Hydro- and Icephobic Properties and Durability of Epoxy Gelcoat Modified with Double-Functionalized Polysiloxanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Matrix Material
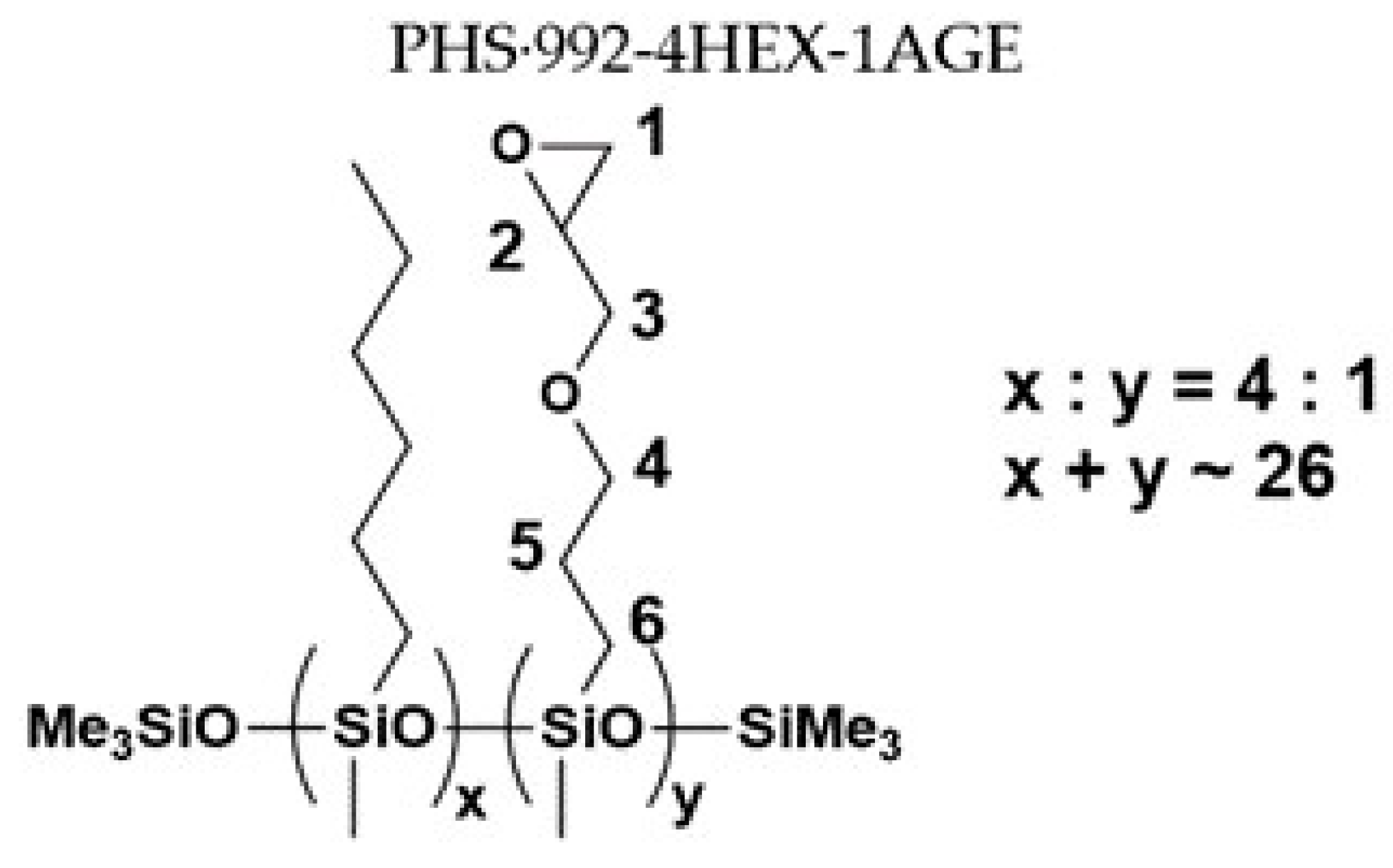
2.2. Synthesis of Chemical Modifiers
2.3. Analysis of Chemical Modifiers
2.4. Preparation of Samples
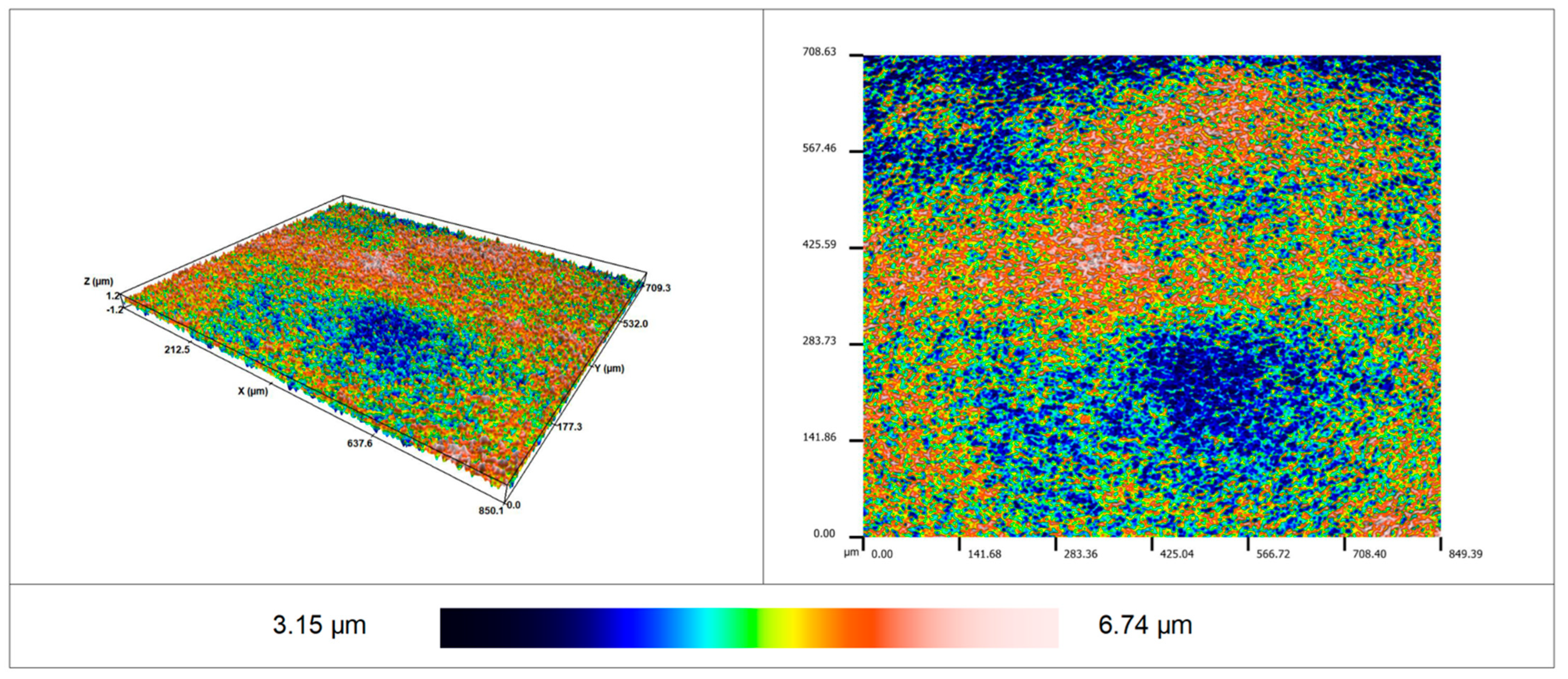
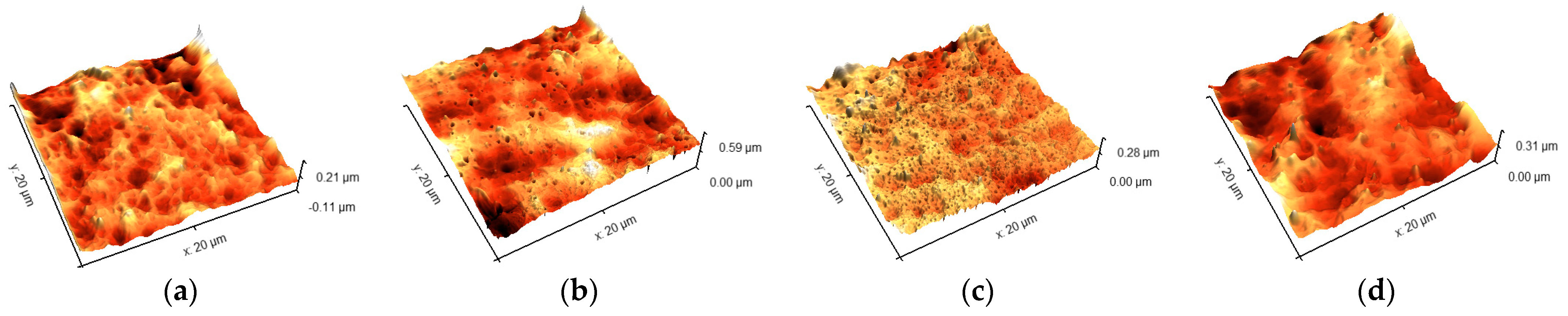
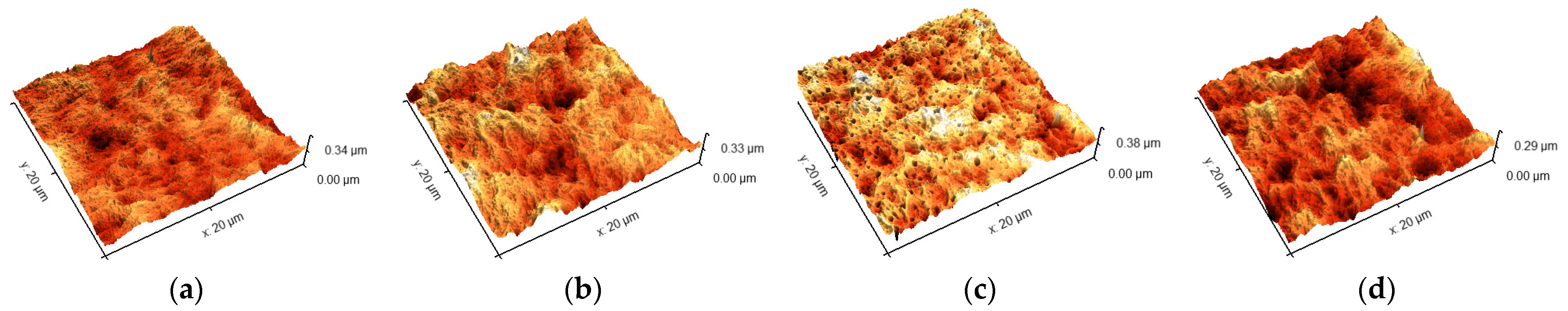
2.5. Determination of Roughness
2.6. Hydrophobicity Measurements
2.7. Ice Adhesion Measurements
2.8. Climate Aging Test
2.9. Gloss and Color Measurements
- 20° geometry for high-gloss surfaces;
