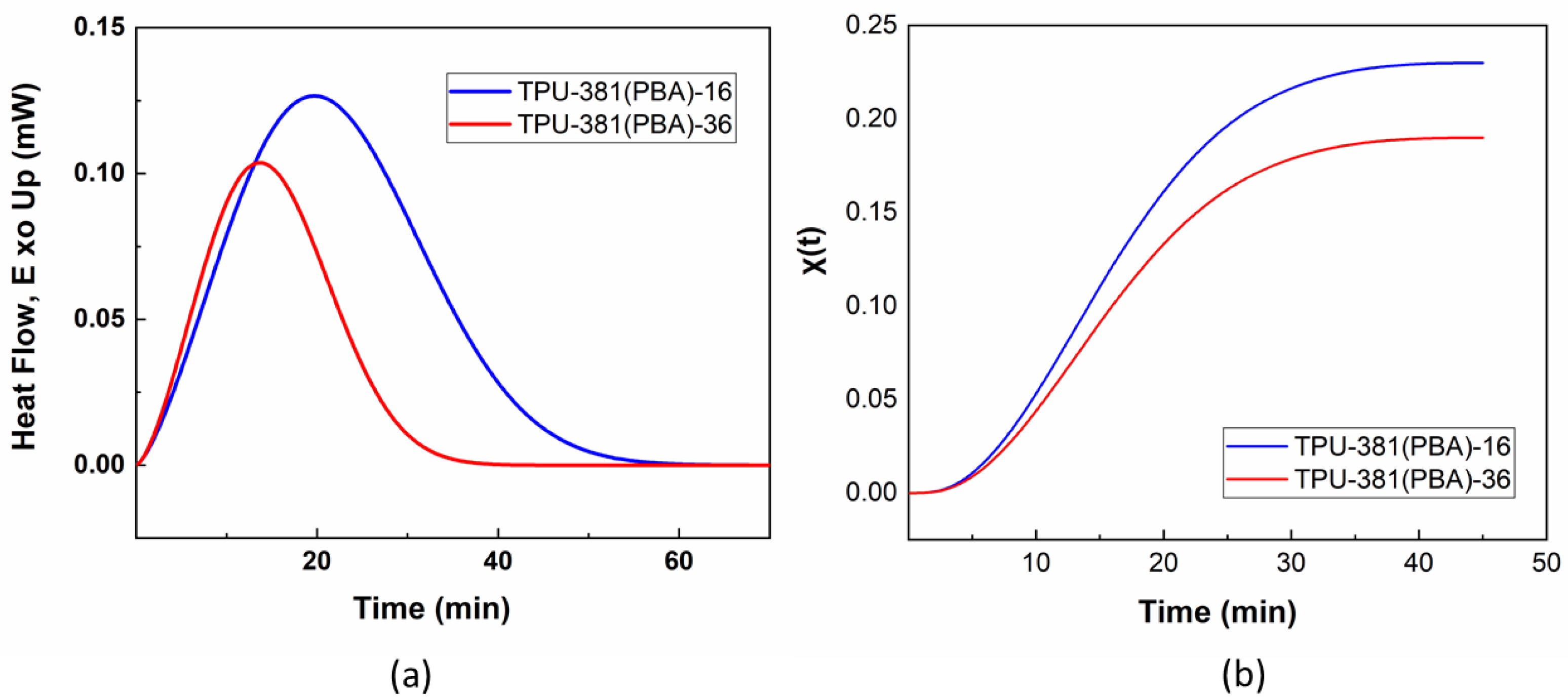
3.3. Isothermal Crystallization Kinetics of TPUs
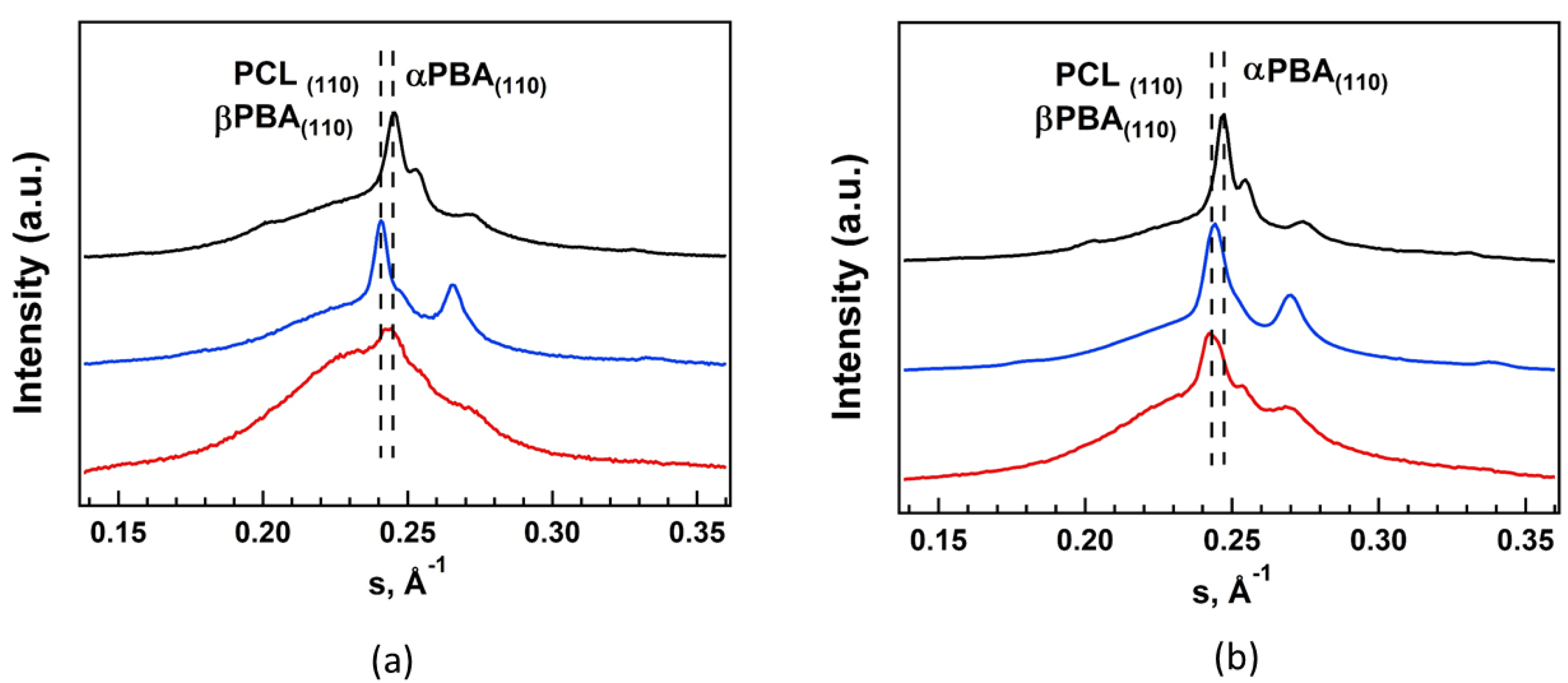
3.4. Crystal Structure and Phase Composition of TPUs
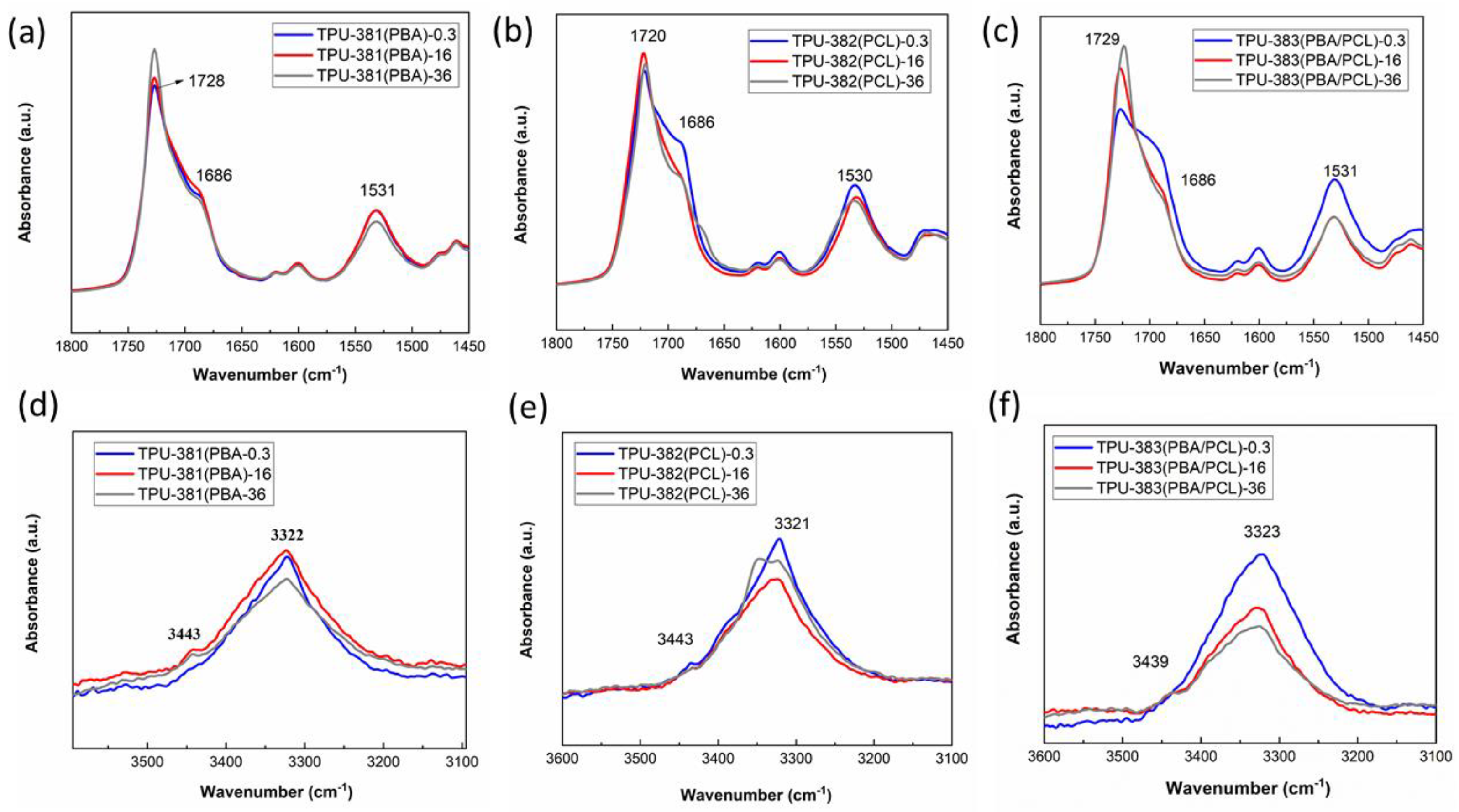
3.5. Microphase Structure and Hydrogen Bonding of TPUs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anokhin, D.V.; Gorbunova, M.A.; Abukaev, A.F.; Ivanov, D.A. Multiblock Thermoplastic Polyurethanes: In Situ Studies of Structural and Morphological Evolution under Strain. Materials 2021, 14, 3009. [Google Scholar] [CrossRef]
- Sabahi, N.; Chen, W.; Wang, C.H.; Kruzic, J.J.; Li, X. A Review on Additive Manufacturing of Shape-Memory Materials for Biomedical Applications. Jom 2020, 72, 1229–1253. [Google Scholar] [CrossRef]
- Chalissery, D.; Pretsch, T.; Staub, S.; Andrä, H. Additive Manufacturing of Information Carriers Based on Shape Memory Polyester Urethane. Polymers 2019, 11, 1005. [Google Scholar] [CrossRef] [Green Version]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing-Characterization and Fabrication. Materials 2019, 16, 887. [Google Scholar] [CrossRef] [Green Version]
- Gorbunova, M.A.A.; Shukhardin, D.M.M.; Lesnichaya, V.A.A.; Badamshina, E.R.R.; Anokhin, D.V. New Polyurethane Urea Thermoplastic Elastomers with Controlled Mechanical and Thermal Properties for Medical Applications. Key Eng. Mater. 2019, 816, 187–191. [Google Scholar] [CrossRef]
- Gorbunova, M.; Komratova, V.; Grishchuk, A.; Badamshina, E.; Anokhin, D. The Effect of Addition of Low-Layer Graphene Nanoparticles on Structure and Mechanical Properties of Polyurethane-Based Block Copolymers. Polym. Bull. 2019, 76, 5813–5829. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, R.-Y.; Ying, W.-B.; Hu, H.; Wang, Y.-B.; Guo, Y.-Q.; Wang, W.-Q.; Tang, Z.-B.; Zhu, J. Polyether-Polyester and HMDI Based Polyurethanes: Effect of PLLA Content on Structure and Property. Chinese J. Polym. Sci. 2019, 37, 1152–1161. [Google Scholar] [CrossRef]
