Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Preparation
2.2. Sample Characterization
2.3. Photodegradation Experiments
3. Results and Discussion
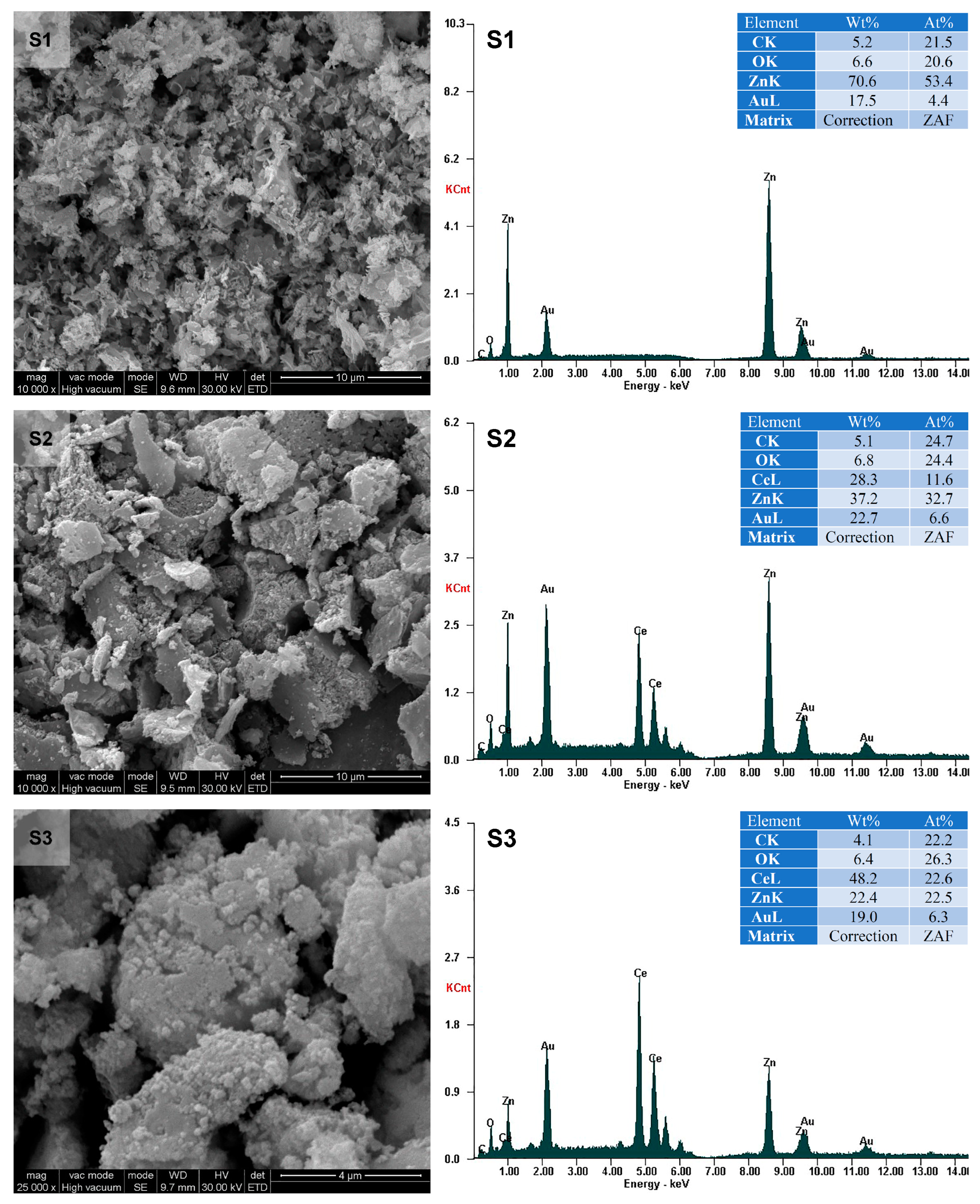
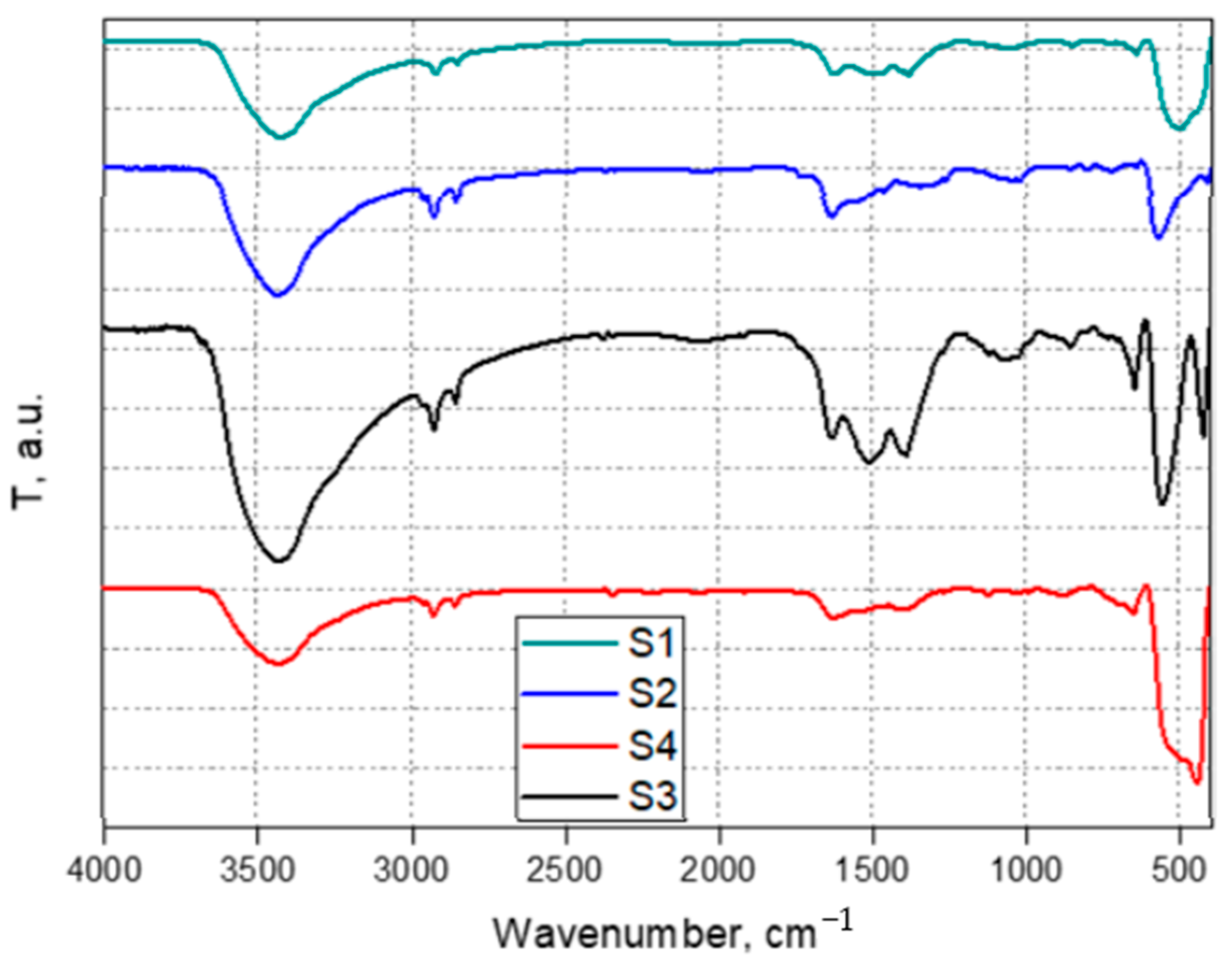
3.1. Characterization of the Photocatalysts
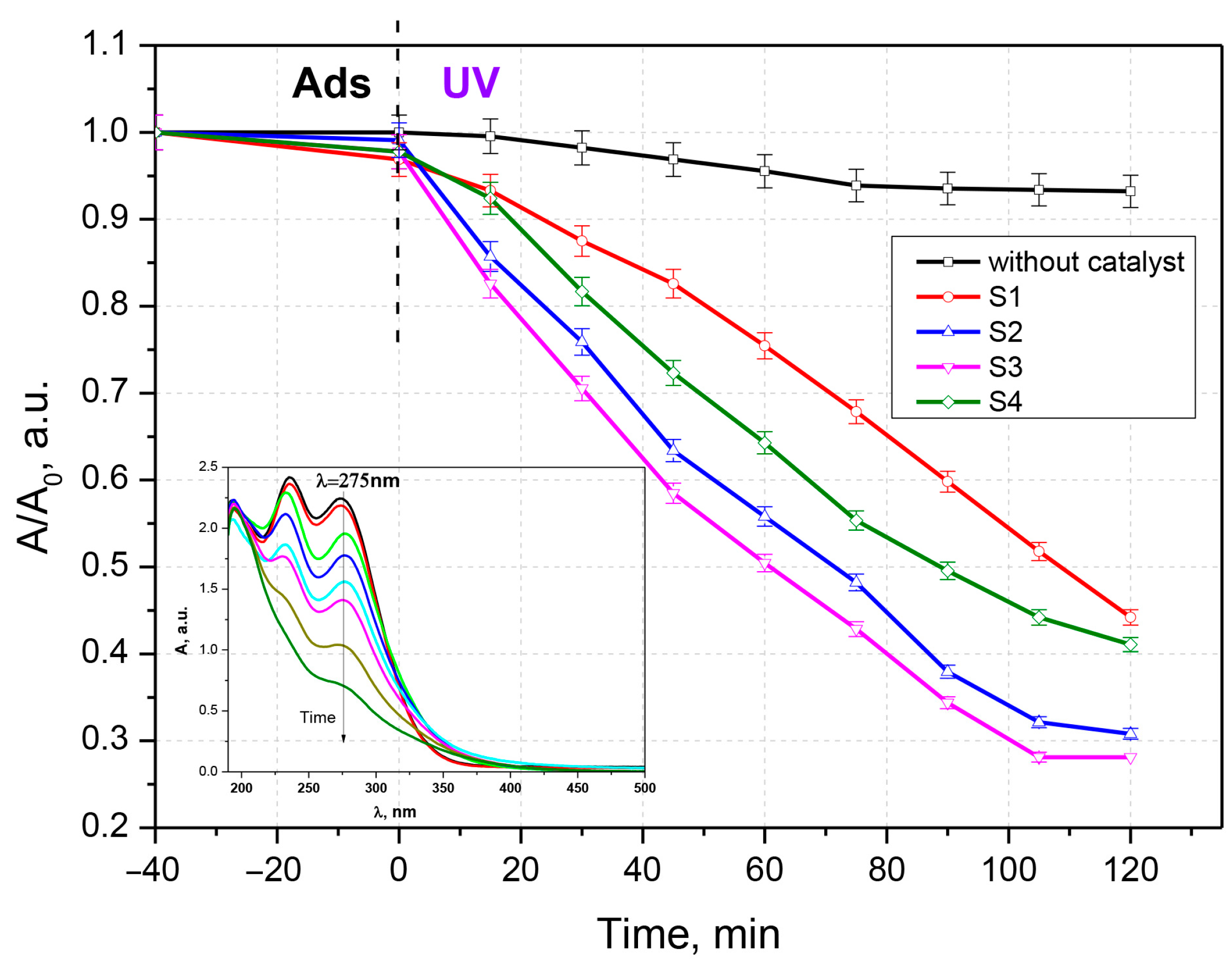