- 60° geometry for semi-gloss surfaces;
- 85° geometry for matt surfaces.
3. Results
3.1. Characterization of Polysiloxane Derivatives
3.2. Roughness of Surface
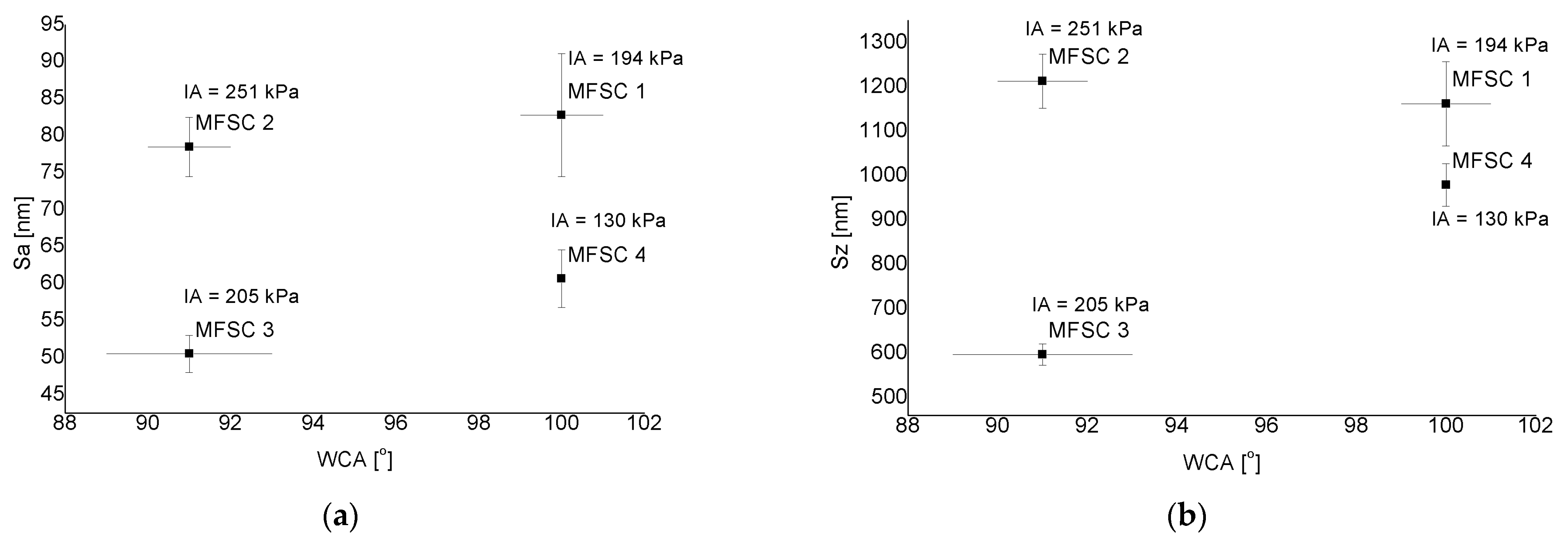
3.3. Wetting Properties
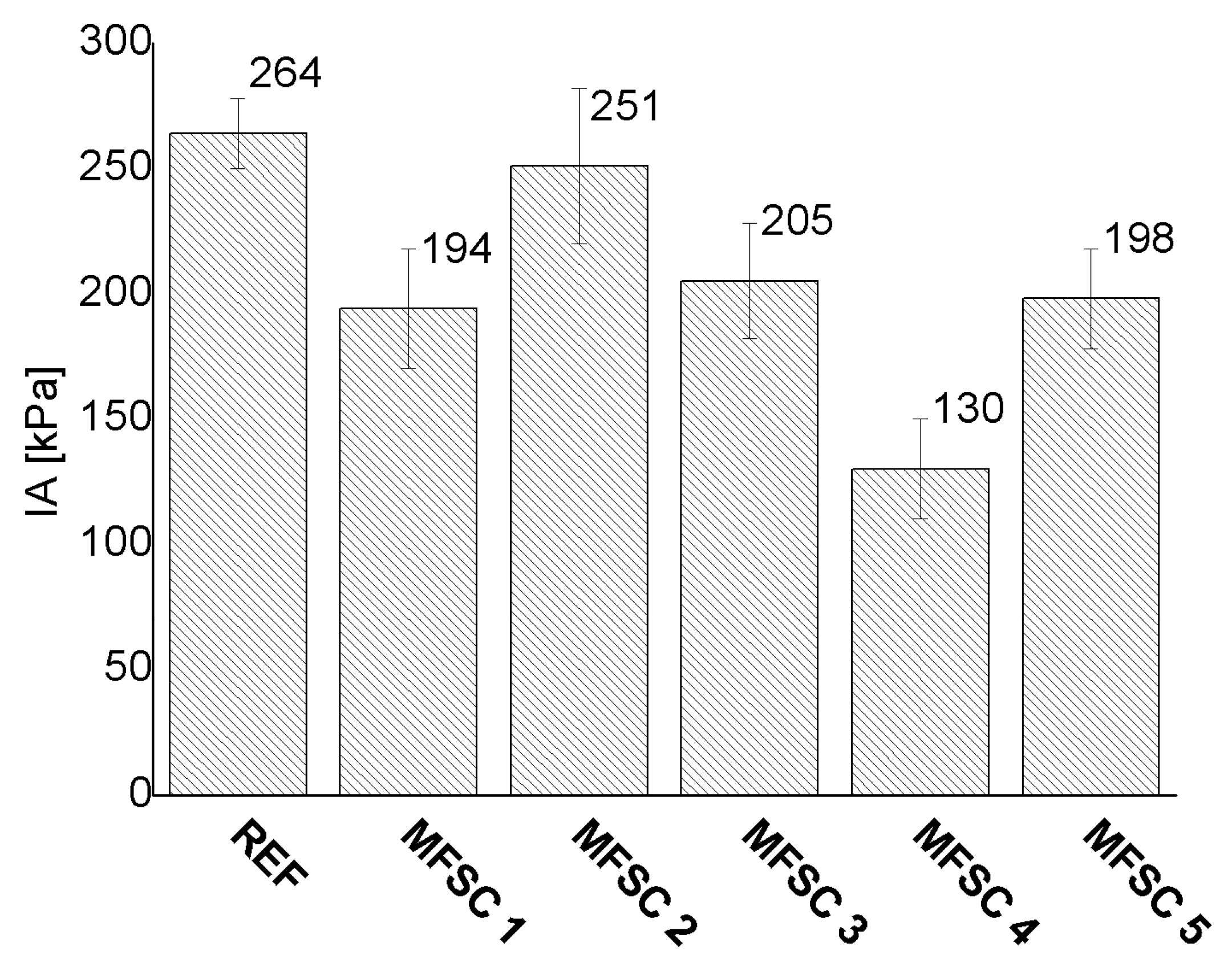
3.4. Ice Adhesion Strength
3.5. Durability of Surfaces
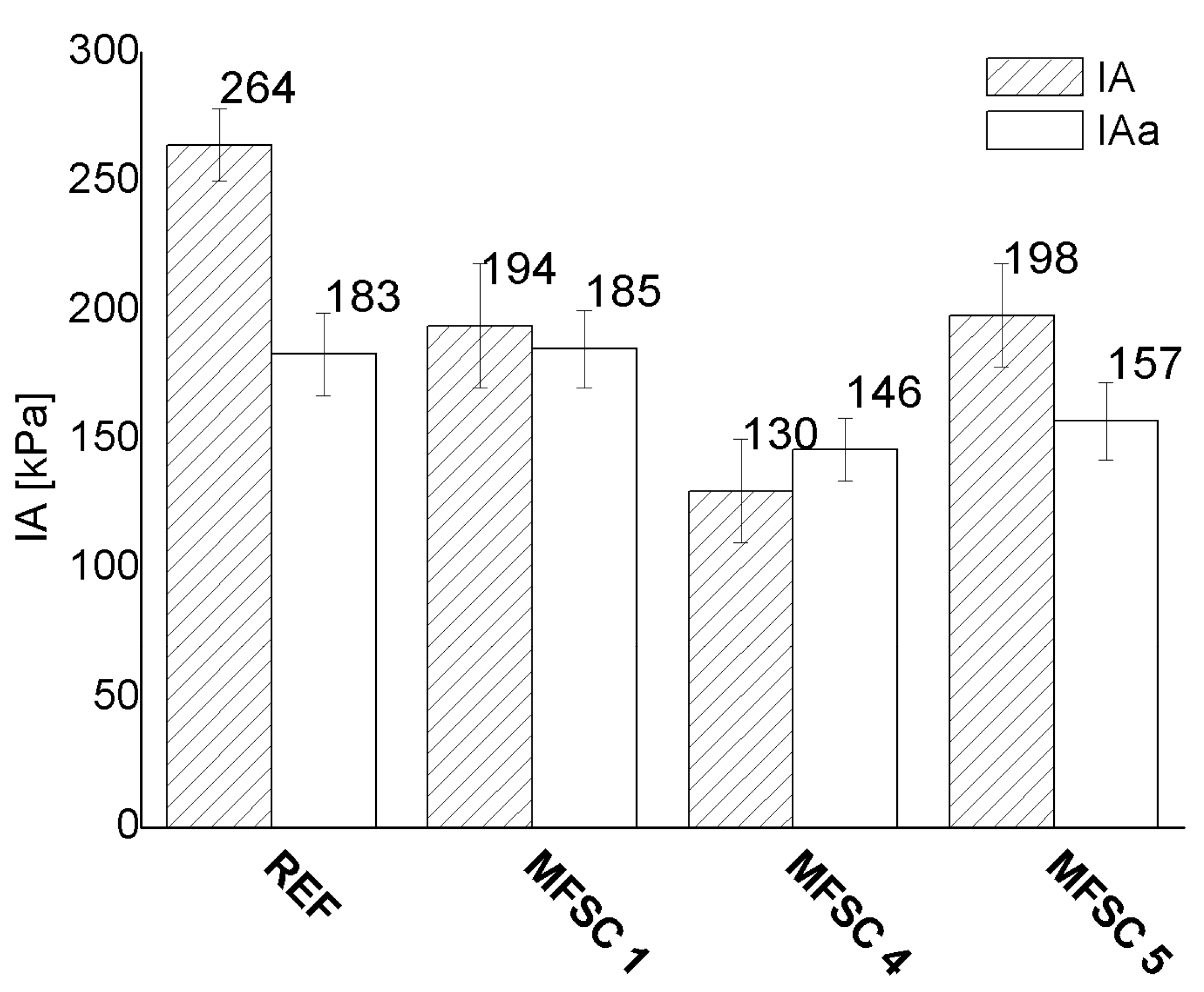
3.5.1. Ice Adhesion Strength
3.5.2. Wettability
3.5.3. Roughness
3.5.4. Gloss and Color
4. Discussion
4.1. Roughness
4.2. Wettability
4.3. Ice Adhesion Strength
4.4. Ice Adhesion/Wettability/Roughness
4.5. Durability
4.5.1. Ice Adhesion Strength
4.5.2. Wettability
4.5.3. Roughness
5. Conclusions
- The highest surface roughness of the epoxy gelcoat after chemical modification was obtained for samples modified with polysiloxanes with a PHS991 core functionalized with AGE, HEX and AGE, OCT functional groups, respectively, with the same molar ratios. It is likely that the additive core and the presence of the AGE group significantly increased the roughness of these samples, even though all of them were produced within the same methodology. In general, the roughness of samples with modified polysiloxanes was increased with higher content of HEX functional groups.