- Schönfeld, D.; Chalissery, D.; Wenz, F.; Specht, M.; Eberl, C.; Pretsch, T. Actuating Shape Memory Polymer for Thermoresponsive Soft Robotic Gripper and Programmable Materials. Molecules 2021, 26, 522–562. [Google Scholar] [CrossRef] [PubMed]
- Puszka, A.; Sikora, J.W. Synthesis and Characterization of New Polycarbonate-Based Poly(Thiourethane-Urethane)S. Polymers 2022, 14, 2933. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical Applications of Polyurethane Materials and Coatings. Trans. Inst. Met. Finish. 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Marcano, A.; Fatyeyeva, K.; Koun, M.; Dubuis, P.; Grimme, M.; Marais, S. Recent Developments in the Field of Barrier and Permeability Properties of Segmented Polyurethane Elastomers. Rev. Chem. Eng. 2019, 35, 445–474. [Google Scholar] [CrossRef]
- Que, Y.H.; Shi, Y.; Liu, L.Z.; Wang, Y.X.; Wang, C.C.; Zhang, H.C.; Han, X.Y. The Crystallisation, Microphase Separation and Mechanical Properties of the Mixture of Ether-Based Tpu with Different Ester-Based Tpus. Polymers 2021, 13, 3475. [Google Scholar] [CrossRef]
- Cheng, B.-X.; Gao, W.-C.; Ren, X.-M.; Ouyang, X.-Y.; Zhao, Y.; Zhao, H.; Wu, W.; Huang, C.-X.; Liu, Y.; Liu, X.-Y.; et al. A Review of Microphase Separation of Polyurethane: Characterization and Applications. Polym. Test. 2022, 107, 107489. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Manas-Zloczower, I. Understanding Phase Separation and Morphology in Thermoplastic Polyurethanes Nanocomposites. Polymer. 2016, 90, 256–263. [Google Scholar] [CrossRef]
