Efficient Production of Segmented Carbon Nanofibers via Catalytic Decomposition of Trichloroethylene over Ni-W Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of 100Ni and 96Ni-4W Catalysts
2.3. Studies on the Metal Dusting Process and Carbon Deposition
2.4. Characterization of Catalysts and Carbon Nanomaterials
3. Results and Discussion
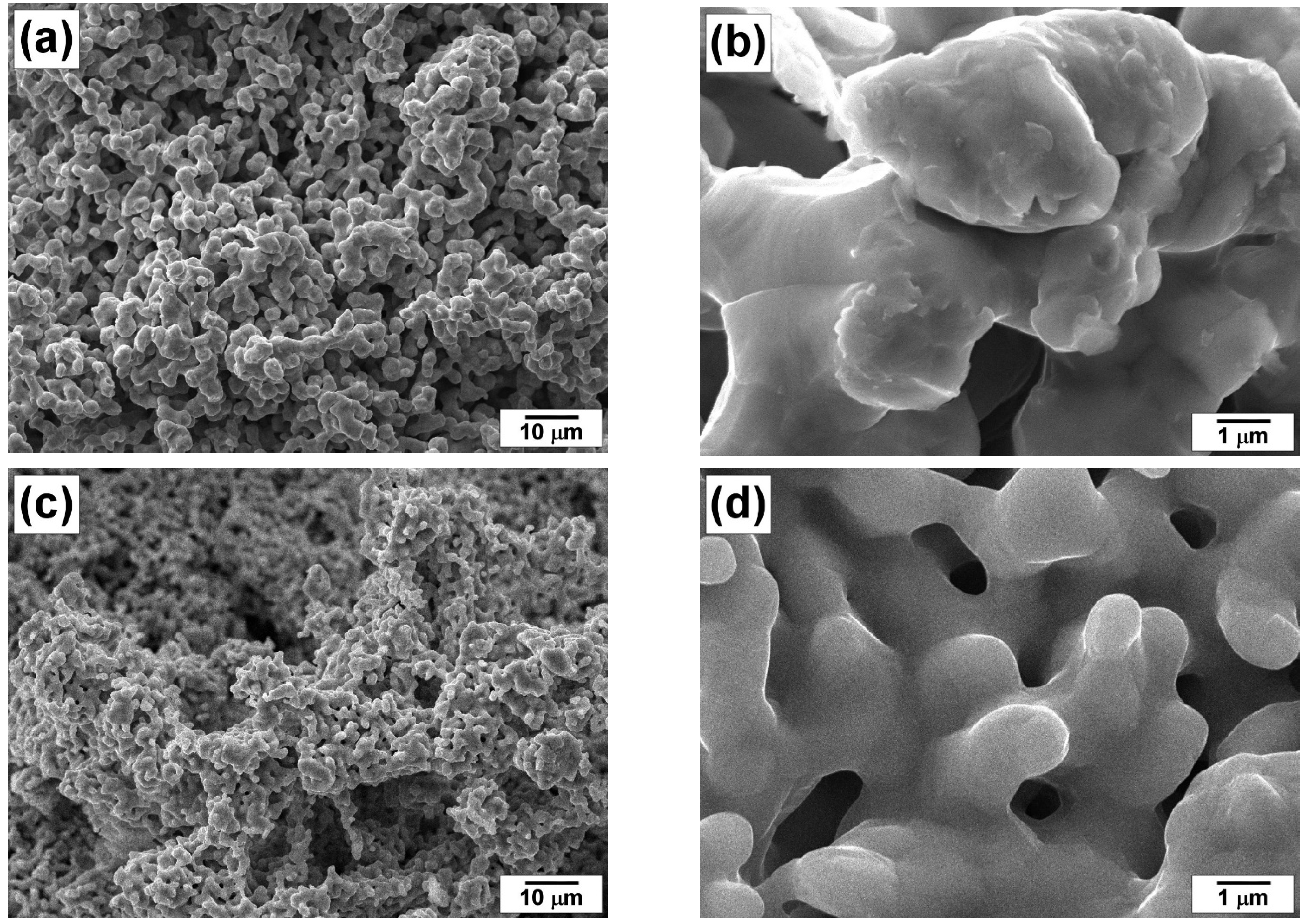
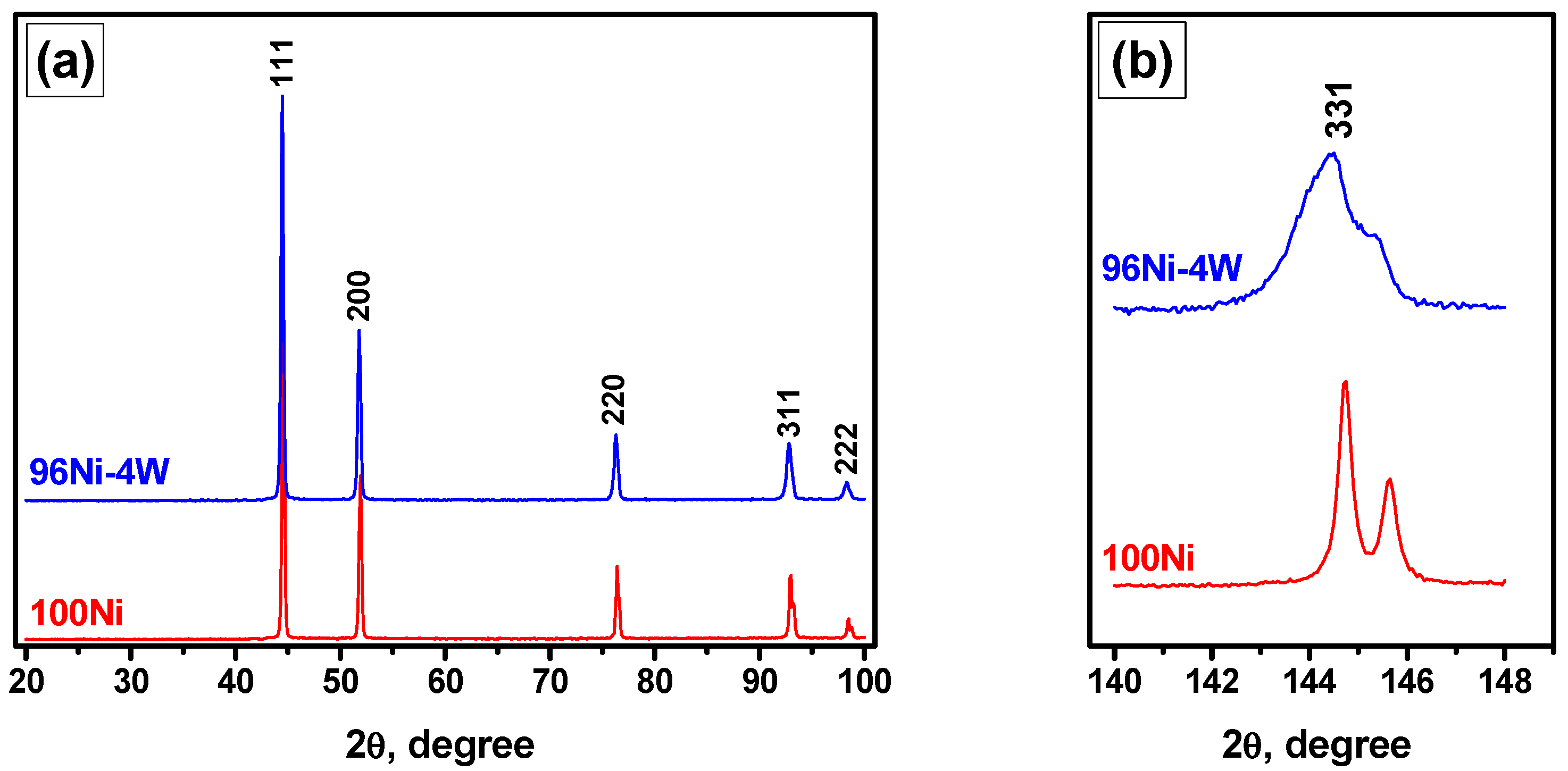
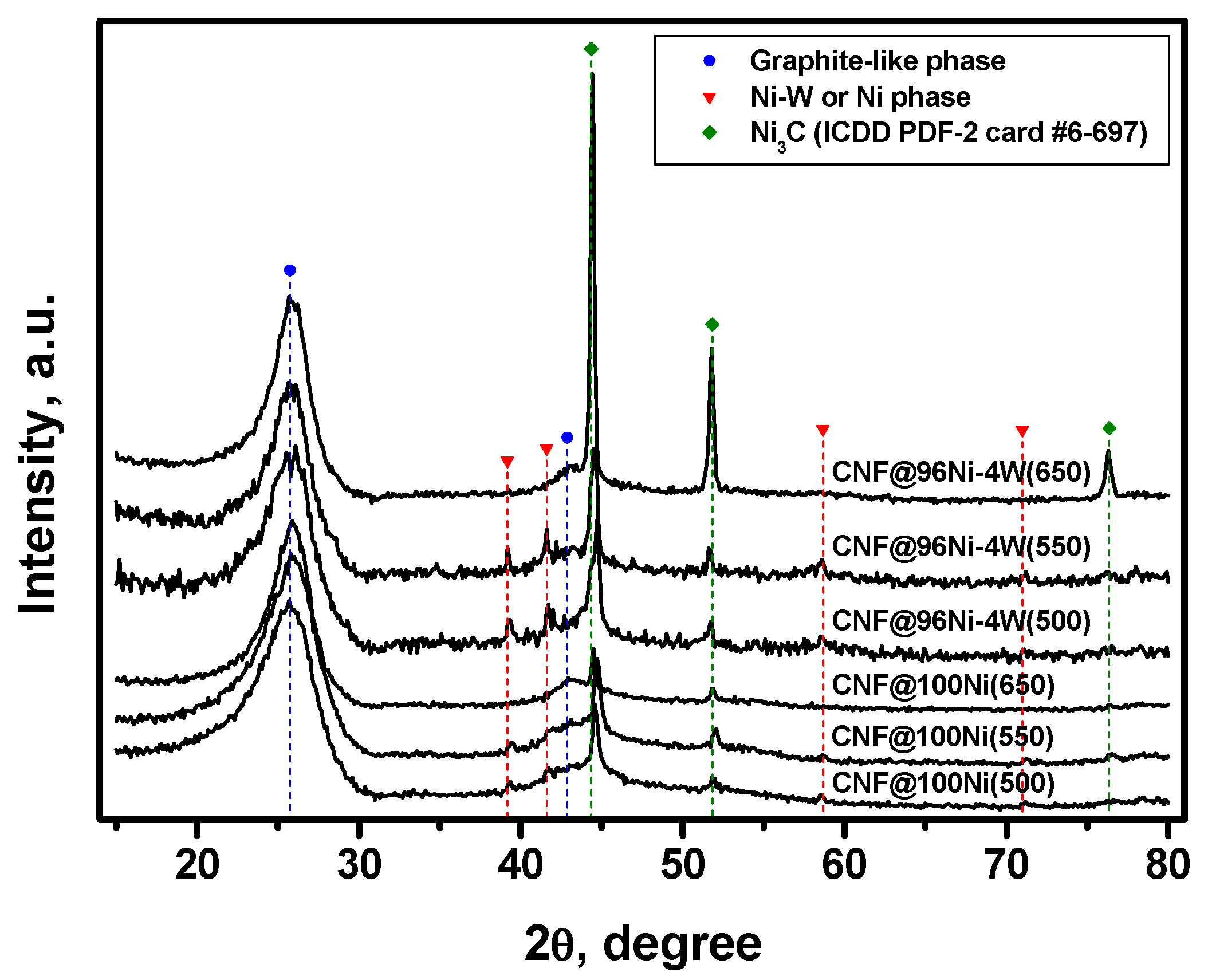
3.1. Study of Microdispersed 100Ni and 96Ni-4W Catalysts by XRD and SEM
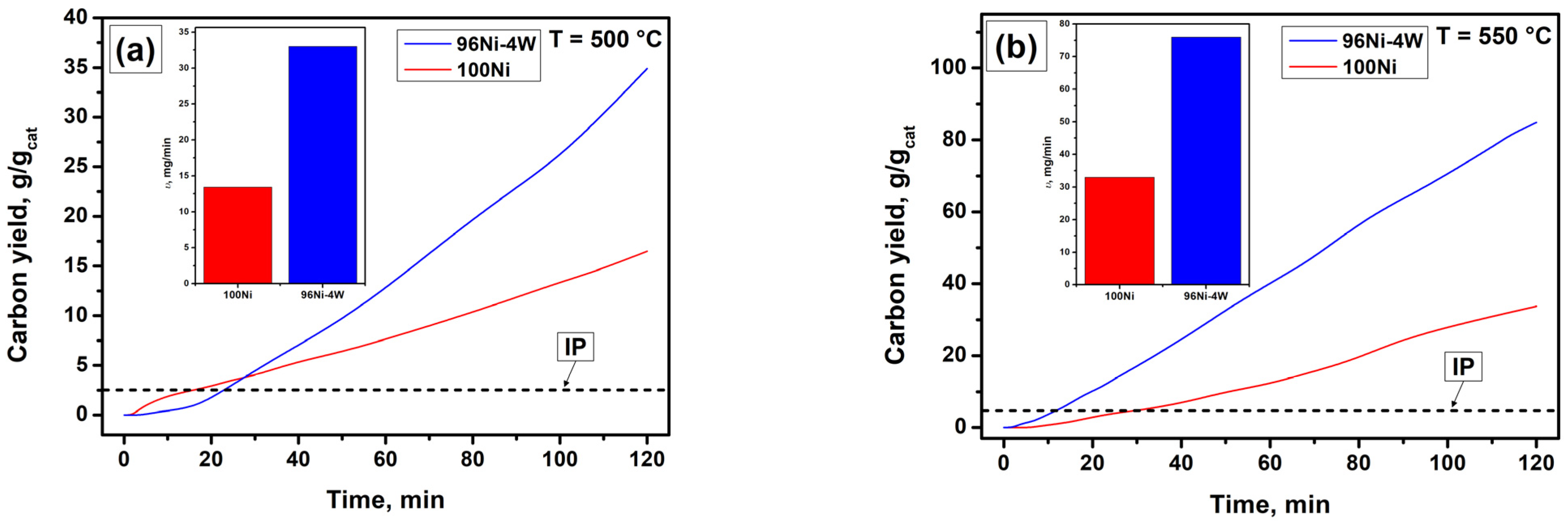
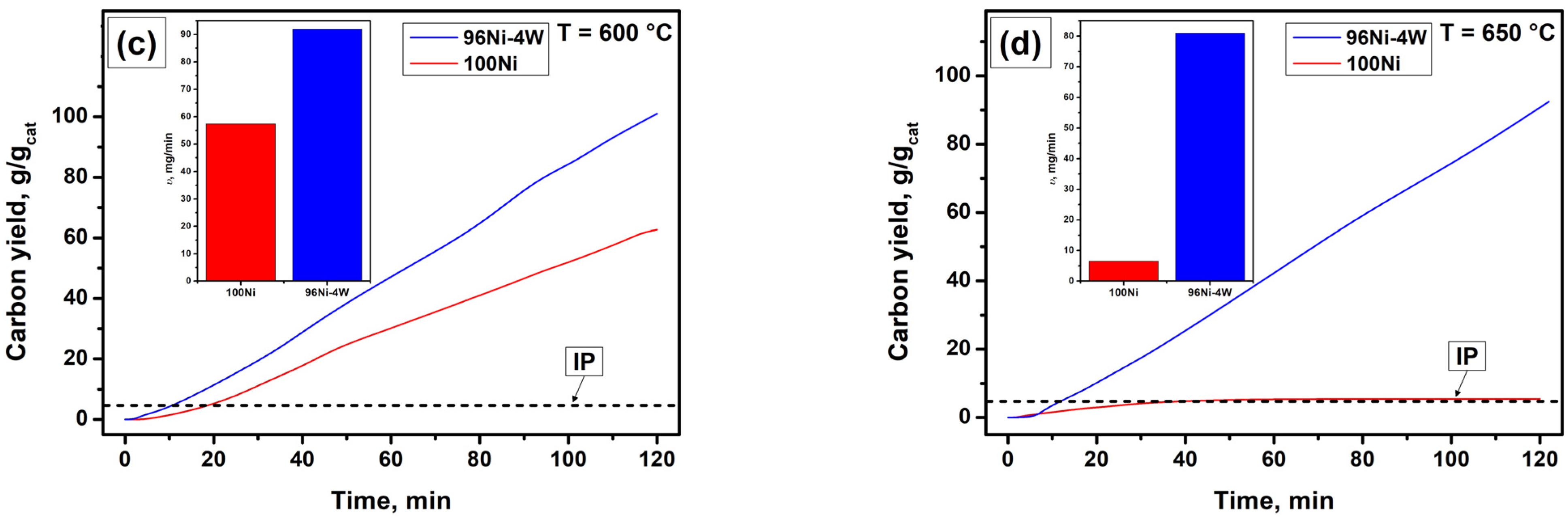
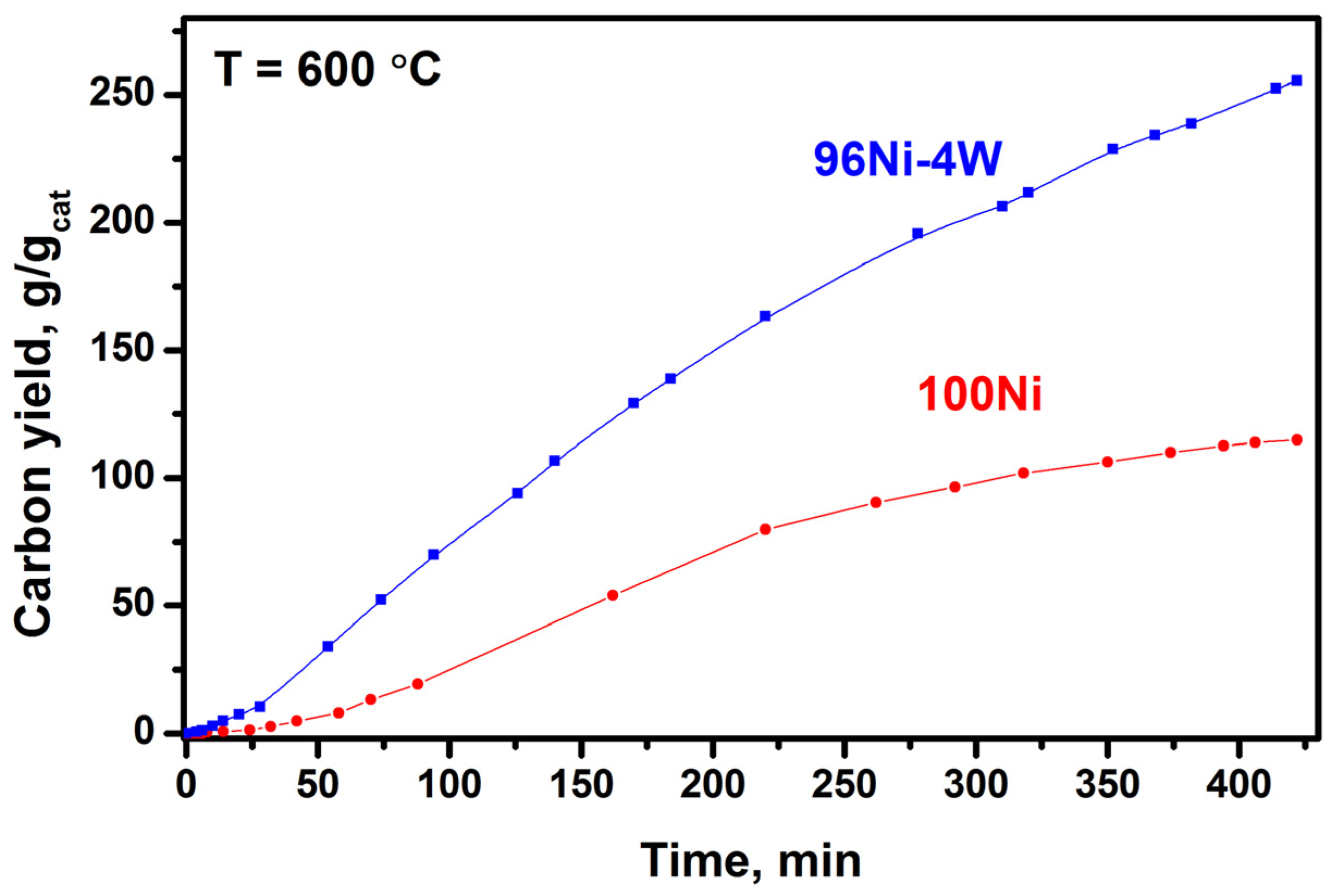
3.2. Catalytic Decomposition of TCE over 100Ni and 96Ni-4W Catalysts
3.3. Study of Morphology and Structure of Carbon Product
3.3.1. XRD Data
3.3.2. Raman Data
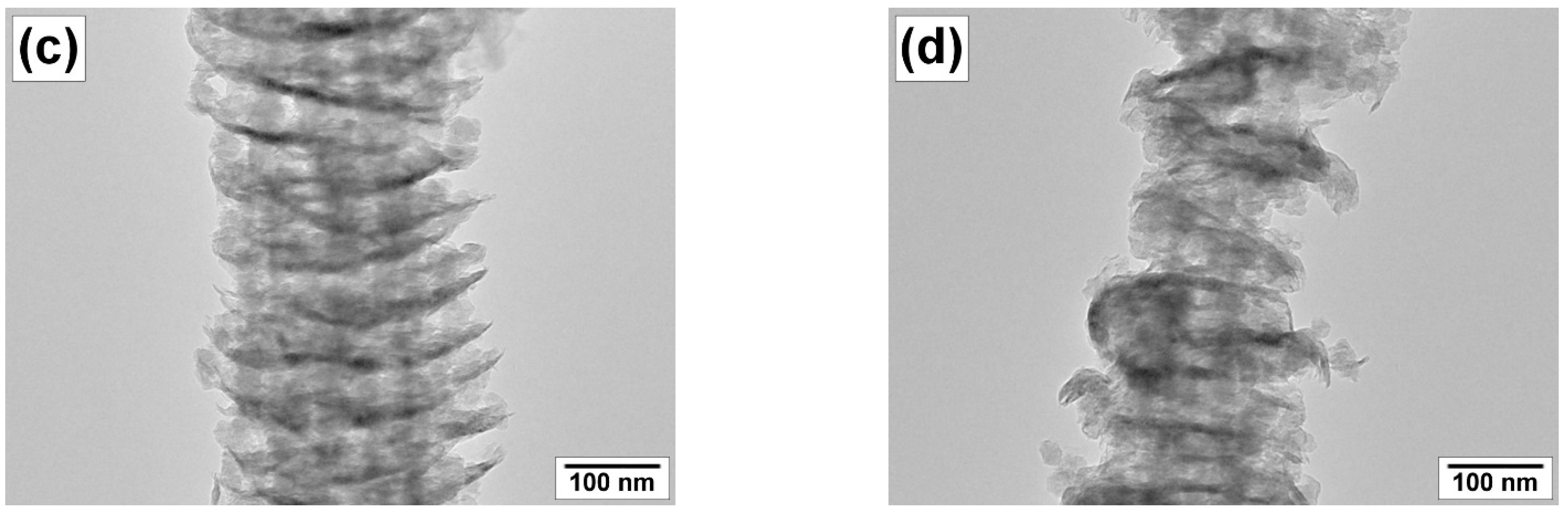
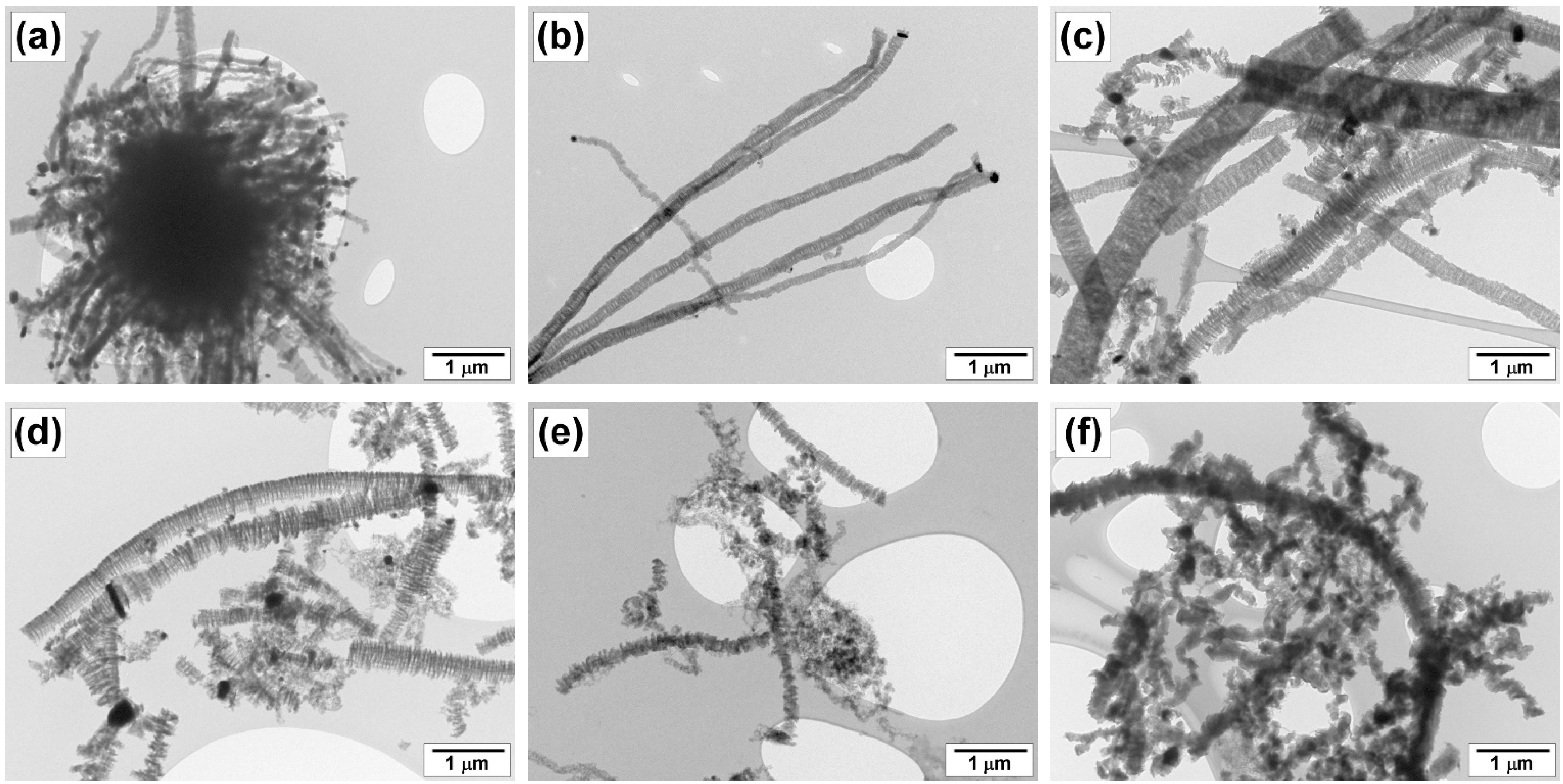
3.3.3. SEM and TEM Data
3.4. Results of Longevity Tests of 100Ni and 96Ni-4W Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, W.; Khayat, K.H. Mechanical properties of ultra-high-performance concrete enhanced with graphite nanoplatelets and carbon nanofibers. Compos. B. Eng. 2016, 107, 113–122. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-based engine oil nanofluids for tribological applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Rauhe, J.C.; Jensen, L.R.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M.Á. Evaluation of the thermal conductivity and mechanical properties of water blown polyurethane rigid foams reinforced with carbon nanofibers. Eur. Polym. J. 2018, 108, 98–106. [Google Scholar] [CrossRef]
- Netskina, O.V.; Komova, O.V.; Tayban, E.S.; Oderova, G.V.; Mukha, S.A.; Kuvshinov, G.G.; Simagina, V.I. The influence of acid treatment of carbon nanofibers on the activity of palladium catalysts in the liquid-phase hydrodechlorination of dichlorobenzene. Appl. Catal. A-Gen. 2013, 467, 386–393. [Google Scholar] [CrossRef]
- Qu, Z.; Mao, C.; Zhu, X.; Zhang, J.; Jiang, H.; Chen, R. Pd-Decorated Hierarchically Porous Carbon Nanofibers for Enhanced Selective Hydrogenation of Phenol. Ind. Eng. Chem. Res. 2022, 61, 13416–13430. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Shubin, Y.V.; Buyanov, R.A. Synthesis of carbon nanofibers via catalytic chemical vapor deposition of halogenated hydrocarbons. In Carbon Nanofibers: Synthesis, Applications and Performance; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 77–182. [Google Scholar]