- The sample modified with the polysiloxane functionalized with AGE and OCT groups with a molar ratio of 1:2 recorded the best icephobic properties. Moreover, this sample was one of two that obtained the highest WCA value. The other samples achieved a significantly lower reduction in IA values compared to the reference sample. It can also be concluded that the PHS992 core improves the icephobic properties more effectively compared to the PHS991 core.
- The following correlation was observed: as the value of the water contact angle increases, the value of ice adhesion decreases. The conducted studies corroborated the well-established assumption that ice adhesion closely depends on the surface roughness, which can lead to the formation of a mechanical blockage between the ice and the substrate.
- The highest stability of surface roughness after aging was observed for the sample modified with the polysiloxane with a PHS991 core with AGE:HEX functional groups in a 1:2 molar ratio. No noticeable color or visible gloss changes were observed.
- Cycles of icing and deicing caused a decrease in the value of the water contact angle. However, the chemical surface modification enabled the retention of its hydrophobicity after aging, despite a decrease in WCA.
- Exposure in the aging chamber did not increase ice adhesion, i.e., deterioration of the icephobic properties, while, in some cases, e.g., the reference sample, which was not chemically modified, we observed a decrease in IA after a decrease in WCA. For the rest of the samples, despite the deterioration of the WCA, the IA remained without significant changes, thus indicating the positive influence of modification on the surface stability and the possible influence of a low WCA as the reason for the IA decrease in the reference sample.
Author Contributions
Funding
Conflicts of Interest
References
- Kraj, A.G.; Bibeau, E.L. Phases of icing on wind turbine blades characterized by ice accumulation. Renew Energ. 2010, 35, 966–972. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Irajizad, P.; Nazifi, S.; Ghasemi, H. Icephobic surfaces: Definition and figures of merit. Adv. Colloid Interface Sci. 2019, 269, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Menini, R.; Farzaneh, M. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xue, J.; Wang, Q.; Chen, Q.; Ding, J. Verification of icephobic/anti-icing properties of a superhydrophobic surface. ACS Appl. Mater. Interfaces 2013, 5, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Golovin, K.; Kobaku, S.P.R.; Lee, D.H.; Diloreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [Green Version]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. The ice repellency of plasma polymerized hexamethyldisiloxane coating. Appl. Surf. Sci. 2013, 284, 459–463. [Google Scholar] [CrossRef]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. Robust icephobic, and anticorrosive plasma polymer coating. Cold Reg. Sci. Technol. 2018, 151, 89–93. [Google Scholar] [CrossRef]