- Liu, W.-K.; Zhao, Y.; Wang, R.; Luo, F.; Li, J.-S.; Li, J.-H.; Tan, H. Effect of Chain Extender on Hydrogen Bond and Microphase Structure of Biodegradable Thermoplastic Polyurethanes. Chinese J. Polym. Sci. 2018, 36, 514–520. [Google Scholar] [CrossRef]
- Prisacariu, C.; Scortanu, E. Influence of Macrodiol on Phase Separation and Crystallization Processes in Hard-Phase Reinforced Polyurethane Elastomers Based on Isocyanates of Variable Conformational Mobility. Int. J. Polym. Anal. Charact. 2010, 15, 277–286. [Google Scholar] [CrossRef]
- Kojio, K.; Nozaki, S.; Takahara, A.; Yamasaki, S. Influence of Chemical Structure of Hard Segments on Physical Properties of Polyurethane Elastomers: A Review. J. Polym. Res. 2020, 27, 64–67. [Google Scholar] [CrossRef]
- Yoon, P.J.; Han, C.D. Effect of Thermal History on the Rheological Behavior of Thermoplastic Polyurethanes. Macromolecules 2000, 33, 2171–2183. [Google Scholar] [CrossRef]
- Xie, K.; Xu, S.; Hao, W.; Wang, J.; Huang, A.; Zhang, Y. Surface Effect of the MgCl2 Support in Ziegler–Natta Catalyst for Ethylene Polymerization: A Computational Study. Appl. Surf. Sci. 2022, 589, 153002. [Google Scholar] [CrossRef]
- Xie, K.; Wang, W.; Li, Y.; Xu, M.; Han, Z.; Zhang, Y.; Gao, W. Study on Structure-Performance Relationship of RGO Enhanced Polypropylene Composites with Improved Atomic Oxygen Resistance. Compos. Part B Eng. 2022, 239, 109970. [Google Scholar] [CrossRef]
- Cao, S.; Ge, W.; Yang, Y.; Huang, Q.; Wang, X. High Strength, Flexible, and Conductive Graphene/Polypropylene Fiber Paper Fabricated via Papermaking Process. Adv. Compos. Hybrid Mater. 2022, 5, 104–112. [Google Scholar] [CrossRef]
- Odegard, G.M.; Bandyopadhyay, A. Physical Aging of Epoxy Polymers and Their Composites. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1695–1716. [Google Scholar] [CrossRef]