- Nasibulina, L.I.; Koltsova, T.S.; Joentakanen, T.; Nasibulin, A.G.; Tolochko, O.V.; Malm, J.E.M.; Karppinen, M.J.; Kauppinen, E.I. Direct synthesis of carbon nanofibers on the surface of copper powder. Carbon 2010, 48, 4559–4562. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Kuvshinov, G.G. Effective catalysts for direct cracking of methane to produce hydrogen and filamentous carbon: Part I. Nickel catalysts. Appl. Catal. A-Gen. 2000, 201, 61–70. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, B.; Huang, M.; Yan, L.; Wang, Z.; Huang, Y.; Shen, S.; Zhang, Q.; Gu, L.; Zhong, W. Realizing Negatively Charged Metal Atoms through Controllable d-Electron Transfer in Ternary Ir1−xRhxSb Intermetallic Alloy for Hydrogen Evolution Reaction. Adv. Energy Mater. 2022, 12, 2200855. [Google Scholar] [CrossRef]
- Ma, S.; Deng, J.; Xu, Y.; Tao, W.; Wang, X.; Lin, Z.; Zhang, Q.; Gu, L.; Zhong, W. Pollen-like self-supported FeIr alloy for improved hydrogen evolution reaction in acid electrolyte. J. Energy Chem. 2022, 66, 560–565. [Google Scholar] [CrossRef]
- Shen, S.; Hu, Z.; Zhang, H.; Song, K.; Wang, Z.; Lin, Z.; Zhang, Q.; Gu, L.; Zhong, W. Highly active Si sites enabled by negative valent Ru for electrocatalytic hydrogen evolution in LaRuSi. Angew. Chem. Int. Ed. 2022, 134, e202206460. [Google Scholar] [CrossRef]
- Chang, J.K.; Tsai, H.Y.; Tsai, W.-T. A metal dusting process for preparing nano-sized carbon materials and the effects of acid post-treatment on their hydrogen storage performance. Int. J. Hydrog. Energy 2008, 33, 6734–6742. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Afonnikova, S.D.; Bauman, Y.I.; Shubin, Y.V.; Trenikhin, M.V.; Serkova, A.N.; Vedyagin, A.A. Carbon Erosion of a Bulk Nickel–Copper Alloy as an Effective Tool to Synthesize Carbon Nanofibers from Hydrocarbons. Kinet. Catal. 2022, 63, 97–107. [Google Scholar] [CrossRef]
- Grabke, H.J.; Schütze, M. Corrosion by Carbon and Nitrogen: Metal Dusting, Carburisation and Nitridation; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–308. [Google Scholar]