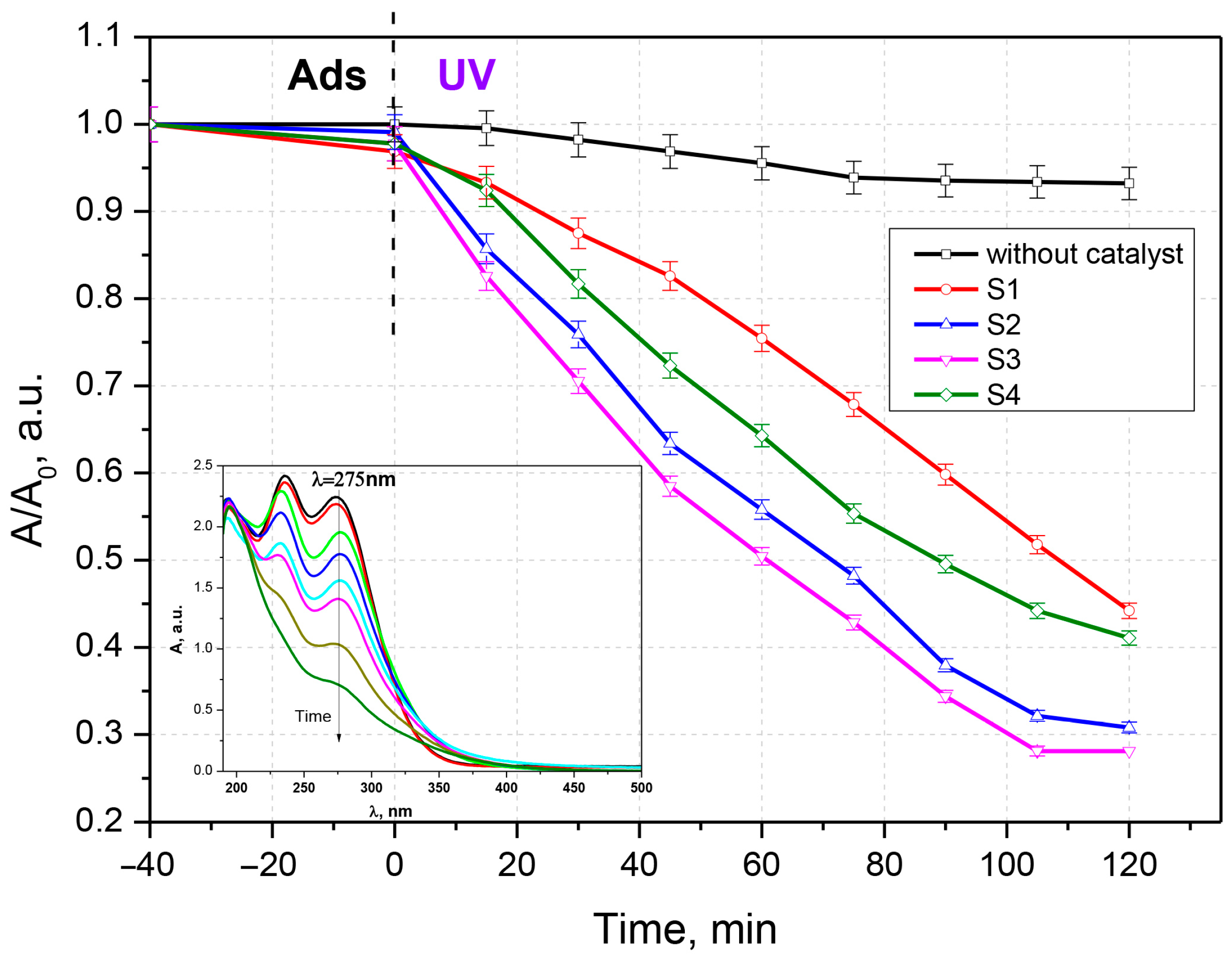
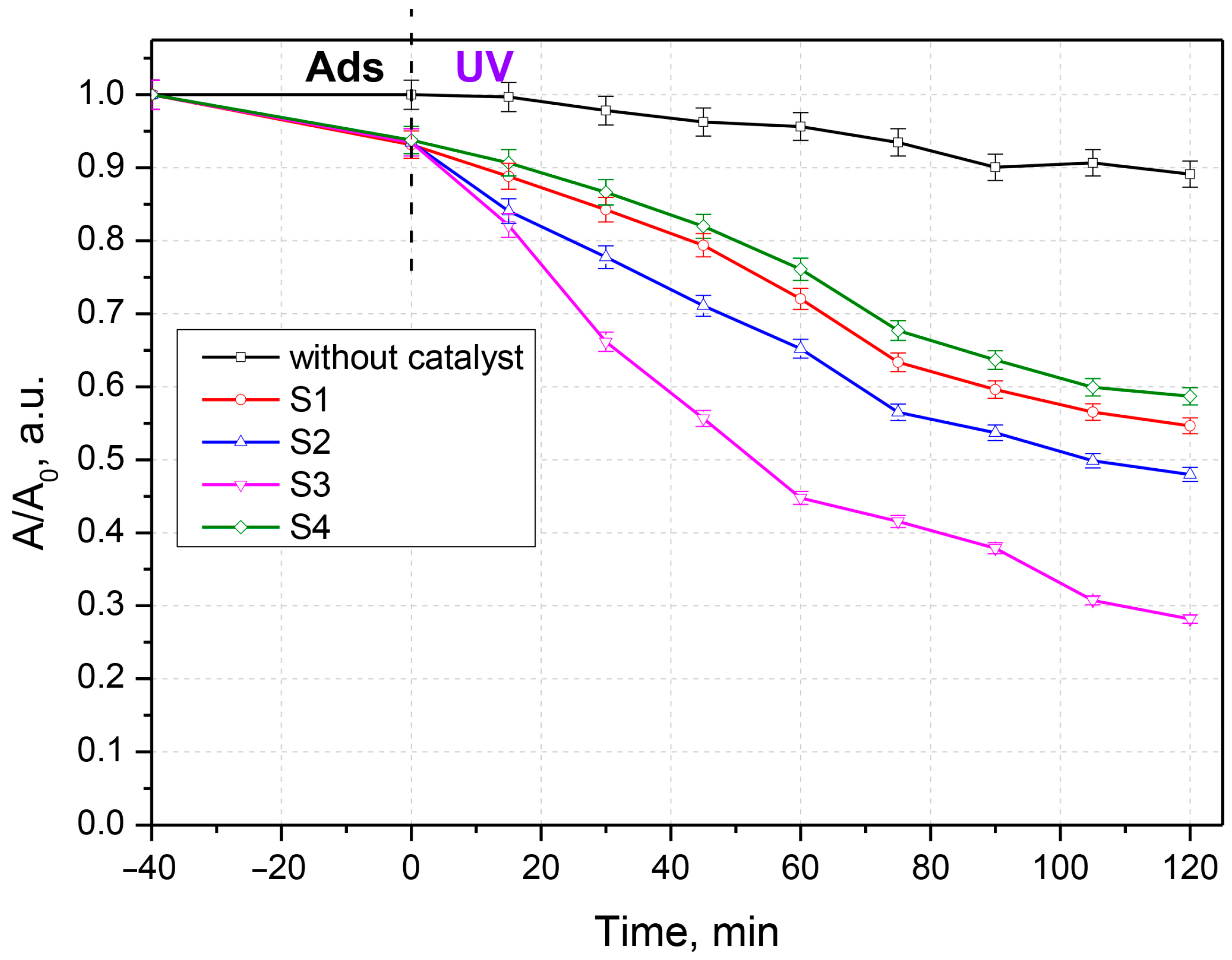
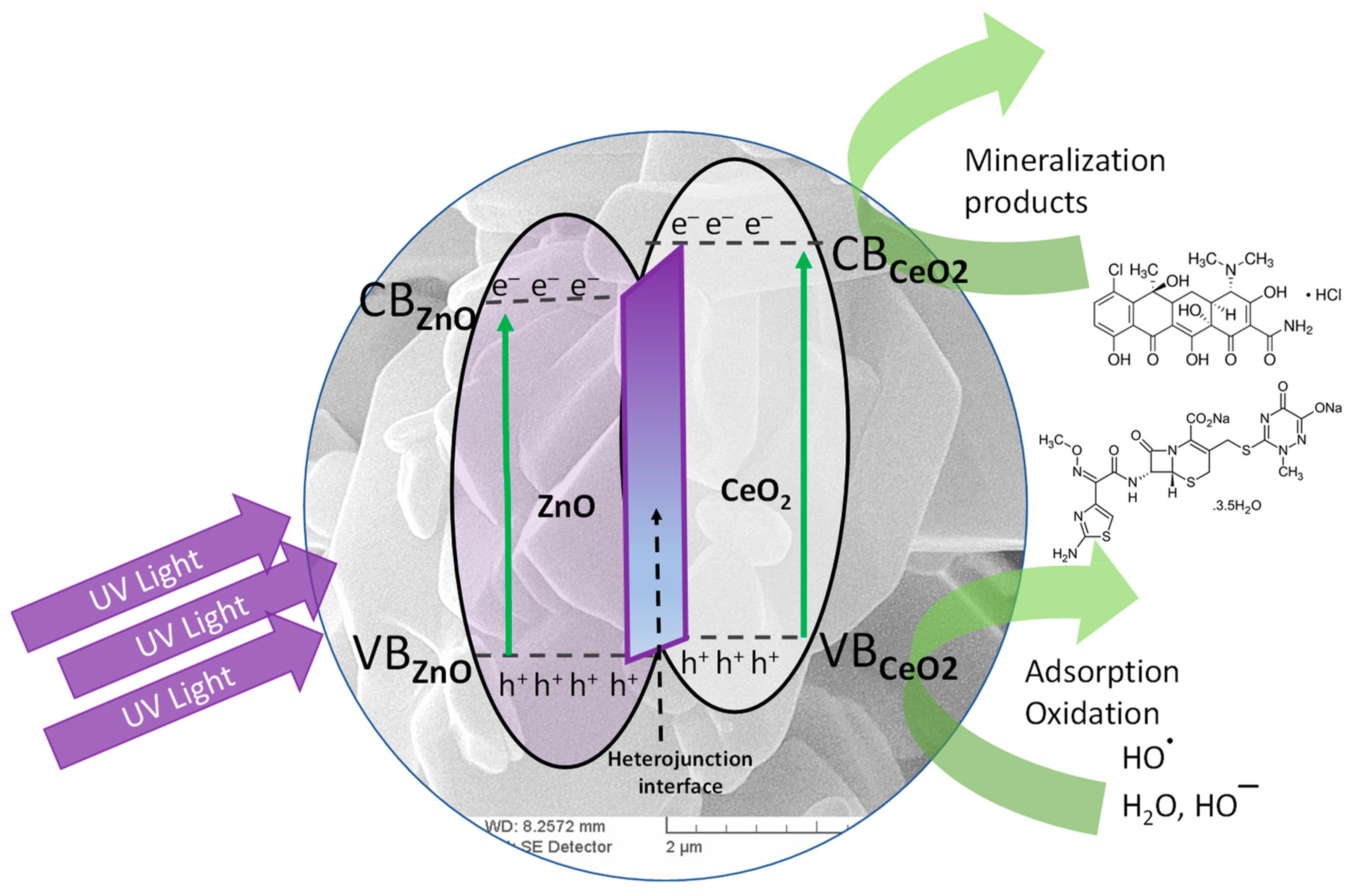
3.2. Antibiotic Photocatalytic Degradation