- Qing, Y.; Long, C.; An, K.; Liu, C. Natural rosin-grafted nanoparticles for extremely-robust and eco-friendly antifouling coating with controllable liquid transport. Compos. Part B 2022, 236, 109797. [Google Scholar] [CrossRef]
- Xu, X.; Qing, Y.; Liu, N.; Long, C.; Ma, J.; Cui, M.; Yao, Y.; Yao, W.; Liu, C. Microskeleton Magnetic Nanofiller Composite with Highly Reliable Superhydrophobic Protection for Long-Lived Electromagnetic Interface Shielding. ACS Appl. Mater. Interfaces 2022, 14, 37039–37050. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tepylo, N.; Pommier-Budinger, V.; Budinger, M.; Bonaccurso, E.; Bennani, P.V.L. A survey of icephobic coatings and their potential use in a hybrid coating/active ice protection system for aerospace applications. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef] [Green Version]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings-screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Xia, Q.; Zhu, L.; Xue, J.; Wang, Q.; Chen, Q. Research on the icephobic properties of fluoropolymer-based materials. Appl. Surf. Sci. 2011, 257, 4956–4962. [Google Scholar] [CrossRef]
- Wong, S.T.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.J.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Volman, V.; Zhu, Y.; Raji, A.O.; Genorio, B.; Lu, W.; Xiang, C.; Kittrell, C.; Tour, J.M. Radio-frequency-transparent, electrically conductive graphene nanoribbon thin film as deicing heating layers. Appl. Mater. Interfaces 2014, 6, 298–304. [Google Scholar] [CrossRef]
- Laforte, C.; Blackburn, C.; Perron, J.; Aubert, R.J. Icephobic coating evaluation for aerospace applications. In Proceedings of the 55th AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics & Materials Conference, National, National Harbor, MD, USA, 13–17 January 2014. [Google Scholar]
- Palacios, J.; Wolfe, D.; Bailey, M.; Szefi, J. Ice testing of a centrifugally powered pneumatic deicing system for helicopter rotor blades. J. Am. Helicopter Soc. 2015, 60, 1–12. [Google Scholar] [CrossRef]
- Arnaldo Del Cerro, D.; Romer, G.R.B.E.; Huis in’t Veld, A.J. Erosion resistant anti-icing surfaces generated by ultra short laser pulses. Phys. Procedia 2010, 5, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xia, H.; Kim, E.; Sun, H.-B. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217–11231. [Google Scholar] [CrossRef]
- Dotan, A.; Dodiuk, H.; Laforte, C.; Kenig, S. The relationship between water wetting and ice adhesion. J. Adhes. Sci. Technol. 2009, 23, 1907–1915. [Google Scholar] [CrossRef]
- Rios, P.F.; Dodiuk, H.; Kenig, S.; McCarty, S.; Dotan, A. The effects of nanostructure and composition on the hydrophobic properties of solid surfaces. J. Adhes. Sci. Technol. 2006, 20, 563–587. [Google Scholar] [CrossRef]
- Shiu, J.Y.; Kuo, C.W.; Chen, P.; Mou, C.Y. Fabrication of Tunable Superhydrophobic Surfaces by Nanosphere Lithography. Chem. Mater. 2004, 16, 561–564. [Google Scholar] [CrossRef]