- Mishakov, I.V.; Korneev, D.V.; Bauman, Y.I.; Vedyagin, A.A.; Nalivaiko, A.Y.; Shubin, Y.V.; Gromov, A.A. Interaction of chlorinated hydrocarbons with nichrome alloy: From surface transformations to complete dusting. Surf. Interfaces 2022, 30, 101914. [Google Scholar] [CrossRef]
- Maboya, W.K.; Coville, N.J.; Mhlanga, S.D. The synthesis of carbon nanomaterials using chlorinated hydrocarbons over a Fe-Co/CaCO3 catalyst. S. Afr. J. Chem. 2016, 69, 15–26. [Google Scholar] [CrossRef]
- Setayesh, S.R.; Waugh, K.C. Kinetic studies of carbon nanofibre and hydrogen evolution via ethane decomposition over fresh and steam regenerated Ni/La2O3 catalyst. Appl. Catal. A-Gen. 2012, 417, 174–182. [Google Scholar] [CrossRef]
- Toebes, M.L.; Bitter, J.H.; Van Dillen, A.J.; de Jong, K.P. Impact of the structure and reactivity of nickel particles on the catalytic growth of carbon nanofibers. Catal. Today 2002, 76, 33–42. [Google Scholar] [CrossRef]
- Malik, J.K.; Aggarwal, M.; Kalpana, S.; Gupta, R.C. Chlorinated hydrocarbons and pyrethrins/pyrethroids. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 641–664. [Google Scholar]
- Rosner, D.; Markowitz, G. Persistent pollutants: A brief history of the discovery of the widespread toxicity of chlorinated hydrocarbons. Environ. Res. 2013, 120, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shaikjee, A.; Coville, N.J. Catalyst restructuring studies: The facile synthesis of tripod-like carbon fibers by the decomposition of trichloroethylene. Mater. Lett. 2012, 68, 273–276. [Google Scholar] [CrossRef]
- Potylitsyna, A.R.; Bauman, Y.I.; Mishakov, I.V.; Plyusnin, P.E.; Vedyagin, A.A.; Shubin, Y.V. The Features of the CCVD of Trichloroethylene Over Microdispersed Ni and Ni–Mo Catalysts. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Rudneva, Y.V.; Popov, A.A.; Rieder, D.; Korneev, D.V.; Serkova, A.N.; Shubin, Y.V.; Vedyagin, A.A. Catalytic synthesis of segmented carbon filaments via decomposition of chlorinated hydrocarbons on Ni-Pt alloys. Catal. Today 2020, 348, 102–110. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Valverde, J.L.; Keane, M.A. Catalytic growth of structured carbon from chloro-hydrocarbons. Appl. Catal. A-Gen. 2007, 332, 237–246. [Google Scholar] [CrossRef]