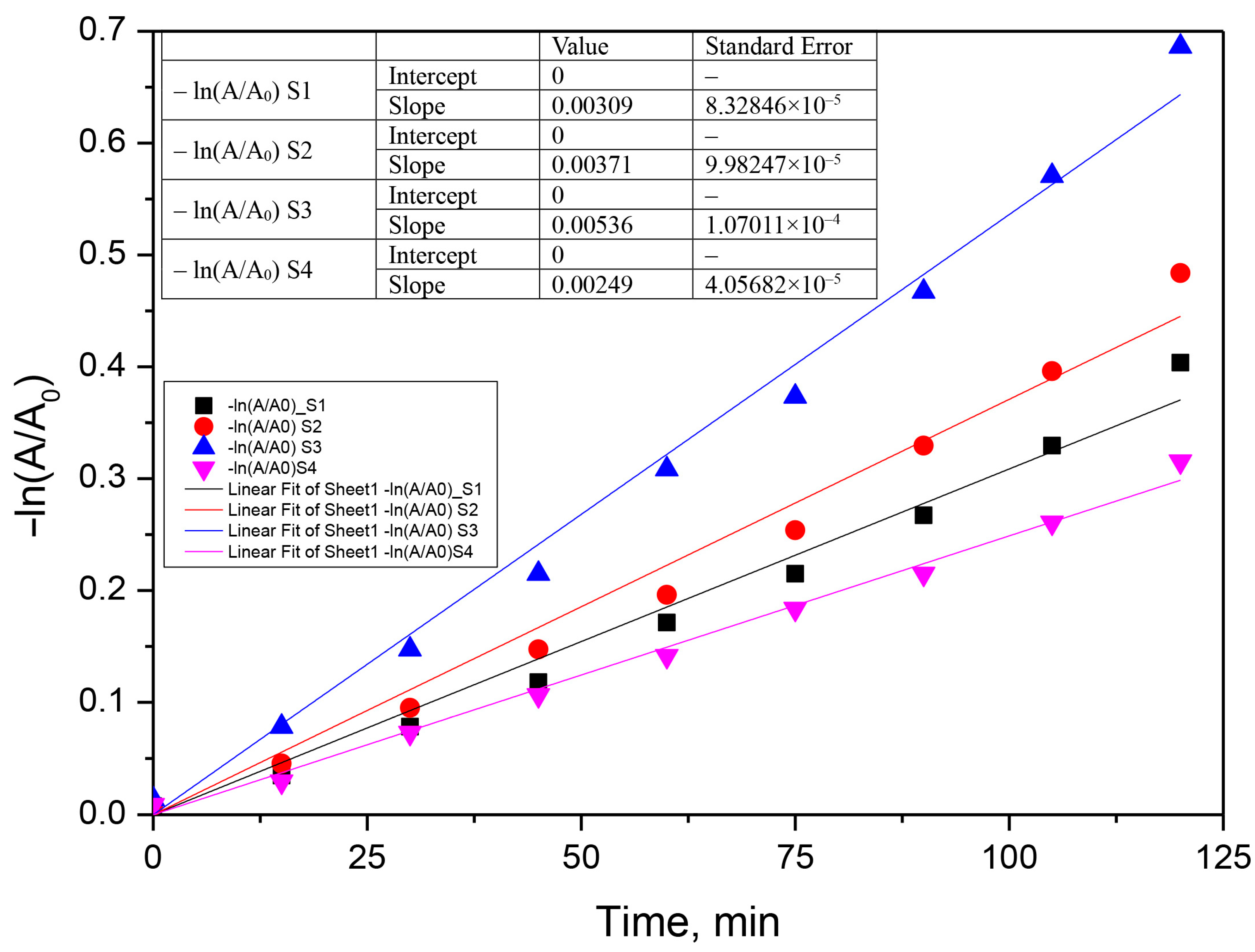
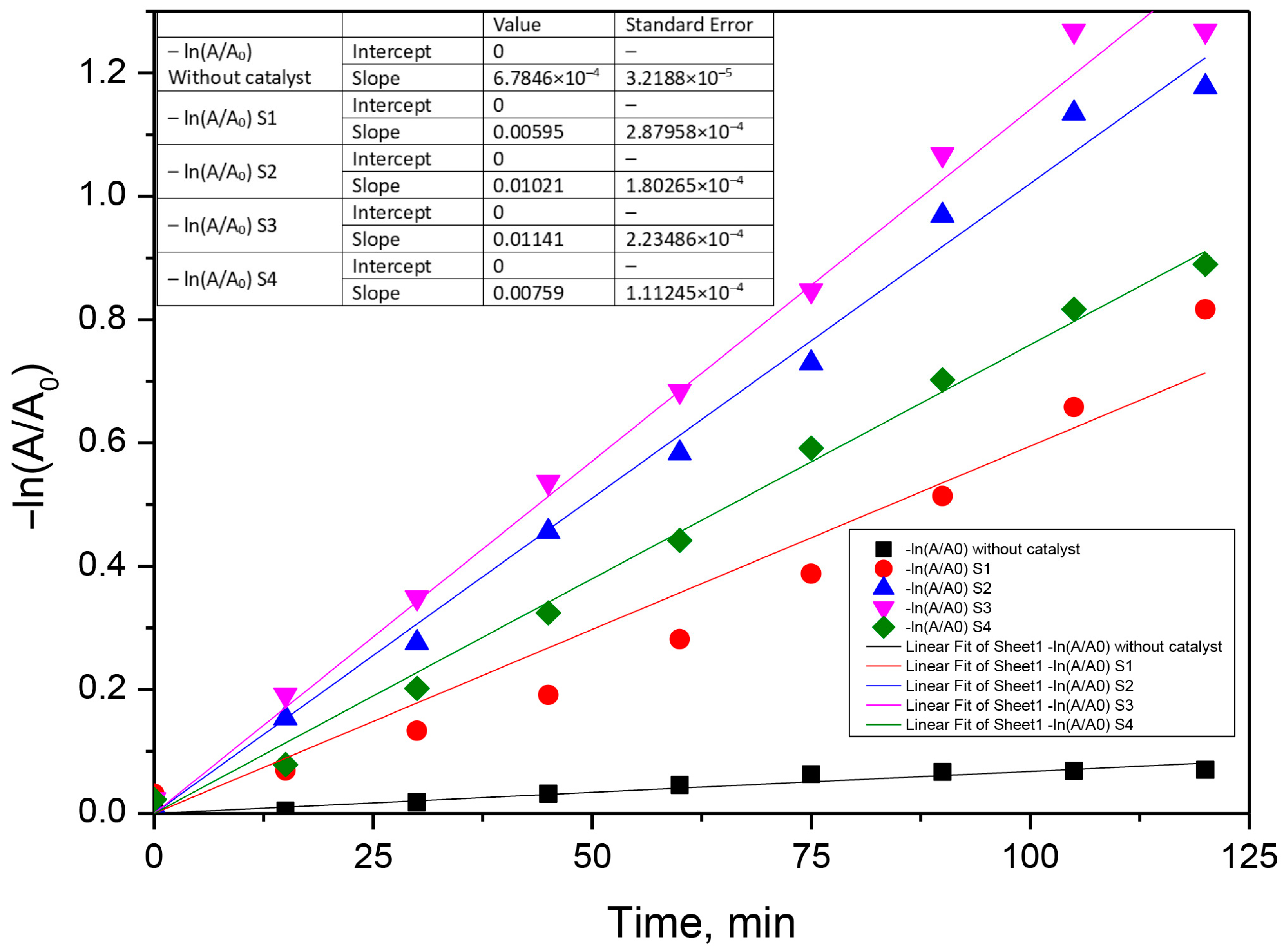
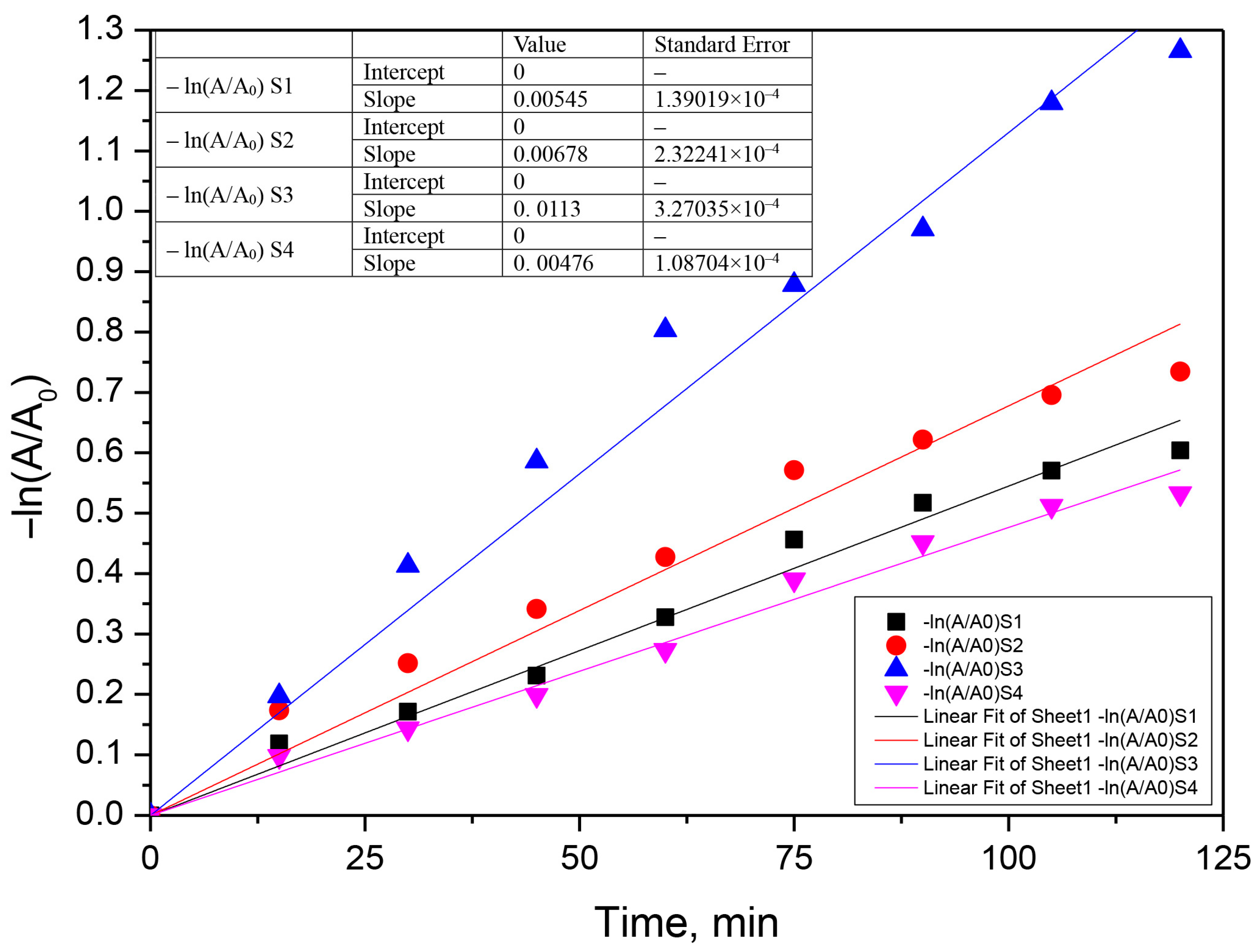
3.3. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO. hdl:10665/325771; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/rest/bitstreams/1237479/retrieve (accessed on 10 May 2020).