- Pozzato, A.; Zilio, S.D.; Fois, G.; Vendramin, D.; Mistura, G.; Belotti, M.; Chen, Y.; Natali, M. Superhydrophobic surfaces fabricated by nanoimprint lithography. Microelectron. Eng. 2006, 83, 884–888. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper, and zinc substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Zhu, L.; Hess, D.W.; Wong, C.P. Hierarchical Silicon Etched Structures for Controlled Hydrophobicity/Superhydrophobicity. Nano Lett. 2007, 7, 3388–3393. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- Hozumi, A.; Takai, O. Preparation of ultra water-repellent films by microwave plasma-enhanced CVD. Thin Solid Film. 1997, 303, 222–225. [Google Scholar] [CrossRef]
- Crick, C.R.; Parkin, I.P. CVD of copper and copper oxide thin films via the in situ reduction of copper(II) nitrate—A route to conformal superhydrophobic coatings. J. Mater. Chem. 2011, 21, 14712–14716. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are Superhydrophobic Surfaces Best for Icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, G.; Tian, Q. Effects of nano-fluorocarbon coating on icing. Appl. Surf. Sci. 2012, 258, 7219–7224. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, M.; Yi, S.; Liu, X.; Li, H.; Huang, C.; Luo, Y.; Li, Y. Preparation and characterization of silica/fluorinated acrylate copolymers hybrid films and the investigation of their icephobicity. Thin Solid Films 2012, 520, 5644–5651. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Hu, J.; Shu, L.; Shi, X. Anti-icing performance of a superhydrophobic PDMS/modified nano-silica hybrid coating for insulators. J. Adhes. Sci. Technol. 2012, 26, 665–679. [Google Scholar] [CrossRef]
- Kozera, R.; Przybyszewski, B.; Żołyńska, K.; Boczkowska, A.; Sztorch, B.; Przekop, R.E. Hybrid Modification of Unsaturated Polyester Resins to Obtain Hydro- and Icephobic Properties. Processes 2020, 8, 1635. [Google Scholar] [CrossRef]
- Nakano, T.; Okamoto, Y. Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hill, R.G. Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Yilgor, E.; Yilgor, I. Hydrogen Bonding: A Critical Parameter in Designing Silicone Copolymers. Polymer 2001, 42, 7953–7959. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Liu, M.; Peng, C.; Wu, Z. Epoxy-functionalized polysiloxane reinforced epoxy resin for cryogenic application. J. Appl. Polym. Sci. 2018, 136, 46930. [Google Scholar] [CrossRef]
- Tong, J.; Bai, R.; Zou, Y.; Pan, C.; Ichimura, S.J. Flexibility improvement of epoxy resin by using polysiloxanes and their derivatives. Appl. Polymer Sci. 1994, 52, 1373–1381. [Google Scholar] [CrossRef]
- Cheng, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Deng, X.; Deng, S.; Cai, X. The investigation of methyl phenyl silicone res-in/epoxy resin using epoxy-polysiloxane as compatibilizer. J. Therm. Anal. Calorim. 2014, 118, 247–254. [Google Scholar] [CrossRef]