- Potylitsyna, A.R.; Mishakov, I.V.; Bauman, Y.I.; Kibis, L.S.; Shubin, Y.V.; Volochaev, M.N.; Melgunov, M.S.; Vedyagin, A.A. Metal dusting as a key route to produce functionalized carbon nanofibers. React. Kinet. Mech. Catal. 2022, 135, 1387–1404. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.I.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Lysakova, A.S.; Rudnev, A.V.; Mishakov, I.V.; Shubin, Y.V.; Vedyagin, A.A.; Buyanov, R.A. Synthesis of nanostructured carbon fibers from chlorohydrocarbons over Bulk Ni-Cr Alloys. Nanotechnol. Russ. 2014, 9, 380–385. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water Purification from Chlorobenzenes using Heteroatom-Functionalized Carbon Nanofibers Produced on Self-Organizing Ni-Pd Catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Modi, A.; Bhaduri, B.; Verma, N. Facile one-step synthesis of nitrogen-doped carbon nanofibers for the removal of potentially toxic metals from water. Ind. Eng. Chem. Res. 2015, 54, 5172–5178. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Shao, Y.; Jiang, H.; Chen, R.; Xing, W. Controllable synthesis of 1D Pd@ N-CNFs with high catalytic performance for phenol hydrogenation. Catal. Lett. 2021, 151, 1013–1024. [Google Scholar] [CrossRef]
- Iwasaki, T.; Makino, Y.; Fukukawa, M.; Nakamura, H.; Watano, S. Low-temperature growth of nitrogen-doped carbon nanofibers by acetonitrile catalytic CVD using Ni-based catalysts. Appl. Nanosci. 2016, 6, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Shalagina, A.E.; Ismagilov, Z.R.; Podyacheva, O.Y.; Kvon, R.I.; Ushakov, V.A. Synthesis of nitrogen-containing carbon nanofibers by catalytic decomposition of ethylene/ammonia mixture. Carbon 2007, 45, 1808–1820. [Google Scholar] [CrossRef]
- Takenaka, S.; Ishida, M.; Serizawa, M.; Tanabe, E.; Otsuka, K. Formation of carbon nanofibers and carbon nanotubes through methane decomposition over supported cobalt catalysts. J. Phys. Chem. B 2004, 108, 11464–11472. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Chesnokov, V.V.; Buyanov, R.A.; Pakhomov, N.A. Decomposition of chlorinated hydrocarbons on iron-group metals. Kinet. Catal. 2001, 42, 543–548. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Rudneva, Y.V.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V.; Korneev, D.V.; Stoyanovskii, V.O.; Vedyagin, A.A.; Buyanov, R.A. Effect of Mo on the catalytic activity of Ni-based self-organizing catalysts for processing of dichloroethane into segmented carbon nanomaterials. Heliyon 2019, 5, e02428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishakov, I.V.; Bauman, Y.I.; Potylitsyna, A.R.; Shubin, Y.V.; Plyusnin, P.E.; Stoyanovskii, V.O.; Vedyagin, A.A. Catalytic properties of bulk (1−x) Ni−xW alloys in the decomposition of 1, 2-Dichloroethane with the production of carbon nanomaterials. Kinet. Catal. 2022, 63, 75–86. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Rouhaghdam, A.R.S. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Yang, R.; Du, X.; Zhang, X.; Xin, H.; Zhou, K.; Li, D.; Hu, C. Transformation of jatropha oil into high-quality biofuel over Ni–W bimetallic catalysts. ACS Omega 2019, 4, 10580–10592. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Qiu, J. An active and coke-resistant dry reforming catalyst comprising nickel–tungsten alloy nanoparticles. Catal. Commun. 2015, 69, 123–128. [Google Scholar] [CrossRef]
- Shekunova, V.M.; Sinyapkin, Y.T.; Didenkulova, I.I.; Tsyganova, E.I.; Aleksandrov, Y.A.; Sinyapkin, D.Y. Catalytic pyrolysis of light hydrocarbons in the presence of ultrafine particles formed by electrically induced explosive dispersion of metal wires. Pet. Chem. 2013, 53, 92–96. [Google Scholar] [CrossRef]
- Jia, J.; Veksha, A.; Lim, T.-T.; Lisak, G.; Zhang, R.; Wei, Y. Modulating local environment of Ni with W for synthesis of carbon nanotubes and hydrogen from plastics. J. Clean. Prod. 2022, 352, 131620. [Google Scholar] [CrossRef]
- Von Brauer, G. Handbuch Der Präparativen Anorganischen Chemie in Drei Bänden; Enke: Omaha, NE, USA, 1978. [Google Scholar]
- Wang, C.; Bauman, Y.I.; Mishakov, I.V.; Stoyanovskii, V.O.; Shelepova, E.V.; Vedyagin, A.A. Scaling up the Process of Catalytic Decomposition of Chlorinated Hydrocarbons with the Formation of Carbon Nanostructures. Processes 2022, 10, 506. [Google Scholar] [CrossRef]
- The Powder Diffraction File 2 (PDF-2): International Centre for Diffraction Data; Newtown Square, PA, USA, 2009.