- Anokhin, D.V.; Gorbunova, M.A.; Estrin, Y.I.; Komratova, V.V.; Badamshina, E.R. The Role of Fast and Slow Processes in the Formation of Structure and Properties of Thermoplastic Polyurethanes. Phys. Chem. Chem. Phys. 2016, 18, 31769–31776. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.E.; Lodygina, V.P.; Komratova, V.V.; Gorbunova, M.A.; Badamshina, E.R. New IR-Spectroscopic Methods for Determining the Hydroxyl Content in Oligomers. J. Appl. Spectrosc. 2017, 84, 211–216. [Google Scholar] [CrossRef]
- Gorbunova, M.A.; Anokhin, D.V.; Lesnichaya, V.A.; Grishchuk, A.A.; Badamshina, E.R. Optimization of Structure of Soft Block for Design of Adaptive Polyurethanes. Key Eng. Mater. 2020, 869, 273–279. [Google Scholar] [CrossRef]
- He, Y.; Xie, D.; Zhang, X. The Structure, Microphase-Separated Morphology, and Property of Polyurethanes and Polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Meares, P. Polymers: Properties Structure and Bulk Covlometric Analysis; Van Nostrand: New York, NY, USA, 1965. [Google Scholar]
- Hay, J.N. Application of the Modified Avrami Equations to Polymer Crystallisation Kinetics. Br. Polym. J. 1971, 3, 74–82. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, H.; He, S.; Liu, X.; Liu, W.; Huang, M.; Zhu, C. Polyurethane as Smart Biocoatings: Effects of Hard Segments on Phase Structures and Properties. Prog. Org. Coatings 2021, 150, 106000. [Google Scholar] [CrossRef]
- Hamley, I.W. Crystallization in Block Copolymers. In Interfaces Crystallization Viscoelasticity; Springer: Berlin/Heidelberg, Germany, 1999; pp. 113–137. ISBN 978-3-540-48836-1. [Google Scholar]
- Christian, J.W. The Theory of Transformations in Metals and Alloys; Elsevier: Oxford, UK, 2002; ISBN 9780080440194. [Google Scholar]
- Xiu, Y.; Zhang, Z.; Wang, D.; Ying, S.; Li, J. Hydrogen Bonding and Crystallization Behaviour of Segmented Polyurethaneurea: Effects of Hard Segment Concentration. Polymer (Guildf) 1992, 33, 1335–1338. [Google Scholar] [CrossRef]