- Kozera, R.; Przybyszewski, B.; Krawczyk, Z.D.; Boczkowska, A.; Sztorch, B.; Przekop, R.E.; Barbucha, R.; Tański, M.; Garcia-Casas, X.; Borras, A. Hydrophobic and Anti-Icing Behavior of UV-Laser-Treated Polyester Resin-Based Gelcoats. Processes 2020, 8, 1642. [Google Scholar] [CrossRef]
- Bessonov, I.V.; Kopitsyna, M.N. New Prepregs Based on Unsaturated Polyester Resins Modified with Polysiloxane Oligomers. Int. Polym. Sci. Technol. 2016, 43, 49–56. [Google Scholar] [CrossRef]
- Rosa, V.M.; Felisberti, M.I. Unsaturated polyester resin modified with poly(organosiloxanes). I. Preparation, dynamic mechanical properties, and impact resistance. J. Appl. Polymer Sci. 2001, 81, 3272–3279. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Amirfazli, A.; He, J.; Zhang, Z. Polysiloxane as icephobic materials—The past, present and the future. Chem. Eng. J. 2020, 405, 127088. [Google Scholar] [CrossRef]
- Raraty, L.E.; Tabor, D. The adhesion and strength properties of ice. Proc. Royal Soc. A Math. Phys. Eng. Sci. 1958, 245, 184–201. [Google Scholar]
- Tesoro, G.; Wu, Y. Silane coupling agents: The role of the organofunctional group. J. Adhes. Sci. Technol. 1991, 5, 771–784. [Google Scholar] [CrossRef]
- Marciniec, B. Hydrosilylation: A Comprehensive Review on Recent Advances; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zheng, Q.; Lv, J.; Zhang, J.; Feng, J. Fabrication and application of icephobic silicone coatings on epoxy substrate. Prog. Org. Coatings 2021, 161, 106483. [Google Scholar] [CrossRef]
- Wang, Y.; Hansen, C.J.; Wu, C.; Robinette, E.J.; Peterson, A.M. Effect of surface wettability on the interfacial adhesion of a thermosetting elastomer on glass. RSC Adv. 2021, 11, 31142–31151. [Google Scholar] [CrossRef]
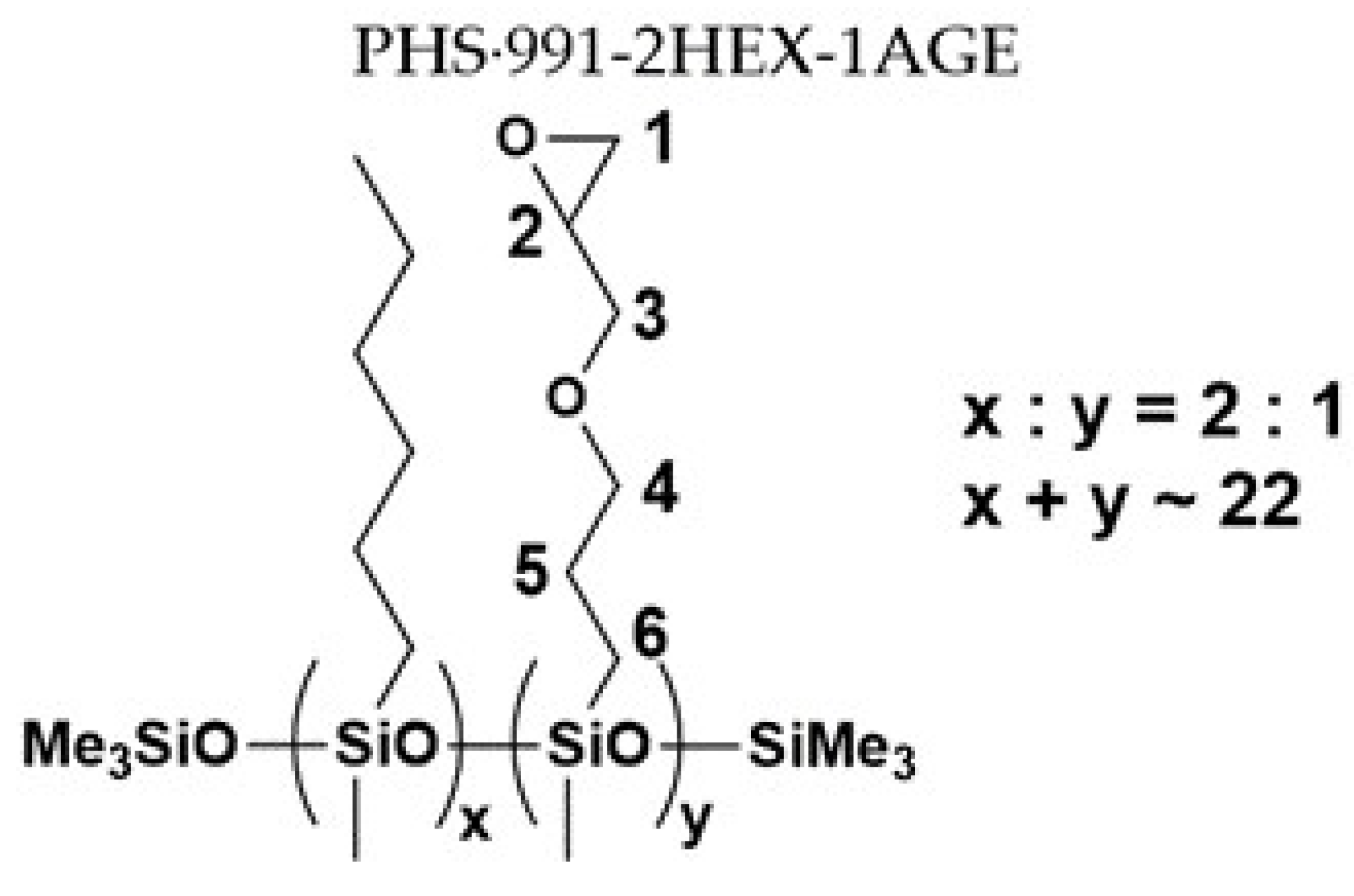
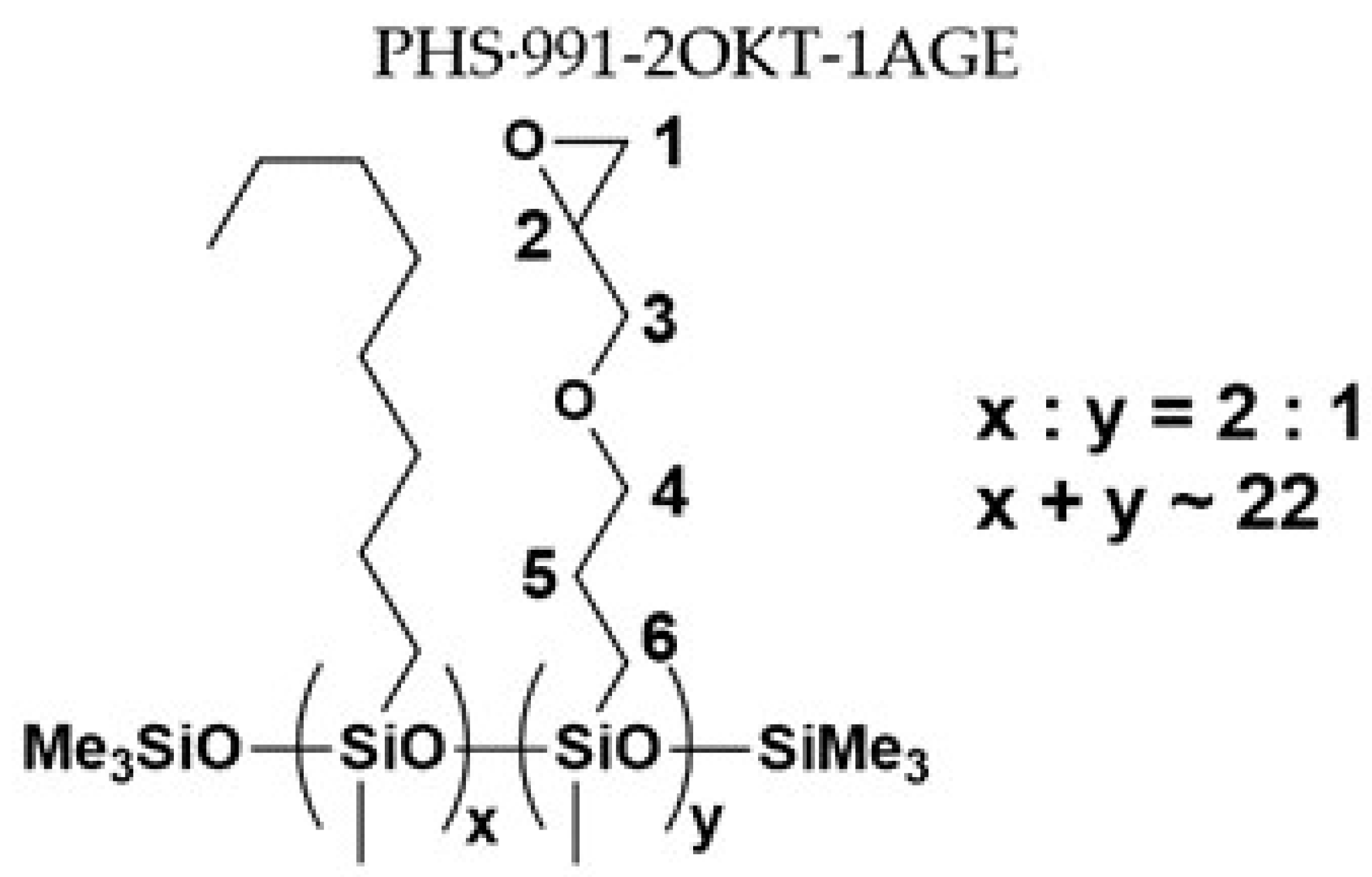
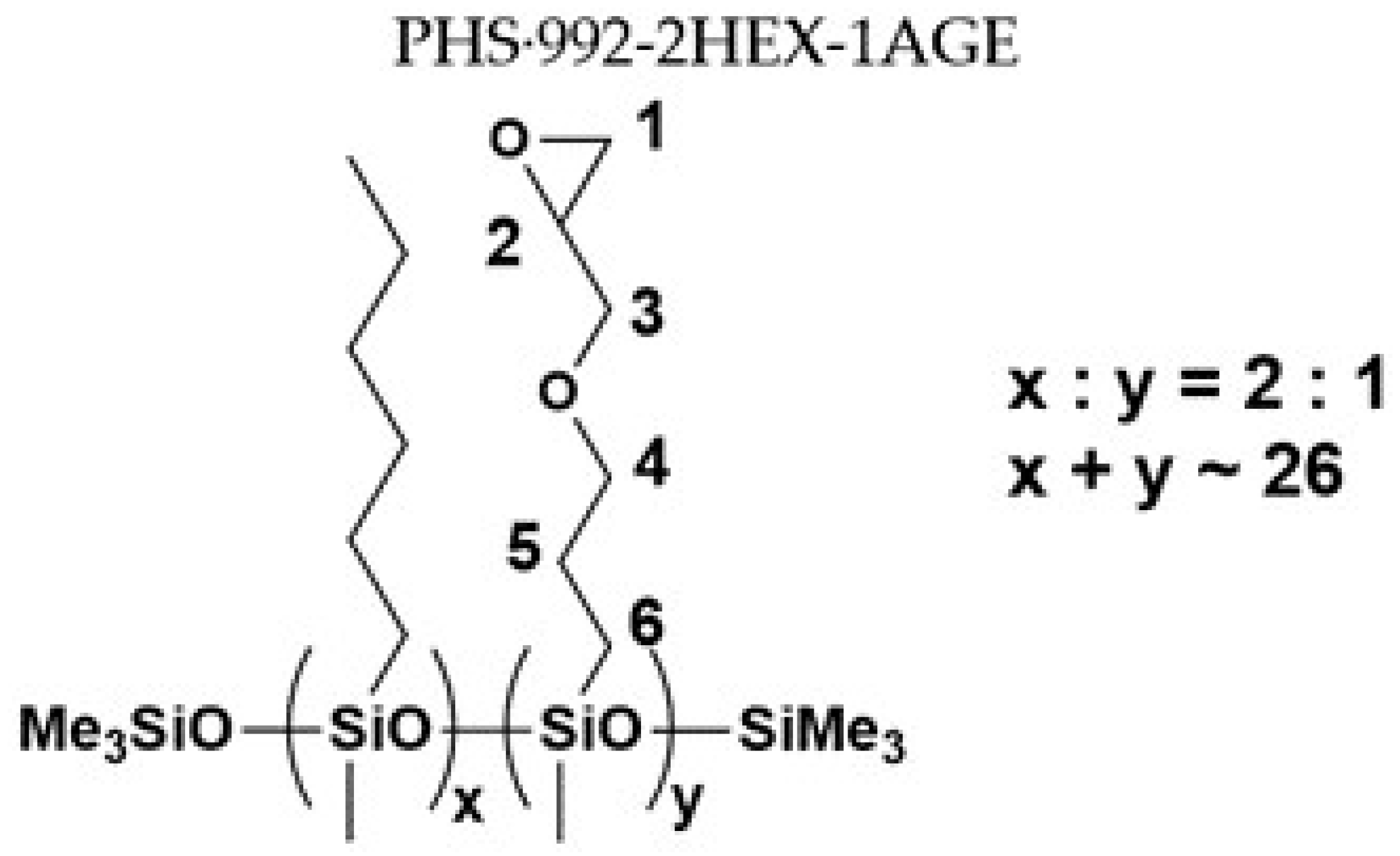
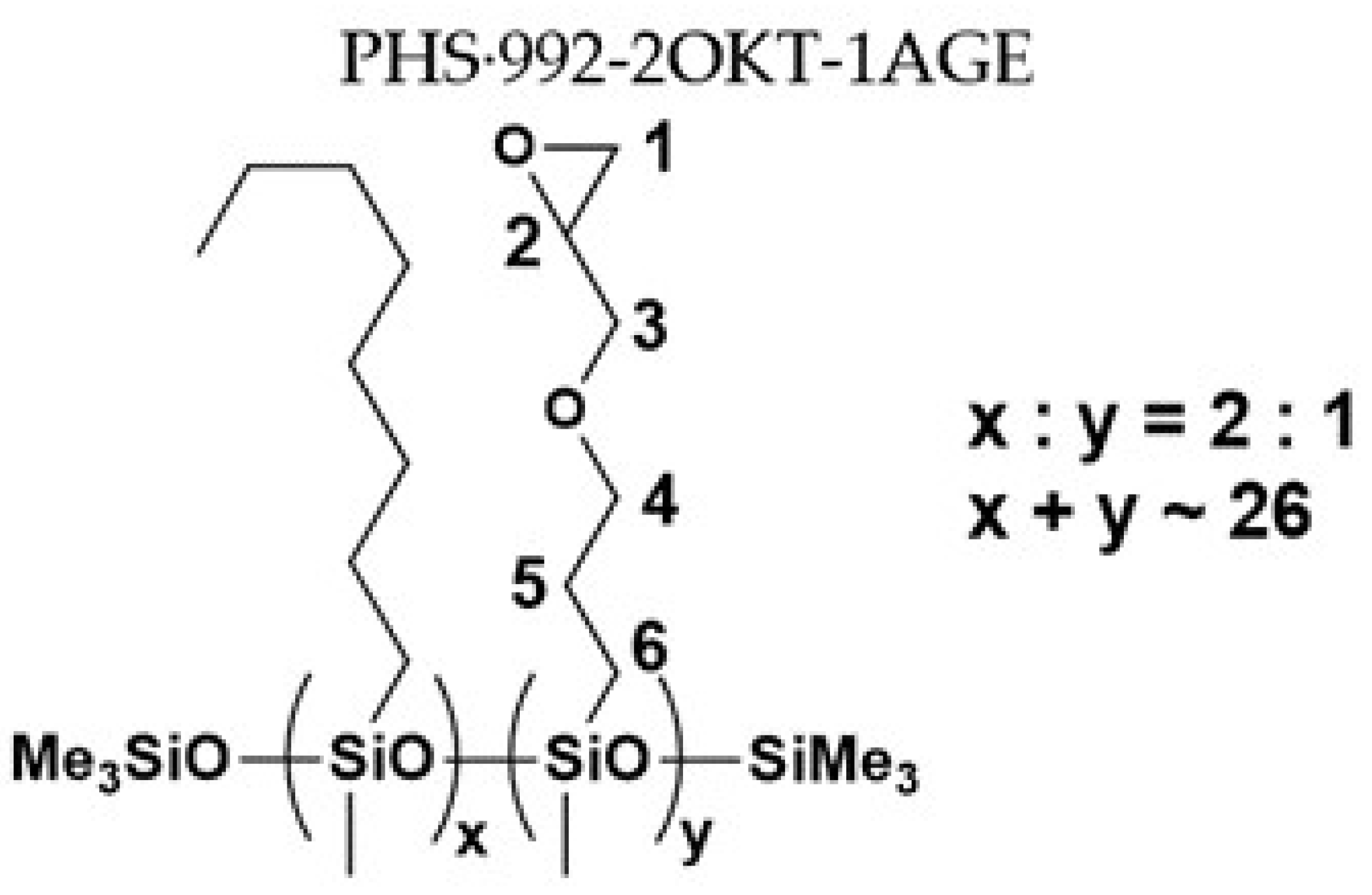


| Sample No. | MFSC Type | PHS | Olefin 1 | Olefin 2 | Molar Ratio |
|---|---|---|---|---|---|
| 1 (REF) | - | - | - | - | - |
| 2 | MFSC 1/2 wt.% | PHS991 | AGE | HEX | 1:2 |
| 3 | MFSC 2/2 wt.% | PHS991 | AGE | OCT | 1:2 |
| 4 | MFSC 3/2 wt.% | PHS992 | AGE | HEX | 1:2 |
| 5 | MFSC 4/2 wt.% | PHS992 | AGE | OCT | 1:2 |
| 6 | MFSC 5/2 wt.% | PHS992 | AGE | HEX | 1:4 |
| Sample No. | MFSC Type | Sa (nm) | ±Sa (nm) | Sz (nm) | ±Sz (nm) | WCA (°) | ±WCA (o) | CAH (°) |
|---|---|---|---|---|---|---|---|---|
| 1 (REF) | - | 55.43 | 5.46 | 1048 | 55 | 88 | 1 | 31 |
| 2 | MFSC 1/2 wt.% | 82.77 | 8.31 | 1162 | 95 | 100 | 1 | 31 |
| 3 | MFSC 2/2 wt.% | 78.49 | 4.00 | 1213 | 61 | 91 | 1 | 34 |
| 4 | MFSC 3/2 wt.% | 50.52 | 2.50 | 597 | 24 | 91 | 2 | 32 |
| 5 | MFSC 4/2 wt.% | 60.68 | 3.89 | 979 | 48 | 100 | 0 | 28 |
| 6 | MFSC 5/2 wt.% | 62.42 | 3.12 | 840 | 38 | 96 | 1 | 38 |
| Sample No. | MFSC Type | Ra (nm) | ±Ra (nm) | Raa (nm) | ±Raa (nm) | RSm (nm) | ±RSm (nm) | RSma (nm) | ±RSma (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 (REF) | - | 33.38 | 5.41 | 25.15 | 2.06 | 43.40 | 6.92 | 32.02 | 2.73 |