- Kraus, W.; Nolze, G. PowderCell 2.0 for Windows. Powder Diffr. 1998, 13, 256. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, Addison; Wesley Mass: Reading, MA, USA, 1978; pp. 127–131. [Google Scholar]
- Krumm, S. An interactive Windows program for profile fitting and size/strain analysis. Mater. Sci. Forum 1996, 228–231, 183–190. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Shorstkaya, Y.V.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V.; Korneev, D.V.; Stoyanovskii, V.O.; Vedyagin, A.A. Catalytic conversion of 1, 2-dichloroethane over Ni-Pd system into filamentous carbon material. Catal. Today 2017, 293, 23–32. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Buyanov, R.A.; Vedyagin, A.A.; Volodin, A.M. Catalytic properties of massive iron-subgroup metals in dichloroethane decomposition into carbon products. Kinet. Catal. 2011, 52, 547–554. [Google Scholar] [CrossRef]
- Modekwe, H.U.; Mamo, M.A.; Moothi, K.; Daramola, M.O. Effect of different catalyst supports on the quality, yield and morphology of carbon nanotubes produced from waste polypropylene plastics. Catalysts 2021, 11, 692. [Google Scholar] [CrossRef]
- Buyanov, R.; Chesnokov, V.; Afanas’ ev, A.; Babenko, V. Carbide mechanism of formation of carbonaceous deposits and their properties on iron-chromium dehydrogenation catalysts. Kin. Cat. 1977, 18, 839–845. [Google Scholar]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Smausz, T.; Kondász, B.; Gera, T.; Ajtai, T.; Utry, N.; Pintér, M.; Kiss-Albert, G.; Budai, J.; Bozóki, Z.; Szabó, G.; et al. Determination of UV–visible–NIR absorption coefficient of graphite bulk using direct and indirect methods. Appl. Phys. A 2017, 123, 633. [Google Scholar] [CrossRef]