- Dan, A.; Zhang, X.; Dai, Y.; Chen, C.; Yang, Y. Occurrence and removal of quinolone, tetracycline, and macrolide antibiotics from urban wastewater in constructed wetlands. J. Clean. Prod. 2020, 252, 119677. [Google Scholar] [CrossRef]
- Tetracycline Pharmacokinetics. Available online: https://www.drugs.com/monograph/tetracycline.html (accessed on 2 November 2022).
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Aggarwal, A.; Singh, S.; Sharma, S.; Sarma, S.J. Current challenges and advancements towards discovery and resistance of antibiotics. J. Mol. Struc. 2022, 1248, 131380. [Google Scholar] [CrossRef]
- Zahedi, S.; Gros, M.; Balcazar, J.L.; Petrovic, M.; Pijuan, M. Assessing the occurrence of pharmaceuticals and antibiotic resistance genes during the anaerobic treatment of slaughterhouse wastewater at different temperatures. Sci. Total Environ. 2021, 789, 147910. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, M.; Zhang, B.; He, Y.; Zhong, Y. Changes of antibiotic resistance genes and bacterial communities in the advanced biological wastewater treatment system under low selective pressure of tetracycline. Water Res. 2021, 207, 117834. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, T.; Su, Z.; Chi, M.; Wang, H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental. Sci. Total Environ. 2021, 788, 147811. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Shigei, M.; Assayed, A.; Hazaymeh, A.; Dalahmeh, S.S. Pharmaceutical and Antibiotic Pollutant Levels in Wastewater and the Waters of the Zarqa River, Jordan. Appl. Sci. 2021, 11, 8638. [Google Scholar] [CrossRef]
- Imwene, K.O.; Ngumba, E.; Kairigo, P.K. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review. J. Environ. Manag. 2022, 322, 116065. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838 (Pt 1), 156010. [Google Scholar] [CrossRef]
- Masood, Z.; Ikhlaq, A.; Farooq, A.; Qi, F.; Javed, F.; Aziz, H.A. Removal of anti-biotics from veterinary pharmaceutical wastewater using combined Electroflocculation and Fe-Zn loaded zeolite 5A based catalytic ozonation process. J. Water Process. Eng. 2022, 49, 103039. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Shuai, M.; Saheed, M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, K.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Wang, J.; He, S.; Chenc, C.; Wojnárovits, L.; Takács, E. Treatment of pharmaceutical wastewater by ionizing radiation: Removal of antibiotics, antimicrobial resistance genes and antimicrobial activity. J. Hazard Mater. 2021, 415, 125724. [Google Scholar] [CrossRef]
- Lan, L.; Kong, X.; Sun, X.; Li, C.; Liu, D. High removal efficiency of antibiotic resistance genes in swine wastewater via nanofiltration and reverse osmosis processes. J. Environ. Manag. 2019, 231, 439–445. [Google Scholar] [CrossRef]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef]
- Guo, Z.; Huo, W.; Cao, T.; Liu, X.; Ren, S.; Yang, J.; Ding, H.; Chen, K.; Dong, F.; Zhang, Y. Heterojunction interface of zinc oxide and zinc sulfide promoting reactive molecules activation and carrier separation toward efficient photocatalysis. J. Colloid Interface Sci. 2021, 588, 826–837. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Studies on adsorption of oxytetracycline from aqueous solutions onto hydroxyapatite. Sci. Total Environ. 2018, 628–629, 36–43. [Google Scholar] [CrossRef]
- Sanguanpak, S.; Shongkittikul, W.; Saengam, C.; Chiemchaisri, W.; Chiemchaisri, C. TiO2-immobilized porous geopolymer composite membrane for removal of antibiotics in hospital wastewater. Chemosphere 2022, 307 (Pt 2), 135760. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Tong, L.; Gao, Y.; Zhang, H.; Zhang, Y.; Wang, Z.; Zhu, S. Non-free Fe dominated PMS activation for enhancing electro-Fenton efficiency in neutral wastewater. J. Electroanal. Chem. 2023, 928, 117062. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, S.; Yu, J.; Tang, X.; Guo, Y.; Guo, Y.; Wang, L.; Dai, S.; Zhan, W. Total Oxidation of Light Alkane over Phosphate-Modified Pt/CeO2 Catalysts. Environ. Sci. Technol. 2022, 56, 9661–9671. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; della Giustina, S.V.; Rocha, J.; Fernandes, T.; Pablos, C.; Encinas, A.; Barceló, D.; Rodríguez-Mozaz, S.; Manaia, C.M.; Marugán, J. Assessment of full-scale tertiary wastewater treatment by UV-C based-AOPs: Removal or persistence of antibiotics and antibiotic resistance genes? Sci. Total Environ. 2019, 652, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Intensification of ceftriaxone degradation under UV and solar light irradiation in presence of phosphors based structured catalyst. Chem. Eng. Process. 2019, 137, 12–21. [Google Scholar] [CrossRef]