- Hu, H.; Dorset, D.L. Crystal Structure of Poly(Iε-Caprolactone). Macromolecules 1990, 23, 4604–4607. [Google Scholar] [CrossRef]
- Gan, Z.; Kuwabara, K.; Abe, H.; Iwata, T.; Doi, Y. Metastability and Transformation of Polymorphic Crystals in Biodegradable Poly (Butylene Adipate). Biomacromolecules 2004, 5, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, M.A.; Komov, E.V.; Grunin, L.Y.; Ivanova, M.S.; Abukaev, A.F.; Imamutdinova, A.M.; Ivanov, D.A.; Anokhin, D.V. The Effect of Separation of Blocks on the Crystallization Kinetics and Phase Composition of Poly (Butylene Adipate) in Multi-Block Thermoplastic Polyurethanes. Phys. Chem. Chem. Phys. 2022, 24, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Piegat, A.; Sonseca Olalla, Á.; El Fray, M. New Approach to Evaluate Microphase Separation in Segmented Polyurethanes Containing Carbonate Macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Ciobanu, L.C.; Ciobanu, C.; Dorohoi, D. ATR-FTIR Studies of the Temperature Effects on Polyurethane Doped with Silver Nanoparticles. High Perform. Polym. 2010, 22, 56–68. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.Y.; Xu, M. Polyurethanes Having Shape Memory Effects. Polymer 1996, 37, 5781–5793. [Google Scholar] [CrossRef]
| Polymer | Polymer Composition | Mass Fraction of Reagents, % | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | Polyol | Diisocyanate | Chain Extender | SS/HS | |
| TPU-381(PBA) | PBA | - | 69 | 23 | 8 | 2.2 |
| TPU-382(PCL) | - | PCL | 69 | 23 | 8 | 2.2 |
| TPU-383(PBA/PCL) | PBA | PCL | 69 | 23 | 8 | 2.2 |
| Samples | Tg, °C | Tonset, °C | Tmax, °C | ΔHm, J/g | χcPBA, % | χcPCL, % |
|---|---|---|---|---|---|---|
| TPU-381(PBA) | ||||||
| TPU-381(PBA)-0.3 | −48 | 43 | 49 | 30 | 31 | - |