| 2 | MFSC 1/2 wt.% | 39.96 | 5.73 | 39.83 | 8.40 | 51.16 | 6.98 | 49.90 | 10.76 |
| 5 | MFSC 4/2 wt.% | 25.21 | 2.65 | 31.54 | 6.97 | 33.20 | 3.35 | 42.25 | 10.07 |
| 6 | MFSC 5/2 wt.% | 41.99 | 3.06 | 35.54 | 4.41 | 55.28 | 4.00 | 44.87 | 4.63 |
| Sample No. | MFSC Type | Before | After |
|---|---|---|---|
| 1 (REF) | - | 90.3 | 86.6 |
| 2 | MFSC 1/2 wt.% | 81.0 | 78.6 |
| 5 | MFSC 4/2 wt.% | 88.1 | 79.8 |
| 6 | MFSC 5/2 wt.% | 83.0 | 75.6 |
| Sample No. | MFSC Type | Color before Aging | Color after Aging | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Δa * | Δb ** | ΔL *** | ΔE | Δa * | Δb ** | ΔL *** | ΔE | ||
| 1 (REF) | - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | MFSC 1/2 wt.% | 0.74 | −0.18 | −0.07 | 0.76 | −0.53 | 0.42 | −0.04 | 0.68 |
| 5 | MFSC 4/2 wt.% | −0.30 | 0.36 | −0.03 | 0.47 | 0.05 | 0.2 | −0.58 | 0.62 |
| 6 | MFSC 5/2 wt.% | 1.62 | −0.35 | −1.10 | 1.99 | −0.76 | 0.61 | −0.26 | 1.01 |
| Delta E | Perception |
|---|---|
| 0 < ΔE < 1 | Not perceptible by the human eye |
| 1 < ΔE < 2 | Perceptible through close observation |
| 2 < ΔE < 3.5 | Average color deviations, recognizable by an inexperienced observer |
| 3.5 < ΔE < 5 | Significant color deviations |
| ΔE > 5 | Noticeable at a glance |
| Sample No. | MFSC Type | Color after Aging | |||
|---|---|---|---|---|---|
| * | ** | *** | |||
| 1 (REF) | - | 0.93 | −0.22 | −1.37 | 1.67 |
| 2 | MFSC 1/2 wt.% | −0.34 | 0.38 | −1.34 | 1.43 |
| 5 | MFSC 4/2 wt.% | 1.28 | −0.38 | −1.92 | 2.34 |
| 6 | MFSC 5/2 wt.% | −1.45 | 0.74 | −0.53 | 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętkowska, K.; Kozera, R.; Przybyszewski, B.; Boczkowska, A.; Sztorch, B.; Pakuła, D.; Marciniec, B.; Przekop, R.E. Hydro- and Icephobic Properties and Durability of Epoxy Gelcoat Modified with Double-Functionalized Polysiloxanes. Materials 2023, 16, 875. https://doi.org/10.3390/ma16020875
Ziętkowska K, Kozera R, Przybyszewski B, Boczkowska A, Sztorch B, Pakuła D, Marciniec B, Przekop RE. Hydro- and Icephobic Properties and Durability of Epoxy Gelcoat Modified with Double-Functionalized Polysiloxanes. Materials. 2023; 16(2):875. https://doi.org/10.3390/ma16020875
Chicago/Turabian StyleZiętkowska, Katarzyna, Rafał Kozera, Bartłomiej Przybyszewski, Anna Boczkowska, Bogna Sztorch, Daria Pakuła, Bogdan Marciniec, and Robert Edward Przekop. 2023. "Hydro- and Icephobic Properties and Durability of Epoxy Gelcoat Modified with Double-Functionalized Polysiloxanes" Materials 16, no. 2: 875. https://doi.org/10.3390/ma16020875
APA StyleZiętkowska, K., Kozera, R., Przybyszewski, B., Boczkowska, A., Sztorch, B., Pakuła, D., Marciniec, B., & Przekop, R. E. (2023). Hydro- and Icephobic Properties and Durability of Epoxy Gelcoat Modified with Double-Functionalized Polysiloxanes. Materials, 16(2), 875. https://doi.org/10.3390/ma16020875