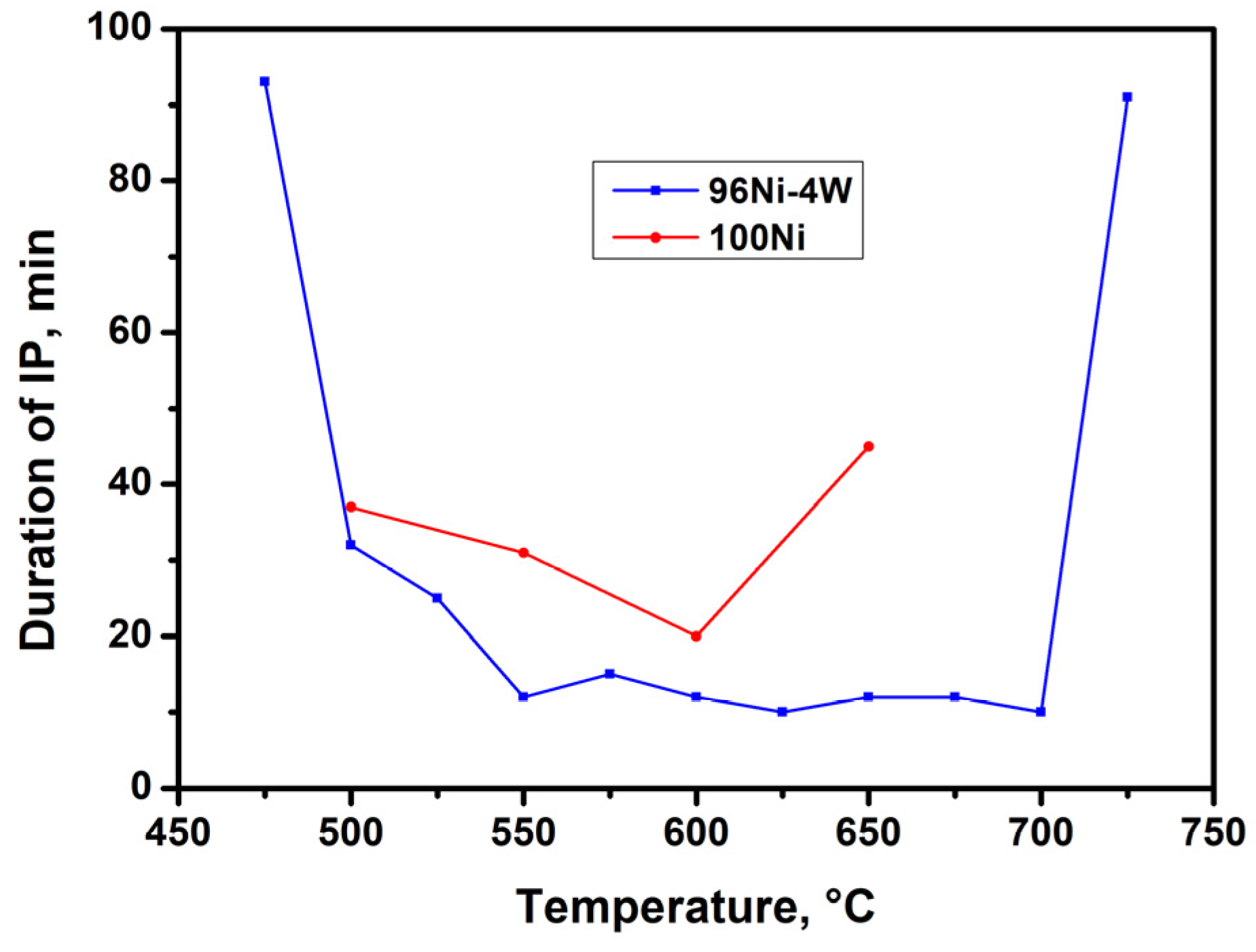
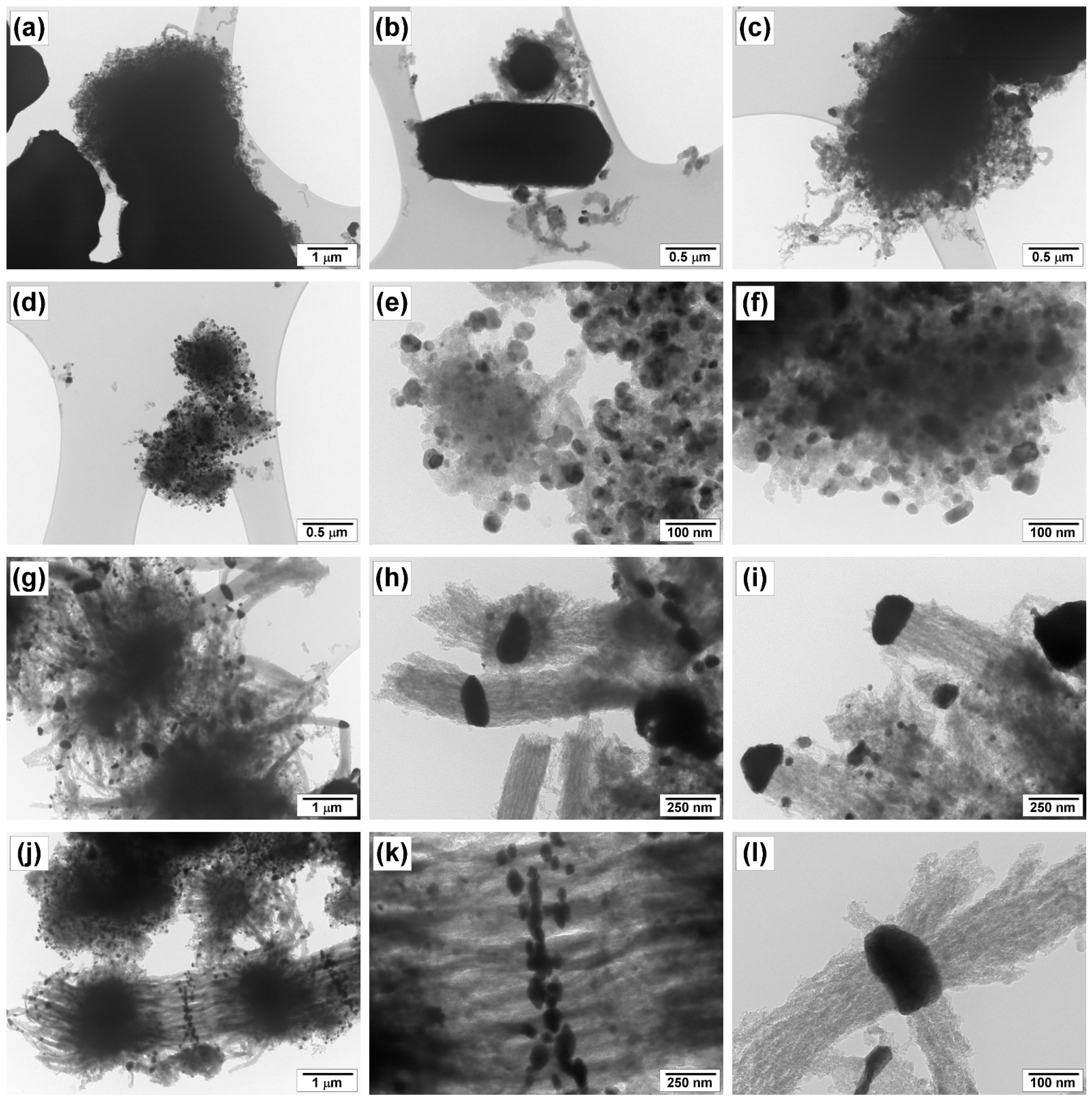
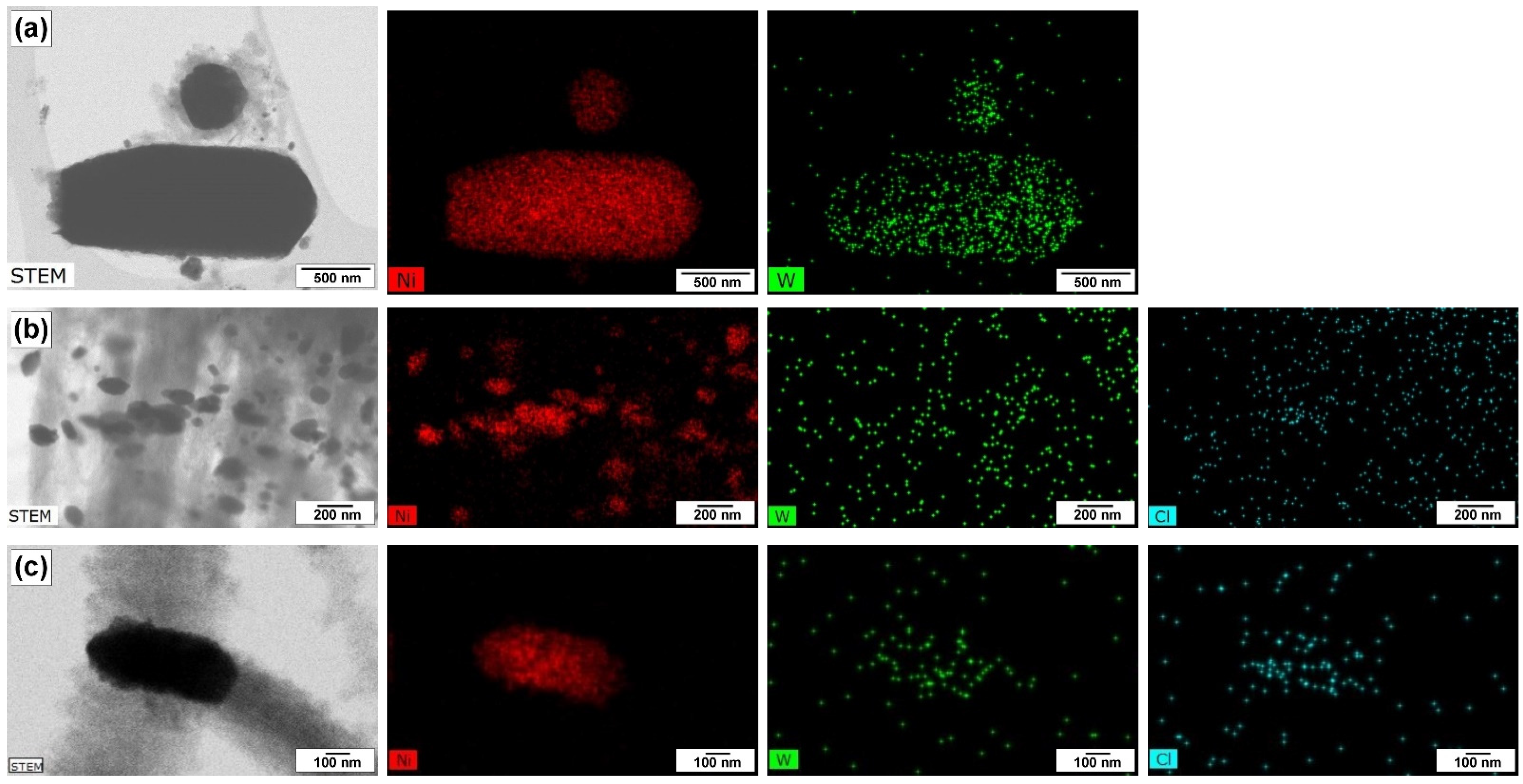
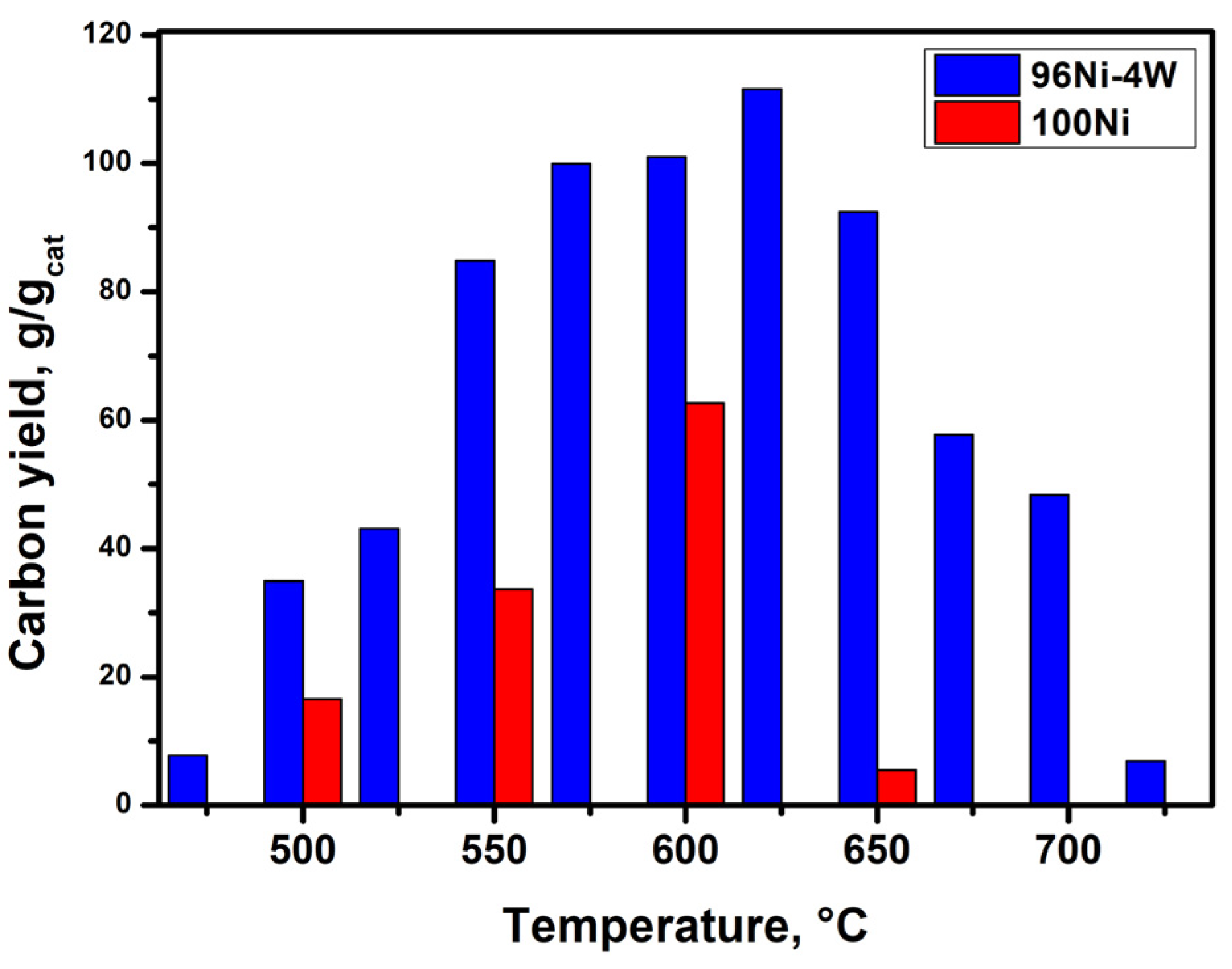






| N | Temperature, °C | 100Ni | 96Ni-4W | ||||
|---|---|---|---|---|---|---|---|
| τ(IP), min | Yield of CNF, g/gcat | v, mg/min | τ(IP), min | Yield of CNF, g/gcat | v, mg/min | ||
| 1 | 475 | - | - | - | 93 | 8 | 9 |
| 2 | 500 | 37 | 16 | 13 | 32 | 35 | 33 |
| 3 | 525 | - | - | - | 25 | 43 | 40 |
| 4 | 550 | 31 | 34 | 33 | 12 | 85 | 76 |
| 5 | 575 | - | - | - | 15 | 100 | 92 |
| 6 | 600 | 20 | 63 | 57 | 12 | 101 | 92 |
| 7 | 625 | - | - | - | 10 | 112 | 99 |
| 8 | 650 | 40 * | 5 | - | 12 | 92 | 81 |
| 9 | 675 | - | - | - | 12 | 58 | 47 |
| 10 | 700 | - | - | - | 10 | 48 | 35 |
| 11 | 725 | - | - | - | 91 | 7 | - |
| Sample | Carbon | Metal Particles (Ni or Ni-W) | S111/S002 | |||||
|---|---|---|---|---|---|---|---|---|
| S002 (*) | d002, Å | D, nm | n | a, Å | D, nm | S111 | ||
| CNF@100Ni(500) | 11400 | 3.46 | 1.7 | 5 | 3.526(2) (140–148°) 3.522(3) (75–78°) | 25 | 600 | 0.05 |
| CNF@100Ni(550) | 9500 | 3.41 | 1.8 | 5 | 3.525(2) (140–148°) 3.523(3) (75–78°) | 25 | 500 | 0.05 |
| CNF@100Ni(650) | 6400 | 3.44 | 2.3 | 6 | 3.526(2) (140–148°) 3.527(3) (75–78°) | >100 | 100 | 0.02 |
| CNF@96Ni-4W(500) | 2400 | 3.42 | 1.8 | 5 | 3.541(4) (75–78°) | 40 | 70 | 0.03 |