| TPU-381(PBA)-0.5 | −48 | 43 | 50 | 32 | 33 | - |
| TPU-381(PBA)-5 | −49 | 49 | 54 | 34 | 35 | - |
| TPU-381(PBA)-16 | −49 | 48 | 54 | 35 | 36 | - |
| TPU-381(PBA)-36 | −49 | 50 | 55 | 35 | 37 | - |
| TPU-382(PCL) | ||||||
| TPU-382(PCL)-0.3 | −51 | - | - | - | - | - |
| TPU-382(PCL)-0.5 | −51 | 37 | 46 | 19 | - | 9 |
| TPU-382(PCL)-5 | −53 | 37 | 42/48 | 24 | - | 12 |
| TPU-382(PCL)-16 | −54 | 40 | 47 | 35 | - | 17 |
| TPU-382(PCL)-36 | −54 | 42 | 48 | 39 | - | 19 |
| TPU-383(PBA/PCL) | ||||||
| TPU-383(PBA/PCL)-0.3 | −49 | - | - | - | - | - |
| TPU-383(PBA/PCL)-0.5 | −49 | 40/53/- | 45/61/- | 2/1/- | 3 | 0.5 |
| TPU-383(PBA/PCL)-5 | −57 | 39/45/57 | 43/49/66 | 4/3/3 | 6 | 2 |
| TPU-383(PBA/PCL)-16 | −58 | 39/47/55 | 44/50/63 | 5/4/4 | 9 | 3 |
| TPU-383(PBA/PCL)-36 | −58 | 40/49/57 | 47/53/67 | 12/6/5 | 12 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbunova, M.A.; Anokhin, D.V.; Abukaev, A.F.; Ivanov, D.A. The Influence of Long-Time Storage on the Structure and Properties of Multi-Block Thermoplastic Polyurethanes Based on Poly(butylene adipate) Diol and Polycaprolactone Diol. Materials 2023, 16, 818. https://doi.org/10.3390/ma16020818
Gorbunova MA, Anokhin DV, Abukaev AF, Ivanov DA. The Influence of Long-Time Storage on the Structure and Properties of Multi-Block Thermoplastic Polyurethanes Based on Poly(butylene adipate) Diol and Polycaprolactone Diol. Materials. 2023; 16(2):818. https://doi.org/10.3390/ma16020818
Chicago/Turabian StyleGorbunova, Marina A., Denis V. Anokhin, Ainur F. Abukaev, and Dimitri A. Ivanov. 2023. "The Influence of Long-Time Storage on the Structure and Properties of Multi-Block Thermoplastic Polyurethanes Based on Poly(butylene adipate) Diol and Polycaprolactone Diol" Materials 16, no. 2: 818. https://doi.org/10.3390/ma16020818