- Kordestani, B.; Yengejeh, R.J.; Takdastan, A.; Neisi, A.K. A new study on photocatalytic degradation of meropenem and ceftriaxone antibiotics based on sulfate radicals: Influential factors, biodegradability, mineralization approach. Microchem. J. 2019, 146, 286–292. [Google Scholar] [CrossRef]
- Abramović, B.F.; Uzelac, M.M.; Armaković, S.J.; Gašić, U.; Četojević-Simin, D.D.; Armaković, S. Experimental and computational study of hydrolysis and photolysis of antibiotic ceftriaxone: Degradation kinetics, pathways, and toxicity. Sci. Total Environ. 2021, 768, 144991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, X.; Wang, Y.; Shi, H.; Liu, E.; Fan, J.; Hu, X. Degradation and removal of Ceftriaxone sodium in aquatic environment with Bi2WO6/g-C3N4 photocatalyst. J. Colloid Interface Sci. 2018, 523, 7–17. [Google Scholar] [CrossRef]
- Kurt, A.; Mert, B.K.; Özengin, N.; Sivrioğlu, Ö.; Yonar, T. Treatment of Antibiotics in Wastewater Using Advanced Oxidation Processes (AOPs). In Physico-Chemical Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Lwin, H.M.; Zhan, W.; Song, S.; Jia, F.; Zhou, J. Visible-light photocatalytic degradation pathway of tetracycline hydrochloride with cubic structured ZnO/SnO2 heterojunction nanocatalyst. Chem. Phys. Lett. 2019, 736, 136806. [Google Scholar] [CrossRef]
- de Moraes, N.P.; de Campos Sanmartin, M.B.; da Silva Rocha, R.; de Siervo, A.; de Vasconcelos Lanza, M.R.; Reddy, D.A.; Lianqing, Y.; Alvares Rodrigues, L. ZnO/CeO2/Carbon xerogel composites with direct Z-scheme heterojunctions: Enhancing the photocatalytic remediation of 4-chlorophenol under visible light. J. Rare Earths 2022, in press. [Google Scholar] [CrossRef]
- Kashmery, H.A.; El-Hout, S.I.; Zak, Z.I. Fast photocatalytic oxidation of ciprofloxacin over Co3O4@CeO2 heterojunctions under visible-light. J. Taiwan Inst. Chem. Eng. 2022, 140, 104563. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, A.A.; Jalila, A.A.; Hassan, N.S.; Aziz, F.F.A.; Azami, M.S.; Hussain, I.; Saravanan, R.; Vo, D.-V.N. A critical review on relationship of CeO2-based photocatalyst towards mechanistic degradation of organic pollutant. Chemosphere 2022, 286, 131651. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.M.; Haq, A.U.; Amin, F.; Ajaz-un-Nabi, M.; Khan, I.; Sabir, N. Synthesis and antimicrobial activities of Manganese (Mn) and iron (Fe) co-doped Cerium dioxide (CeO2) Nanoparticles. Physica B Condens. Matter. 2021, 600, 412562. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of Cerium oxide nanoparticles—A Review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, S.; Subramanian, P.; Karthick, S.A.; Subbaiya, R. The structural, optical, antimicrobial and anticancer properties of biocompatible astaxanthin coated ZnO and CeO2 nanoparticles. Mater. Lett. 2022, 312, 131669. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.; Dong, J. Immobilized ZnO based nanostructures and their environmental applications. Prog. Nat. Sci. Mater Int. 2021, 31, 821–834. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and applications of ZnO nanostructures (ZONSs): A review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 99–141. [Google Scholar] [CrossRef]
- Sescu, A.M.; Harja, M.; Favier, L.; Berthou, L.O.; Gomez de Castro, C.; Pui, A.; Lutic, D. Zn/La mixed oxides prepared by coprecipitation: Synthesis, characterization and photocatalytic studies. Materials 2020, 13, 4916. [Google Scholar] [CrossRef]
- Khankhuean, A.; Kuratsameethong, W.; Santibenchakul, S.; Laobuthee, A.; Sugimoto, M.; Srisawat, N.; Jamnongkan, T. Oriented ZnO nanoflowers obtained after calcination of electrospinning poly(vinyl alcohol)/zinc oxide/zinc acetate composite mats. S. Afr. J. Chem. Eng. 2021, 37, 179–185. [Google Scholar] [CrossRef]
- Apostolescu, N.; Cernătescu, C.; Tătaru-Fărmuș, R.E.; Cobzaru, C.; Apostolescu, G.A. Promoting Effect of Ceria on the Catalytic Activity of CeO2-ZnO Polycrystalline Materials. Environ. Eng. Manag. J. 2018, 17, 765–770. [Google Scholar]
- Vojtová, V.; Urbánek, K. Pharmacokinetics of tetracyclines and glycylcyclines. Klin. Mikrobiol. Infekc. Lek. 2009, 15, 17–21. [Google Scholar] [PubMed]
- SigmaAldrich. Available online: https://www.sigmaaldrich.com/RO/en/sds/sigma/c4881 (accessed on 11 December 2021).