| CNF@96Ni-4W(550) | 1300 | 3.45 | 2.7 | 7 | 3.533(3) (75–78°) | 30 | 120 | 0.09 |
| CNF@96Ni-4W(650) | 5400 | 3.43 | 2.5 | 7 | 3.529(2) (140–148°) 3.531(3) (75–78°) | 35 | 1000 | 0.19 |
| Reaction Time, min | 100Ni | 96Ni-4W | ||
|---|---|---|---|---|
| Yield of CNF, g/gcat | v, mg/min | Yield of CNF, g/gcat | v, mg/min | |
| 120 | 35 | 35 | 91 | 88 |
| 180 | 61 | 44 | 136 | 77 |
| 240 | 85 | 44 | 174 | 64 |
| 300 | 99 | 23 | 204 | 51 |
| 360 | 108 | 15 | 232 | 49 |
| 420 | 114 | 11 | 256 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potylitsyna, A.R.; Rudneva, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A.; Shubin, Y.V.; Mishakov, I.V. Efficient Production of Segmented Carbon Nanofibers via Catalytic Decomposition of Trichloroethylene over Ni-W Catalyst. Materials 2023, 16, 845. https://doi.org/10.3390/ma16020845
Potylitsyna AR, Rudneva YV, Bauman YI, Plyusnin PE, Stoyanovskii VO, Gerasimov EY, Vedyagin AA, Shubin YV, Mishakov IV. Efficient Production of Segmented Carbon Nanofibers via Catalytic Decomposition of Trichloroethylene over Ni-W Catalyst. Materials. 2023; 16(2):845. https://doi.org/10.3390/ma16020845
Chicago/Turabian StylePotylitsyna, Arina R., Yuliya V. Rudneva, Yury I. Bauman, Pavel E. Plyusnin, Vladimir O. Stoyanovskii, Evgeny Y. Gerasimov, Aleksey A. Vedyagin, Yury V. Shubin, and Ilya V. Mishakov. 2023. "Efficient Production of Segmented Carbon Nanofibers via Catalytic Decomposition of Trichloroethylene over Ni-W Catalyst" Materials 16, no. 2: 845. https://doi.org/10.3390/ma16020845