- Patel, I.H.; Kaplan, S.A. Pharmacokinetic profile of ceftriaxone in man. Am. J. Med. 1984, 77, 17–25. [Google Scholar] [PubMed]
- Abbas, K.K.; AbdulkadhimAl-Ghaban, A.M.H.; Rdewi, E.H. Synthesis of a novel ZnO/TiO2-nanorod MXene heterostructured nanophotocatalyst for the removal pharmaceutical ceftriaxone sodium from aqueous solution under simulated sunlight. J. Environ. Chem. Eng. 2022, 10, 108111. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Han, L.; Li, X.; Xu, Y. Highly sensitive and selective triethylamine gas sensor based on hierarchical radial CeO2/ZnO n-n heterojunction. Sens. Actuators B Chem. 2022, 367, 132031. [Google Scholar] [CrossRef]
- Meenakshi, G.; Sivasamy, A. Enhanced photocatalytic activities of CeO2@ZnO core-shell nanostar particles through delayed electron hole recombination process. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128920. [Google Scholar] [CrossRef]
- Rosi, H.; Janci, M.; Preethi, M. Phyto-mediated green synthesis of CeO2–ZnO nanoparticles using glycosmis mauritiana leaf extract: Antibacterial activity and photodegradative applications. Mater. Today Proc. 2022, 48, 561–567. [Google Scholar] [CrossRef]
- Duarte, F.d.S.; Melo, A.L.M.d.S.; Ferro, A.d.B.; Zanta, C.L.d.P.e.S.; Duarte, J.L.d.S.; Oliveira, R.M.P.B. Magnetic Zinc Oxide/Manganese Ferrite Composite for Photodegradation of the Antibiotic Rifampicin. Materials 2022, 15, 8185. [Google Scholar] [CrossRef]
- Ma, L.; Ai, X.; Jiang, W.; Liu, P.; Chen, Y.; Lu, K.; Song, X.; Wu, X. Zn/Ce metal organic framework-derived ZnO@CeO2 nano-heterojunction for enhanced photocatalytic activity. Colloid Interface Sci. Commun. 2022, 49, 100636. [Google Scholar] [CrossRef]
- Ye, Z.; Li, J.; Zhou, M.; Wang, H.; Ma, Y.; Huo, P.; Yu, L.; Yan, Y. Well-dispersed nebula-like ZnO/CeO2@HNTs heterostructure for efficient photocatalytic degradation of tetracycline. Chem. Eng. J. 2016, 304, 917–933. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Telfah, M.; Ahmad, A.A.; Alsaad, A.M.; Qattan, I.A.; Baaziz, H.; Charifi, Z.; Telfah, A. Synthesis, Crystallography, Microstructure, Crystal Defects, Optical and Optoelectronic Properties of ZnO:CeO2 Mixed Oxide Thin Films. Photonics 2020, 7, 112. [Google Scholar] [CrossRef]
- Sizykh, M.; Batoeva, M.; Matafonova, G. Enhanced catalyst-free degradation and mineralization of ceftriaxone by UV/H2O2 and UV/S2O82− processes using KrCl excilamp (222 nm). J. Photochem. Photobiol. A Chem. 2023, 436, 114357. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.-W.; Huang, C.P.; Chuang, Y.; Nguyen, T.B.; Dong, C.D. A visible-light sensitive MoSSe nanohybrid for the photocatalytic degradation of tetracycline, oxytetracycline, and chlortetracycline. J. Colloid Interface Sci. 2022, 616, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Jácome-Acatitla, G.; Tzompantzi, F.; López-González, R.; García-Mendoza, C.; Alvaro, J.M.; Gomez, R. Photodegradation of sodium naproxen and oxytetracycline hydrochloride in aqueous medium using as photocatalysts Mg-Al calcined hydrotalcites. J. Photochem. Photobiol. A Chem. 2014, 277, 82–89. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Z.; Li, J.; Bai, B.; Jia, Y.; Wang, Q.; Cheng, H. Selective adsorption and photocatalytic degradation of chlortetracycline hydrochloride using a La2Ti2O7/acid-modified coal-bearing strata kaolinite composite. Appl. Surf. Sci. 2023, 609, 155489. [Google Scholar] [CrossRef]




| Chemical Composition and Structure | Properties |
|---|---|
| CT—Chlortetracycline hydrochloride C22H23ClN2O8‧HCl 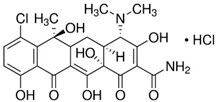 | The first antibiotic discovered and used; 515.34 g‧mol−1 Solubility: 0.5–0.6 mg‧mL−1 (20 °C) Biological half-life: 5.6–9 h Excretion mode: 60% renal, less than 10% biliary [44] Toxicology: 2.31 mg‧kg−1 (LD50, mouse, oral) [45] |
| CFTX—Ceftriaxone C18H16N8Na2O7S3·3.5H2O 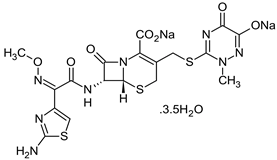 | A third-generation antibiotic of the cephalosporin family; 661.60 g‧mol−1 Melting point: 155 °C Solubility: 0.105 g·L−1 Biological half-life: 5.8–8.7 h Excretion mode: 33–67% renal, 35–45% biliary [46] |
| Antibiotic (Target Pollutant) | Catalyst Type | Irradiation Source Power Intensity, Exposure Time, Mineralization Degree | Ref |
|---|---|---|---|
| Ceftriaxone | ZnO nanospherical particles supported TiO2-nanorod MXene | Solar simulator 2000 550 W Max Lamp, 100 mW‧cm−2, 99.4% | [47] |
| Ceftriaxone C0 = 16.5–66 μM | Bi2WO6 and g-C3N4 nanosheets | KrCl excilamp, 222 nm 23 W, incident irradiance—0.74 mW‧cm−2, 60 min | [55] |
| Ceftriaxone 20–100 mg∙L−1 | Fenton-like oxidation process, persulphate activator, iron dosage—0.1–0.5 g‧L−1 + scavengers (tert-butyl alcohol and isopropanol) | 60 min, pH influence: 54.4% (pH: 5–6, Fe2+ dosage: 0.2 g‧L−1, PS concentration: 3 mM, initial antibiotic concentration: 20 mg‧L−1, UV power: 8 W: 20 °C) 95.7% (pH: 4.0, Fe2+ dosage: 0.3 g‧L−1, PS concentration: 4 Mm, UV, power: 8 W, 20 °C) | [27] |
| Ceftriaxone | Bi2WO6/g-C3N4 | 300 W Xe lamp, 120 min, 94.5% | [29] |
| Tetracycline, Oxytetracycline, Chlortetracycline | MoSSe nanohybrids | 60 min 48.6% for TC 51.1% for OTC 56.5% for CTC | [56] |
| Oxytetracycline | MgAl calcined hydrotalcites | Pen Ray Power Supply 2.16 W MgAl-2.0, 59.32% for 5 h MgAl-2.5, 65.82% for 5 h MgAl-3, 63.87% for 5 h | [57] |
| Tetracycline | CeO2-ZnO hetero photocatalyst | 300 W Xenon lamp, 60 min, 87.25% | [53] |
| Tetracycline | La2Ti2O7/AK—acid-modified coal-bearing strata kaolinite | 300 W Xenon lamp La2Ti2O7, 60 min, 57.11% La2Ti2O7/CK, 60 min, 83.07% La2Ti2O7/AK, 60 min, 88.61% | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolescu, N.; Tataru Farmus, R.E.; Harja, M.; Vizitiu, M.A.; Cernatescu, C.; Cobzaru, C.; Apostolescu, G.A. Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction. Materials 2023, 16, 850. https://doi.org/10.3390/ma16020850
Apostolescu N, Tataru Farmus RE, Harja M, Vizitiu MA, Cernatescu C, Cobzaru C, Apostolescu GA. Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction. Materials. 2023; 16(2):850. https://doi.org/10.3390/ma16020850
Chicago/Turabian StyleApostolescu, Nicolae, Ramona Elena Tataru Farmus, Maria Harja, Mihaela Aurelia Vizitiu, Corina Cernatescu, Claudia Cobzaru, and Gabriela Antoaneta Apostolescu. 2023. "Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction" Materials 16, no. 2: 850. https://doi.org/10.3390/ma16020850
APA StyleApostolescu, N., Tataru Farmus, R. E., Harja, M., Vizitiu, M. A., Cernatescu, C., Cobzaru, C., & Apostolescu, G. A. (2023). Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction. Materials, 16(2), 850. https://doi.org/10.3390/ma16020850






